Abstract
Low-voltage-activated calcium channels are reexpressed in ventricular myocytes in pathological conditions associated with hypoxic episodes, but a direct relation between oxidative stress and T-type channel function and regulation in cardiomyocytes has not been established. We aimed to investigate low-voltage-activated channel regulation under oxidative stress in neonatal rat ventricular myocytes. RT-PCR measurements of voltage-gated Ca2+ (Cav)3.1 and Cav3.2 mRNA levels in oxidative stress were compared with whole cell patch-clamp recordings of T-type calcium current. The results indicate that hypoxia reduces T-type current density at −30 mV (the hallmark of this channel) based on the shift of the voltage dependence of activation to more depolarized values and downregulation of Cav3.1 at the mRNA level. Upon reoxygenation, both Cav3.1 mRNA levels and the voltage dependence of total T-type current are restored, although differently for activation and inactivation. Using Ni2+, we distinguished different effects of hypoxia/reoxygenation on the two current components. Long-term incubation in the presence of 100 μM CoCl2 reproduced the effects of hypoxia on T-type current activation and inactivation, indicating that the chemically induced oxidative state is sufficient to alter T-type calcium current activity, and that hypoxia-inducible factor-1α is involved in Cav3.1 downregulation. Our results demonstrate that Cav3.1 and Cav3.2 T-type calcium channels are differentially regulated by hypoxia/reoxygenation injury, and, therefore, they may serve different functions in the myocyte in response to hypoxic injury.
Keywords: low-voltage-activated calcium channel, oxidative stress, cardiomyocytes
since the initial biophysical (34) and molecular characterization (5, 27, 36) of low-voltage-activated T-type calcium currents in heart and neurons, these channels have been studied in various systems and pathological conditions. T-type currents are reexpressed in the adult hypertrophied ventricle and in postmyocardial infarction (18), in the monocrotaline model of pulmonary hypertension and heart failure (24, 44), and in vitro in adult ventricular myocytes exposed to 10% FBS (9) and endothelin-1 (19), relating the T-type current to the pathology rather than to normal physiological function of the myocytes. The mechanisms for regulation of the T-type channels are incompletely characterized, largely due to the lack of specific inhibitors and the presence of the more robust L-type and store-operated calcium channels, which make it difficult to isolate T-type Ca2+ channel functions, such as secretion (29), excitation (52), or transcription regulation (4).
The lesions at the site of ischemia-reperfusion injury can trigger structural and functional cardiac remodeling. Due to the complex paracrine and autocrine regulation of cells as a response to injury (30), there are many potential mediators at the injury site. Both low-oxygen conditions and the subsequent reoxygenation phase perturb normal function in cardiac myocytes. Hypoxia/reoxygenation (H/R) injury alters the activity of ionic transport systems by inhibiting K+ channels (38), voltage-dependent L-type Ca2+ channels (10), Na+/Ca2+ exchanger (47), and sarcoplasmic reticulum Ca2+-ATPase pump (40), and by stimulating store-operated channels (32) and ryanodine receptors (22).
Multiple lines of evidence suggest that factors secreted at the site of injury can modulate the T-type calcium channel, such as growth hormone secreted by atrial tumor cells (50), endothelin-1 and IGF-I in ventricular myocytes (45), and PDGF in fibroblasts (48). Of the two cardiovascular α-subunits of the T-type channel [voltage-gated Ca2+ (Cav)3.1 and Cav3.2], Cav3.2 regulation has been observed more often than Cav3.1, since its expression is increased in response to hypoxia (7), high glucose (31), aldosterone (26), and angiotensin II (12). Transcription factors proposed to regulate Cav3.2 include hypoxia-inducible factor (HIF)-1α (7), neuron restrictive silencer factor (25, 51), and Csx/Nkx2.5 (46). The effects of hypoxia on T-type calcium channels in renal proximal tubule (1), neurons (20, 21), PC12 cells (7), and in a heterologous expression system (11) implicate Cav3.2 as the primary hypoxia sensitive α-subunit.
Less is known about the pathways involved in Cav3.1 α-subunit regulation, although there is evidence that Cav3.1 mRNA is increased in adult ventricular myocytes exposed to endothelin-1 (19). Also, heterologously expressed Cav3.1 membrane currents can be increased when coexpressed with the L-type calcium channel auxiliary α2δ-subunit (13) or decreased by the γ6-subunit (16).
The present study is based on the hypothesis that oxidative stress that is present in different forms under pathological conditions induces the reexpression of T-type current. We have investigated the effects of H/R conditions on T-type channels in neonatal rat ventricular myocytes (NRVM), and we show that both Cav3.1 and Cav3.2 α-subunits are regulated in oxidative stress conditions, possibly involving different mechanisms. Our results show that cardiomyocytes downregulate Cav3.1 in hypoxic conditions, and the window current is shifted toward more depolarized intervals, which might explain the decrease in spontaneous activity of neonatal myocytes in hypoxic conditions by decreasing the influx of calcium for diastolic depolarization. The changes induced in vitro by H/R conditions suggest that Cav3.2 can increase via a negative shift in activation, which may explain the reappearance of the T-type current in hypertrophy, and thus our in vitro observations may be applicable to the regulation of T-type calcium channels under pathological conditions in the native system.
MATERIALS AND METHODS
Cell culture.
Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Loyola University of Chicago, Stritch School of Medicine. Ventricular myocytes (NRVM) were isolated from newborn rats (1–3 days old), as described elsewhere (42) and kindly supplied by Dr. Allen Samarel (Cardiovascular Institute, Loyola University of Chicago). After dissociation, NRVM were plated overnight in supplemented PC1 medium (Lonza, Walkersville, MD). The PC1 medium was changed to serum-free Dulbecco's modified Eagle's medium (Mediatech, Herndon, VA) plus antibiotic, and experiments were initiated 24 h later. Hypoxic conditions were induced using an anaerobic microbiological system with indicator (1% oxygen, Becton Dickinson, Sparks, MD) for 18–24 h. After this time interval, cells were returned to normal oxygen levels in a 5% CO2 incubator for 1–6 days. In parallel, NRVM were cultured in normoxia for the same length of time as control cells. Chemical hypoxia was induced for 24 h with 100 μM CoCl2 (Sigma, St. Louis, MO). Chemical reoxygenation was induced with 100 μM H2O2 (Sigma) for 24 h.
Quantitative RT-PCR.
