Abstract
Efficient removal of apoptotic cells is essential for resolution of inflammation. Failure to clear dying cells can exacerbate lung injury and lead to persistent inflammation and autoimmunity. Here we show that TNFα blocks apoptotic cell clearance by alveolar macrophages and leads to proinflammatory responses in the lung. Compared with mice treated with intratracheal TNFα or exogenous apoptotic cells, mice treated with the combination of TNFα plus apoptotic cells demonstrated reduced apoptotic cell clearance from the lungs and increased recruitment of inflammatory leukocytes to the air spaces. Treatment with intratracheal TNFα had no effect on the removal of exogenous apoptotic cells from the lungs of TNFα receptor-1 (p55) and -2 (p75) double mutant mice and no effect on leukocyte recruitment. Bronchoalveolar lavage from mice treated with TNFα plus apoptotic cells contained increased levels of proinflammatory cytokines IL-6, KC, and MCP-1, but exhibited no change in levels of anti-inflammatory cytokines IL-10 and TGF-β. Administration of TNFα plus apoptotic cells during LPS-induced lung injury augmented neutrophil accumulation and proinflammatory cytokine production. These findings suggest that the presence of TNFα in the lung can alter the response of phagocytes to apoptotic cells leading to inflammatory cell recruitment and proinflammatory mediator production.
Keywords: apoptosis, phagocytosis
the removal of apoptotic cells is a tightly controlled process critical to development, tissue remodeling, and immunity. Rapid clearance of apoptotic cells by macrophages and other phagocytes prevents the release of toxic intracellular contents from dying cells and leads to the production of anti-inflammatory mediators such as TGF-β, PGE2, platelet-activating factor, and IL-10 (8). These mediators act in an autocrine/paracrine manner to actively suppress the production of proinflammatory growth factors, cytokines, and eicosanoids (8, 9, 16). Impaired clearance of apoptotic cells can result in the accumulation of secondarily necrotic cells, which may exacerbate inflammatory responses and contribute to chronic inflammatory states and autoimmunity (7, 10, 20).
Efficient removal of dying cells may be particularly important for the resolution of lung injury. Instillation of apoptotic cells into LPS-injured lungs results in rapid abrogation of the inflammatory response following phagocytosis (16). Conversely, defects in apoptotic cell clearance have been implicated in the pathogenesis of a number of chronic inflammatory lung diseases including chronic obstructive pulmonary disease, cystic fibrosis, bronchiectasis, and asthma (13, 14, 15, 17, 30, 31).
In our ongoing studies of the mechanisms by which macrophages recognize and remove apoptotic cells, we recently showed that TNFα had a pleomorphic effect on apoptotic cell clearance in vitro. In immature monocytes and macrophages, TNFα enhanced phagocytosis (see also Ref. 26), but in mature macrophages, TNFα acted as a potent inhibitor of apoptotic cell engulfment (22). These observations raised important questions about the potential role of TNFα in modifying apoptotic cell clearance in vivo and the possible effect of TNFα on the normally anti-inflammatory consequences of the apoptotic cell recognition. Accordingly, a series of in vivo experiments was conducted in which apoptotic thymocytes were instilled into naïve mouse lungs in the presence or absence of exogenous TNFα. These studies confirmed that TNFα reduces the ability of alveolar macrophages to clear apoptotic cells. This resulted in an enhanced rather than suppressed inflammatory response.
MATERIALS AND METHODS
Reagents.
RPMI 1640, HBSS, and PBS were purchased from Cellgro Mediatech (Herndon, VA). X-vivo 10 medium was obtained from BioWhittaker (Walkersville, MD), and endotoxin-free FCS was obtained from HyClone Laboratories (Logan, UT). LPS (from Escherichia coli 0111:B4) was provided by List Biological Laboratories (Campbell, CA). Avertin (2,2,2-tribromoethanol), tert-amyl alcohol, red blood cell lysing buffer, and PKH26 Red Fluorescent Cell Linker Mini Kit was obtained from Sigma-Aldrich (St. Louis, MO). Polystyrene flasks and plates for tissue culture were from Becton Dickinson Labware (Franklin Lakes, NJ). TNFα was obtained from Calbiochem (San Diego, CA). APC-conjugated rat anti-mouse F4–80 and APC-conjugated rat IgG isotype control was supplied by Serotec (Raleigh, NC). Rat anti-mouse Fc block (CD16/CD32) IgG2b antibody and rat anti-mouse CD3 (clone 17A2) were purchased from Becton Dickinson. Popper animal feeding needles (22 G, 1.5 in) were obtained from Fisher Scientific (Pittsburgh, PA). Pentobarbital was purchased from Abbott Laboratories (North Chicago, IL).
Animals.
Female Balb/c mice were obtained from Taconic (Germantown, NY). Mice 9–10 wk old (20–25 g) were used for in vivo instillation assays. Thymocytes were harvested from mice 3–4 wk of age. Wild-type C57BL/6 and TNFα receptor-1 (p55) and -2 (p75)-deficient mice (25) were purchased from Jackson Laboratories (Bar Harbor, ME). The Animal Care and Use Committee of the National Jewish Health approved all experimental procedures.
Induction of apoptosis.
Mouse thymocytes were prepared by removing thymuses from 3- to 4-wk-old mice and gently pushing the tissue through a 40-μm strainer to separate individual cells. Apoptosis was induced by exposing the thymocytes to ultraviolet irradiation (254 nm) for 10 min followed by culture in RPMI 1640 with 10% FCS at 37°C in 5% CO2 for 3 h. Thymocytes prepared in this fashion were ∼90% annexin V positive and ∼20% propidium iodide positive by FACS analysis.
