Abstract
Ongoing investigation of the electrophysiology and pathophysiology of the human atria requires an accurate representation of the membrane dynamics of the human atrial myocyte. However, existing models of the human atrial myocyte action potential do not accurately reproduce experimental observations with respect to the kinetics of key repolarizing currents or rate dependence of the action potential and fail to properly enforce charge conservation, an essential characteristic in any model of the cardiac membrane. In addition, recent advances in experimental methods have resulted in new data regarding the kinetics of repolarizing currents in the human atria. The goal of this study was to develop a new model of the human atrial action potential, based on the Nygren et al. model of the human atrial myocyte and newly available experimental data, that ensures an accurate representation of repolarization processes and reproduction of action potential rate dependence and enforces charge conservation. Specifically, the transient outward K+ current (It) and ultrarapid rectifier K+ current (IKur) were newly formulated. The inwardly recitifying K+ current (IK1) was also reanalyzed and implemented appropriately. Simulations of the human atrial myocyte action potential with this new model demonstrated that early repolarization is dependent on the relative conductances of It and IKur, whereas densities of both IKur and IK1 underlie later repolarization. In addition, this model reproduces experimental measurements of rate dependence of It, IKur, and action potential duration. This new model constitutes an improved representation of excitability and repolarization reserve in the human atrial myocyte and, therefore, provides a useful computational tool for future studies involving the human atrium in both health and disease.
Keywords: ionic model, repolarization, potassium current
accurate representation of the ionic currents of the human atrial myocyte is essential for the ongoing investigation of human atrial electrophysiology and pathophysiology. It is now well established that the elucidation of mechanisms underlying the complex phenomena that occur in highly integrative systems, such as atrial tissue, requires that experimental data be incorporated such that models are both accurate and biophysically based. Within the past decade, advances in experimental methods have resulted in a much-improved characterization of repolarizing currents in the human atria (21, 50, 59–61). New insights into the underlying channel isoforms responsible for these K+ currents are also now available (32, 41, 53, 54, 56). However, findings regarding essential repolarization processes in the human atria have been incorporated into none of the available mathematical models of the action potential (AP) (7, 40).
Of particular interest are the recent experimental findings regarding the transient outward K+ current (It; Kv4.3) and ultrarapid delayed rectifier K+ current (IKur; Kv1.5). It contributes significantly to the early repolarization phase of the human atrial AP, which is analogous to its contribution to phase 1 repolarization in human ventricular myocytes. IKur, a sustained outward current that is expressed in human atrial but not ventricular myocytes, contributes to both early and late repolarization in the human atrial myocyte. The rapid and slow delayed rectifier K+ currents (IKr and IKs, respectively), which are essential for late repolarization in the human ventricle, are very small (or even undetectable) in the human atria (18). The inward rectifier K+ current (IK1), which contributes to late repolarization and stabilizes the resting membrane potential (RMP), is expressed at a much lower density in the human atria compared with the ventricles; IK1 has been measured in the human atria at densities that are 10–50% of those found in the human ventricle (30).
The main goals of the present study were twofold: 1) to create a new model of the human atrial AP that accurately represents repolarization in the human atrial myocyte, which will be accomplished by the reformulation of It and IKur, and 2) to analyze the contribution of repolarization processes to the rate dependence of the human atrial AP. The result was a well-quantified model of the human atrial myocyte that ensures the availability of a physiologically relevant and robust model for cell-, tissue-, and, eventually, organ-level studies of atrial electrophysiological phenomena, including arrhythmogenesis.
METHODS
Basic Assumptions
Many fundamental aspects of the mathematical model presented in this article are based on a previous model of the adult human atrial myocyte AP, that of Nygren et al. (40). Our model will be referred to as the human atrial myocyte with new repolarization (hAMr) model. It consists of an electric equivalent circuit for the sarcolemma, including ionic currents based on Hodgkin-Huxley formalisms, as well as a fluid compartment model to account for changes in ionic concentrations, as described previously (40). The dimensions and capacitance of the human atrial myocyte are the same as those in the original article (40); specifically, total myocyte volume (Voli) and cell membrane capacitance (Cm) were considered to be 5.884 μl and 50 pF, respecitively.
Transmembrane Ionic Currents
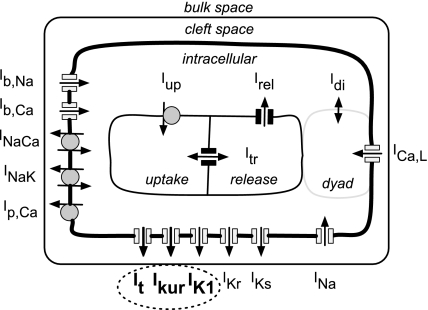
Figure 1 is a schematic of the hAMr model of the human atrial myocyte. The formulation includes all time- and voltage-dependent ion currents that contribute to the generation of the human atrial AP: the Na+ current (INa), the L-type Ca2+ current (ICa,L), and the K+ currents It, IKur, IK1, IKr, and IKs. In addition, our model includes electrogenic pump and exchanger currents: the sarcolemmal Ca2+ pump currrent (ICa,P), the Na+/Ca2+ exchanger current (INaCa), and the Na+-K+ pump current (INaK), which are responsible for the maintenance of intracellular ion concentrations. Na+ and Ca2+ time-independent or background currents (IBNa and IBCa, respectively) are also included. The K+ currents that are the focus of the present study, It, IKur, and IK1, are shown in bold and indicated by the dashed ellipse in Fig. 1; the formulations of the remaining currents and ion fluxes are based on the formulation of Ref. 40, with some parameters updated. In the present model, It and IKur are the transient and sustained components, respectively, of the outward K+ current. The significant reformulation and novel contribution of these key repolarization currents are described in detail in the next two sections.
Fig. 1.
Schematic representation of the mathematical model of the human atrial myocyte (hAMr model). Ion channel-mediated currents as well as electrogenic pump and ion exchanger currents are shown. Significant compartments include the extracellular, intracellular, and cleft spaces as well as the uptake and release compartments within the sarcoplasmic reticulum (40). The dashed ellipse indicates the currents that are the main focus of this new model of the human atrial AP. The myocyte was modeled as a cylindrical cell with a length of 130 μm and a diameter of 11 μm. Cell capacitance was 50 pF. See the Glossary for definitions of abbreviations used in this and subsequent figures.
It
Due to a lack of experimental data, the Nygren et al. (40) formulation of It in the human atrial myocyte was based on experimental data obtained from rabbit atrial myocytes (16). It is now well accepted that the K+ channel underlying It in the rabbit atria is the Kv1.4 gene product, whereas in the human atria, the α-subunit encoded by Kv4.3 underlies this current (14, 16, 42, 53, 54, 56). In human atria, It activates very rapidly (∼10 ms) (46) and then inactivates more slowly (∼20 ms) (56). The recovery from inactivation is strongly voltage dependent (56) and relatively rapid (on the order of 10s of ms) (55, 56). In contrast, in the model of Nygren et al. (40), the time course of recovery was on the order of ∼300 ms at hyperpolarized potentials.
