Abstract
This study identified, on the integrative level, two components of the ANG II signaling pathway that lay downstream from the ANG II type 1 (AT1) receptor and are critically involved in maintaining vascular relaxation in cerebral resistance arteries. In these experiments, the relaxation of isolated middle cerebral arteries (MCA) in response to ACh (10−9-10−5 M), iloprost (10−16-10−11 g/ml), and reduced PO2 was lost and the ratio of phospho-ERK/ERK1/2 was significantly reduced in aortas of male Sprague-Dawley rats fed a high-salt (HS; 4% NaCl) diet to suppress plasma ANG II levels. In salt-fed rats, relaxation of MCA in response to these vasodilator stimuli was restored by chronic (3 days) intravenous infusion of either ANG II (5 ng·kg−1·min−1) or epidermal growth factor (EGF; 2 μg/h). The protective effect of ANG II infusion to restore vascular relaxation was eliminated by coinfusion of either the EGF receptor kinase inhibitor AG-1478 (20 μg/h), the ERK1/2 inhibitor PD-98059 (10 μg/h), or the protein synthesis inhibitor cycloheximide (5 μg/h). In rats fed a low-salt (0.4% NaCl) diet, MCA relaxation in response to ACh, reduced PO2, and iloprost was eliminated by intravenous infusion of AG-1478, PD-98059, or cycloheximide. In ANG II-infused rats fed HS diet, and in rats fed LS diet, vasodilator responses to reduced PO2 and iloprost were unaffected by the p38 MAP kinase inhibitor SB-203580 and the phosphatidylinositol 3-kinase inhibitor wortmannin. These findings indicate that maintenance of normal vascular relaxation mechanisms by ANG II in rat MCA requires activation of the EGF receptor kinase and ERK1/2.
Keywords: hypertension, salt, cell signaling, oxidative stress, renin-angiotensin system, epidermal growth factor, extracellular signal-regulated kinase
elevated dietary salt intake adversely affects vascular structure and function in several circulatory beds (4; 17; 21–23; 29; 37; 38). Vascular relaxation in response to several endothelium-dependent and -independent vasodilator stimuli is eliminated or dramatically attenuated in middle cerebral arteries (MCA) (23) and other vascular beds (21; 22; 37; 38) of rats fed a high-salt (HS) diet. This loss of vascular relaxation is due to salt-induced suppression of ANG II, because vasodilator responses can be completely restored when the salt-fed rats receive a chronic intravenous infusion of a low (subpressor) dose of ANG II for 3 days to restore plasma ANG II levels to the normal physiological range (37; 38; 42).
The beneficial effect of physiological levels of ANG II to restore vascular relaxation in rats fed HS diet is mediated via the ANG II type 1 (AT1) receptor subtype, since it can be blocked by losartan (38). In addition, chronic AT1 receptor blockade with losartan attenuates vasodilator responses in MCA (31) and skeletal muscle resistance arteries (30) of animals maintained on a normal salt diet. These findings indicate that tonic activation of angiotensin AT1 receptors is necessary to maintain vascular relaxation under normal physiological conditions and to restore vascular function that is lost during elevated dietary salt intake.
AT1 receptor activation requires transactivation of the epidermal growth factor (EGF) receptor to stimulate protein synthesis (8; 35). In many systems, the ERK1/2 pathway (41) plays an important role in protein synthesis following EGF receptor transactivation. This pathway could be a mediator of the ANG II-dependent maintenance and restoration of vascular function, since recent findings indicate that HS diet reduces the expression of at least one key antioxidant enzyme (Cu/Zn SOD) in a manner that can be reversed by low-dose ANG II infusion (24).
The downstream signaling components necessary for ANG II to maintain vascular relaxation via AT1 receptor activation have yet to be determined. Moreover, although many studies have investigated AT1 receptor signaling in vitro, there are no in vivo studies investigating the role of specific signaling pathways in mediating the action of ANG II to maintain normal vascular relaxation. The goal of the present study was to evaluate, on the integrative level, the possible role of the ERK1/2 pathway versus other signal transduction pathways [phosphatidylinositol 3-kinase (PI3K) and p38 MAP kinase] as a potential downstream mediator responsible for the restoration of vascular relaxation by low-dose ANG II infusion in Sprague-Dawley rats fed HS diet. The study tested two hypotheses. The first was that transactivation of the EGF receptor plays a crucial role in the protective effect of ANG II to restore vascular relaxation in normotensive rats fed HS diet. The second was that the ERK1/2 protein kinase pathway acts downstream from the EGF receptor to mediate the restoration of vascular relaxation in cerebral resistance arteries of salt-fed rats receiving low-dose ANG II infusion and to maintain vascular responses to vasodilator stimuli in rats maintained on low-salt (LS) diet.
MATERIALS AND METHODS
Experimental animals.
Age-matched (8–10 weeks old) male Sprague-Dawley rats (Harlan Teklad, Madison, WI) were used for all studies (Table 1), and all experiments were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. Rats receiving an intravenous infusion of peptides (ANG II, EGF), dimethylsulfoxide (DMSO) vehicle, or pharmacological inhibitors (AG-1478, PD-98059, wortmannin, SB-203580, or cycloheximide) were anesthetized with an injection of a 70:30 mixture of ketamine:acepromazine (1 mg/kg im). A catheter (microrenathane PE10; Braintree Scientific, Braintree, MA) was inserted into a femoral vein under aseptic conditions, tunneled subcutaneously to the midscapular region, externalized, and attached to a swivel to allow free movement of the conscious animal during drug infusion. An infusion of sterile isotonic saline (0.5 ml/h) was begun after surgery to maintain catheter patency, and the animals were fed a LS diet and allowed a 3-day recovery period. Following this recovery period, one of the diet and infusion protocols described below was begun.
Table 1.
