Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease of excess vasoconstriction and vascular cell proliferation that results in increased pulmonary vascular resistance and right heart failure. We have previously shown (66) that tissue factor expression is increased in the abnormal vessels of patients and rats with PAH. We hypothesized that tissue factor and its downstream mediator, thrombin, would promote migration of endothelial cells (EC) and the vascular pathology of PAH. Immunostaining revealed EC and a fibronectin-enriched matrix within the “plexiform-like” lesions in a rat model of severe PAH. In a modified Boyden assay, protease-activated receptor 1 (PAR1; thrombin receptor) stimulation by agonist peptide or thrombin induced pulmonary microvascular EC (PMVEC) migration when the cells were interacting with fibronectin, but not with other extracellular matrix proteins. Thrombin/fibronectin-induced migration was confirmed in wound healing and angiogenesis assays and was abrogated by the PAR1 antagonist SCH79797 and soluble RGD peptide. This fibronectin dependence was unique to PAR1 activation; other EC agonists evaluated did not induce migration on any matrix, and 10% FBS stimulated similar levels of migration on all matrix proteins tested. Thrombin/fibronectin stimulated autophosphorylation of calcium/calmodulin dependent protein kinase II (CaMKII) in PMVEC, and inhibitors of CaMKII blocked thrombin-induced migration on fibronectin, but had no effect on migration induced by 10% FBS. In contrast, EC isolated from the proximal pulmonary artery migrated in response to most agonists independent of the matrix substrate. Our findings illustrate EC heterogeneity in a single tissue and indicate a novel role for CaMKII in mediating EC migration. Because PMVEC have been shown to have impressive proliferative potential, thrombin/fibronectin-stimulated migration of these cells to a site of injured endothelium is a potential mechanism by which thrombin contributes to the development of vascular lesions in PAH.
Keywords: pulmonary hypertension, plexiform lesions, fibronectin
cellular migration is a highly organized process that involves cell adhesion, polarization, and coordinated cytoskeletal rearrangement (37, 52, 56). Migration plays a critical role in normal physiology, including embryological development, wound healing, and the immune response (32, 45). Endothelial cell (EC) migration is a vital component of angiogenesis, the growth of new blood vessels from existing vasculature (e.g., for revascularizing tissue following ischemic injury) (20, 36, 59). However, cellular migration and angiogenesis may also contribute to a variety of pathological conditions, including rheumatoid arthritis, diabetic retinopathy, and tumor growth and metastasis (1, 17, 19). Cytokines and growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor, are classically considered the most important inducers of angiogenesis (40, 49, 57). But the extracellular matrix (ECM) milieu also plays a vital role in modulating EC function and angiogenesis, primarily through interaction with cell surface adhesion receptors called integrins (27, 61). ECM ligation of specific integrins strongly influences cell fate (growth vs. differentiation vs. apoptosis) and migration (23, 51); more recently, integrin ligation was shown to modulate VEGF signaling directly (68). Therefore, both classic growth factor receptor stimulation and ECM components are important determinants of cell behavior.
Pulmonary arterial hypertension (PAH) is a progressive, often fatal, disease of vasoconstriction and vascular cell proliferation, resulting in increased pulmonary vascular resistance. PAH occurs in idiopathic form, both sporadically and in families; it is also associated with a variety of other conditions, including collagen vascular disease, human immuodeficiency virus infection, cirrhosis, and anorexigen exposure (16, 18). Regardless of etiology, the common vascular pathology includes medial hypertrophy and neointimal formation in precapillary arterioles, eventually leading to luminal obliteration (47, 54). Most patients also have unique proliferative vascular structures, called “plexiform lesions”, composed of endothelial and smooth muscle cells that may form through a process of disordered angiogenesis (10, 63). Because PAH mortality remains unacceptable [60% 5-yr survival with contemporary therapy (31)], novel treatment strategies built on improved understanding of disease mechanisms must be found (50).
Our laboratory has found recently that tissue factor (TF), the transmembrane glycoprotein that initiates the coagulation cascade, is highly expressed in the hypertrophied arteries, obliterated arterioles, and proliferative vascular lesions of PAH patients and in a rat model of severe PAH (66). Membrane-bound TF forms a complex with factor VIIa, cleaving FX to FXa, and subsequently generates the serine protease thrombin from its precursor prothrombin. In addition to cleaving fibrinogen, thrombin (and TF) influences a myriad of cellular functions by acting on protease-activated receptors (PARs) on vascular cells (7, 30). PARs are G protein-coupled receptors that are activated when they undergo NH2-terminal cleavage by specific proteases (11). Intrareceptor binding of the newly exposed “tethered ligand” allows intracellular activation of classic G protein-coupled receptor-mediated signaling pathways that mediate processes, such as cellular activation, proliferation, and migration. In the systemic circulation, TF mediates vascular pathology, including neointimal formation after arterial injury and pathological angiogenesis (28, 43, 48, 55). Because a prothrombotic diathesis, arteriolar obliteration by neointima, and aberrant angiogenesis are components of PAH, we hypothesized that TF and downstream thrombin contribute to PAH pathophysiology.
In this study, we show that EC populate the vascular lesions within a fibronectin (FN)-rich matrix in a rat model of severe PAH, consistent with observations in human PAH lesions (10, 29, 64). In culture, PAR1 activation induced pulmonary microvascular EC (PMVEC) migration, wound healing, and an angiogenic phenotype in cells interacting with FN, but not with collagen. Inhibiting calcium/calmodulin-dependent protein kinase II (CaMKII) blocked thrombin/FN-induced PMVEC migration without impacting FBS-induced migration, suggesting a specific role for CaMKII downstream of thrombin/FN stimulation. Thrombin activation of PAR1 on PMVEC in the context of an injured, FN-rich vascular wall matrix may contribute to the vascular pathophysiology of PAH.
METHODS
Cells and reagents.