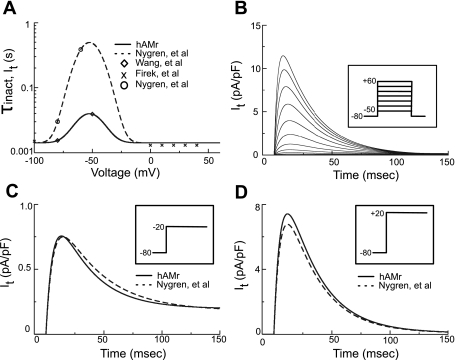
Figure 2 shows the new implementation of It in the hAMr model. Figure 2A shows implementations made to achieve improved kinetic descriptions for the time constant of inactivation (τs). These were based on newer experimental data from the human atrium (56). Figure 2B shows a family of current traces of It generated by our new formulation when subjected to the voltage-clamp protocol shown in the inset; note that the current is both rapidly activating and inactivating. Figure 2, C and D, compare current traces generated by the hAMr model and the model of Nygren et al. when subjected to identical voltage clamps from −80 to −20 and +20 mV, respectively (as indicated in the insets). Note the more rapid decay of the updated It compared with the same current in the model of Nygren et al. for negative (Fig. 2C) versus positive (Fig. 2D) holding potentials. A complete mathematical description of the new formulation of It can be found in the appendix.
Fig. 2.
Modification of It. A: data describing the modification of voltage-dependent kinetics of reactivation in Ref. 40 (○). The time constant of inactivation/recovery (τinact) is shown by the dashed trace. The present model is based on more complex recovery data [◊ (56)]. This, combined with inactivation data [“X”s (18)], led to a new formulation for the time constant of inactivation/recovery in the hAMr model (solid trace). B: a family of current recordings of It simulated using a holding potential of −80 mV and a series of 750-ms voltage clamp steps from −50 to +60 mV in 10-mV increments applied at 0.1 Hz (as shown in the inset). C: simulated currents after a voltage clamp step from a holding potential of −80 to −20 mV (as shown in the inset) for the hAMr model (solid trace) and the model of Nygren et al. (dashed trace). D: simulated currents after a voltage clamp step from a holding potential of −80 to +20 mV (as shown in the inset) for the hAMr model (solid trace) and the model of Nygren et al. (dashed trace).
IKur
In the Nygren et al. (40) model of the human atrial myocyte AP, recovery from inactivation of the “sustained outward K+ current” (Isus) was represented via a time course on the order of ∼300 ms and included a partial inactivation of 40%. Isus is now known to be IKur, a member of the family of ultrarapidly delayed rectifier K+ currents encoded by the Kv1.5 gene (13). IKur activates very rapidly (<10 ms) (1) and inactivates very slowly (∼3 s) but completely.
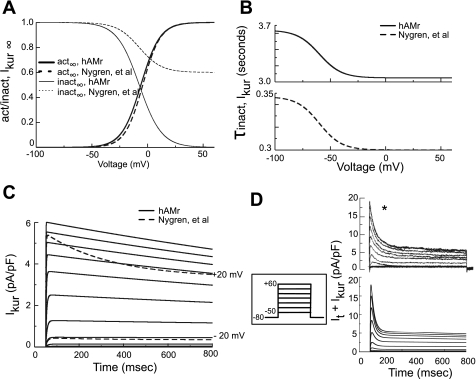
Figure 3 shows the major changes to IKur made to accurately simulate the kinetics of inactivation and recovery of this current. Figure 3A shows the new implementations of the steady-state activation and inactivation of IKur. Steady-state activation was adjusted to match data from human atrial myocytes (13). The partial steady-state inactivation present in the original model (40) was replaced by a complete inactivation process in the hAMr model (58). Figure 3B shows the new voltage dependence of the time constant of inactivation at hyperpolarized potentials; it was increased by ∼10-fold (15) (Fig. 3B, top, solid black trace). Figure 3C shows current traces of IKur generated by the voltage clamp steps shown in the inset. For holding potentials of ±20 mV, current traces of Isus, the analogous current from the model of Nygren et al., are also shown for comparison (Fig. 3C, dashed traces); note that Isus decays much more rapidly compared with IKur. A comparison of the sum of It and IKur elicited via the voltage-clamp protocol shown in the inset revealed a close correspondence between the recent experiment (Fig. 3D, top) (21) and hAMr model (Fig. 3D, bottom). A complete mathematical description of the formulation of IKur in the present model can be found in the appendix.
Fig. 3.
Modification of IKur. A: steady-state activation and inactivation variables for IKur. Parameters for both the Nygren et al. and hAMr models are shown. B: time constants for the inactivation of IKur for the hAMr model (top) and the model of Nygren et al. (bottom). C: current traces of IKur (solid traces) simulated using a holding potential of −80 mV and a series of 750-ms-duration depolarizing steps from −50 to +60 mV applied in 10-mV increments (as shown in the inset between C and D). Additionally, for depolarizing steps to −20 and +20 mV, simulated current traces of Isus, the analogous current in the model of Nygren et al., are overlaid for comparison (dashed traces). D: a family of currents corresponding to the sum of It and IKur recorded from experiments [top [*Reprinted from Eur J Pharmacol 497, Gluais P. et al. (21), Risperidone reduces K+ currents in human atrial myocytes and prolongs repolarization in human myocardium, p. 215–222, 2004, with permission of Elsevier. Permission also granted by authors (21)]. These results were compared with the same simulated traces (bottom) generated by an identical voltage-clamp protocol in silico (shown in the inset between C and D).
Optimization of Parameters
Individual parameter values in each mathematical formulation in the hAMr model were optimized to reproduce physiologically relevant experimental observations. The specific magnitudes of these changes are discussed in the following section; two prime examples are IK1 and INa. Please reference the appendix for the exact parameter values.
IK1
While the mathematical formulation of IK1 remains the same as in Ref. 40, the maximum conductance of IK1 (gK1) was increased by ∼3%; this change maintains the RMP at experimentally measured values and results in IK1 densities within the range of experimentally validated values for the human atria (59, 61).
INa
It was necessary to increase the peak magnitude of this current by 12.5% by adjusting the Na+ permeability (PNa) to agree with experimental measurements of the maximum upstroke velocity (dV/dtmax) of the human atrial AP. Specifically, this change increased dV/dtmax from ∼120 to ∼150 V/s when stimulated at 1.5 times the threshold for activation, which falls within the experimentally measured range of values for human atrial myocytes (8).
It and IKur
The maximum conductance of It (gt) was increased by ∼9% in the hAMr model to match values reported in recent experiments (21, 60). The maximum channel conductance of IKur (gKur) was decreased by ∼11% in the hAMr model to fit recent experimental data (21).
Material Balance: Elimination of the Electroneutral Na+ Influx
The model of Nygren et al. (40) incorporated an electroneutral Na+ influx (Φen) to achieve long-term stability in ionic concentrations over long periods of stimulation at a specific rate (1 Hz) and stimulus strength (280 pA, or 5.6 pA/pF). A consequence of the incorporation of this flux is a lack of charge conservation as well as the absence of any true mathematical steady states (25). These factors limit the ability of any model to accurately reproduce long-term physiological phenomena and thus decrease its predictive power. In the hAMr model, Φen was removed, and the stimulus current delivered to the intracellular space was attributed to the influx of potassium ions, as suggested by Hund et. al. (24). As a result, charge is conserved within the system for the hAMr model for any stimulation protocol.
RESULTS
The simulation results presented here had two goals: 1) to illustrate the effects of incorporation of essential new K+ current data and 2) to further explore the mechanisms underlying the repolarization reserve in the human atrial myocyteby by examining both early and late repolarizationin in the hAMr model.