Body weight, mean arterial pressure, resting tone, and maximum vessel diameter for each experimental group
| Treatment | n | Body Weight, g | Mean Arterial Pressure, mmHg | Maximum Diameter, μm | Resting Tone, % |
|---|---|---|---|---|---|
| HS | 8 | 300±5.4* | 122±3.0 | 241±4.4 | 43±3.3 |
| HS + DMSO | 5 | 300±12.7* | 112±5.0 | 239±5.0 | 37±2.8 |
| HS + ANG II | 5 | 250±7.3 | 130±4.7 | 233±2.4 | 50±1.5 |
| HS + EGF | 5 | 247±7.4 | 125±3.1 | 251±7.2 | 47±2.6 |
| HS + ANG II + AG | 6 | 277±6.5 | 129±1.2 | 242±5.6 | 48±2.8 |
| HS + EGF + AG | 6 | 253±10.8 | 123±4.3 | 249±10.2 | 48±1.2 |
| HS + ANG II + Wort | 6 | 262±8.8 | 137±11.7 | 238±1.9 | 43±2.7 |
| HS + ANG II + PD | 7 | 290±9.2* | 134±4.5 | 249±3.4 | 46±2.2 |
| HS + ANG II + SB | 6 | 293±9.8* | 134±2.8 | 243±2.2 | 43±3.2 |
| HS + ANG II + CHX | 6 | 242±10.3 | 118±4.7 | 236±4.2 | 49±2.8 |
| LS | 9 | 299±5.9* | 121±2.8 | 242±3.6 | 39±3.2 |
| LS + AG | 6 | 260±9.0 | 118±3.5 | 250±3.5 | 42±2.7 |
| LS + Wort | 6 | 251±9.1 | 143±5.5† | 238±2.7 | 38±4 |
| LS + PD | 7 | 273±6.4 | 117±3.1 | 243±3.6 | 37±3.1 |
| LS + SB | 6 | 257±8.9 | 114±6.5 | 236±2.9 | 44±3.8 |
| LS + CHX | 6 | 256±8.6 | 101±3.9 | 239±4.2 | 48±1.6 |
Values are means ± SE. HS, high salt; LS, low salt; DMSO, dimethylsulfoxide; AG, AG-1478; Wort, wortmannin; PD, PD-98059; SB, SB-203580; CHX, cycloheximide.
P < 0.05 vs. HS + ANG II.
P < 0.05 vs. LS.
Western blotting experiments.
To evaluate ERK1/2 phosphorylation, aortas and cerebral resistance arteries from the ventral surface of the brain were removed and the relative phosphorylation of ERK1/2 was determined by immunoblot analysis. In those studies, the vessels were removed at the time the MCA was isolated for the diameter measurements (see below) and quickly frozen in liquid nitrogen. The aortas were homogenized on ice using a tissue protein extraction reagent (Pierce, Rockford, IL). The protein concentration was determined using a Bradford protein assay, and 10 μg of each sample was loaded onto a 10% SDS-polyacrylamide gel and separated by electrophoresis. The samples were then transferred to a nitrocellulose membrane (0.45 μm) and blocked overnight with 10% nonfat dried milk. The next morning, membranes were incubated with a polyclonal antibody against ERK1/2 or phospho-ERK1/2 (Thr202/Tyr204) for 2 h. The membranes were then washed and incubated with a horse radish peroxidase-conjugated goat anti-rabbit antibody for 2 h. After incubation with the secondary antibody, the membranes were washed and protein bands were visualized using chemiluminescence (Super Signal; Pierce) and quantified using scanning densitometry (UNSCAN-IT software; Silk Scientific, Orem, UT). All antibodies were purchased from Cell Signaling Technologies (Danvers, MA).
Evaluation of signaling pathways.
To identify the AT1 receptor signaling pathway(s) necessary for ANG II to restore vascular relaxation in animals fed HS diet, rats were fed a HS diet (4% NaCl) diet for 3 days and assigned to one of the following intravenous infusion groups for 3–5 additional days before acute in vitro experiments: 1) control animals receiving either no infusion or an intravenous infusion of either 2) ANG II (5 ng·kg−1·min−1), 3) EGF (2 μg/h) in DMSO vehicle (20 μl/h), 4) ANG II + the EGF receptor kinase inhibitor AG-1478 (20 μg/h), 5) EGF + AG-1478, 6) ANG II + the MEK inhibitor PD-98059 (10 μg/h), 7) ANG II + the PI3K inhibitor wortmannin (2 μg/h, 8) ANG II + the p38 MAPK inhibitor SB-203580 (10 μg/h), 9) ANG II + the eukaryotic ribosome inhibitor cycloheximide (CHX-5; μg/h) to block de novo protein synthesis, and 10) DMSO vehicle alone (20 μl/h). For experiments to characterize the effect of inhibiting the AT1 receptor signaling pathway in animals fed a LS diet, rats maintained on LS diet (0.4% NaCl) were assigned to one of the following intravenous infusion groups for 4–5 days before acute in vitro experiments: 1) control animals receiving no infusion, 2) AG-1478 infusion, 3) PD-98059 infusion, 4) wortmannin infusion, 5) SB-203580 infusion, or 6) cycloheximide infusion.
Cannulated MCA preparation.
On the day of the experiment, animals were anesthetized with pentobarbital sodium (60 mg/kg ip), mean arterial pressures were determined via carotid artery catheterization, and the brain was removed and immersed in physiological salt solution (PSS) having the following ionic composition (in mM): 119.0 NaCl, 4.7 KCl, 1.6 CaCl2, 1.18 NaH2PO4, 1.17 MgSO4, 24.0 NaHCO3, 5.5 D-glucose, and 0.03 EDTA. The MCA was carefully excised under a dissecting microscope (Leica, Buffalo, NY), cannulated at the proximal and distal ends using glass micropipettes (80–120 μm; FHC, Brunswick, ME), and extended to its approximate in situ length. Side branches were ligated to prevent leaks and to allow the vessel to be pressurized. The vessel was continuously perfused and superfused with PSS (37°C) equilibrated with a 21% O2-5% CO2-74% N2 gas mixture, and the intraluminal pressure was maintained at 80 mmHg to approximate in vivo conditions. The internal diameter was measured using television microscopy and a video micrometer (model IV-550; FOR-A Company, Tokyo, Japan). Vessels lacking intrinsic resting tone were excluded from analysis.