NRVM were harvested, and RNA was purified using RNeasy Plus Mini kit (Qiagen, Valencia, CA), according to the manufacturer's protocol, and reverse transcription was carried out starting from 1 μg of total RNA using iScript kit (Bio-Rad, Hercules, CA). mRNA levels were measured by quantitative RT-PCR with an Applied Biosystems 7300 cycler (Applied Biosystems, Foster City, CA) using Platinum Taq Quantitative Super Mix with ROX (Invitrogen, Carlsbad, CA) or SYBR Green master mix with ROX (Fermentas, Glen Burnie, MD). Cycling conditions were 2 min at 50°C, 10 min at 95°C, and 50 cycles of 15 s at 95°C and 1 min at 60°C. RNA content was normalized to 18S ribosomal RNA, and relative changes in gene expression were quantified using the threshold cycle (ΔΔCt) method with RQ software (Applied Biosystems).
Electrophysiology.
Whole cell recordings using borosilicate glass capillaries (Kimble Glass, Vineland, NJ) of 2- to 3-MΩ resistance were used to record barium currents. Data are sampled at 10 kHz, filtered at 3 kHz with an EPC-7 amplifier (HEKA Electronics, Cologne, Germany), and digitized with a Digidata 1200 under the control of pClamp8 software (Axon Instruments, Union City, CA). For barium current recordings, pipette solution contains the following (in mM): 120 CsCl, 1 MgCl2, 10 EGTA, and 10 HEPES, pH 7.2. The bath solution contains the following (in mM): 130 N-methyl-d-glucamine, 10 BaCl2, 10 CsCl, 10 HEPES, and 10 glucose, pH 7.4. The activation protocol consisted of voltage pulses of 350 ms between −100 and +50 mV in 10-mV increments, applied after 5-s prepulse to −100 mV or −50 mV. The protocols were applied in the absence and presence of 5 μM nifedipine, with the gradual addition of 50 μM NiCl2. T-type current amplitudes were analyzed after voltage protocol subtraction in the presence of 5 μM nifedipine. L-type current was analyzed after subtraction of the traces elicited from −100 mV in the presence and absence of 5 μM nifedipine. Due to the partial reversibility of NiCl2 block, only one cell was recorded per cover slip. Current amplitudes are normalized to membrane capacitance and plotted against voltage. Voltage dependence of activation was determined from the current amplitude during the activation protocol, and conductance/maximal conductance (G/Gmax) was fitted with Boltzmann equation: G/Gmax = 1/{1+ exp[(V1/2 − V)/k]}, where V is voltage, V1/2 is half-activation potential, and k is slope factor, for nifedipine sensitive, nifedipine resistant, and 50 μM Ni2+ sensitive and resistant currents. The apparent reversal potential was calculated from the linear part of the current-voltage (I-V) plots (+43 ± 1.3 mV). Voltage dependence of inactivation was determined from the peak currents at −20 mV recorded after 5-s prepulses between −100 and −30 mV in 10-mV increments. For L-type channels, the prepulse voltages ranged from −100 to +10 mV, followed by a pulse to 0 mV. The currents are normalized to maximum (I/Imax) and fitted with Boltzmann equation: I/Imax = 1/{1+ exp[(V1/2 − V)/k]}.
Statistics.
Data are expressed as means ± SE. Statistical analyses were performed using Student's t-test and considered significant at P < 0.05. For RT-PCR data, Student's t-test was applied to ΔCt values.
RESULTS
Recording of T-type channels in NRVM.
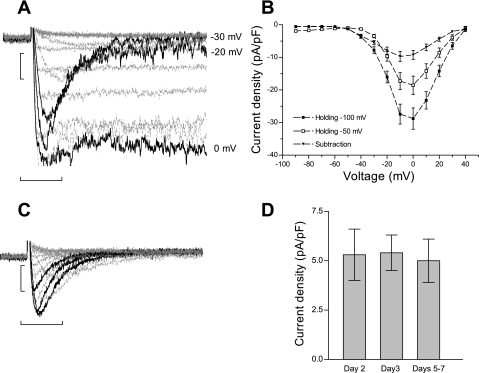
In NRVM, calcium currents are dominated by the robust high-voltage-activated L-type calcium current. The most conventional method used to measure low-voltage-activated T-type currents is by subtracting the voltage protocols from two different holding potentials: −100 mV (both T- and L-type currents) and −50 mV (L-type current). In NRVM, traces derived after the subtraction of the voltage protocols recorded in 10 mM BaCl2 indicated that, at − 30 mV, the inward barium current is predominantly carried by a fast inactivating channel, with a significant fraction of L-type channels already inactivated at −50 mV (Fig. 1A). The I-V plots (Fig. 1B) show considerable overlap of the T- and L-type currents between −20 and 0 mV, which affects the accurate assessment of T-type current in NRVM. Therefore, we have chosen to record T-type current by voltage subtraction method of the protocols from −100 and −50 mV applied in the presence of 5 μM nifedipine. Figure 1C shows typical current traces obtained after voltage protocol subtraction in the presence of 5 μM nifedipine, with a peak current density between −30 and −20 mV. Because further experiments required extended culture times, we investigated whether T-type current density changes with time in culture. Figure 1D shows that the peak current density for T-type current did not change with time in culture (up to 7 days). However, there was a reproducible positive shift in the peak current from −20 mV to −10 mV over time. Because the currents from days 2 and 3 in culture were not different from each other, the results were pooled together and considered as “early” time in culture, while the results from days 5–7 are referred to as “late” cultured cells.
Fig. 1.
T-type current recordings in cultured neonatal rat ventricular myocytes (NRVM). A: representative traces for T-type currents recorded in 10 mM BaCl2 using the subtraction of voltage protocols from holding potentials of −100 mV and −50 mV. The black traces represent −30, −20, and 0 mV. Scale bars represent 250 pA and 50 ms. B: current-voltage relation of the maximal current amplitude vs. command voltage, for traces elicited from −100 mV (■), −50 mV (□), and the subtracted current (▾). Values are means ± SE for n = 25 myocytes maintained in culture for 2 and 3 days. C: representative traces for T-type currents recorded in 10 mM BaCl2 using the subtraction of voltage protocols in the presence of 5 μM nifedipine. The black traces represent −30, −20, and 0 mV. Scale bars represent 250 pA and 50 ms. D: bar graph of the peak current density of nifedipine-resistant current at different time points in culture. The peak currents at −20 mV are −5.3 ± 1.3 pA/pF at day 2 (n = 6; ▪), −5.4 ± 0.9 pA/pF at day 3 (n = 11; ○), and − 5.5 ± 1.2 pA/pF at days 5–7 (n = 11; ▾) in culture. Values are means ± SE.