Rat PMVEC (RPMVEC) and rat pulmonary artery EC (PAEC) were a generous gift from Troy Stevens at the University of South Alabama. PMVEC are highly proliferative, vasculogenic cells isolated from the periphery of the rat lung and positively selected for binding of the microvascular EC-specific plant lectin Griffonia simplicifolia; PAEC are isolated from the proximal pulmonary arteries and characterized by binding of the lectin Helix pomatia (35). Primary cultures of EC from two different rats were used between passages 5 and 9; cells had a typical cobblestone endothelial morphology and generally grew to confluence within 5 days after being passaged at 1:10 dilution. PAR1 agonist peptide/thrombin receptor agonist peptide (SFLLRN-NH2), PAR2 agonist peptide (SLIGRL-NH2), VEGF, basic fibroblast growth factor, endothelin-1, KN-93, SCH-79797, W-7, RGD/RGE peptides, FN, vitronectin, and lectins were from Sigma Aldrich (St. Louis, MO). Collagen was from Cohesion (Palo Alto, CA). Human α-thrombin was from Enzyme Research Laboratories (South Bend, IN), with a minimum activity of 2,700 U/mg. Sphingosine-1-phosphate was from Avanti Polar Lipids (Alabaster, AL).
Histochemistry.
Rat lung tissue from previous experiments was utilized for the lectin and FN staining. In brief, young male rats underwent left pneumonectomy and received 60 mg/kg monocrotaline (MCT) 1 wk later; details of the methods and results were published previously (66). Those experiments and use of the tissue have ongoing local Institutional Animal Care and Use Committee approval. Formalin-fixed, paraffin-embedded rat lung tissue was sectioned at 5 μm, baked at 60°C for 1 h, deparaffinized, and rehydrated through graded ethanol. The sections were blocked with 3% hydrogen peroxide followed by serum-free blocking solution (Dako, Carpinteria, CA) and then incubated with biotin-labeled lectins Griffonia simplicifolia or Helix pomatia (100 μg/ml) for 30 min at 37°C. After washing, horseradish peroxidase-labeled streptavidin was added before incubation for 30 min at room temperature. Staining was visualized with Nova Red. Trichrome staining for collagen and elastin (“CME”) and immunohistochemistry for TF, von Willebrand factor, and FN were performed, as described previously (66).
Modified Boyden chamber migration assay.
ChemoTx modified Boyden chambers (Neuro Probe, Gaithersburg, MD), with 8-μm pores, were coated on both sides with ECM protein (FN, collagen, or vitronectin) for 2 h. PMVEC at 80–90% confluence were released with 1 mM EDTA in PBS, and then rinsed and resuspended in DMEM with 0.1% BSA at a density of 1.25 × 106 cells/ml. Agonists were diluted in 0.1% BSA/DMEM, and a 29-μl aliquot was loaded into each lower well of the chamber. Antagonists or vehicle controls were added to cells 30 min before loading 20 μl aliquots of cells on top of the filter. The chamber was incubated at 37°C, and, after 6 h, nonmigrating cells were cleaned off the top of the filter. Cells on the underside of the filter were fixed in methanol and visualized by Romanowski staining. The assay was quantified by counting the cells in five high-powered fields per well. All conditions were performed in triplicate for a given experiment, and findings were confirmed on at least one other occasion.
Scratch wound assay.
PMVEC in culture were prepared as above and plated at high density on chamber slides (Nalge Nunc, Rochester, NY), which had been precoated with the relevant ECM protein (FN or collagen, 10 μg/ml). After cells had formed a confluent monolayer, a scratch was created using a pipette tip, and the edges of the wound were marked. The plate was rinsed to remove detached cells, and agonists/antagonists were added in 0.1% BSA/DMEM. After 12–16 h (an overnight incubation), the cells were fixed with 10% neutral buffered formalin, stained with hematoxylin/eosin or fluorescently labeled phalloidin (Molecular Probes, Eugene, OR), and photographed. The wound areas before and after healing were measured using Spot Advanced digital imaging software.
Matrigel assay.
Phenol red-free, reduced growth factor Matrigel (BD Biosciences, San Jose, CA) was thawed on ice overnight and diluted 1:2 in phosphate-buffered saline. FN or collagen was added to the liquid Matrigel to produce a final concentration of 10 μg/ml. Chilled eight-well chamber slides were coated with the enriched Matrigel (50 μl/cm2, thin gel method) and placed in a 37°C incubator for 30 min. Cells were prepared as described above and plated with or without agonists/antagonists. After 4–6 h, slides were fixed with 10% formalin, stained with hematoxylin/eosin, and photographed; in determining the duration (4–6 h), tube formation with serum was monitored as a positive control during the experiment, and the experiment was terminated when there had been robust serum-induced tube formation. The assay was quantified by counting the number of intersections per high-powered field.
Western blot.
RPMVEC were plated on collagen or FN (10 μg/ml)-coated dishes in DMEM supplemented with 0.1% BSA overnight. Cells were treated with inhibitor or vehicle control for 30 min, followed by 15 min of thrombin/control treatment. Cells were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors, and total cellular protein was collected and quantified by Bradford assay. Twenty-five micrograms of protein per sample were resolved by PAGE on a 12% gel and transferred to nitrocellulose. The membranes were blocked with 1% BSA in Tris-buffered saline for 1 h at 37°C and then incubated with anti phospho-Thr286 CaMKII-α antibody (Promega, Madison, WI) at a dilution of 1:5,000 in 0.1% BSA/Tris-buffered saline-Tween for 2 h at room temperature. After washing, membranes were incubated with horseradish peroxidase-labeled donkey anti-rabbit antibody at 1:10,000 for 1 h at room temperature. Bands were visualized with ECL Plus (GE Healthcare, Piscataway, NJ) and imaged with on Biomax Light film (Eastman Kodak, Rochester, NY). Membranes were then stripped, blocked with 5% nonfat milk in PBS, reprobed using an antibody to total CaMKII-α, and visualized as above. Densitometry was performed, and phosphorylated CaMKII-α was normalized to total.
Statistical analysis.
Data were evaluated by two-way ANOVA followed by post hoc t-testing. A P value of 0.05 was considered statistically significant. Graphs present means and standard deviations.