RMP
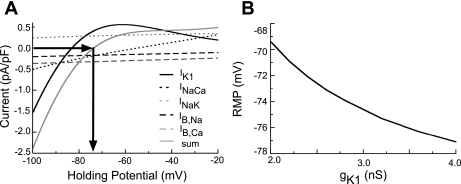
A detailed understanding of the factors that control the RMP is one of the essential elements of any physiologically accurate model (33). This reflects a dynamic balance between the time-independent background, pump, and exchanger currents (IK1, IB,Na, IB,Ca, INaK, ICaP, and INaCa). In Fig. 4A, the current-voltage relationships of five of the key ion-trafficking mechanisms that are responsible for determining the RMP in the quiescent atrial myocyte (IK1, INaCa, INaK, IB,Ca, and IB,Na) are shown. The resulting net current and RMP in our model are also shown. As indicated by the arrows, the net membrane current is zero at approximately −74.0 mV, in agreement with experimental measurements of the RMP (8, 12, 20, 46, 52). We note that RMP exhibits strong dependence on IK1; for example, a 10% increase in this conductance (gK1) will cause a decrease in RMP of ∼1 mV in the absence of other alterations in the hAMr model. The dependence of the RMP on gK1 for a wider range of conductances is shown in Fig. 4B.
Fig. 4.
A: current-voltage relationships of the currents underlying the stability of RMP (IK1, INaCa, and IB,Na) in the hAMr model along with the sum of these currents (solid shaded trace), which can be used to predict the RMP of the model. B: examination of the sensitivity of RMP to changes in gK1.
Long-Term Steady State of Simulated Ion Fluxes
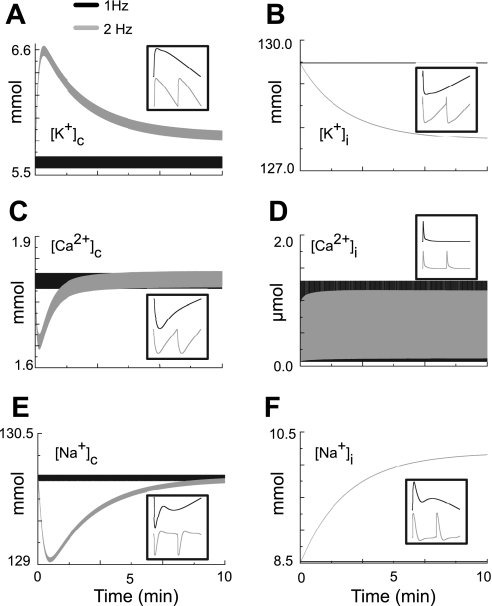
The long-term steady-state ionic concentrations in our model are shown in Fig. 5 for pacing rates of 1 and 2 Hz and for a simulation time of 10 min (600 and 1,200 cycles, respectively). Initial conditions for pacing at both rates were steady-state values following pacing at a rate of 1 Hz. The cleft concentrations of K+, Ca2+, and Na+ (Fig. 5, A, C, and E, respectively, solid traces) showed steady-state ionic balance through the entire time course of stimulation at a pacing rate of 1 Hz. When the pacing rate was elevated to 2 Hz (Fig. 5, A, C, and E, shaded traces), the cleft concentrations initially changed somewhat. [K+]c initially rose by ∼1 mmol/l and then returned to a steady-state value as a result of INaK activity and diffusion, consistent with experimental observations (31). The intracellular concentrations of K+, Ca2+, and Na+ (Fig. 5, B, D, and F, respectively, solid traces) also demonstrated steady-state ionic balance through the entire time course of stimulation at a pacing rate of 1 Hz. When the pacing rate was elevated to 2 Hz, [K+]i and [Na+]i (Fig. 5, B and F, respectively, shaded traces) reached steady state within the time course of simulation, decreasing by 2.0 mmol/l and increasing by 1.5 mmol/l, respectively. The peak magnitude of [Ca2+]i (Fig. 5D, shaded trace) decreased slightly by 0.1 μmol/l at steady state for pacing at 2 Hz, and diastolic [Ca2+]i increased by ∼0.025 μmol/l, as observed in experiments (49, 51).
Fig. 5.
Evaluation of the ability of the model to maintain ionic stability when paced at either 1 or 2 Hz (10 min of simulation shown). The insets in A–F are the selected ion concentrations as they change during a single AP when the model is paced at either 1 or 2 Hz. A: [K+]c. B: [K+]i. C: [Ca2+]c. D: [Ca2+]i. E: [Na+]c. F: [Na+]i.
The hAMr model reveals a critical difference with the original model of Nygren et al. in terms of long-term stability of both intracellular and cleft space ion concentrations. The elimination of the constant background flux ΦNa,en allows the hAMr model to adhere to fundamental physical principles of conservation of charge and ionic species, which results in the existence of long-term mathematical steady states (25). Moreover, the changes in intracellular and cleft ionic concentrations with an increase in pacing frequency to 2 Hz in the hAMr model are consistent with rate-dependent concentration alterations observed in experiments (40).
AP Morphology
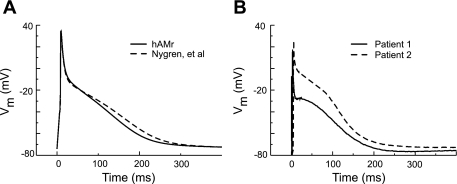
Figure 6A shows the AP waveform generated by our model and the original Nygren et al. model at a rate of 1 Hz. The change to the hAMr model AP was relatively small; the AP in our model had a more rapid rate of late repolarization. We note that in the Nygren et al. model, the AP morphology was tuned to match a subset of recorded data almost exactly. In the hAMr model, in contrast, we chose not to make this requirement upon the AP morphology. This is because recorded traces from human atrial myocytes, even when from the same region of the atria, as shown in Fig. 6B (from the right atrial appendage; unpublished observations by U. Ravens, used with permission), often show heterogeneity in AP morphology from one patient to the next. Thus, rather than require adherence to a single experimental recording, we chose to focus on the reproduction of key kinetic properties of individual K+ currents while also requiring AP morphological features that fall well within the range of experimental observations in human atrial myocytes.
Fig. 6.
A: APs generated by the hAMr and Nygren et al. (40) models. B: APs recorded from human atrial myocytes from two different patients (unpublished data from U. Ravens, used with permission) in response to a 1-Hz stimulus train at 37°C. Vm, transmembrane voltage.
Early Repolarization of the AP
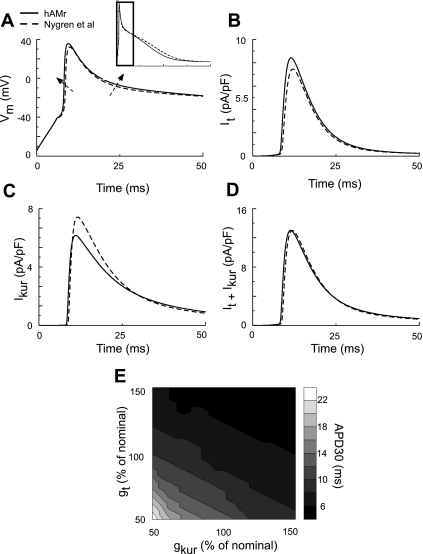
Figure 7 shows early repolarization of the AP in our model. In Fig. 7A, APs from both the Nygren et al. (40) and hAMr models are shown. The AP upstroke and early repolarization are emphasized. The major changes in our model were 1) an increased AP upstroke velocity and 2) a slight decrease in the rate of early repolarization, as indicated by the dashed arrows. The increased rate of rise of the AP is a result of an increase in peak INa, as discussed previously; the slight decrease in the rate of early repolarization in the hAMr model can be attributed to the alteration in the net contribution of It and IKur. Figure 7B shows It during the first 50 ms of the cardiac AP for both hAMr and Nygren et al. (40) models. It activated more quickly in our hAMr formulation. This was due to the increased rate of rise of the AP; peak It density was also larger. In contrast, the peak density of IKur (Fig. 7C) was decreased, and the inactivation of this current was slower in the hAMr model than in the original model.