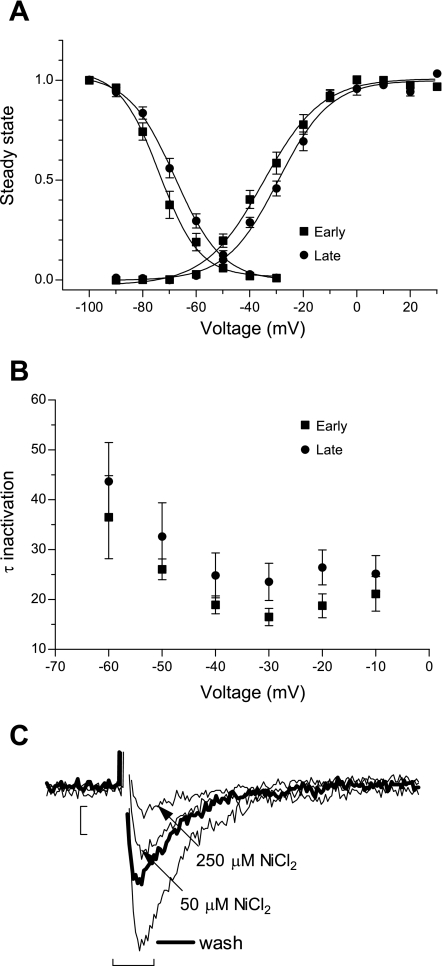
An earlier report of T-type current in NRVM (37) characterized a nifedipine-resistant component of the high-voltage activated L-type current, and, therefore, we sought to establish that the nifedipine-resistant current recorded in our experimental conditions passes through low-voltage-activated T-type channels, by analyzing in more detail the current properties. Figure 2A shows that the voltage dependence of activation and inactivation in the early time are typical for T-type current. At a later time in culture, there is an average 5-mV shift of the voltage dependence of activation and inactivation to more depolarized values. The parameters are summarized in Table 1. In Fig. 2B, the voltage dependence of the inactivation kinetic was faster with depolarization at both early and late times in culture, which is a hallmark of T-type channels (early vs. late: 26 ± 2.1 vs. 32.6 ± 6.7 ms at −50 mV, and 18.7 ± 2.4 vs. 26 ± 3.5 ms, P < 0.05, at −20 mV).
Fig. 2.
Electrophysiological characterization of nifedipine-resistant current in NRVM. A: voltage dependence of activation and inactivation at early (days 2–3, n = 17) and late (days 5–7, n = 11) time in culture, in normal conditions. Values are means ± SE fitted with Boltzmann equation. B: voltage dependence of the inactivation kinetic at early and late time of culturing. Values are means ± SE. C: representative traces showing sensitivity to 50 and 250 μM NiCl2 of the T-type current recorded at −20 mV pulse from −100 mV and the partial reversibility of NiCl2 block (wash). Scale bars represent 25 pA and 10 ms.
Table 1.
Parameters of the voltage dependence of activation and inactivation for T-type and L-type Ca2+ currents
| Voltage Dependence | T-type Current |
L-type Current |
||
|---|---|---|---|---|
| Activation | Inactivation | Activation | Inactivation | |
| Control “Early” | ||||
| V1/2 | −33.8±2.2 (17) | −72.4±1.7 (17) | −14.7±1.3 (13) | −38.7±8 (9) |
| k | 9.5±0.8 | −5.7±0.6 | 4.9±0.3 | −10.3±0.8 |
| AR | ||||
| V1/2 | −14.3±1.3‡ (12) | −63.9±2.8† (12) | −11.2±2.7 (11) | −33.7±8 (4) |
| k | 8±0.7 | −7.8±0.9* | 4.3±0.2 | −10.2±2.8 |
| T24R | ||||
| V1/2 | −25.1±1.9† (6) | −68.2±2.8 (6) | −17.1±1.3 (10) | −39.7±3 (5) |
| k | 9.8±0.6 | −7.7±0.6* | 4.5±0.4 | −12.6±3.4 |
| Control “Late” | ||||
| V1/2 | −28.5±1.8*(11) | −67.7±1.4*(11) | −17.5±1.7 (9) | −28.3±0.5 (9) |
| k | 9.7±0.8 | −8.4±0.7* | 5.1±0.4 | −9.8±1.3 |
| CR | ||||
| V1/2 | −26.8±2.5*(17) | −63.7±2 (17) | −19.3±0.9 (12) | −34.8±2.8 (12) |
| k | 7.3±0.7* | −6.9±0.6 | 4.4±0.5 | −8.2±1 |
Values are means ± SE for (n) recordings. AR, acute reoxygenation; T24R, 24 h of reoxygenation; CR, chronic reoxygenation; V1/2, half-activation voltage; k, slope. V1/2 and k values are derived from Boltzmann fit.
P < 0.05,
P < 0.01,
P < 0.001. Statistical significance is calculated vs. “early” control myocytes.
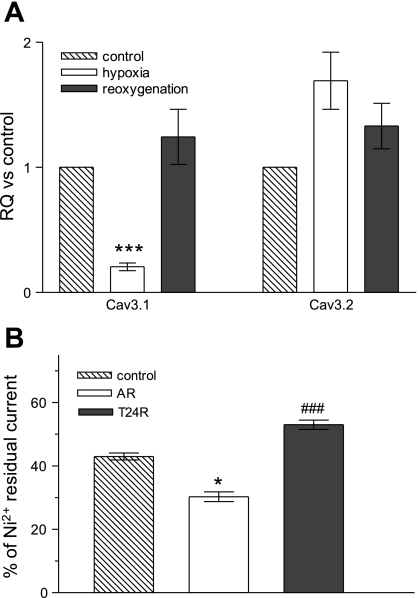
Pharmacological characterization of T-type channel α-subunits in heterologous expression systems has shown that 50 μM NiCl2 blocks 66% of Cav3.2 and 10–15% of Cav3.1, while 250 μM NiCl2 completely blocks Cav3.2 and blocks 50% of Cav3.1 (28). Figure 2C shows representative traces of the nifedipine-resistant current in the presence of NiCl2, showing the sensitivity of the current to 50 μM NiCl2 and further to 250 μM NiCl2, in a partially reversible manner, all characteristics of the T-type channel. When 50 μM NiCl2 was applied, the residual current represented 43 ± 3.5% of total T-type current (n = 10) at early times, and 52.9 ± 9.6% (n = 5) at later times in culture. These results indicate that the nifedipine-resistant current corresponds to the T-type Ca2+ channel and comprises both Cav3.1 and Cav3.2 α-subunits at both early and late culture times.
Effect of H/R conditions on calcium currents in cardiomyocytes.