RESULTS
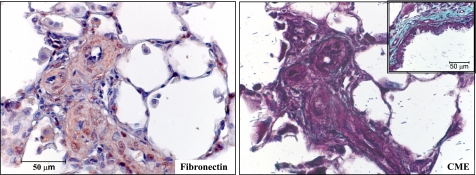
In the pulmonary vasculature, the EC of the large, conduit pulmonary arteries are distinct from the distal microvascular ECs in terms of embryological origin, functional phenotype, and physiological role in processes such as barrier maintenance (22). We have previously shown that EC are present in the distal vascular pathology in a rat model of severe PAH, including plexiform-like lesions and neointimal obliteration of precapillary arterioles (66). To identify the ECM milieu of the lesions in the pneumonectomy and MCT rat model of severe PAH, we stained archived rat lung tissue. The animals used had received 60 mg/kg MCT 1 wk after a left pneumonectomy, and lungs were harvested 3 wk later. All four rats whose tissue was examined had mean pulmonary artery pressures under anesthesia exceeding 50 mmHg. Vascular lesions were enriched with FN and had little collagen deposition, as assessed by immunostaining and a modified trichrome stain (“CME”), respectively (Fig. 1). Most previous studies of cell migration have been conducted on plastic or collagen. In light of this staining showing FN enrichment, we studied pulmonary EC migration in the context of different matrix proteins.
Fig. 1.
Rat proliferative vascular lesions are enriched with fibronectin (FN). Lung sections from rats with severe proliferative pulmonary arterial hypertension (PAH) were stained for FN (immunostaining) or collagen (CME, see methods). Serial sections show that proliferative vascular lesions contain abundant FN (left) with very little collagen (right). As a positive control for collagen staining from the same section, a bronchus is shown in the inset with abundant collagen.
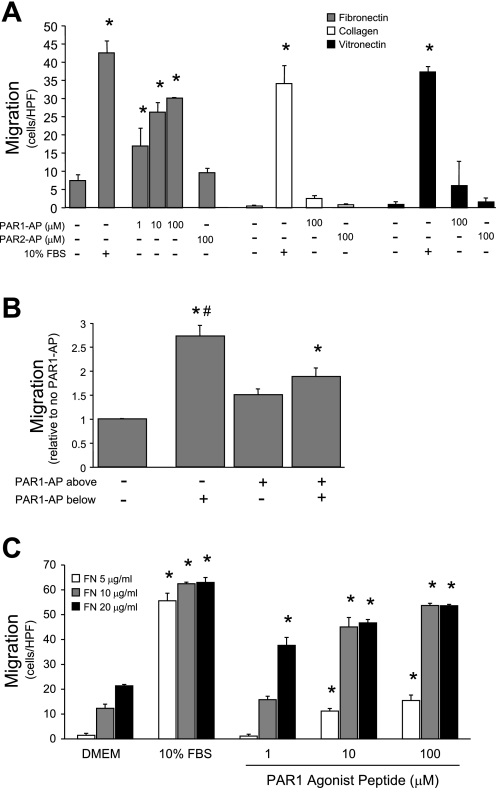
TF and downstream components of the coagulation cascade, including thrombin, can exert direct cellular effects by acting through PARs. To determine whether PAR signaling plays a role in mediating PMVEC migration, we performed modified Boyden chamber migration assays (Fig. 2 A). PAR1 agonist peptide (SFLLRN, PAR1-AP), which mimics activation of PAR1 by its tethered ligand, induced dose-dependent PMVEC migration in cells on FN, but not on other ECM proteins. PAR2 agonist peptide (SLIGRL, PAR2-AP) did not stimulate migration under any conditions. Interestingly, 10% FBS, used as a maximal stimulus for migration, induced a similar degree of migration on all matrix coatings; thus the matrix dependence for PAR1-AP is not a general characteristic of PMVEC migration, but is specific to PAR1-induced migration.
Fig. 2.
Protease-activated receptor 1 (PAR1) activation induces FN-dependent rat pulmonary microvascular endothelial cell (RPMVEC) migration. Modified Boyden chamber filters were coated on both sides with the indicated extracellular matrix (ECM) protein. A: activation of the PAR1 receptor with PAR1 agonist peptide (SFLLRN, PAR1-AP) stimulated dose-dependent chemotaxis of RPMVEC when the cells were interacting with FN, but not with any other ECM protein tested. Migration in response to 10% FBS (positive control) did not differ significantly among the ECM proteins (*P < 0.05 vs. no agonist, n = 3). B: checkerboard assay performed by including PAR1-AP in the lower well (analogous to 2A), upper well (with cells), or both. PAR1-induced migration has both chemotactic and chemokinetic components (*P < 0.01 vs. no PAR1-AP; #P < 0.02 vs. PAR1-AP above and below, n = 3). C: modified Boyden assay with varying concentrations of FN coating. Increasing concentration of FN coating potentiated migration in response to low amounts of PAR1-AP (*P < 0.05 vs. DMEM, n = 3). HPF, high-powered field.
To determine the relative contribution of chemotaxis and chemokinesis to PAR1-induced migration, we performed a checkerboard assay (Fig. 2B). The most robust migratory response occurred with PAR1-AP only in the lower well, indicating that this stimulus is largely chemotactic. However, adding PAR1-AP to both the upper and lower wells induced a modest increase in PMVEC migration above the basal level. Therefore, PAR1 activation also exerts a chemokinetic effect, stimulating migration even in the absence of an agonist gradient. Finally, to further explore the interaction between FN and PAR1, a migration assay was performed using a range of FN coating concentration (Fig. 2C). Even in the absence of a chemotactic agonist, PMVEC migration was proportional to FN concentration, indicating that FN exerts a chemokinetic effect. With low concentration (5 μg/ml) of FN coating, PAR1-AP only stimulated migration at doses above 10−5 mol/l. Increasing the concentration of FN coating potentiated the effect of PAR1 stimulation, with significant induction of migration at 10−6 mol/l of PAR1-AP. These data suggest a supra-additive interaction between the PAR1 receptor and a FN-binding integrin.