Fig. 7.
Illustration of early AP repolarization in the hAMr model and in the model of Nygren et al. (40). A: comparison of AP morphology during early repolarization in the two models. The inset shows the time course of portion of the AP examined. B and C: traces of both It and IKur during the first 50 ms of a single AP. D and E: contribution of It and IKur to early repolarization of the human atrial AP. D: sum of It and IKur, which was similar for the hAMr and Nygren et al. (40) models. E: APD30 as a function of gt and gKur; nominal values are those used in the hAMr model.
Figure 7D shows the sum of It and IKur. Although each of the two currents were significantly different in the models, their sum was quite similar. In the hAMr model, the sum of It and IKur decayed more rapidly and thus resulted in a slightly slower early repolarization. Figure 7E shows the roles of gt and gKur on APD30; gt and gKur are expressed as percentages of the nominal gt and gKur, defined as those in the hAMr model. Note that this AP property is dependent on both gt and gKur; for instance, if gt and gKur are both decreased by 25%, the APD30 lengthens by ∼6 ms; if both gt and gKur increase by 25%, the APD30 decreases by ∼1 ms. Early repolarization in the hAMr model is thus especially sensitive to the collective decrease in It and IKur.
Late Repolarization of the AP
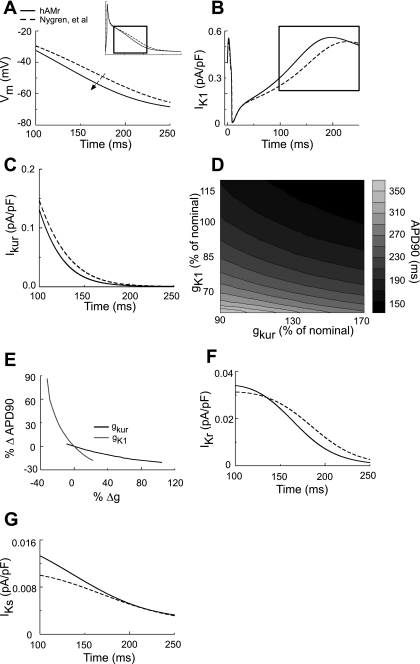
Figure 8 shows the revised simulation of late repolarization of the AP. Figure 8A shows AP traces during late repolarization for each model. The inset shows the entire time course of the APs, whereas the box indicates the latter 150 ms of the cardiac cycle. The major waveform change in the hAMr model compared with the original model of Nygren et al. was an increased rate of late repolarization, as shown by the dashed arrow. IK1 is shown during the entire time course of the AP in Fig. 8B (the box indicates the last 150 ms of the AP). Although the densities of IK1 in early repolarization were quite similar between the two models, the density of IK1 in our modified formulation was, on average, ∼12% greater than that of the parent model during late repolarization. This corresponds to a difference in the average current density of ∼0.06 pA/pF during the last 150 ms of the AP between the two models. Figure 8C shows IKur during late repolarization of the AP. Note that IKur during late repolarization was decreased in the hAMr model. IKur was ∼24% smaller on average in the hAMr model; the difference in average current density during the time course shown was ∼0.007 pA/pF. Although it was an order of magnitude smaller than IK1 during late repolarization, the density of IKur can have a marked influence on APD90.This is shown in Fig. 8D. Here, gK1 and gKur are expressed as percentages of nominal gK1 and gKur in the hAMr model. APD90 is dependent on both gK1 and gKur, as shown in Fig. 8E. Note that a decrease in gKur (from its nominal value) has a greater influence on increases in APD90 compared with a similar increase in gKur (and subsequent APD90 decreases). For nominal gK1, a 10% decrease in the nominal value of gKur causes an increase in APD90 on the order of 10 ms, whereas a 30% increase in nominal gKur is necessary to lengthen the AP by the same amount. Deviations from the nominal value in gK1 exert a greater influence on APD90 than gKur. However, similarly to gKur, increases in gK1 from its nominal value exert less influence on APD90 than decreases: for a nominal gKur, a 15% decrease and increase in gK1 will increase and decrease APD90 by ∼20 and ∼10 ms, respectively.
Fig. 8.
AP waveform of the human atrial myocyte during late repolarization. A: late repolarization occurred more rapidly in the hAMr model compared with the model of Nygren et al. (40), as shown by the arrow. B: note that IK1 during late repolarization in the hAMr model was increased compared with the model of Nygren et al. (40). C: IKur during the late repolarization phase of the AP. The magnitude and time courses of IKur in the hAMr and Nygren et al. (40) models were very similar. D: modulation of the time course of late repolarization as a function of the relative conductance of gK1 and gKur. E: percent change in APD90 with changes in gK1 or gKur compared with nominal values (those used in the hAMr model). The relative size and time course of the two additional K+ currents active during late repolarization in the human atrial myocyte (IKr and IKs) are shown in F and G, respectively. Note the small density of these currents compared with IK1 and IKur in B and C.
In addition to IK1 and IKur, the role of smaller K+ conductances was examined during late repolarization of the AP. Figure 8F shows the role of IKr (human ether-a-go-go-related gene) during late repolarization; the density of this current was ∼31% smaller for the interval of 150–250 ms from the beginning of a single cardiac cycle. However, this corresponds to a difference in the average density of IKr in the two models of only ∼0.004 pA/pF (∼0.5% of total membrane current) during the same time course. IKs (Fig. 8G) was ∼14% greater in the hAMr model until 200 ms of the AP; this was only a difference of ∼0.001 pA/pF (∼0.2% of total membrane current) in the average current density between the two models for the same time course.