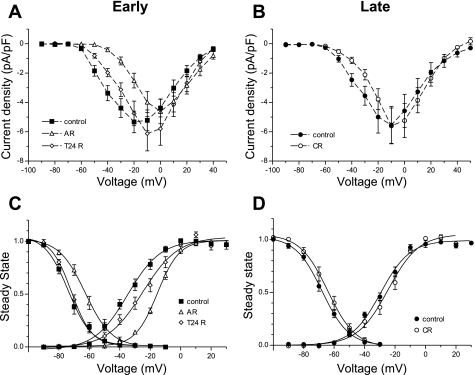
We next sought to test the effect of H/R injury on T-type channels in NRVM. Myocytes were exposed to hypoxic conditions for 18 h, followed by 24 h of normoxia, representing the reoxygenation phase. Currents were recorded 1–4 h after the hypoxic episode [acute reoxygenation (AR)], after 24 h of reoxygenation (T24R), and at 4–6 days of reoxygenation [chronic reoxygenation (CR)] and compared with time-matched control myocytes (Fig. 3). The I-V plots for early cultures (Fig. 3A) indicate that the peak current density was shifted in AR from −20 to 0 mV and not changed at T24R compared with control conditions. Notably, the reduction of T-type current density during hypoxia was significant at lower voltages, where these channels are typically active (−30 mV and below), without significantly altering the current density at higher voltages (∼0 mV and above).
Fig. 3.
Effects of hypoxia-reoxygenation (H/R) injury on T-type currents in NRVM. Current-voltage relation is shown for T-type current densities recorded in the first hours [acute reoxygenation (AR), peak: −4.6 ± 0.8 pA/pF at 0 mV, n = 112] and after 24 h of reoxygenation (T24R; peak: −6.1 ± 1.2 pA/pF at −10 mV, n = 14) (A) and chronic reoxygenation (CR; peak: −5.6 ± 1.2 pA/pF at −10 mV, n = 19) (B), compared with control myocytes. Values are means ± SE. C and D: corresponding changes in the voltage dependence of activation and inactivation at an early (C) and late (D) time in culture. Values are means ± SE fitted with Boltzmann equation.
In the context of the reversibility of the injury, we addressed the changes in T-type current properties at a later time in culture after reoxygenation. In Fig. 3B, there was no significant difference in the current density between treated and untreated myocytes and no noticeable shift in peak current density.
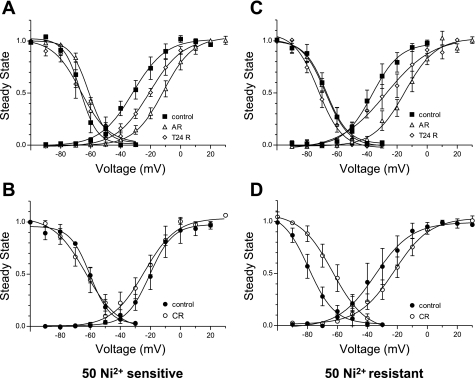
Next we analyzed in more detail the effect of H/R conditions on the voltage dependence of activation and inactivation of T-type current, as suggested from the shift in the peak current. Figure 3C shows that AR induces a robust positive shift of 20 and 11 mV, respectively, in the voltage dependence of activation and inactivation, meaning that more channels are in a closed state at resting membrane potential but would require stronger depolarization to activate. Reoxygenation partially reversed the activation shift and completely reversed inactivation. With CR (Fig. 3D), both activation and inactivation recovered to values similar to control myocytes. The parameters of voltage dependence of activation and inactivation are summarized in Table 1.
Compared with the effects of H/R injury on T-type currents in NRVM, L-type currents, recorded as nifedipine-sensitive current, did not change significantly with treatment or over time. Figure 4 shows similar analyses of the effect of H/R injury on L-type current at the early time in culture. Figure 4A shows that the peak current density varies with H/R injury, being decreased by hypoxia, partially recovered in the first hours of reoxygenation, and increased and shifted at T24R, with no significant changes in voltage dependence of activation or inactivation (Fig. 4B). With CR, there was no difference in the L-type current density or activation and inactivation compared with time-matched control myocytes. The parameters are summarized in Table 1.
Fig. 4.
Effects of H/R injury on L-type currents in NRVM. A: bar graph representation of the L-type peak current densities at 0 mV recorded in the first hours (AR, −27.2 ± 4.4 pA/pF, n = 13) and after T24R (−39.2 ± 5.6 pA/pF, n = 10) (A) compared with corresponding control myocytes (−33.8 ± 4.8 pA/pF, n = 13). Values are means ± SE. B: corresponding changes in the voltage dependence of activation and inactivation at an early time in culture. Values are means ± SE fitted with Boltzmann equation.
Effect of H/R conditions on voltage dependence of Ni2+ sensitive and resistant T-type currents.
Next, we attempted to distinguish whether the effect of hypoxia is mainly on Cav3.1 or Cav3.2 α-subunit. Starting from the assumption that the T-type current has a Ni2+-sensitive component that is composed mostly of Cav3.2 and a Ni2+-resistant component that is composed mainly of Cav3.1, we applied 50 μM NiCl2 to estimate the effect of H/R injury on each α-subunit. Figure 5, A and B, shows the analyses of activation and inactivation of the Ni2+-sensitive component of T-type current with H/R injury treatment. The results show that, during AR, both activation and inactivation were shifted to more depolarized membrane potentials, similar to total T-type current (Fig. 5A). After T24R, inactivation is completely reversed, while activation remains shifted to more depolarized values (Fig. 5A). At a later time in culture, there is no difference in the inactivation, but the activation is shifted to more hyperpolarized potentials (Fig. 5B), indicating increased probability of opening the channel at lower voltages. For the Ni2+-resistant component, only the activation was sensitive to hypoxic conditions and shifted to more depolarized membrane potentials, while inactivation was unchanged; reoxygenation reversed the effect of hypoxia on activation and shifted the inactivation to more hyperpolarized values (Fig. 5C), therefore decreasing the probability of opening the channels at lower voltages. At a later time in culture, the Ni2+ residual current displayed robust shifts in both activation and inactivation compared with time-matched control myocytes (Fig. 5D), indicating more availability of the channels at resting membrane potential, but requiring higher depolarization to open. The parameters of activation and inactivation are summarized in Table 2. Overall, these data suggest that Cav3.1 and Cav3.2 have different sensitivities to oxidative stress, and the total T-type Ca2+ current properties can be altered, depending on the balance between these two components.
Fig. 5.
Effects of H/R on voltage dependence of NiCl2-sensitive and resistant T-type currents. Voltage dependence of activation and inactivation for 50 μM Ni2+-sensitive T-type current was analyzed at early (A) and late (B) time in culture, as a tool to estimate the behavior of voltage-gated Ca2+ (Cav)3.2 in H/R conditions. Similar analysis is shown of voltage dependence of activation and inactivation for 50 μM Ni2+-resistant T-type current at early (C) and late (D) time in culture, as a tool to estimate the behavior of Cav3.1 in H/R conditions. Values are means ± SE fitted with Boltzmann equation.
Table 2.