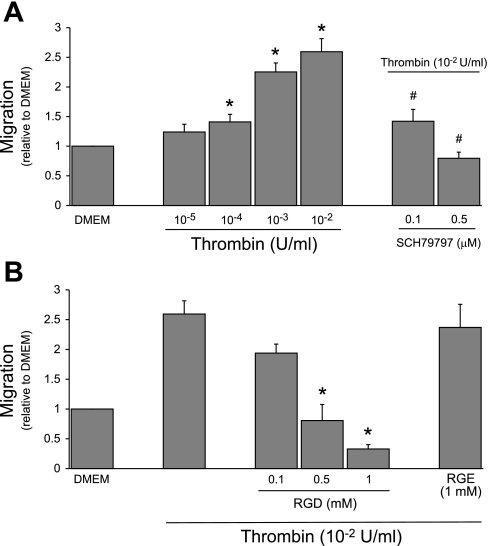
Thrombin, generated downstream of TF during activation of the coagulation cascade, is the canonical endogenous agonist for PAR1. Therefore, we evaluated the ability of thrombin to stimulate PMVEC migration. Like PAR1-AP, thrombin induced dose-dependent PMVEC migration on FN (Fig. 3A). Migration was abrogated by a PAR1-selective antagonist (SCH79797), confirming that this effect is mediated by thrombin acting on its receptor. Soluble arginine-glycine-aspartate (RGD) peptide can act as a competitive antagonist blocking cell surface integrins from ligating the RGD motif on FN (21, 25, 53). Soluble RGD blocked thrombin-induced PMVEC migration (Fig. 3B), emphasizing the absolute requirement of FN interaction with RGD-binding integrins to facilitate PAR1-induced migration. As a control, soluble arginine-glycine-glutamate (RGE) peptide did not block migration.
Fig. 3.
Thrombin induces PAR1-specific, FN-dependent RPMVEC migration. A: thrombin treatment resulted in dose-dependent RPMVEC chemotaxis that was inhibited by the PAR1-selective antagonist SCH79797 (*P < 0.05 vs. DMEM; #P < 0.05 vs. thrombin 10−2 U/ml, n = 3). B: migration was also prevented by blocking RPMVEC association with the FN arg-gly-asp (RGD) motif; using soluble RGD peptide soluble arg-gly-glut (RGE) peptide did not block migration (*P < 0.05 vs. thrombin, n = 3).
To determine whether PAR1 stimulation was unique in its matrix-dependent induction of PMVEC migration, we tested a variety of other classic EC agonists (Table 1). Surprisingly, none of these other agonists induced migration on either FN or collagen, suggesting that PMVEC are generally resistant to migration. To ensure that the cells were capable of responding to VEGF, we confirmed the presence of phosphorylated VEGF receptor 2 and downstream ERK phosphorylation by Western blot, and we attenuated receptor phosphorylation using a tyrosine kinase inhibitor (data not shown). PAEC are isolated from proximal, conduit pulmonary arteries and are functionally distinct from PMVEC with respect to characteristics such as proliferative capacity and barrier function (9, 33). To compare the migratory behavior of PAEC to that of PMVEC, we performed Boyden assays with a panel of agonists (Table 1). PAEC exhibited greater basal and FBS-induced migration than PMVEC. In striking contrast to PMVEC, PAEC migrated in response to several different agonists, on both collagen and FN. Therefore, especially compared with PAEC, PMVEC are generally resistant to migration induced by commonly used endothelial agonists but display a robust response after PAR1 ligation in the context of a FN matrix.
Table 1.
Microvascular and macrovascular pulmonary endothelial cells display contrasting migratory behavior
| DMEM | 10% FBS | VEGF | ET-1 | S-1-P | PAR1-AP | PAR2-AP | |
|---|---|---|---|---|---|---|---|
| RPMVEC | |||||||
| Collagen | 1.0±0.3 | 44.6±9.9 | 0.7±0.4 | 1.1±0.1 | 0.8±0.8 | ||
| Fibronectin | 11.1±2.3 | 52.4±8.6* | 9.4±4.3 | 9.1±6.3 | 9.7±3.5 | ||
| RPAEC | |||||||
| Collagen | 19.5±3.0 | 100.3±1.2* | 38.3±3.3* | 23.4±3.7 | 45.9±1.4* | 66.1±11.4* | 53.3±4.0* |
| Fibronectin | 21.9±2.1 | 105.9±3.4* | 40.8±5.4* | 23.9±3.2 | 54.9±4.6* | 57.9±3.5* | 59.9±7.0* |
Values are mean cells per high-powered field ± SD, n = 3. Rat pulmonary microvascular endothelial cells (RPMVEC) did not migrate in response to classic endothelial cell agonists: VEGF (vascular endothelial growth factor, 100 ng/ml); basic fibroblast growth factor (100 ng/ml); ET-1 (endothelin-1, 10−7 mol/l); S-1-P (sphingosine-1-phosphate, 10−6 mol/l). In contrast, rat pulmonary artery endothelial cells (RPAEC) migrated with classic agonists compared with DMEM. Migration was insensitive to the extracellular matrix coating [agonist concentrations same as above; protease-activated receptor 1 agonist peptide (PAR1-AP) (SFLLRN, 10−4 mol/l); PAR2-AP (SLIGRL, 10−4 mol/l].
P < 0.05 vs. DMEM.
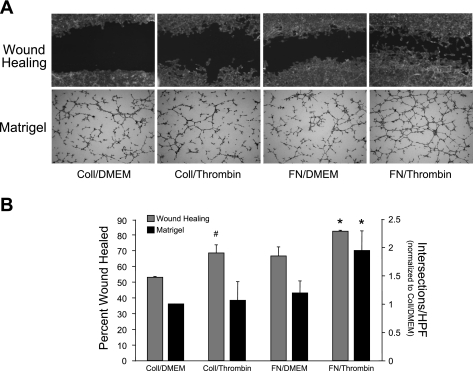
To confirm our findings from the modified Boyden assays, we performed alternate in vitro assays of cellular migration/angiogenesis. As shown in Fig. 4A and quantified in Fig. 4B, healing of a scratch wound was accelerated in cells plated on FN and treated with thrombin compared with thrombin-treated cells on collagen or unstimulated cells on FN. Similarly, in a Matrigel assay of angiogenesis, thrombin-treated PMVEC formed a more extensive network of cords and plexi when plated on FN-spiked Matrigel than on collagen-spiked Matrigel. In both assays, 10% FBS produced a robust response (wound closure or tube formation), regardless of matrix protein treatment (data not shown).