Response to reduced PO2.
After the cannulated vessel was allowed a minimum 1-h equilibration period at 21% O2, the response to reduced PO2 was assessed by simultaneous equilibration of the perfusion and superfusion solutions with a 0% O2-5% CO2-95% N2 gas mixture for 10 min. Under these conditions, the PO2 of PSS equilibrated with 21% O2 is ∼140 mmHg, and this value decreases to 35–45 mmHg during equilibration with 0% O2 (11).
Responses to vasodilator stimuli and Ca2+-free solution:.
Vascular diameter changes in response to the endothelium-dependent vasodilator agonist ACh (10−9 M-10−5 M) and the stable prostacyclin analog iloprost (10−16 g/ml-10−11 g/ml) were also assessed in MCA from each group of rats. At the end of the experiment, resting tone and maximum diameter of the artery (Table 1) were assessed by superfusing the vessel with Ca2+-free PSS having the following composition (in mM): 119.0 NaCl, 20.0 MgCl2, 4.7 KCl, 1.18 NaH2PO4, 1.17 MgSO4, 24.0 NaHCO3, 5.5 D-glucose, and 2.0 EGTA. Resting tone in percentage was calculated as [(Dmax − Drest)/Dmax] × 100, where Dmax is the maximum diameter in Ca2+-free solution and Drest is the resting control diameter.
Statistical methods.
Data are presented as mean values ± SE. For all concentration-response curves, differences between groups at each concentration were determined using a two-way, repeated-measures ANOVA, and differences in other variables were evaluated using one-way ANOVA. Differences between individual means following ANOVA were evaluated using a post hoc Student-Newman-Keuls test. A P value of <0.05 was considered to be statistically significant.
RESULTS
Arterial pressures, vessel diameter, and resting tone.
Mean arterial pressures, resting diameters, and maximum diameters of MCA in the various groups are summarized in Table 1. There were no differences in maximum vessel diameter or in active resting tone between any of the groups.
Role of the EGF receptor in ANG II-mediated restoration of cerebrovascular relaxation.
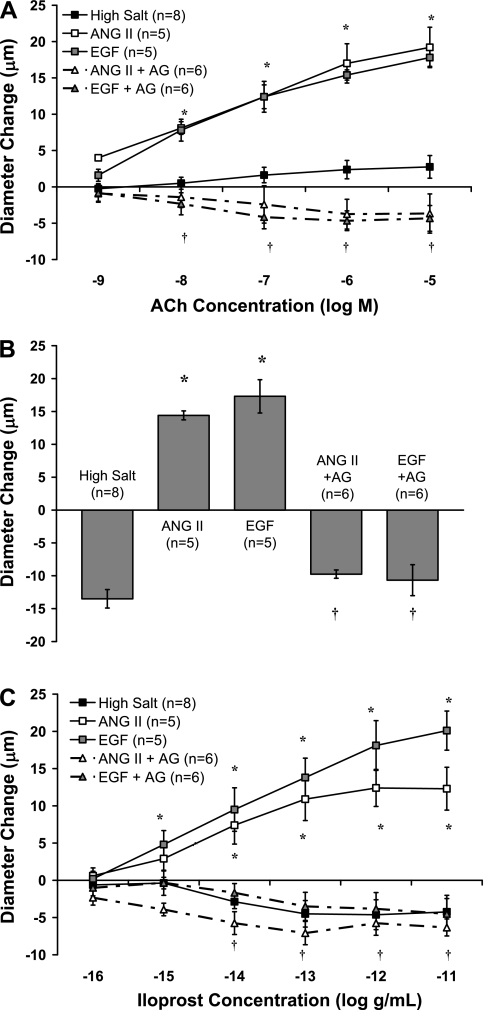
Figure 1 compares MCA responses with ACh (Fig. 1A), reduced PO2 (Fig. 1B), and the stable prostacyclin analog iloprost (Fig. 1C) in animals that were fed a HS diet and received either no infusion (HS; control group) or an infusion of either ANG II or EGF (±the EGF receptor kinase inhibitor AG-1478) for 3 days. As previously reported (23), HS diet eliminated the dilation of MCA in response to ACh, reduced PO2, and iloprost. Infusion of either ANG II or EGF restored vascular relaxation in response to all three vasodilator stimuli in MCA from salt-fed rats, and the protective effects of both ANG II and EGF infusion to restore vascular relaxation were eliminated by coinfusion of AG-1478 to inhibit the EGF receptor kinase.
Fig. 1.
Responses of middle cerebral arteries (MCA) to ACh (A), reduced PO2 (B), and iloprost (C) in rats fed a high-salt (HS) diet and receiving an infusion of ANG II or EGF, with or without the EGF receptor kinase inhibitor AG-1478 (AG). Data are summarized as means ± SE. *P < 0.05: HS + ANG II and HS + EGF vs. HS alone; †P < 0.05: HS + ANG II + AG or HS + EGF + AG vs. HS + ANG II alone and HS + EGF alone.