Parameters of the voltage dependence of activation and inactivation for currents separated by 50 μM NiCl2
| Voltage Dependence | 50 μM Ni2+ Sensitive |
50 μM Ni2+ Resistant |
||
|---|---|---|---|---|
| Activation | Inactivation | Activation | Inactivation | |
| Control “Early” | ||||
| V1/2 | −30.5±3.7 (8) | −66.6±2.8 (6) | −32.3±3.9 (8) | −68.8±3.7 (5) |
| k | 10.4±1.4 | −4±1 | 8.3±2.4 | −7.5±0.9 |
| AR | ||||
| V1/2 | −10.5±2.9‡ (8) | −61.6±1* (7) | −15.7±4.4† (7) | −67.7±2.2 (6) |
| k | 8.9±0.8 | −5.7±0.6 | 8.9±0.8 | −7.5±0.8 |
| T24R | ||||
| V1/2 | −20.9±3.4 (5) | −66.6±4 (4) | −22.5±6.8 (5) | −72.4±3 (4) |
| k | 10.9±1.1 | −7.5±2.5 | 14.4±2.1 | −7.4±0.4 |
| Control “Late” | ||||
| V1/2 | −17.5±5.1 (3) | −58.6±1.9 (5) | −31.8±6.6 (4) | −78.3±4.7 (5) |
| k | 8.8±1.1 | −5.5±1.7 | 9.2±2.9 | −8.6±2.1 |
| CR | ||||
| V1/2 | −25.1±4.7 (6) | −61.1±4.9 (7) | −22.2±4.6 (7) | −62.8±4.9 (6)* |
| k | 8.6±1.1 | −8.6±1.1 | 9.3±1.4 | −6.9±0.6 |
Values are means ± SE for (n) recordings. V1/2 and k values are derived from Boltzmann fit.
P < 0.05,
P < 0.01,
P < 0.001. Statistical significance is calculated vs. “early” control myocytes.
Effect of H/R conditions on T-type calcium channel mRNA levels in cardiomyocytes.
To determine whether H/R injury regulates expression of Cav3.1 and/or Cav3.2 at the transcriptional level, we measured mRNA expression levels in NRVM subjected to the same H/R injury conditions. Figure 6A shows that, in hypoxic conditions, the Cav3.1 α-subunit mRNA level was decreased nearly fivefold [relative quantification (RQ): 0.21 ± 0.03, n = 20, P < 0.001], while Cav3.2 was not significantly increased (RQ: 1.69 ± 0.23, n = 17). After T24R, mRNA level returned to normal for Cav3.1 (RQ: 1.24 ± 0.22, n = 12) and remained unchanged for Cav3.2 (RQ: 1.54 ± 0.27, n = 12). To compare mRNA expression and T-type current levels, Figure 6B summarizes changes in the Ni2+ residual current, as a measure of Cav3.1 current at the same time points, showing a corresponding decrease in the Cav3.1 current following hypoxia (from 43 ± 3.5%, n = 10, to 30.3 ± 4.3%, n = 8, P < 0.05) with recovery in the first T24R (53 ± 3.9%, n = 7, P < 0.001 compared with AR). The mRNA data correlate well with the regulation of Ni2+ sensitive T-type currents, corroborating downregulation of Cav3.1 during the hypoxic phase and its upregulation during the reoxygenation phase. Taken together, molecular and electrophysiological measurements of T-type Ca2+ channel expression and function show that both Cav3.1 and Cav3.2 are differentially regulated in conditions of hypoxia and reoxygenation in cultured cardiac myocytes.
Fig. 6.
Effects of H/R injury on mRNA levels of T-type channel α-subunits in NRVM. A: bar graphs representing the relative expression of mRNA for Cav3.1 and Cav3.2 after 18–24 h of incubation in hypoxia followed by 24 h of reoxygenation. Relative quantification (RQ) was estimated based on the threshold cycle (ΔΔCT) method, using 18S rRNA as endogenous control vs. NRVM maintained in normoxic conditions for the same length of time. Values are means ± SE. B: bar graph representing the percentage of 50 μM Ni2+-resistant current out of total T-type current. Values are means ± SE for n = 10, 8, and 7 myocytes. *P < 0.05 and ***P < 0.001 calculated vs. control. ###P < 0.001 calculated vs. AR.
Effect of chemical H/R conditions on T-type calcium channels in cardiomyocytes.
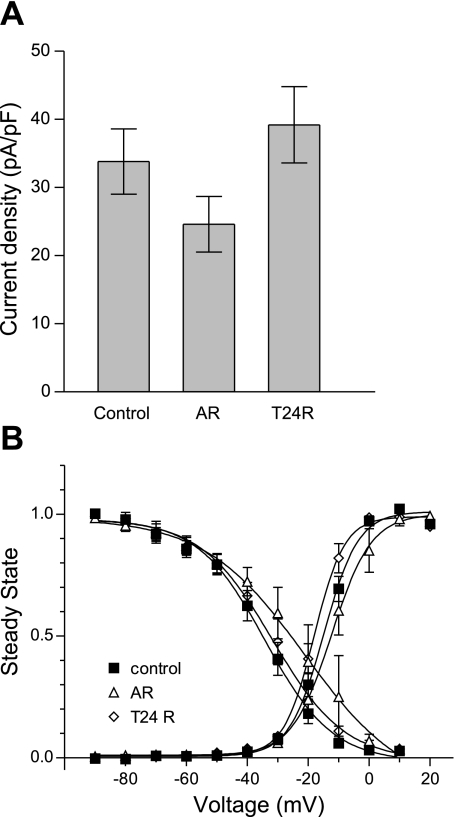
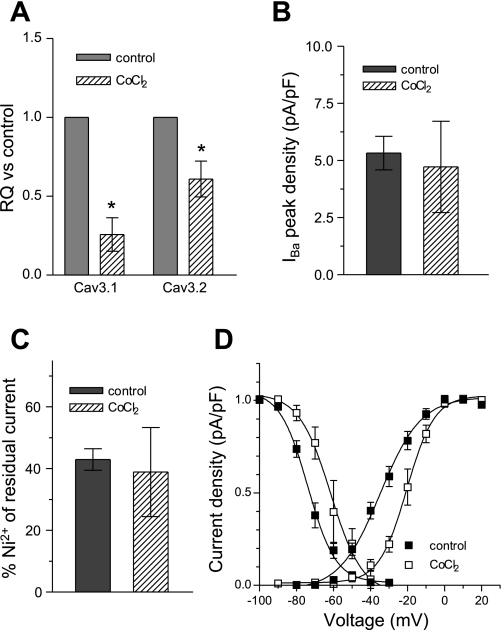
The regulation of Cav3.1 mRNA during H/R injury led us to study the possible effectors involved in T-type channel transcriptional regulation. To test whether Cav3.1 regulation is mediated by HIF-1α, we induced conditions of chemical hypoxia by incubating NRVM with 100 μM CoCl2 for 48 h, which increases the levels of HIF-1α via the inhibition of prolyl hydroxylase (7). The results presented in Fig. 7A show that 48 h of incubation in CoCl2 decreased the mRNA levels of both Cav3.1 and Cav3.2 (RQ: 0.26 ± 0.1 and 0.61 ± 0.11). These results suggest that, in NRVM, HIF-1α contributes to the downregulation of Cav3.1 during hypoxia, but is not likely to mediate the effects observed on Cav3.2. Incubation of NRVM with 100 μM CoCl2 for 48 h did not alter the peak amplitude (Fig. 7B) or Ni2+ sensitivity (Fig. 7C) of the T-type current, whereas both voltage dependence of activation and inactivation were shifted to more depolarized values (15 and 12 mV, respectively) (Fig. 7D), with a similar trend to that seen in hypoxic conditions.