Fig. 4.
Thrombin/FN stimulation induces an angiogenic phenotype in RPMVEC. A: classic wound healing and Matrigel assays after treatment with collagen/DMEM, collagen/thrombin, FN/DMEM, or FN/thrombin. Concurrent FN and thrombin treatment resulted in enhanced wound closure and increased vascular plexus formation compared with control conditions or either stimulus alone. B: quantification of wound healing and Matrigel assays. (*P < 0.05 vs. coll/DMEM, coll/thrombin, and FN/DMEM; #P < 0.05 vs. coll/DMEM, n = 3).
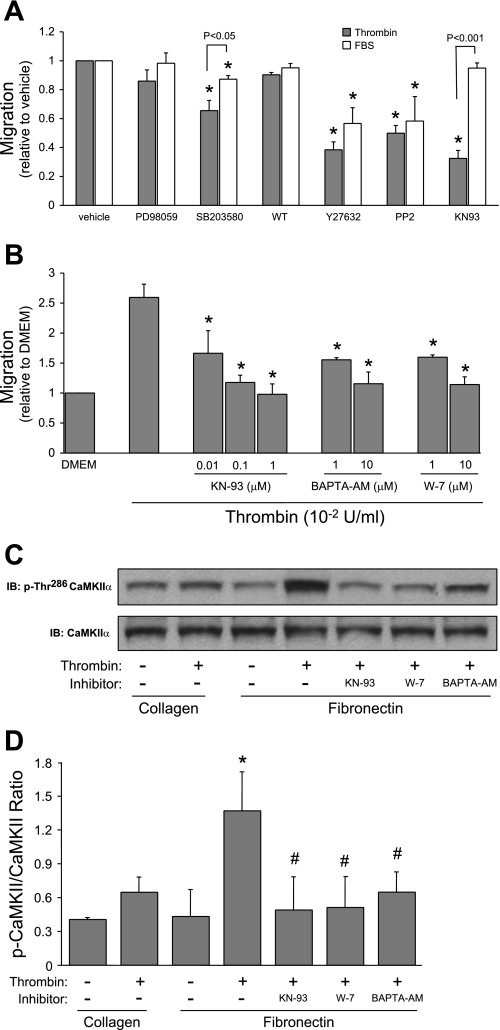
To elucidate signaling events mediating the interaction between FN and PAR1 stimulation, we inhibited several pathways involved in cellular migration. Because we were interested in the FN dependence of PAR1-induced migration, we sought to identify inhibitors that blocked migration in response to this stimulus while leaving matrix-independent FBS-induced migration intact. Some inhibitors (e.g., wortmannin to inhibit phosphatidylinositol 3-kinase) had minimal effect on migration in response to thrombin or FBS, while others (e.g., Y27632, Rho kinase) interfered with both stimuli, suggesting that they blocked a common, distal signal in PMVEC migration. Borbiev et al. (4) had previously demonstrated an important role for CaMKII in mediating thrombin's effects on lung EC. We investigated the role of CaMKII in mediating migration and found that KN-93 produced robust inhibition of thrombin/FN-induced migration with no effect on migration to FBS (Fig. 5A). This result suggests that CaMKII is specifically important to PMVEC migration in response to thrombin/FN. The importance of CaMKII was further supported by the finding that antagonizing the two major activators of its activity, calcium (with BAPTA) and calmodulin (W-7), also resulted in strong inhibition of thrombin/FN-induced migration (Fig. 5B). These more general inhibitors caused a small decrease in FBS-induced migration (data not shown), likely due to the importance of calcium/calmodulin-dependent proteins other than CaMKII.
Fig. 5.
Calcium/calmodulin-dependent protein kinase II (CaMKII) activity is required for thrombin/FN-induced pulmonary microvascular endothelial cell migration. A: we interrogated signaling pathways classically involved in migration to evaluate the relative contribution to thrombin/FN- and FBS-induced migration: PD-98059 (MEK1/ERK1/2 inhibitor, 2 × 10−5 mol/l); SB-203580 (p38 kinase, 2 × 10−5 mol/l); Y-27632 (rho kinase, 10−5 mol/l); wortmannin (WT; phosphatidylinositol 3-kinase, 10−8 mol/l); PP2 (src kinase, 10−5 mol/l); and KN-93 (CaMKII, 10−5 mol/l). KN-93 produced specific inhibition of thrombin/FN-induced migration, with no effect on FBS-induced migration (*P < 0.05 vs. vehicle, n = 3). B: antagonizing CaMKII signaling with W-7 (calmodulin antagonist) or BAPTA (Ca2+ chelator) produced robust inhibition of thrombin/FN-induced migration (*P < 0.05 vs. thrombin, n = 3). C: representative Western blots for phospho-thr286 and total CaMKII-α. Thrombin induced FN-specific CaMKII-α phosphorylation that was blocked by inhibitors of the CaMKII pathway. D: quantification of Western blot densitometry (*P < 0.05 vs. collagen/no thrombin; #P < 0.05 vs. FN/thrombin, n = 3).
An important event in CaMKII activation is autophosphorylation of threonine-286; we assessed activation by Western blot using an antibody specific to CaMKII-α phosphorylated on this residue. As shown in Fig. 5C and quantified in Fig. 5D, thrombin induced phosphorylation of CaMKII-α in a FN-dependent fashion. This phosphorylation was blocked by KN-93, W-7, and BAPTA-AM. Therefore, CaMKII-α is activated in PMVEC under the conditions that promote migration.