Rate Dependence of the hAMr Model
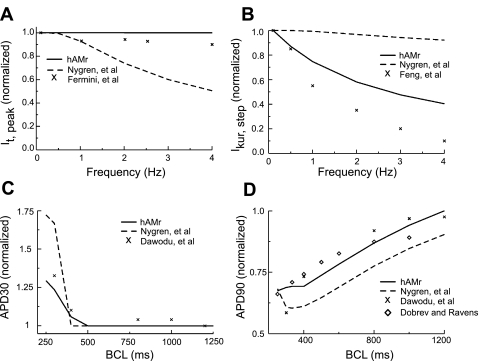
Figure 9 shows the rate dependence of key parameters in our new model compared with experimental data. For reference, we also show the rate dependence of the same parameters in the Nygren et al. model (40). In Fig. 9, A and B, peak It and end-pulse (as measured at the end of the voltage clamp) IKur were normalized to the peak and step current density, respectively, for a stimulation frequency of 0.1 Hz. Figure 9A shows the rate dependence of peak It compared with the experimental data of Fermini et al. (16). It as formulated in the original Nygren et al. model (dashed trace) was strongly rate dependent. The reformulation of recovery kinetics in the hAMr model resulted in much less rate dependence of It (black trace), resulting in a close match to experimental data. Figure 9B shows the rate dependence of end-pulse IKur compared with the experimental results of Feng et al. (15). The alteration of inactivation kinetics in the hAMr model improved the representation of the rate dependence of IKur.The new formulation reduced the amount of available IKur after an increase in pacing rate (15). The rate dependence of APD30 together with data from the experiments by Dawodu et al. [black “X”s (8)] are shown in Fig. 9C. APD30 was normalized to its value at a basic cycle length of 1,600 ms for the model of Nygren et al., the hAMr model, and the experimental data. While the original model qualitatively replicated the experimental observation that APD30 increases in response to an increase in stimulation rate, the present simulations more closely reproduced experimental results. The rate dependence of APD90 is shown in Fig. 9D and was compared with experiments (8, 9). APD90 was normalized to its value at a rate of 1,200 ms. The rate dependence of APD90 in the hAMr model was a much closer match to the available experimental values; moreover, in the hAMr model, APD90 continued to decrease for pacing rates of >2 Hz, a behavior that has been observed in experiments but was not duplicated by the previous model (40). It is important to note that there was a close agreement between experimental measurements and the improved rate dependence. This is an emergent feature of the hAMr model rather than a pattern of behavior to which the model was fit (see discussion).
Fig. 9.
Changes in K+ currents and APD as a function of basic cycle length (BCL). A: rate dependence of normalized peak It for both models and experimental data (16). From a holding potential of −60 mV, 100 steps to 0 mV of 200-ms duration were applied at frequencies of 0.1, 0.5, 1, 2, 3, and 4 Hz. The voltage-clamp protocol was identical for the experiment and simulation. B: rate dependence of normalized IKur at the end of test voltage pulses for both models and experimental data (15). From a holding potential of −80 mV, 100 steps to +40 mV of 200-ms duration were applied at frequencies of 0.1, 0.5, 1, 2, 3, and 4 Hz. The voltage-clamp protocol was identical for the experiment and simulation. C: rate dependence of APD30 in both models compared with the experiment (8). D: rate dependence of APD90 in both models compared with experiments (8, 9).
Utility of the hAMr Model in Applications
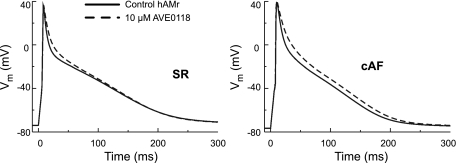
Unlike other models of the human atrial myocyte, the hAMr model features new repolarization kinetics and rate dependence and thus is appropriate for a wide range of cellular, tissue-level, and organ-level studies of the APs in human atria under normal and pathophysiological conditions. Of particular utility at the cellular level is the simulation of pharmacological interventions. Figure 10 shows the results of the use of the hAMr model to reproduce the effects of the It/IKur/IK(ACh) blocker AVE0118 on human atrial myocyte APs, as described by Christ et al. (6). For sinus rhythm (or healthy) atrial myocyte APs, simulations used the hAMr model, whereas for chronic atrial fibrillation APs, a version of the hAMr model modified to represent chronic atrial fibrillation based on experimental findings (6, 9) was used. The experimentally measured effects of AVE0118 on diverse K+ currents (6) were used in both sets of simulations. The simulations reproduced experimental findings (6) showing that AVE0118 administered in cells at sinus rhythm increased APD20 but not APD90, whereas in cells with chronic atrial fibrillation, both APD20 and APD90 increased with the administration of AVE0118. The increase in APD90 afforded by AVE0118 in human atrial myocytes with chronic atrial fibrillation has been suggested to underlie the efficacy of this compound to convert atrial fibrillation to sinus rhythm in goats (2), but the potential effects in a clinical setting to treat atrial fibrillation is unknown. As a forerunner and complement to clinical testing, the hAMr model could prove a useful tool to investigate the tissue-level effects of AVE0118 as well as numerous other pharmacokinetic therapies targeting human atrial myocytes.
Fig. 10.
Use of the hAMr model to investigate the effects of the drug AVE0118 on the human atrial AP waveform during sinus rhythm (SR) and chronic atrial fibrillation (cAF). The specific differential effects of AVE0118 in SR versus cAF human atrial myocytes described by Ref. 6 are shown here, illustrating the potential utility of the hAMr model in the simulation of pharmacological interventions.
DISCUSSION
The primary goal of the present study was to provide a computational framework in which the repolarization of the human atrial myocyte could be examined. This was achieved via the development of a new human atrial myocyte model based on the model of Nygren et al. (40), which incorporated recent experimental K+ current data and then characterized the emergent rate dependence of the new model. The results presented here demonstrate the ways in which select properties of major repolarizing K+ currents can result in the ability of the model to reproduce experimental measurements of AP properties of adult human atrial myocytes. In addition, the RMP matches available experimental data from human atrial myocytes. Importantly, our model now satisfies fundamental physical constraints of conservation of charge and ionic species and thereby ensures unique, long-term steady states of ionic concentrations in both the intracellular and cleft spaces. The morphology of the AP in the hAMr model resembles experimental APs from adult human atrial myocytes. This is the case despite the fact that no attempt was made to systematically match any such recordings. APD values for both early and late repolarization more closely approximated those measured in experiments, and an emergent feature of the hAMr model is its improved rate dependence of both individual currents and APD. The model presented in this article provides insight into repolarization processes and results in a much-improved match of current and AP behaviors to available experiments, an essential characteristic for the future use of the model in the context of human atrial rhythm disturbances.
IK1 and RMP
IK1 is essential for the maintenance of the resting potential in all myocytes and for late repolarization (17, 43). This is true even though the conductance of IK1 in the atria (compared with the ventricles) is relatively small (30). Some studies have indicated that IK1 in the atria does not exhibit strong rectification, i.e., the current-voltage relation shows little, if any, negative slope (30). These studies would suggest that, at high levels of depolarization, IK1 would maintain a current density close to its maximum value at less depolarized potentials and would remain at this density throughout the time course of the AP. It is possible that limitations in methodology (i.e., seal resistance changes for human atrial myocytes) lead to leakage currents, which may be interpreted as incomplete rectification but are an experimental artifact (39). A strong rectification of IK1 allows the reproduction of experimentally observed current densities and the maintenance of a stable RMP and has thus been included in the hAMr model.
One important consequence of the relatively small conductance of IK1 in the human atria is a slower rate of final repolarization as well as a somewhat more depolarized RMP (∼5–10 mV) compared with human ventricular myocytes (45). The RMP maintained in the hAMr model is −74.0 mV, which agrees with the majority of experimental studies using isolated adult human atrial myocytes (8, 21, 45, 46, 52, 60). The model of Courtemanche et al. (7) of the human atrial myocyte has a relatively hyperpolarized RMP of −81.2 mV. This is more typical of the maximum diastolic potential found in recordings from isolated human atrial tissue preparations (20, 45). We believe that accurate representation of the RMP is important not only in the context of steady-state phenomena but in the case of rate accommodation, wherein the maximum diastolic potential is typically depolarized compared with RMP and may affect both the availability and time dependence of ionic currents.