Fig. 7.
Effect of 100 μM CoCl2 on T-type current in NRVM. Bar graphs represent the changes in T-type current mRNA (A; n = 4), current density at peak (B), and the percentage of 50 μM Ni2+ residual current (C) after 48 h of 100 μM CoCl2. D: analysis of voltage dependence of activation and inactivation of T-type current in the presence of CoCl2. IBa, barium current. Values are means ± SE for 5 myocytes. *P < 0.05.
While many cellular signaling pathways are affected during H/R injury, both hypoxia and reoxygenation share a common effector that is generated from different sources: reactive oxygen species (ROS). We attempted to simulate the reoxygenation phase by incubating NRVM in 100 μM H2O2 for 24 h, to test whether the upregulation of T-type channels observed during the reoxygenation phase might be ROS dependent. Similar real-time PCR measurements of mRNA levels for Cav3.1 and Cav3.2 indicated that, in the presence of 100 μM H2O2, these genes were downregulated compared with control conditions (0.61 ± 0.31 for Cav3.1, 0.55 ± 0.07, P < 0.05 for Cav3.2), confirming results reported previously (35). These results suggest that increased levels of ROS alone do not induce the upregulation of T-type channels recorded during reoxygenation, and, therefore, other pathways activated during injury might be responsible. Another explanation might be that the sources of ROS as well as the active pathway at the time of ROS generation are important for the trend of T-type channel regulation.
DISCUSSION
In the cardiovascular system, T-type Ca2+ channels are formed by either Cav3.1 or Cav3.2 α-subunits. When expressed in heterologous cells, each α-subunit cDNA can form individual functional low-voltage-activated channels with similar electrophysiological properties and differential sensitivity to nickel (5, 28, 36). Although T-type Ca2+ channels have been reexpressed in pathology associated with hypoxic conditions, such as heart failure (17), little is known about the effect of hypoxia and reoxygenation on the regulation of these channels. In this study, we investigated the effects of H/R injury on T-type Ca2+ channels at the mRNA and current levels. Patch clamp electrophysiology in the presence of NiCl2 was used to distinguish the two subtypes, which allowed us to discern differential effects of H/R on the two T-type channels at the functional level. Hypoxic conditions led to decreased expression of Cav3.1 mRNA and reproducible shifts in the voltage dependence of activation and inactivation of the T-type Ca2+ current. The effects of CoCl2-induced hypoxic injury on Cav3.1 mRNA and the T-type Ca2+ current resembled the effects of physical hypoxia, suggesting the involvement of a HIF-1α-dependent mechanism in the negative regulation of Cav3.1. Upon reoxygenation, the Cav3.1 mRNA and current voltage dependence were restored to control, which could not be explained by the direct actions of ROS on the channel. Therefore, these experiments implicate distinct regulation of Cav3.1 and Cav3.2 T-type channel expression and function during H/R injury in cardiomyocytes.
A decrease in the amplitude of the T-type calcium current with time in culture has been observed previously (15). Because our study required prolonged culture times up to 10 days, we characterized the expression of T-type currents during this time interval, and we show that NRVM cultured in serum-free medium maintain T-type calcium currents up to 7 days in culture, with no change in the peak current density. However, we noted changes in the voltage dependence of T-type currents in early vs. late time of culturing. The observed increase in the residual Ni2+-resistant current component in late vs. early cultures suggests that the Cav3.1 current becomes more prominent with prolonged culture of NRVM. These observations are consistent with isoform switching previously reported in developing cardiomyocytes (33). Furthermore, our unpublished observations in mouse embryonic stem cells differentiating to cardiomyocytes suggest that, while Cav3.2 may be necessary during the early differentiation phase, the expression of Cav3.1 becomes more predominant as the cardiomyocytes mature, supporting the idea that the Cav3.1 and Cav3.2 channels are differentially regulated and have distinct functions.
The effect of hypoxia on T-type channel regulation has been investigated in PC12 cells (7) and adrenal chromaffin cells (3), but not in cardiomyocytes. In our experimental conditions, both physical and chemical oxidative stress (CoCl2) induced similar shifts in the voltage dependence of T-type currents in cardiomyocytes, suggesting that different forms of oxidative stress can induce changes in T-type channel expression and function. The analyses of voltage dependence indicate that activation gating of both Cav3.1 and Cav3.2 is sensitive to hypoxic conditions, whereas, for inactivation, only Cav3.2 is affected by hypoxia. The effect of hypoxia on the activation (positive shift) might be related to the loss of spontaneous activity with hypoxia. Since T-type calcium channels are involved in late diastolic depolarization in pacemaker cells (52), this relation can be established in particular for NRVM. For example, when hypoxia shifts the activation of T-type channels, neonatal myocytes require more time to initiate the action potential, therefore decreasing the spontaneous activity. Also, our results suggest that T-type current is not completely blocked in hypoxic conditions, but the window current is shifted to higher voltages, based on the positive shift of both activation and inactivation. Without this shift, Cav3.2 would be completely inactive at hypoxic resting membrane potentials and would have no contribution.
While relatively little is known about the posttranslational modulation of T-type channels, it is possible that such modifications could alter the gating properties of these channels. For example, calmodulin-regulated protein kinase II shifts Cav3.2 activation to more hyperpolarized values without altering the inactivation in human embryonic kidney (HEK)-293 cells, increasing the T-type current at lower voltages (2). It is also conceivable that, in pathological conditions such as H/R injury, T-type calcium channel α-subunits interact with auxiliary subunits to regulate their function, as in the modulation of high-voltage-activated P/Q-type Ca2+ channels by Ca2+ channel β4-subunits (14). Also, recent studies revealed selective upregulation of the β3 auxiliary subunit in atrial myocytes isolated from a rat model of pulmonary hypertension-induced heart failure (24). These observations warrant further investigation of possible modulation of T-type calcium channels, specifically in pathological conditions.