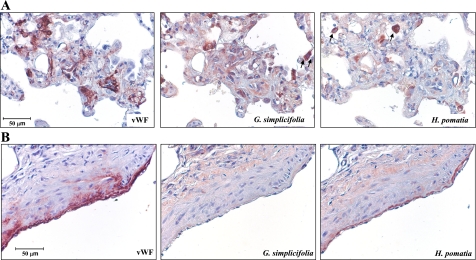
Having demonstrated a substantial difference in the migration phenotype between PAEC and PMVEC, we stained rat lung tissue to identify the EC in the pneumonectomy and MCT model of severe PAH. Figure 6 shows representative histochemistry of lung sections taken from PAH rats using lectins that selectively recognize microvascular (Griffonia simplicifolia) or macrovascular (Helix pomatia) EC. As seen in Fig. 6A, von Willebrand factor-positive EC in plexiform-like lesions bound G. simplicifolia with less H. pomatia staining. Conversely, the EC lining large-conduit pulmonary arteries showed the opposite pattern of lectin binding with more macrovascular staining (Fig. 6B). These limited data confirm our previous report that EC contribute to the pathological lesions in rat PAH and suggest that microvascular cells may be overrepresented in these lesions.
Fig. 6.
Rat proliferative vascular lesions contain endothelial cells. Lung sections from rats with severe proliferative PAH were stained with the lectins Helix pomatia or Griffonia simplicifolia, which specifically bind to macrovascular or microvascular endothelial cells, respectively. Von Willebrand factor (vWF) was used as a marker of endothelial cells. A: proliferative vascular lesions were populated by cells that bound G. simplicifolia more so than H. pomatia, suggesting a microvascular origin. Both lectins cross reacted strongly with leukocytes (arrows mark stained cells in alveolar spaces). B: in contrast to the endothelial cells in proliferative lesions, those lining conduit pulmonary arteries bound H. pomatia less than G. simplicifolia.
DISCUSSION
We have identified an apparently unique role for thrombin in directing the migration of a highly proliferative, vasculogenic microvascular EC isolated from the pulmonary circulation, but the stimulus is only effective in the context of a FN matrix. We have further identified a specific role for CaMKII in mediating thrombin/FN-induced migration. This EC is of particular interest because the cell's phenotype in culture (2, 9) (see discussion below) suggests a role for the cell in the poorly understood vascular pathology of PAH.
The FN dependence of thrombin-induced PMVEC migration indicates that binding and activation of specific integrins are necessary events; the sensitivity to RGD implies integrin interaction with the RGD motif of FN. On PMVEC, the most likely integrins involved are αvβ3, αvβ5, and α5β1, all of which have been implicated in modulating EC behavior and regulating angiogenesis (15, 34, 38). α5β1 is the classic FN receptor; αvβ5 and αvβ3 are typically considered vitronectin receptors, but both also bind FN (8, 13). αvβ3 is one of the earliest integrins identified during angiogenesis, especially in tumors and ischemic tissue (24, 26). In addition to the direct effects of ECM/integrin interaction on intracellular signaling and cellular behavior, there is extensive signaling cross talk between integrins and growth factor receptors (44, 67, 68). Although it is well established that thrombin activation of platelets leads to integrin inside-out signaling and activation, our finding of a functional interaction between the PAR1 receptor and FN-binding integrins in EC migration is novel.
Our results also indicate an important, strikingly specific role for CaMKII in thrombin/FN-induced PMVEC migration. CaMKII has been studied most thoroughly in the nervous system, where it is a central mediator for many processes, including synaptic plasticity, learning and memory, long-term potentiation, and ion channel function (41, 60). More relevant to the present study, CaMKII has been implicated in integrin inside-out signaling to control the following: the affinity of α5β1 for FN (6); migration in vascular smooth muscle cells (3); and the integrin-dependent cytoskeletal reorganization necessary for dendritic spine elongation in mammalian neurons (58). Our initial focus on CAMKII was partially motivated by Borbiev et al. (4, 5), who identified a role for CaMKII in mediating thrombin-induced barrier dysfunction in cultured bovine pulmonary EC. In those experiments, CaMKII phosphorylated caldesmon and nonmuscle filamin to promote cellular contraction and barrier dysfunction; the authors did not investigate the matrix specificity of their findings. Further experiments will be required to determine whether CaMKII plays a critical role in integrin activation or in downstream cytoskeletal rearrangement (or both) in our system. In addition, FN-specific autophosphorylation (activation) of CaMKII-α by thrombin suggests fundamental differences in PMVEC calcium handling, depending on ECM interaction.
We have previously shown that TF expression is increased in humans and rats with PAH (66). While TF overexpression in PAH may explain the thrombotic diathesis in these patients, we propose that TF contributes more directly to the vascular pathology of PAH, as it does in diseases of the systemic circulation. The present study suggests that thrombin generation and subsequent stimulation of PAR1 on PMVEC is one possible mechanism by which TF plays a role in PAH pathogenesis; our data also strengthen the hypothesis that inhibition of the TF/thrombin/PAR1 axis may represent an effective, novel treatment of the disease. We propose that one important effect of TF blockade is to reduce thrombin-induced PMVEC migration to sites of pulmonary vascular injury where FN is often abundant (29).
In addition to the TF/thrombin/PAR1 axis, the current study highlights integrin signaling as another potential therapeutic target. Generation of provisional ECM and subsequent integrin signaling have been implicated in conditions such as intimal hyperplasia, autoimmune disease, and cancer (14, 42, 62). These events have been especially well studied in a rare brain tumor called glioblastoma multiforme (24); interestingly these tumors form disordered vascular networks resembling the plexiform lesions of PAH (64). Cowan et al. (12) postulated a role for integrin αvβ3 in PAH. They found that the increased serine elastase activity seen in the context of PAH was associated with clustering of αvβ3 on smooth muscle cells, and, further, that antibody blockade of the integrin leads to regression of medial hypertrophy in PAH organ culture explants (12). However, the RGD analog cilengitide failed to affect mortality when administered to rats with PAH (46). Importantly, those investigators used the standard MCT model of PAH. In contrast to human PAH, MCT produces a pathology dominated by smooth muscle cell hypertrophy. Although anti-αvβ3 therapy failed to reduce the medial hypertrophy in that model, our current study suggests that targeting relevant integrins may prove to be an effective treatment when neointima and plexiform lesion formation containing both endothelial and smooth muscle cells is part of the vascular pathology (as it is in human PAH and the pneumonectomy/MCT rat model).