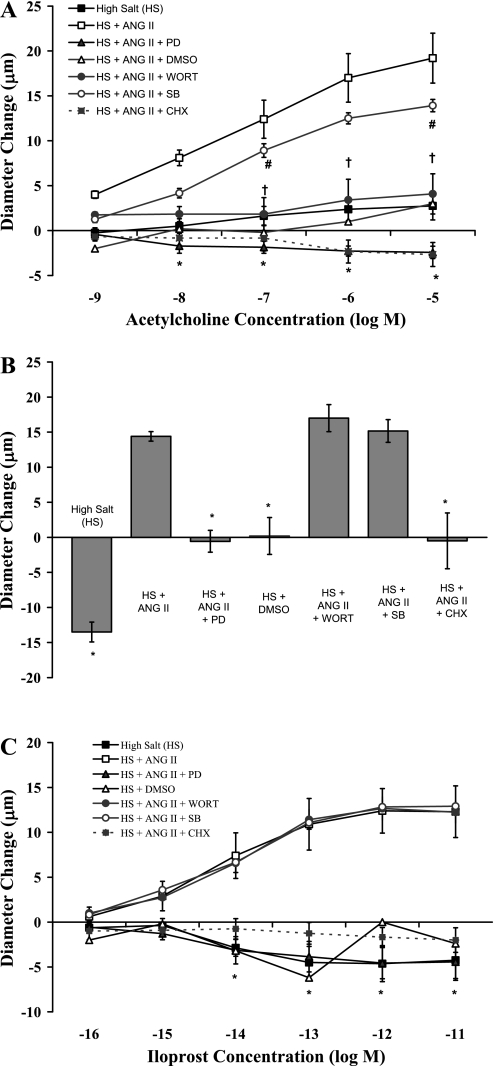
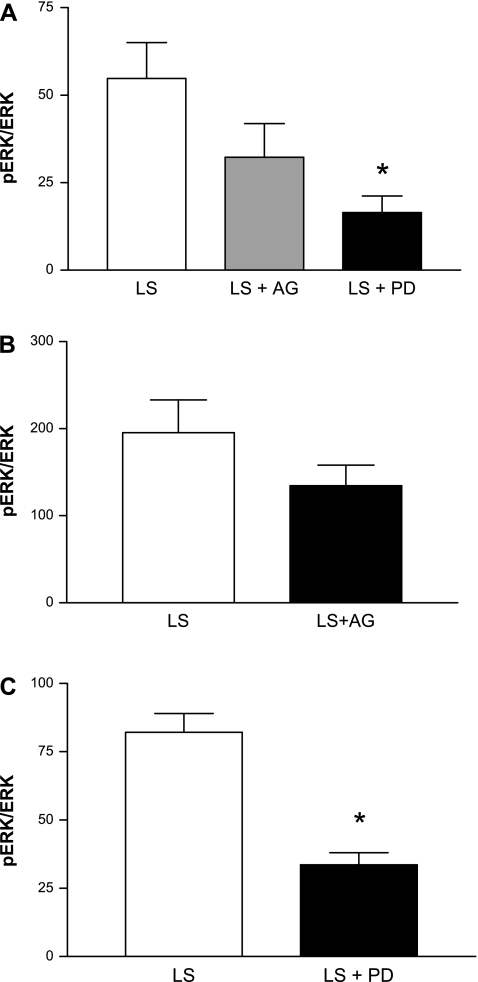
Role of ERK1/2 in ANG II-mediated restoration of vascular relaxation.
Figure 2 compares MCA responses with ACh (Fig. 2A), reduced PO2 (Fig. 2B), and iloprost (Fig. 2C) in animals fed a HS diet and receiving either 1) no infusion, 2) infusion of the DMSO vehicle alone, 3) ANG II infusion alone (ANG II) or simultaneous infusion of 4) ANG II plus the MEK inhibitor PD-98059 (ANG II + PD), 5) ANG II + the PI3K inhibitor wortmannin, 6) ANG II + the p38 MAP kinase inhibitor SB-203580, or 7) ANG II + the eukaryotic protein synthesis inhibitor cycloheximide (CHX). As previously reported (23; 37; 38), low-dose ANG II infusion restored vascular relaxation in response to all three vasodilator stimuli in salt-fed rats. This protective effect of ANG II infusion to restore vascular relaxation was prevented when the animals received a simultaneous coinfusion of PD-98059 to inhibit the ERK1/2 signaling pathway or cycloheximide to inhibit protein synthesis. Wortmannin inhibited ACh-induced dilation in ANG II-infused animals but had no effect on ANG II-induced restoration of vasodilator responses to reduced PO2 or iloprost in salt-fed rats. Vascular relaxation in response to all three vasodilator stimuli was still present in rats receiving a coinfusion of ANG II + SB-203580, whereas infusion of the DMSO vehicle alone failed to restore vascular relaxation in response to any of the dilator stimuli in rats fed HS diet.
Fig. 2.
Responses of MCA to ACh (A), reduced PO2 (B), and iloprost (C) in rats fed a HS diet (n = 8), HS diet with an intravenous infusion of dimethylsulfoxide (DMSO) vehicle alone (n = 5), HS diet with ANG II infusion (n = 5), HS diet with a simultaneous infusion of ANG II plus either the MEK inhibitor PD-98059 (PD; n = 7), the phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin (Wort; n = 6), the P38 MAPK inhibitor SB-203580 (SB; n = 6), or the eukaryotic protein synthesis inhibitor cycloheximide (CHX; n = 6). Data are summarized as mean changes from control diameter (in μm) ± SE. *P < 0.05: HS, HS + DMSO, HS + ANG II + PD, and HS + ANG II + CHX vs. HS + ANG II; †P < 0.05: ACh response with HS + ANG II + Wort vs. HS + ANG II alone; #P < 0.05: ACh response with HS + ANG II + SB vs. HS + ANG II alone.