Demonstration of Long-term Pacing and Steady-State Ion Concentrations in the hAMr Model
Intracellular and cleft ion concentrations are at steady states for >10 min of simulation at a pacing rate of 1 Hz in the hAMr model. A small but notable change occurs in the steady-state value of [K+]c in the hAMr model compared with the original model of Nygren et al. (40); basal diastolic [K+]c was increased to 5.6 from 5.4 mmol/l (∼3.7% increase). This may be explained as resulting from the elimination of ΦNa in the hAMr model: the stimulus current that enters the intracellular space is now carried by potassium ions (24). These potassium ions are then extruded via sarcolemmal ion channels and accumulate in the cleft space surrounding the cell, whereas the bulk K+ concentration remains unchanged. Experimental measurements of extracellular ion concentrations are taken from the bulk fluid surrounding the cell or preparation, and there may exist locally higher concentrations of ions in an extracellular cleft space surrounding a myocyte than may be measured in the bulk plasma.
When the stimulation frequency was increased to 2 Hz, [K+]c accumulated, increased to values up to ∼1 mmol/l greater than its basal value (∼18% increase), and then returned to a unique steady state close to its initial value. [Na+]c and [Ca2+]c initially decreased by ∼0.8% and ∼8%, respectively, and then returned to steady-state values. At a stimulation rate of 2 Hz, [Na+]i increased by ∼18% to a new steady-state value. [K+]i reached a new steady-state value, which was a 1.5% decrease. [Ca2+]i, in contrast, underwent very little change in steady-state value at a stimulation rate of 2 Hz: the peak magnitude of the transient was decreased by ∼6% (peak magnitude), and peak diastolic [Ca2+]i increased. It is difficult to provide a comprehensive comparison of our results due of the lack of experimental data for many of these quantities. However, an increase in [K+]c with an increase in stimulation rate to 2 Hz has been recorded in experiments (31). In addition, peak diastolic [Ca2+]i has also been shown to increase with an increase in stimulation frequency in experimental models (49, 51).
The elimination of ΦNa results in the existence of a mathematical steady state in the hAMr model, as discussed briefly in Jacquemet et al. (25). The concentration of nonspecific, monovalent cations in the intracellular space, constant for any stimulation protocol in the hAMr model, was ∼138.4 mmol/l. Based on the elegant stability analysis provided in Jacquemet et al. (25), it can be determined that the initial condition for the transmembrane potential (initial condition in the appendix) in the hAMr model ensures that the system is dynamically stable for any stimulation protocol.
AP Waveforms
The AP in the hAMr model is characterized by a slightly slowed early (phase 1) repolarization as well as a more rapid late (phase 3) repolarization compared with the model of Nygren et al. (40) and fits well within the range of AP morphologies seen in recordings from experiments with adult human atrial myocytes (U. Ravens, unpublished observations). It is well known that there exist heterogeneities in human atrial AP shape. AP morphology and characteristics can vary both from patient to patient and from region to region in the atria (45) and have variously been classified into “types” or patterns (8, 10, 27, 57). Rather than require that the resultant AP morphology adhere to a specific experimental recording as in previous models of the adult human atrial myocyte (7, 40), we have chosen to arrive at a morphology for which accurate reproduction of observed behaviors of individual currents has been the focus. Although an exact match to an atrial AP shape was not required in our development, the result of the hAMr model is an AP morphology that is markedly similar to many experimental traces.
Repolarization Reserve Revisited
In the human atrial myocyte, It and IKur, followed by IK1, are the main time- and voltage-dependent currents responsible for repolarization. It is active during phase 1 (early) repolarization of the AP only, whereas IKur inactivates very slowly and thus contributes to repolarization during phases 1, 2, and 3 of the human atrial AP. IK1 figures prominently during late repolarization; because of the small densities of IKr and IKs compared with IK1, neither current plays a major role during late repolarization in the hAMr model.
In the human atria, myocytes may have a relatively small safety factor for repolarization due to decreased densities of outward currents compared with ventricular myocytes. “Repolarization reserve” has previously been defined as the ability of myocardial cells to repolarize when normal repolarizing currents are altered or reduced via either physiological perturbations or pathological challenges (22). This concept has been applied as a measure of the safety factor for repolarization (17) and, in practice, has been defined as the sum of the K+ currents IK1, IKr, and/or IKs, which can be activated under physiological conditions (17). However, the concept needs to be operationally defined with reference to particular contexts and situations. For example, outward currents that are active relatively late in the cardiac cycle may compensate for the low availability of other repolarizing currents when pacing rates increase, due to residual activation (i.e., IKur). The comparatively low density of outward K+ currents in the human atrial myocyte may result in low repolarization reserve, particularly in the context of drug therapies and pathologies that may interfere with K+ currents and repolarization processes. The underlying complexity of outward current remodeling, which contributes to varying degrees of the repolarization reserve, has been demonstrated recently (44). These new findings highlight the dynamic nature of the repolarization reserve and suggest that even outward currents active in early repolarization, i.e., It and IKur, may be critically important for repolarization in the human atria.
Rate Response of the AP Waveform
As shown in Fig. 9, the rate dependence of both It and IKur agree closely with experimental data. In addition, the frequency dependence of the AP waveform in the present model reproduces recent experimental results (Fig. 9) (8, 9).This improved representation of current availability and AP behavior at a variety of pacing rates is essential not only for the simulation of behaviors in healthy tissue but for the capacity of the model to lend its predictive power to disease state phenomena.
It is of interest to simulate the behavior of atrial myocytes and tissue at increased rates of stimulation so that atrial tachycardic rhythm disturbances (such as atrial flutter and atrial fibrillation) can be examined. For instance, in both paroxysmal and chronic atrial fibrillation, structural remodeling including progressive fibrosis has been widely implicated in the initiation and maintenance of the arrhythmia (3, 11). However, despite a wealth of investigation in recent years (29, 34, 48), a complete picture of the mechanisms underlying fibrosis-linked atrial arrhythmia has yet to emerge. Recent investigations have suggested that a critical aspect of atrial fibrotic remodeling may be functional: electrotonic coupling between fibroblasts and myocytes may play a crucial role at the cellular level (4, 5, 19, 26, 28, 35, 37, 38). Fibroblasts that are present within and maintain the extracellular matrix adjacent to myocytes can have a large influence on local myocyte activation and repolarization. To appropriately address questions regarding the potential involvement of electronic coupling between fibroblasts and human atrial myocytes in the development and maintenance of atrial arrhythmias, it is essential that the repolarization processes of the latter cell type be realistically represented at a variety of physiological and pathophysiological stimulation rates. The hAMr model was used in a recent study from our group (36) to investigate the effects of fibroblast-myocyte coupling on excitability and repolarization in the adult human atrial myocyte under a variety of conditions, including increased rates of stimulation.
Limitations of This Study
There are important limitations to the present work that should be recognized.
In Fig. 3D, computational traces of the sum of It and IKur appear to decay more rapidly than the experimental voltage clamp results. A cause of this apparent disparity is that the experimental traces available are from a recent publication (21), which, however, was not used in the new model, as the focus of the study of Gluais et al. was characterization of the AP and steady-state current density properties of human atrial myocytes after treatment with risperidone compared with control. The experimental datasets used to formulate the kinetics included in the model (15, 56) unfortunately did not include multiple voltage-clamp traces of the currents and thus could not be presented for a comparison to the simulated data shown in Fig. 3D.