Although the pathogenesis of PAH remains enigmatic, Cool and colleagues (10, 39, 63) have presented human autopsy data to support the hypothesis that aberrant EC proliferation in the pulmonary microvasculature plays a key role. Detailed three-dimensional reconstruction of serial sections from a small group of patients suggests that the process of cell proliferation starts distally at branch points (plexiform lesions) before progressing proximally into larger vessels to form classic “onion skin” concentric lesions obliterating the lumen (10). As recently reviewed, concentric lesions appear in ∼25% of pulmonary vessels sized between 25 and 200 μm (65). In rats, the transition from macrovascular cells staining with Helix to microvascular cells staining with Griffonia occurs at vessels between 25 and 50 μm in diameter (Troy Stevens, personal communication). Detailed reconstructions of the rat vascular pathology have not been performed, but our previous work shows a striking similarity between the pathological features of human disease and those of the pneumonectomy/MCT model (66). The data in Fig. 6 confirm our previous data that EC populate proliferative lesions in the rat model, and we believe that further study of these cells is likely to shed light on the pathogenesis of PAH.
Recently, two papers from Stevens and colleagues (2, 9) have further highlighted the phenotypic and functional differences between microvascular and macrovascular lung EC. The authors identified a subpopulation of PMVEC (binding G. simplicifolia) that expressed classic EC antigens and progenitor cell markers. Consistent with this antigenic classification as endothelial progenitor cells, these “resident microvascular endothelial progenitor cells” displayed both normal EC barrier function and highly proliferative/vasculogenic behavior with long telomeres. In further studies, the authors found that PMVEC displayed much greater proliferative capacity and expressed higher levels of nucleosome assembly protein-1 (NAP-1) than did PAEC. Inhibiting expression of NAP-1 in PMVEC abrogated their highly proliferative phenotype, while heterologous NAP-1 expression in PAEC conferred on them a greater proliferative capacity. Combined with our present findings, this work indicates that the pulmonary microvasculature is populated by EC that have high proliferative capacity but are relatively resistant to migration. After an insult resulting in endothelial damage and death, we hypothesize that surviving cells, including a significant proportion of endothelial progenitors, are stimulated to proliferate and repopulate the pulmonary endothelium on a provisional, FN-rich matrix. A concurrent increase in TF expression and thrombin generation would stimulate migration for these cells, which already have an intrinsic, highly proliferative behavior. The net result would be the aberrant angiogenesis thought to underlie generation of plexiform lesions in PAH. (63)
In summary, we have identified a functional interaction between PAR1 activation and FN binding in which both are essential to stimulate PMVEC migration. Among common endothelial agonists, thrombin stimulation was uniquely effective in promoting migration of this highly proliferative, vasculogenic EC isolated from the distal pulmonary circulation. Our data suggest a critical role for excess thrombin stimulation in the vascular pathology of PAH and underscore the importance of studying thrombin inhibitors and thrombin receptor antagonists as novel treatments for this devastating disease.
GRANTS
R. J. White was supported by a Parker B. Francis Fellowship in Pulmonary Biology and holds an American Heart Association Scientist Development Grant (073540N). D. F. Meoli is supported by National Heart, Lung, and Blood Institute Multidisciplinary Training in Pulmonary Research Grant 5T32HL066988.
ACKNOWLEDGMENTS
We are grateful to Drs. Mark Taubman and Jane Sottile for mentorship as this project developed and to Christine Miller for assistance with immunohistochemistry.
REFERENCES
- 1.Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 118: 445–450, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 294: L419–L430, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Andersen R, Li Y, Resseguie M, Brenman JE. Calcium/calmodulin-dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila. J Neurosci 25: 8878–8888, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borbiev T, Verin AD, Birukova A, Liu F, Crow MT, Garcia JG. Role of CaM kinase II and ERK activation in thrombin-induced endothelial cell barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 285: L43–L54, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Borbiev T, Verin AD, Shi S, Liu F, Garcia JG. Regulation of endothelial cell barrier function by calcium/calmodulin-dependent protein kinase II. Am J Physiol Lung Cell Mol Physiol 280: L983–L990, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bouvard D, Molla A, Block MR. Calcium/calmodulin-dependent protein kinase II controls alpha 5 beta 1 integrin-mediated inside-out signaling. J Cell Sci 111: 657–665, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA 97: 5255–5260, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng YF, Clyman RI, Enenstein J, Waleh N, Pytela R, Kramer RH. The integrin complex alpha v beta 3 participates in the adhesion of microvascular endothelial cells to fibronectin. Exp Cell Res 194: 69–77, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Clark J, Alvarez DF, Alexeyev M, King JAC, Huang L, Yoder MC, Stevens T. Regulatory role for nucleosome assembly protein-1 in the proliferative and vasculogenic phenotype of pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 294: L431–L439, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 155: 411–419, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost 86: 298–307, 2001 [PubMed] [Google Scholar]
- 12.Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest 105: 21–34, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha 5 beta 1 and alpha v beta 3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol 159: 1071–1086, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufourcq P, Couffinhal T, Alzieu P, Daret D, Moreau C, Duplaa C, Bonnet J. Vitronectin is up-regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc Res 53: 953–963, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin alpha v beta 3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol 140: 1255–1263, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Fearon U, Griosios K, Fraser A, Reece R, Emery P, Jones PF, Veale DJ. Angiopoietins, growth factors, and vascular morphology in early arthritis. J Rheumatol 30: 260–268, 2003 [PubMed] [Google Scholar]
- 18.Fishman AP. Etiology and pathogenesis of primary pulmonary hypertension: a perspective. Chest 114: 242S–247S, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29: 15–18, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Folkman J, Shing Y. Angiogenesis. J Biol Chem 267: 10931–10934, 1992 [PubMed] [Google Scholar]