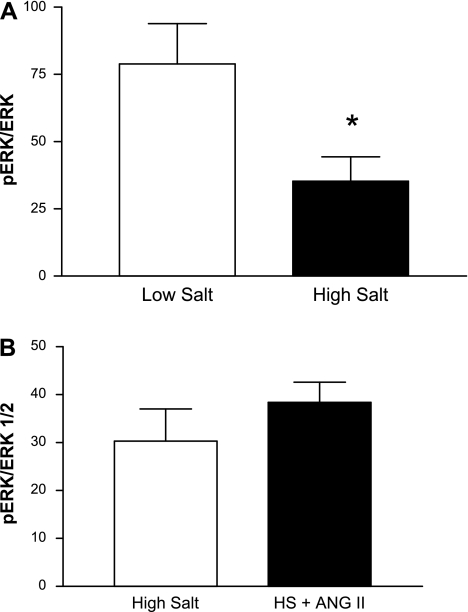
Consistent with the hypothesis that reduced activation of the ERK1/2 pathway plays a role in the loss of vasodilator responses in the salt-fed rats, pERK-to-ERK ratios in aortas from rats fed HS diet were significantly lower than those in aortas from rats maintained on LS diet; ANG II infusion tended to increase pERK-to-ERK ratios (by ∼27%) in rats fed HS diet compared with salt-fed animals not receiving ANG II (Fig. 3). Basal expression of ERK1/2 (mean pixels × 103 ± SE) was not significantly different in aortas from the various groups: 128.9 ± 47.0 in LS (n = 11) vs. 151.1 ± 55.0 in HS (n = 11) and 155.3 ± 27.5 in HS-saline infused (n = 8) vs. 147.9 ± 24.0 in HS-ANG II infused (n = 8).
Fig. 3.
pERK-to-ERK1/2 ratios in aortas of rats fed low-salt (LS; n = 11) or HS (n = 11) diet (A) or HS diet ± low dose ANG II infusion (n = 8/group; B). Data are summarized as means ± SE. *P < 0.05 vs. low salt.
Role of the EGF receptor and the ERK1/2 pathway in maintaining vascular relaxation in rats fed LS diet.
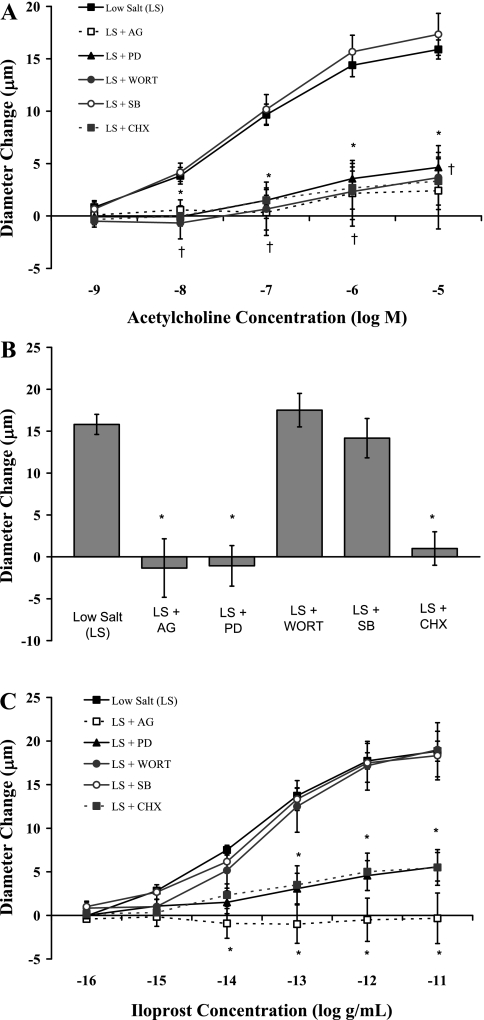
To determine the role of the EGF receptor and the ERK1/2 pathway in maintaining MCA relaxation in the absence of elevated dietary salt intake, we determined the effect of intravenous infusion of the same inhibitors on the responses to ACh, reduced PO2, and iloprost in isolated MCA from rats fed LS diet (Fig. 4). In those experiments, relaxation of the MCA in response to ACh (Fig. 4A), hypoxia (Fig. 4B), and iloprost (Fig. 4C) was eliminated or dramatically reduced in rats receiving an intravenous infusion of AG-1478, PD-98059, or cycloheximide. Vasodilator responses to ACh, but not to reduced PO2 or iloprost, were also reduced by wortmannin. Responses to ACh, reduced PO2, and iloprost were unaffected by SB-203580 in MCA of rats fed LS diet.
Fig. 4.
Responses of MCA to ACh (A), reduced PO2 (B), and iloprost (C) in rats fed a LS diet alone (n = 9) or LS diet with an intravenous infusion of either the EGF receptor kinase inhibitor AG-1478 (AG; n = 6), the MEK inhibitor PD-98059 (PD; n = 7); the PI3K inhibitor wortmannin (Wort; n = 6), the P38 MAPK inhibitor SB-203580 (SB; n = 6), or the eukaryotic protein synthesis inhibitor cycloheximide (CHX; n = 6). Data are summarized as mean changes from control diameter (in μm) ± SE. *P < 0.05: LS + AG, LS + PD, and LS + CHX vs. LS alone; †P < 0.05: ACh response in LS + Wort vs. LS alone.
When compared with that in untreated control animals, ERK1/2 phosphorylation in the aorta and in cerebral arteries was reduced by ∼30–40% in the LS + AG group and by ∼60–70% in the LS + PD group (Figs. 5 and 6). In contrast with the effects of AG and PD, ERK1/2 phosphorylation was unaffected by chronic infusion of wortmannin, SB-203580, or cycloheximide for 3 days (Fig. 5). Basal expression of ERK1/2 was not significantly different in cerebral arteries [(mean pixels × 103 ± SE) = 63.9 ± 26.3 in LS-DMSO (n = 5) vs. 112.8 ± 5.5 in LS + PD (n = 5) and 55.0 ± 20.7 in LS-DMSO (n = 6) vs. 94.4 ± 11.6 in LS + AG (n = 5)] or in aortas from the various groups [(mean pixels × 102) ± SE for (n = 6) = 16.9 ± 3.1 in LS, 17.4 ± 2.2 in LS + AG, 15.4 ± 2.2 in LS + PD, 15.5 ± 3.1 in LS + wortmannin, 17.7 ± 2.2 in LS + SB, and 15.6 ± 2.8 in LS + CHX].