The use of Hodgkin-Huxley formalism in our model employs first-order kinetics. Activation/deactivation and inactivation/recovery from inactivation are each governed by a single time constant, obtained, where possible, from fits to experimental measurements at a variety of holding potentials. However, many of these processes exhibit very complex kinetics. Markovian models allow for the existence of a multiplicity of channel states, each with specific transitional rate constants; however, these rate constants are often very difficult to determine. In addition, Markovian models often result in comprehensive AP models that are computationally expensive, particularly when more than one channel is involved. Additionally, Markovian models for the ion channels in the human atrium that were the main focus of this study (It and IKur) have not yet been developed. Future development and incorporation of such models will lead to further accuracy in the representation of ionic currents.
The mathematical formulations that account for Ca2+ homeostasis in the model of Nygren et al. (40) remained unchanged in the hAMr model. Although detailed models of Ca2+ cycling have emerged in recent years (23, 47), such models focus on ventricular rather than atrial tissue. Because of the lack of appropriate experimental data and because incorporation of a detailed model of Ca2+ cycling may be computationally prohibitive, particularly with respect to tissue and organ models, the formulation of Ca2+ cycling used in the model of Nygren et al. (40) was maintained in the hAMr model.
GRANTS
The laboratory of N. A. Trayanova gratefully acknowledges support of this work by National Heart, Lung, and Blood Institute Grants R01-HL-063195, R01-HL-082729, and R01-HL-067322 and National Science Foundation Grant CBET-0601935. The W. R. Giles laboratory is funded by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. In addition, W. Giles holds a Medical Scientist Award from the Alberta Heritage Foundation for Medical Research.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Prof. Ursula Ravens and members of her laboratory for providing action potential recording data from human atrial myocytes. These are included in Fig. 4B.
APPENDIX: MODEL EQUATIONS
This section contains all the equations, parameter values, and initial conditions necessary to be the basis for a full and accurate implementation of the hAMr model when combined with the kinetic descriptions available in the model of Nygren et al. (40). Unless otherwise noted, the units are as follows: time (in s), voltage (in mV), concentrations (in mmol/L), current (in pA), conductance (in nS), capacitance (in pF), volume (in nl), and temperature (in K). The stimulus used to evoke an AP was a rectangular current pulse (Istim) with amplitude of 280 pA and duration of 6 ms.
It
IKur
Intracellular Ion Concentrations
Parameter Values
Maximum Conductance Values
Initial Conditions
Footnotes
- aur
- Activation gating variable for IKur
- AP
- Action potential
- APD
- AP duration
- APD20
- APD at 20% repolariation
- APD30
- APD at 30% repolarization
- APD90
- APD at 90% repolarization
- [Ca2+]c
- Ca2+ concentration in the extracellular cleft space
- [Ca2+]d
- Ca2+ concentration in the restricted subsarcolemmal space
- [Ca2+]i
- Intracellular Ca2+ concentration
- [Ca2+]rel
- Ca2+ concentration in the sarcoplasmic reticulum release compartment
- [Ca2+]up
- Ca2+ concentration in the sarcoplasmic reticulum uptake compartment
- Cm
- Cell capacitance
- dL
- Activation gating variable for ICa,L
- dV/dtmax
- Maximum upstroke velocity
- EK
- Equilibrium (Nernst) potential for K+
- fL1
- Fast inactivation gating variable for ICa,L
- fL2
- Slow inactivation gating variable for ICa,L
- F
- Faraday's constant
- F1
- Relative amount of “inactive precursor” in the Irel formulation
- F2
- Relative amount of “activator” in the Irel formulation
- gKur
- Maximum conductance for IKur
- gt
- Maximum conductance for It
- gK1
- Maximum conductance for IK1
- h1
- Fast inactivation variable for INa
- h2
- Slow inactivation variable for INa
- hAMr model
- Human atrial myocyte with new repolarization model
- iur
- Inactivation gating variable for IKur
- IBCa
- Background Ca2+ current
- IBNa
- Background Na+ current
- ICa,L
- L-type Ca2+ current
- ICaP
- Ca2+ pump current, sarcolemmal
- IK1
- Inwardly rectifying K+ current
- IK(ACh)
- ACh-sensitive K+ current
- IKr
- Rapid delayed rectifier K+ current (human ether-a-go-go-related gene)
- IKs
- Slow delayed rectifier K+ current
- IKur
- Ultrarapid delayed rectifier K+ current
- INa
- Na+ current
- INaCa
- Na+/Ca2+ exchange current
- INaK
- Na+-K+ pump current, sarcolemmal
- INaK,max
- Maximum Na+-K+ pump current
- Istim
- Stimulus current
- It
- Transient outward K+ current
- kCa
- Half-maximum Ca2+ binding concentration for fCa
- [K+]c
- K+ concentration in the extracellular cleft space
- [K+]i
- Intracellular K+ concentration
- m
- Activation gating variable for INa
- n
- Activation gating variable for IKs
- [Na+]c
- Na+ concentration in the extracellular cleft space
- [Na+]i
- Intracellular Na+ concentration
- OC
- Fractional occupancy of the calmodulin-Ca2+ buffer by Ca2+
- OCalse
- Fractional occupancy of the calsequestrin-Ca2+ buffer by Ca2+
- OTC
- Fractional occupancy of the troponin-Ca2+ buffer by Ca2+
- OTMgC
- Fractional occupancy of the troponin-Mg2+ buffer by Ca2+
- OTMgMg
- Fractional occupancy of the troponin-Mg2+ buffer by Mg2+
- pa
- Activation gating variable for IKr
- PNa
- Permeability for INa
- r
- Activation gating variable for It
- R
- Universal gas constant
- RMP
- Resting membrane potential
- s
- Inactivation gating variable for It
- T
- Absolute temperature
- V
- Membrane voltage
- Voli
- Total myocyte volume
- ΦNa,en
- Electroneutral Na+ influx
- τaur
- Activation time constant for IKur
- τiur
- Inactivation time constant for IKur
- τr
- Activation time constant for It
- τs
- Inactivation time constant for It
REFERENCES
- 1.Amos GJ, Wettwer E, Metzger F, Li Q, Himmel HM, Ravens U. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol 491: 31–50, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaauw Y, Schotten U, van Hunnik A, Neuberger HR, Allessie MA. Cardioversion of persistent atrial fibrillation by a combination of atrial specific and nonspecific class III drugs in the goat. Cardiovasc Res 75: 89–98, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 51: 802–809, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res 62: 415–425, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Camelliti P, McCulloch AD, Kohl P. Microstructured cocultures of cardiac myocytes and fibroblasts: a two-dimensional in vitro model of cardiac tissue. Microsc Microanal 11: 249–259, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Christ T, Wettwer E, Voigt N, Hala O, Radicke S, Matschke K, Varro A, Dobrev D, Ravens U. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br J Pharmacol 154: 1619–1630, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol Heart Circ Physiol 275: H301–H321, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Dawodu AA, Monti F, Iwashiro K, Schiariti M, Chiavarelli R, Puddu PE. The shape of human atrial action potential accounts for different frequency-related changes in vitro. Int J Cardiol 54: 237–249, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Dobrev D, Ravens U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res Cardiol 98: 137–148, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Escande D, Loisance D, Planche C, Coraboeuf E. Age-related changes of action potential plateau shape in isolated human atrial fibers. Am J Physiol Heart Circ Physiol 249: H843–H850, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Everett TH, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm 4: S24–S27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabiato A, Fabiato F. The two components of the human atrial action potential. Circ Res 29: 296–305, 1971 [DOI] [PubMed] [Google Scholar]
- 13.Fedida D, Wible B, Wang Z, Fermini B, Faust F, Nattel S, Brown AM. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ Res 73: 210–216, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Feng J, Wang Z, Li GR, Nattel S. Effects of class III antiarrhythmic drugs on transient outward and ultra-rapid delayed rectifier currents in human atrial myocytes. J Pharmacol Exp Ther 281: 384–392, 1997 [PubMed] [Google Scholar]