- 21.Fujii H, Nishikawa N, Komazawa H, Orikasa A, Ono M, Itoh I, Murata J, Azuma I, Saiki I. Inhibition of tumor invasion and metastasis by peptidic mimetics of Arg-Gly Asp (RGD) derived from the cell recognition site of fibronectin. Oncol Res 8: 333–342, 1996 [PubMed] [Google Scholar]
- 22.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res 68: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol 9: 691–700, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest 88: 1924–1932, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hocking DC, Sottile J, McKeown-Longo PJ. Activation of distinct alpha 5 beta 1-mediated signaling pathways by fibronectin's cell adhesion and matrix assembly domains. J Cell Biol 141: 241–253, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoshiga M, Alpers CE, Smith LL, Giachelli CM, Schwartz SM. Alpha-v beta-3 integrin expression in normal and atherosclerotic artery. Circ Res 77: 1129–1135, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91: 877–887, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Jang Y, Guzman LA, Lincoff AM, Gottsauner-Wolf M, Forudi F, Hart CE, Courtman DW, Ezban M, Ellis SG, Topol EJ. Influence of blockade at specific levels of the coagulation cascade on restenosis in a rabbit atherosclerotic femoral artery injury model. Circulation 92: 3041–3050, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 150: 1349–1360, 1997 [PMC free article] [PubMed] [Google Scholar]
- 30.Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng YW, Cheng A, Griffin C, Coughlin SR. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood 102: 3224–3231, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Kawut SM, Horn EM, Berekashvili KK, Garofano RP, Goldsmith RL, Widlitz AC, Rosenzweig EB, Kerstein D, Barst RJ. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol 95: 199–203, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298: 1950–1954, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol Lung Cell Mol Physiol 274: L810–L819, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Harris M, Varner JA. Regulation of integrin alpha v beta 3-mediated endothelial cell migration and angiogenesis by integrin alpha 5 beta 1 and protein kinase A. J Biol Chem 275: 33920–33928, 2000 [DOI] [PubMed] [Google Scholar]
- 35.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67: 139–151, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res 100: 782–794, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell 84: 359–369, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Lee BH, Ruoslahti E. Alpha 5 beta 1 integrin stimulates Bcl-2 expression and cell survival through Akt, focal adhesion kinase, and Ca2+/calmodulin-dependent protein kinase IV. J Cell Biochem 95: 1214–1223, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest 101: 927–934, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175–190, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Lobb RR, Hemler ME. The pathophysiologic role of alpha 4 integrins in vivo. J Clin Invest 94: 1722–1728, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marmur JD, Rossikhina M, Guha A, Fyfe B, Friedrich V, Mendlowitz M, Nemerson Y, Taubman MB, Marmur JD, Rossikhina M, Guha A, Fyfe B, Friedrich V, Mendlowitz M, Nemerson Y, Taubman MB. Tissue factor is rapidly induced in arterial smooth muscle after balloon injury. J Clin Invest 91: 2253–2259, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mawatari K, Liu B, Kent KC. Activation of integrin receptors is required for growth factor-induced smooth muscle cell dysfunction. J Vasc Surg 31: 375–381, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Melchers F, Rolink AG, Schaniel C. The role of chemokines in regulating cell migration during humoral immune responses. Cell 99: 351–354, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Merklinger SL, Jones PL, Martinez EC, Rabinovitch M. Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation 112: 423–431, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Meyrick B. The pathology of pulmonary artery hypertension. Clin Chest Med 22: 393–404, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res 66: 307–314, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13: 9–22, 1999 [PubMed] [Google Scholar]
- 50.Newman JH, Fanburg BL, Archer SL, Badesch DB, Barst RJ, Garcia JG, Kao PN, Knowles JA, Loyd JE, McGoon MD, Morse JH, Nichols WC, Rabinovitch M, Rodman DM, Stevens T, Tuder RM, Voelkel NF, Gail DB. Pulmonary arterial hypertension: future directions: report of a National Heart, Lung and Blood Institute/Office of Rare Diseases workshop. Circulation 109: 2947–2952, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385: 537–540, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Parent CA, Devreotes PN. A cell's sense of direction. Science 284: 765–770, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309: 30–33, 1984 [DOI] [PubMed] [Google Scholar]
- 54.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Levy PS, Reid LM, Vreim CE, Williams GW. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the NHLBI Primary Pulmonary Hypertension Registry. Circulation 80: 1198–1206, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Pyo RT, Sato Y, Mackman N, Taubman MB. Mice deficient in tissue factor demonstrate attenuated intimal hyperplasia in response to vascular injury and decreased smooth muscle cell migration. Thromb Haemost 92: 451–458, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Schweigerer L. Fibroblast growth factor and angiogenesis. Z Kardiol 78, Suppl 6: 12–15, 1989 [PubMed] [Google Scholar]
- 58.Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci 26: 1813–1822, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359: 843–845, 1992 [DOI] [PubMed] [Google Scholar]
- 60.Soderling TR. CaM-kinases: modulators of synaptic plasticity. Curr Opin Neurobiol 10: 375–380, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim Biophys Acta 1654: 13–22, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Trikha M, Zhou Z, Timar J, Raso E, Kennel M, Emmell E, Nakada MT. Multiple roles for platelet GPIIb/IIIa and alpha v beta 3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res 62: 2824–2833, 2002 [PubMed] [Google Scholar]
- 63.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195: 367–374, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994 [PMC free article] [PubMed] [Google Scholar]
- 65.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 28: 23–42, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White RJ, Meoli DF, Swarthout R, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, Cool CD, Taubman MB. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L583–L590, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Wijelath ES, Murray J, Rahman S, Patel Y, Ishida A, Strand K, Aziz S, Cardona C, Hammond WP, Savidge GF, Rafii S, Sobel M. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res 91: 25–31, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, Groopman JE, Wang JF. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha 5 beta 1. J Cell Physiol 202: 205–214, 2005 [DOI] [PubMed] [Google Scholar]