Fig. 5.
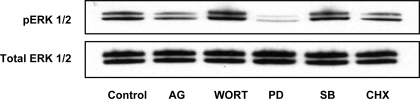
Western blots illustrating the effect of inhibitors on phosphorylation of ERK1/2 in aortas from rats fed low-salt diet. Control, untreated; AG, AG-1478; Wort, wortmannin; PD, PD-98059; SB, SB-203580; CHX, cycloheximide.
Fig. 6.
pERK-to-ERK1/2 ratios in aortas (A) and cerebral arteries (B and C) of rats fed LS diet alone or receiving an infusion of either the EGF receptor kinase inhibitor AG-1478 (AG) or the MEK inhibitor PD-98059 (PD). Data are summarized as means ± SE for 6 aortas/group and 5 to 6 cerebral artery samples/group. *P < 0.05 vs. LS.
DISCUSSION
It is well known in humans and in multiple animal models that elevated levels of ANG II are detrimental to vascular function, due in large part to the effect of ANG II to increase superoxide production (16; 18; 28; 34). Therefore, clinical treatments and the vast majority of animal studies are largely based on the notion that a reduction in ANG II is universally favorable to vascular reactivity. Although this is clearly true with supranormal levels of ANG II, this dogma apparently does not apply in the setting of physiological reductions of ANG II with HS diet, where there is a growing body of evidence indicating that physiological suppression of the renin-angiotensin system by elevated dietary salt intake adversely affects vascular relaxation mechanisms (23; 24; 37; 38; 42). This study is the first to examine changes in intracellular signaling with salt-induced ANG II suppression in an attempt to elucidate the mechanisms by which low ANG II levels affect vascular function. Another important question addressed in the present study is whether the protective effect of ANG II to restore vascular relaxation that is lost during salt-induced ANG II suppression is similar to or distinct from the role of ANG II to maintain vascular relaxation mechanisms when circulating ANG II levels are in the normal physiological range.
Based upon the effects of the inhibitors that we employed in rats fed LS diet and in rats fed HS diet with ANG II infusion (see below), the results of this study indicate that the actions of ANG II to maintain normal responses to vasodilator stimuli under physiological conditions and to rescue vascular relaxation in salt-fed rats utilize identical mechanisms and represent the same protective process. We also found that none of the experimental perturbations had any significant effect on active resting tone in the MCA (Table 1), demonstrating that the protective effect of ANG II infusion (and EGF infusion) to restore vasodilator responses in salt-fed rats is mediated via effects on vascular relaxation mechanisms themselves, and not via effects on active resting tone, e.g., generation of active tone where it was previously absent or increasing the reserve capacity for vasodilation.
As noted above and also shown in the present experiments (Figs. 1 and 2), restoring normal levels of circulating ANG II rescues vasodilator responses that are lost during elevated dietary salt intake (23; 37; 38). Responses to different dilator stimuli are also reduced in MCA (31) and skeletal muscle resistance arteries (30) of rats receiving losartan to block ANG II AT1 receptors and in skeletal muscle arterioles of captopril-treated rats (13; 14).
In the present study, we report the novel finding that the restoration of cerebral vascular relaxation in response to ACh, reduced PO2, and the prostacyclin analog iloprost by low-dose ANG II infusion in salt-fed rats requires transactivation of the EGF receptor tyrosine kinase by the AT1 receptor. This conclusion is based on two key observations. First, the effect of ANG II to restore vasodilator responses in salt-fed rats is mimicked by EGF infusion. Finally, the protective effect of both ANG II infusion and EGF infusion to restore vascular relaxation can be blocked by coinfusion of AG-1478 to block phosphorylation of the EGF receptor.
The present study also indicates that the protective effect of ANG II infusion to restore vascular relaxation in salt-fed animals requires activation of the ERK1/2 pathway, and protein synthesis as ANG II-induced restoration of vascular relaxation is prevented by blocking this pathway with PD-98059 and cycloheximide. The results of this study also suggest that the same signaling components are required to maintain vascular function under physiological (LS) conditions, because both AG-1478 and PD-98059 inhibit vasodilator responses in rats maintained on LS diet (Fig. 4). Taken together, these new findings provide further support for the hypothesis that physiological levels of ANG II play an important role in maintaining normal vascular relaxation mechanisms.
In these experiments, we employed three vasodilator stimuli (ACh, reduced PO2, and iloprost) that have been shown to be eliminated by HS diet and restored with ANG II infusion (23; 37; 38). Two of these stimuli require functional endothelial cells: the nitric oxide (NO)-dependent agonist ACh and the cyclooxygenase-dependent stimulus of reduced PO2 (12). Iloprost, on the other hand, acts directly on prostanoid receptors on the vascular smooth muscle (VSM) cell membrane. In this regard, it is interesting that ANG II not only maintains or restores reactivity to each of these vasodilator stimuli, but also that this effect requires activation of similar signal transduction components, namely the EGF receptor and ERK1/2. These observations suggest that ANG II plays a common permissive role to maintain mechanisms of vascular relaxation in both endothelial and VSM cells.