- 15.Feng J, Xu D, Wang Z, Nattel S. Ultrarapid delayed rectifier current inactivation in human atrial myocytes: properties and consequences. Am J Physiol Heart Circ Physiol 275: H1717–H1725, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Fermini B, Wang Z, Duan D, Nattel S. Differences in rate dependence of transient outward current in rabbit and human atrium. Am J Physiol Heart Circ Physiol 263: H1747–H1754, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Fink M, Giles WR, Noble D. Contributions of inwardly rectifying K+ currents to repolarization assessed using mathematical models of human ventricular myocytes. Philos Transact A Math Phys Eng Sci 364: 1207–1222, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Firek L, Giles WR. Outward currents underlying repolarization in human atrial myocytes. Cardiovasc Res 30: 31–38, 1995 [PubMed] [Google Scholar]
- 19.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac orgin. Circ Res 93: 421–428, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Gelband H, Bush HL, Rosen MR, Myerburg RJ, Hoffman BF. Electrophysiologic properties of isolated preparations of human atrial myocardium. Circ Res 30: 293–300, 1972 [DOI] [PubMed] [Google Scholar]
- 21.Gluais P, Bastide M, Grandmougin D, Fayad G, Adamantidis M. Risperidone reduces K+ currents in human atrial myocytes and prolongs repolarization in human myocardium. Eur J Pharmacol 497: 215–222, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Han W, Chartier D, Li D, Nattel S. Ionic remodeling of cardiac Purkinje cells by congestive heart failure. Circulation 104: 2095–2100, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Hinch R, Greenstein JL, Tanskanen AJ, Xu L, Winslow RL. A simplified local control model of calcium-induced calcium release in cardiac ventricular myocytes. Biophys J 87: 3723–3736, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hund TJ, Kucera JP, Otani NF, Rudy Y. Ionic charge conservation and long-term steady state in the Luo-Rudy dynamic cell model. Biophys J 81: 3324–3331, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacquemet V. Steady-state solutions in mathematical models of atrial cell electrophysiology and their stability. Math Biosci 208: 241–269, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: insights from a microstructure model. Am J Physiol Heart Circ Physiol 294: H2040–H2052, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakob H, Oelert H, Rupp J, Nawrath H. Functional role of cholinoceptors and purinoceptors in human isolated atrial and ventricular heart muscle. Br J Pharmacol 97: 1199–1208, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohl P, Camelliti P, Burton F, Smith G. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J Electrocardiol 38: 45–50, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res 54: 361–379, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Koumi S, Backer CL, Arentzen CE. Characterization of inwardly rectifying K+ channel in human cardiac myocytes. Alterations in channel behavior in myocytes isolated from patients with idiopathic dilated cardiomyopathy. Circulation 92: 164–174, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Kunze DL. Rate-dependent changes in extracellular potassium in the rabbit atrium. Circ Res 41: 122–127, 1977 [DOI] [PubMed] [Google Scholar]
- 32.Lagrutta A, Wang J, Fermini B, Salata JJ. Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J Pharmacol Exp Ther 317: 1054–1063, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Lindblad DS, Murphey CR, Clark JW, Giles WR. A model of the action potential and underlying membrane currents in a rabbit atrial cell. Am J Physiol Heart Circ Physiol 271: H1666–H1696, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Luo MH, Li YS, Yang KP. Fibrosis of collagen I and remodeling of connexin 43 in atrial myocardium of patients with atrial fibrillation. Cardiology 107: 248–253, 2007 [DOI] [PubMed] [Google Scholar]
- 35.MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J 92: 4121–4132, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. Electrotonic coupling between human atrial myocytes and fibroblasts alters myocyte excitability and repolarization. Biophys J In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res 98: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res 101: 755–758, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Newell EW, Schlichter LC. Integration of K+ and Cl− currents regulate steady-state and dynamic membrane potentials in cultured rat microglia. J Physiol 567: 869–890, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, Giles WR. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circ Res 82: 63–81, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet 15: 2185–2191, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Radicke S, Cotella D, Graf EM, Banse U, Jost N, Varro A, Tseng GN, Ravens U, Wettwer E. Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc Res 71: 695–703, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Rasmusson RL, Clark JW, Giles WR, Shibata EF, Campbell DL. A mathematical model of a bullfrog cardiac pacemaker cell. Am J Physiol Heart Circ Physiol 259: H352–H369, 1990 [DOI] [PubMed] [Google Scholar]
- 44.Roden DM. Repolarization reserve: a moving target. Circulation 118: 981–982, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res 90: 939–950, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Shibata EF, Drury T, Refsum H, Aldrete V, Giles W. Contributions of a transient outward current to repolarization in human atrium. Am J Physiol Heart Circ Physiol 257: H1773–H1781, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Shiferaw Y, Watanabe MA, Garfinkel A, Weiss JN, Karma A. Model of intracellular calcium cycling in ventricular myocytes. Biophys J 85: 3666–3686, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spach MS, Heidlage JF, Dolber PC, Barr RC. Mechanism of origin of conduction disturbances in aging human atrial bundles: experimental and model study. Heart Rhythm 4: 175–185, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stefenelli T, Wikman-Coffelt J, Wu ST, Parmley WW. Intracellular calcium during pacing-induced ventricular fibrillation. Effects of lidocaine. J Electrocardiol 25: 221–228, 1992 [DOI] [PubMed] [Google Scholar]
- 50.Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ, Hatem SN. Regulation of the transient outward K+ current by Ca2+/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res 85: 810–819, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Thandroyen FT, Morris AC, Hagler HK, Ziman B, Pai L, Willerson JT, Buja LM. Intracellular calcium transients and arrhythmia in isolated heart cells. Circ Res 69: 810–819, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Trautwein W, Kassebaum DG, Nelson RM, Hecht HH. Electrophysiological study of human heart muscle. Circ Res 10: 306–312, 1962 [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Bondarenko VE, Qu Y, Morales MJ, Rasmusson RL, Strauss HC. Activation properties of Kv4.3 channels: time, voltage and [K+]o dependence. J Physiol 557: 705–717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S, Bondarenko VE, Qu YJ, Bett GC, Morales MJ, Rasmusson RL, Strauss HC. Time- and voltage-dependent components of Kv4.3 inactivation. Biophys J 89: 3026–3041, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Xu H, Kumar R, Tipparaju SM, Wagner MB, Joyner RW. Differences in transient outward current properties between neonatal and adult human atrial myocytes. J Mol Cell Cardiol 35: 1083–1092, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Feng J, Shi H, Pond A, Nerbonne JM, Nattel S. Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circ Res 84: 551–561, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res 73: 276–285, 1993 [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res 73: 1061–1076, 1993 [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation 98: 2422–2428, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res 52: 226–235, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Garratt CJ, Zhu J, Holden AV. Role of up-regulation of IK1 in action potential shortening associated with atrial fibrillation in humans. Cardiovasc Res 66: 493–502, 2005 [DOI] [PubMed] [Google Scholar]