Our observations in NRVM differ from those obtained for T-type channels heterologously expressed in HEK-293 cells exposed to acute hypoxia (20 mmHg) (11), which showed no significant effects on the current amplitude and no change in the voltage dependence of activation and inactivation. This might be explained by different experimental models of H/R injury, the use of different mechanisms by NRVM vs. HEK-293 cells in response to H/R injury, and/or possible modulatory effects on the T-type α-subunits that might depend on the cell background.
Our data show that, in NRVM, T-type Ca2+ channels are regulated at the transcriptional level during H/R injury. Hypoxic conditions had a robust negative effect on Cav3.1 mRNA expression in NRVM via HIF-1α (as suggested by CoCl2 stimulation), an effect that was not previously reported in other systems. The hypoxia effect on Cav3.2 is in agreement neither with the HIF-1α-dependent upregulation reported in PC12 and chromaffin cells, nor with the robust upregulation of Cav3.2 mRNA that we have observed in resistance pulmonary artery smooth muscle cells in the same conditions (data not published), indicating possible tissue or developmental stage-specific mechanisms for regulation of Cav3.2.
Compared with its decrease in the hypoxic phase, Cav3.1 mRNA was significantly upregulated in the first T24R. Notably, our experimental culture conditions of chronic (up to 4 days) reoxygenation failed to reproduce the long-term upregulation of either Cav3.1 or Cav3.2 previously observed in vivo in pathological conditions associated with ischemia (18, 43, 44). Future studies are needed to address additional factors induced during in vivo cardiac ischemia-reperfusion, but not present in cardiomyocyte cultures, that may influence T-type Ca2+ channel expression and function.
These studies were conducted in ventricular myocytes isolated from newborn rats, which are a suitable system for studying T-type channel regulation and modulation for two main reasons: 1) NRVM express endogenous T-type currents due to their developmental stage; and 2) they can be maintained in culture for up to 2 wk, creating an in vitro system to study the regulation of T-type channels in response to cumulative pathological stimuli. While it is likely that T-type Ca2+ channels are reexpressed in adult ventricular myocytes in H/R injury, these cells are less amenable to longer term culture that is required for mechanistic gene regulation studies. Nevertheless, most of the pathways active in NRVM are reactivated in injured adult myocytes, and, furthermore, our data derived from immature cardiomyocytes may still apply to cardiovascular complications associated with hypoxia in early development.
In hypoxic conditions, the metabolism of the cell shifts to glycolysis, and the ATP-dependent processes are slowed down with a direct impact on intracellular calcium regulation. In these conditions, the downregulation of calcium channels can be considered a protective mechanism to counteract the calcium overload. Our observed shift of the T-type window current to more depolarized values may represent a compensatory mechanism for the impaired function of L-type channels, but it could also be detrimental by providing a sustained source of calcium influx in myocytes with impaired ability to pump it out, due to the reduction of ATP. In this context, the observed functional regulation of T-type Ca2+ channel subunits in NRVM subjected to H/R injury suggests that they may play an increasing role in calcium homeostasis in pathological conditions.
From these studies, we conclude that T-type channels can be differentially modulated by changes in the redox state of the cardiomyocyte. Elucidating the mechanisms involved will address the open question of the interactions and function of this class of Ca2+ channels in cardiomyocytes.
GRANTS
The studies were supported by the Falk Fellowship Foundation (Cardiovascular Institute, Loyola University of Chicago) and National Heart, Lung, and Blood Institute R01 HL075115.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Allen Samarel and Rekha Iyengar for providing us with the NRVM.
REFERENCES
- 1.Barac-Nieto M, Constantinescu A, Pina-Benabou MH, Rozental R. Hypoxic rise in cytosolic calcium and renal proximal tubule injury mediated by a nickel-sensitive pathway. Exp Biol Med (Maywood) 229: 1162–1168, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Barrett PQ, Lu HK, Colbran R, Czernik A, Pancrazio JJ. Stimulation of unitary T-type Ca2+ channel currents by calmodulin-dependent protein kinase II. Am J Physiol Cell Physiol 279: C1694–C1703, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Carabelli V, Marcantoni A, Comunanza V, de Luca A, Diaz J, Borges R, Carbone E. Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol 584: 149–165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, Chen CC. The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res 104: 522–530, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Cribbs LL, Lee JH, Daud A, Satin J, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of α1H from human heart, a member of the T-type calcium channel gene family. Circ Res 83: 103–109, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflügers Arch 450: 363–371, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Del Toro R, Levitsky KL, Lopez-Barneo J, Chiara MD. Induction of T-type calcium channel gene expression by chronic hypoxia. J Biol Chem 278: 22316–22324, 2003 [DOI] [PubMed] [Google Scholar]
- 8.El Jamali A, Freund C, Rechner C, Scheidereit C, Dietz R, Bergmann MW. Reoxygenation after severe hypoxia induces cardiomyocyte hypertrophy in vitro: activation of CREB downstream of GSK3beta. FASEB J 18: 1096–1098, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Fares N, Gomez JP, Potreau D. T-type calcium current is expressed in dedifferentiated adult rat ventricular cells in primary culture. C R Acad Sci III 319: 569–576, 1996 [PubMed] [Google Scholar]
- 10.Fearon IM, Palmer AC, Balmforth AJ, Ball SG, Varadi G, Peers C. Modulation of recombinant human cardiac L-type Ca2+ channel alpha1C subunits by redox agents and hypoxia. J Physiol 514: 629–637, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearon IM, Randall AD, Perez-Reyes E, Peers C. Modulation of recombinant T-type Ca2+ channels by hypoxia and glutathione. Pflügers Arch 441: 181–188, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Ferron L, Capuano V, Ruchon Y, Deroubaix E, Coulombe A, Renaud JF. Angiotensin II signaling pathways mediate expression of cardiac T-type calcium channels. Circ Res 93: 1241–1248, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Gao B, Sekido Y, Maximov A, Saad M, Forgacs E, Latif F, Wei MH, Lerman M, Lee JH, Perez-Reyes E, Bezprozvanny I, Minna JD. Functional properties of a new voltage-dependent calcium channel alpha(2)delta auxiliary subunit gene (CACNA2D2). J Biol Chem 275: 12237–12242, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geib S, Sandoz G, Mabrouk K, Matavel A, Marchot P, Hoshi T, Villaz M, Ronjat M, Miquelis R, Leveque C, de Waard M. Use of a purified and functional recombinant calcium-channel beta4 subunit in surface-plasmon resonance studies. Biochem J 364: 285–292, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez JP, Potreau D, Branka JE, Raymond G. Developmental changes in Ca2+ currents from newborn rat cardiomyocytes in primary culture. Pflügers Arch 428: 241–249, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Hansen