The role of the EGF receptor and ERK1/2 pathway in regulating different components of vascular function has been extensively studied in cultured cells (especially endothelial cells). However, there are far fewer studies of the role of these pathways in regulating the function of intact blood vessels, especially in regard to the regulation of active tone in the vessel. Earlier studies investigating the acute effects of EGF on rat aorta identified it as a potent vasoconstrictor in experimental hypertension (9; 10). Aramoto et al. (1) reported that although ERK1/2 activation plays a crucial role in mediating the increase in microvascular permeability that occurs in response to VEGF in the hamster cheek pouch, it does not contribute to the NO-mediated arteriolar dilation in response to VEGF in this tissue. Other studies suggest that activation of the EGF receptor and the ERK1/2 pathway play a crucial role in cell proliferation and angiogenesis (tube formation) in response to cytochrome P-450 epoxygenase products in cultured human endothelial cells (26). Of particular interest to the present study is the report by Watts et al. (36) that stimulation of ERK phosphorylation by AT1 receptor activation is not important for ANG II-induced contraction of rat aorta. However, to our knowledge, the present study is the first to investigate the long-term role of the EGF receptor and ERK1/2 in regulating vascular relaxation mechanisms in intact blood vessels.
One observation that stands out in contrast with the idea of ERK1/2 as a common permissive signaling pathway for vasodilation in endothelial and VSM cells is the effect of the PI3K inhibitor wortmannin to attenuate ACh-induced vasodilation in animals maintained on LS diet and in animals fed HS diet and receiving ANG II infusion. However, PI3K directly controls the activation of the endothelial NO synthase (eNOS) enzyme through Akt-mediated phosphorylation (15), and acute exposure to wortmannin abrogates ACh-induced cerebral vasodilation (20; 40). These observations indicate that wortmannin directly attenuates the ACh response by reducing eNOS activation, rather than chronic blockade of an obligatory signaling pathway.
Although further experiments are necessary to clarify the mechanisms by which the EGF receptor and ERK1/2 maintain vascular relaxation in these vessels, it is clear that inhibiting EGF receptor phosphorylation or the ERK1/2 pathway eliminates vasodilator responses to ACh, reduced PO2, and iloprost in MCA of rats fed LS diet and blocks the protective effect of ANG II infusion to restore vascular relaxation in response to all these vasodilator stimuli in rats fed HS diet. This response is identical to that seen in ANG II-infused rats in which the AT1 receptors are blocked by coinfusion of losartan (38). As such, these observations argue in favor of the EGF receptor and the ERK1/2 pathway as crucial mediators of ANG II-mediated restoration of vasodilator responses in rats fed a HS diet and for the maintenance of vascular relaxation mechanisms in rats fed a LS diet.
The EGF receptor and ERK1/2 pathway have been implicated as mediators of ANG II-induced protein synthesis following AT1 receptor activation (8; 35; 41). The ability of chronic intravenous infusion of ANG II to restore vascular relaxation in vessels from salt-fed animals is not mimicked by acute addition of ANG II to the tissue bath (38), suggesting that this protective effect of ANG II infusion involves protein synthesis in some way. Although there is some evidence that the protective effect of low-dose ANG II infusion in salt-fed animals is mediated, at least in part, by maintenance of antioxidant defense mechanisms in the vessels (24, 43), elevated dietary salt intake and low-dose ANG II infusion in the background of HS diet could affect the expression of multiple genes and signal transduction pathways. The latter possibility emphasizes the importance of investigating the effect of HS diet and ANG II suppression on all potential signaling pathways in the vasculature.
Perspectives
Elevated salt intake is a critical risk factor not only for hypertension (7; 19; 39) but also for cerebrovascular complications such as stroke, myocardial infarction, coronary artery bypass grafts, percutaneous transluminal coronary angioplasty, and cardiovascular death (5; 6; 19; 27; 33). Recent reviews (2; 3; 25) have noted that defensive authoritarianism (25) in support of entrenched positions (3) in the debate about dietary salt and blood pressure has detracted from the wider and more important question of whether HS diet has adverse consequences on vascular function independent of its effects on blood pressure. The possibility of adverse effects of dietary salt intake in the absence of hypertension is further emphasized by clinical studies showing that salt-sensitive normotensive individuals not only have a greater risk of developing hypertension than salt-resistant subjects but also have a higher mortality rate than salt-resistant subjects, even if they do not develop hypertension (39).
As noted above, the results of this study suggest that EGF receptor activation and the ERK1/2 pathway are required for the restoration of vascular relaxation by low-dose ANG II infusion in salt-fed animals and for the maintenance of vascular relaxation in animals fed LS diet. These findings support the idea that tonic activation of this pathway plays a critical role to maintain cerebrovascular relaxation under normal physiological conditions. Of particular interest regarding the physiological versus the pathophysiological aspects of ANG II signaling is a recent report by Reed and coworkers (32) showing that AT1 receptor blockade with candesartan is detrimental to coronary collateral growth following repeated ischemia in Wistar-Kyoto (WKY) rats, in contrast with its beneficial effect to improve coronary collateral growth in JCR rats, a rodent model of syndrome X. Another important finding in that study was that subpressor ANG II infusion improves blood flow in the collateral-dependent zone in WKY rats, whereas higher doses of ANG II reduce blood flow (32). Taken together, the results of that study suggest that the overall effect of AT1 receptor activation, angiotensin receptor blockade, and possibly angiotensin converting enzyme inhibition may differ, depending on the presence or absence of cardiovascular disease. They also emphasize the need to obtain a more complete understanding of the signaling pathways involved in both the physiological and the pathophysiological actions of ANG II.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-65289 and HL-72920.
ACKNOWLEDGMENTS
We thank Lynn Dondlinger for her outstanding technical assistance.