JP, Chen RS, Larsen JK, Chu PJ, Janes DM, Weis KE, Best PM. Calcium channel gamma6 subunits are unique modulators of low voltage-activated (Cav3.1) calcium current. J Mol Cell Cardiol 37: 1147–1158, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Huang B, Deng L, Qin D, Boutjir M, El-Sharif N. Reexpression of T-type Ca2+ channel gene and current in post-infarction remodeled rat left ventricle. Circ Res 98, Suppl: I-840, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Huang B, Qin D, Deng L, Boutjir M, El-Sherif N. Reexpression of T-type Ca2+ channel gene and current in post-infarction remodeled rat left ventricle. Cardiovasc Res 46: 442–449, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Izumi T, Kihara Y, Sarai N, Yoneda T, Iwanaga Y, Inagaki K, Onozawa Y, Takenaka H, Kita T, Noma A. Reinduction of T-type calcium channels by endothelin-1 in failing hearts in vivo and in adult rat ventricular myocytes in vitro. Circulation 108: 2530–2535, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J Neurophysiol 99: 3151–3156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joksovic PM, Doctor A, Gaston B, Todorovic SM. Functional regulation of T-type calcium channels by s-nitrosothiols in the rat thalamus. J Neurophysiol 97: 2712–2721, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kawakami M, Okabe E. Superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum through ryanodine receptor Ca2+ channel. Mol Pharmacol 53: 497–503, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol 41: 256–264, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Koyama T, Ono K, Watanabe H, Ohba T, Murakami M, Iino K, Ito H. Molecular and electrical remodeling of L- and T-type Ca(2+) channels in rat right atrium with monocrotaline-induced pulmonary hypertension. Circ J 73: 256–263, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Kuwahara K, Takano M, Nakao K. Pathophysiological significance of T-type Ca2+ channels: transcriptional regulation of T-type Ca2+ channel–regulation of CACNA1H by neuron-restrictive silencer factor. J Pharm Sci 99: 211–213, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lalevee N, Rebsamen MC, Barrere-Lemaire S, Perrier E, Nargeot J, Benitah JP, Rossier MF. Aldosterone increases T-type calcium channel expression and in vitro beating frequency in neonatal rat cardiomyocytes. Cardiovasc Res 67: 216–224, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Daud A, Cribbs LL, Lacerda AE, Pereverzev A, Klockner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci 19: 1912–1921, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type Ca channels: low concentrations selectively block α1H. Biophys J 77: 3034–3042, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leuranguer V, Monteil A, Bourinet E, Dayanithi G, Nargeot J. T-type calcium currents in rat cardiomyocytes during postnatal development: contribution to hormone secretion. Am J Physiol Heart Circ Physiol 279: H2540–H2548, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 282: C227–C241, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Li M, Zhang M, Huang L, Zhou J, Zhuang H, Taylor JT, Keyser BM, Whitehurst RM., Jr T-type Ca2+ channels are involved in high glucose-induced rat neonatal cardiomyocyte proliferation. Pediatr Res 57: 550–556, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Mizuta E, Miake J, Yano S, Furuichi H, Manabe K, Sasaki N, Igawa O, Hoshikawa Y, Shigemasa C, Nanba E, Ninomiya H, Hidaka K, Morisaki T, Tajima F, Hisatome I. Subtype switching of T-type Ca2+ channels from Cav3.2 to Cav31 during differentiation of embryonic stem cells to cardiac cell lineage. Circ J 69: 1284–1289, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Nilius B, Hess P, Lansman JB, Tsien RW. A novel type of cardiac calcium channel in ventricular cells. Nature 316: 443–446, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Pastukh V, Wu S, Schaffer S. A114. Regulation of T-type calcium channel in rat cardiomyocytes by oxidative stress. J Mol Cell Cardiol 40: 891–892, 2006 [Google Scholar]
- 36.Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low voltage-activated T-type calcium channel. Nature 391: 896–900, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Pignier C, Potreau D. Characterization of nifedipine-resistant calcium current in neonatal rat ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol 279: H2259–H2268, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases K(V) channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol 280: L801–L812, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Razeghi P, Essop MF, Huss JM, Abbasi S, Manga N, Taegtmeyer H. Hypoxia-induced switches of myosin heavy chain iso-gene expression in rat heart. Biochem Biophys Res Commun 303: 1024–1027, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Rowe GT, Manson NH, Caplan M, Hess ML. Hydrogen peroxide and hydroxyl radical mediation of activated leukocyte depression of cardiac sarcoplasmic reticulum. Participation of the cyclooxygenase pathway. Circ Res 53: 584–591, 1983 [DOI] [PubMed] [Google Scholar]
- 41.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J 12: 1681–1692, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samarel AM, Engelmann GL. Contractile activity modulates myosin heavy chain-beta expression in neonatal rat heart cells. Am J Physiol Heart Circ Physiol 261: H1067–H1077, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Sen L, Smith TW. T-type Ca2+ channels are abnormal in genetically determined cardiomyopathic hamster hearts. Circ Res 75: 149–155, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Takebayashi S, Li Y, Kaku T, Inagaki S, Hashimoto Y, Kimura K, Miyamoto S, Hadama T, Ono K. Remodeling excitation-contraction coupling of hypertrophied ventricular myocytes is dependent on T-type calcium channels expression. Biochem Biophys Res Commun 345: 766–773, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Vassort G, Talavera K, Alvarez JL. Role of T-type Ca2+ channels in the heart. Cell Calcium 40: 205–220, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Morishima M, Zheng M, Uchino T, Mannen K, Takahashi A, Nakaya Y, Komuro I, Ono K. Transcription factors Csx/Nkx2.5 and GATA4 distinctly regulate expression of Ca2+ channels in neonatal rat heart. J Mol Cell Cardiol 42: 1045–1053, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Wang YX, Dhulipala PK, Kotlikoff MI. Hypoxia inhibits the Na+/Ca2+ exchanger in pulmonary artery smooth muscle cells. FASEB J 14: 1731–1740, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Estacion M, Mordan LJ. Ca2+ influx via T-type channels modulates PDGF-induced replication of mouse fibroblasts. Am J Physiol Cell Physiol 265: C1239–C1246, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16: 1151–1162, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Xu XP, Best PM. Increase in T-type calcium current in atrial myocytes from adult rats with growth hormone-secreting tumors. Proc Natl Acad Sci USA 87: 4655–4659, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasui K, Niwa N, Takemura H, Opthof T, Muto T, Horiba M, Shimizu A, Lee JK, Honjo H, Kamiya K, Kodama I. Pathophysiological significance of T-type Ca2+ channels: expression of T-type Ca2+ channels in fetal and diseased heart. J Pharm Sci 99: 205–210, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Zhou Z, Lipsius SL. T-type calcium current in latent pacemaker cells isolated from cat right atrium. J Mol Cell Cardiol 26: 1211–1219, 1994 [DOI] [PubMed] [Google Scholar]