REFERENCES
- 1.Aramoto H, Breslin JW, Pappas PJ, Hobson RW, Duran WN. Vascular endothelial growth factor stimulates differential signaling pathways in in vivo microcirculation. Am J Physiol Heart Circ Physiol 287: H1590–H1598, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Aviv A. Salt and hypertension: the debate that begs the bigger question. Arch Intern Med 161: 507–510, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Aviv A. Salt consumption, reactive oxygen species and cardiovascular ageing: a hypothetical link. J Hypertens 20: 555–559, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Boegehold MA. Effect of dietary salt on arteriolar nitric oxide in striated muscle of normotensive rats. Am J Physiol Heart Circ Physiol 264: H1810–H1816, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334: 885, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyle P. High NaCl predisposes Dahl rats to cerebral infarction after middle cerebral artery occlusion. Hypertension 12: 96–101, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Dahl LK, Love RA. Evidence for relationship between sodium (chloride) intake and human essential hypertension. AMA Arch Intern Med 94: 525–531, 1954 [DOI] [PubMed] [Google Scholar]
- 8.Eguchi S, Iwasaki H, Hirata Y, Frank GD, Motley ED, Yamakawa T, Numaguchi K, Inagami T. Epidermal growth factor receptor is indispensable for c-Fos expression and protein synthesis by angiotensin II. Eur J Pharmacol 376: 203–206, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Florian JA, Dorrance A, Webb RC, Watts SW. Mineralocorticoids upregulate arterial contraction to epidermal growth factor. Am J Physiol Regul Integr Comp Physiol 281: R878–R886, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Florian JA, Watts SW. Epidermal growth factor: a potent vasoconstrictor in experimental hypertension. Am J Physiol Heart Circ Physiol 276: H976–H983, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Fredricks KT, Liu Y, Lombard JH. Response of extraparenchymal resistance arteries of rat skeletal muscle to reduced PO2. Am J Physiol Heart Circ Physiol 267: H706–H715, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Fredricks KT, Liu Y, Rusch NJ, Lombard JH. Role of endothelium and arterial K+ channels in mediating hypoxic dilation of middle cerebral arteries. Am J Physiol Heart Circ Physiol 267: H580–H586, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC, Lombard JH. Short-term angiotensin converting enzyme inhibition reduces basal tone and dilator reactivity in skeletal muscle arterioles. Am J Hypertens 13: 389–395, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Frisbee JC, Weber DS, Lombard JH. Chronic captopril administration decreases vasodilator responses in skeletal muscle arterioles. Am J Hypertens 12: 705–715, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiadoni L, Versari D, Magagna A, Kardasz I, Plantinga Y, Giannarelli C, Taddei S, Salvetti A. Ramipril dose-dependently increases nitric oxide availability in the radial artery of essential hypertension patients. J Hypertens 25: 361–366, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hernandez I, Cowley AW, Jr, Lombard JH, Greene AS. Salt intake and angiotensin II alter microvessel density in the cremaster muscle of normal rats. Am J Physiol Heart Circ Physiol 263: H664–H667, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol 22: 311–315, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis 49: 59–75, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kitayama J, Kitazono T, Ibayashi S, Wakisaka M, Watanabe Y, Kamouchi M, Nagao T, Fujishima M. Role of phosphatidylinositol 3-kinase in acetylcholine-induced dilatation of rat basilar artery. Stroke 31: 2487–2493, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol 279: H7–H14, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Fredricks KT, Roman RJ, Lombard JH. Response of resistance arteries to reduced PO2 and vasodilators during hypertension and elevated salt intake. Am J Physiol Heart Circ Physiol 273: H869–H877, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 284: H1124–H1133, 2003 [DOI] [PubMed] [Google Scholar]
- 24.McEwen ST, Schmidt JR, Somberg L, de la Cruz L, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirc 16: 220–234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messerli FH, Schmieder RE. Salt and hypertension: going to the heart of the matter. Arch Intern Med 161: 505–506, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J 17: 770–772, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke 35: 1543–1547, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Ogawa S, Mori T, Nako K, Kato T, Takeuchi K, Ito S. Angiotensin II type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathy. Hypertension 47: 699–705, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Peterson MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high-salt diet. Am J Physiol Heart Circ Physiol 291: H114–H120, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Phillips SA, Drenjancevic-Peric I, Frisbee JC, Lombard JH. Chronic AT1 receptor blockade alters mechanisms mediating responses to hypoxia in rat skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 287: H545–H552, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Phillips SA, Lombard JH. Chronic AT1 receptor blockade alters the mechanisms mediating hypoxic dilation in middle cerebral arteries. J Cardiovasc Pharmacol 46: 706–712, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol 28: 61–67, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Tobian L, Hanlon S. High sodium chloride diets injure arteries and raise mortality without changing blood pressure. Hypertension 15: 900–903, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 19: 1245–1254, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Voisin L, Foisy S, Giasson E, Lambert Moreau P, Meloche S. EGF receptor transactivation is obligatory for protein synthesis stimulation by G protein-coupled receptors. Am J Physiol Cell Physiol 283: C446–C455, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Watts SW, Florian JA, Monroe KM. Dissociation of angiotensin II-stimulated activation of mitogen-activated protein kinase kinase from vascular contraction. J Pharmacol Exp Ther 286: 1431–1438, 1998 [PubMed] [Google Scholar]
- 37.Weber DS, Lombard JH. Elevated salt intake impairs dilation of skeletal muscle resistance arteries via angiotensin II suppression. Am J Physiol Heart Circ Physiol 278: H500–H506, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 280: H2196–H2202, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Xu HL, Feinstein DL, Santizo RA, Koenig HM, Pelligrino DA. Agonist-specific differences in mechanisms mediating eNOS-dependent pial arteriolar dilation in rats. Am J Physiol Heart Circ Physiol 282: H237–H243, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Chalothorn D, Jackson LF, Lee DC, Faber JE. Transactivation of epidermal growth factor receptor mediates catecholamine-induced growth of vascular smooth muscle. Circ Res 95: 989–997, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol 291: H929–H938, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res 44: 382–390, 2007 [DOI] [PubMed] [Google Scholar]