In vivo assays.
For all experiments, mice were anesthetized with 0.3 ml of intraperitoneal Avertin (20 mg/ml). Tracheotomy was performed by making a midline incision in the anterior neck followed by blunt dissection. The trachea was cannulated with a 22-gauge needle through which all treatments were administered (2). To test the effect of TNFα on efferocytosis in vivo, mice were divided into four groups as follows: 1) PBS alone; 2) TNFα alone; 3) PBS + apoptotic cells; 4) TNFα + apoptotic cells. All treatments were administered in a volume of 50 μl. PBS was supplemented with 0.1% BSA. TNFα was administered at a dose of 50 ng in 50 μl PBS plus 0.1% BSA. Apoptotic cells were given as a second injection immediately following PBS or TNFα. The apoptotic cells were murine thymocytes at a concentration of 107 cells in 50 μl of PBS. For some experiments, intratracheal LPS (20 μg in 50 μl of PBS) was administered 48 h before treatment with apoptotic thymocytes and/or TNFα. For all experiments, whole lung lavage was performed with a total of 5 ml of ice-cold HBSS containing 5 mM EDTA. Leukocytes from bronchoalveolar lavage (BAL) were quantified using a hemacytometer and also processed by cytospin. Slides were fixed and stained with modified Wright-Giemsa stain (Fisher Scientific), and the differential counts were calculated by multiplying cell percentage by total numbers in the lavage. Phagocytosis was quantified microscopically and expressed as the phagocytic index (PI) (16). The PI was calculated by dividing the number of ingested apoptotic bodies by the number of macrophages counted and multiplying by 100. A minimum of 400 alveolar macrophages were counted blindly. BAL supernatant was stored at −70°C for future cytokine and chemokine analysis.
Fluorescent cell labeling and flow cytometry.
Apoptotic thymocytes were labeled with PKH26 Red Fluorescent Cell Linker Mini Kit according to the manufacturer's instructions. Following induction of apoptosis by UV light, thymocytes were washed with RPMI 1640 and resuspended in Diluent C and PKH26 (10 μM) at a concentration of 5 × 107 cells/ml. Cells were incubated at 37°C with gentle shaking for 15 min. RPMI 1640 with 10% FCS was added to stop the reaction. Thymocytes were washed once in RPMI 1640 and then resuspended in PBS with 0.1% BSA for instillation. Leukocytes from the airways and alveoli were obtained by BAL. Cells were washed twice in PBS with 1% BSA and incubated with Fc block (1:100) for 20 min on ice. Alveolar macrophages were identified using APC-conjugated rat anti-mouse F4/80 (1:100 dilution) with an APC-conjugated rat IgG isotype as control (see Fig. 1). Lymphocytes were identified by staining with a monoclonal rat anti-mouse CD3 antibody. Samples were washed twice after staining and then fixed with 2% paraformaldehyde in PBS. Flow cytometry was performed on a Facscalibur flow cytometer (BD Biosciences) using Cell Quest Pro (BD Bioscience) software. Final data analysis was performed with FloJo (TreeStar, Ashland, OR).
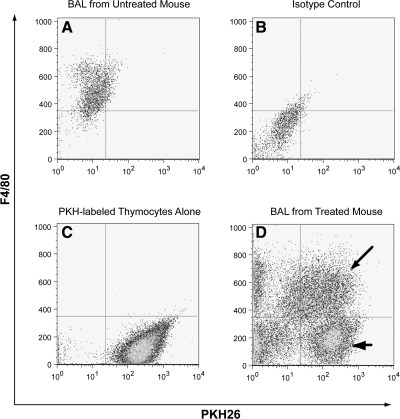
Fig. 1.
Quantification of apoptotic cell clearance from the lungs using flow cytometry. Following induction of apoptosis with UV irradiation, murine thymocytes were labeled with fluorescent dye (PKH26-GL) and then instilled directly into the trachea (107 per mouse). Whole lung lavage was performed 90 min later. Alveolar macrophages were identified using the macrophage-specific marker F4/80. A: F4/80 staining of bronchoalveolar lavage (BAL) from an untreated mouse. Note that nearly all of the cells are macrophages. B: F4/80 isotype control of BAL from the same animal. C: apoptotic thymocytes labeled with PKH26-GL. Unlabeled thymocytes demonstrated minimal autofluorescence (not shown). D: BAL from a mouse treated with apoptotic thymocytes. Cells double positive for F4/80 and PKH26 (arrow) were considered to be alveolar macrophages that had ingested or bound apoptotic cells. Thymocytes were defined as F4/80−/PKH+ events (arrowhead). Events in the bottom left quadrant represent nonspecific debris.
ELISA.
The levels of TGF-β, IL-10, IL-6, KC, and MCP-1 in BAL fluid were measured by ELISA using Quantikine immunoassays manufactured by R&D Systems (Minneapolis, MN) according to the instructions provided with each kit. For TGF-β, the supernatant was acid-activated according to the manufacturer's instructions. Color development was assessed using a microplate autoreader (EL309) by Bio-Tek Instruments (Winooski, VT). Data were analyzed using a log/log curve fit option from Delta Soft 3 ELISA analysis software for Macintosh (BioMetallics, Princeton, NJ).
Statistics.
All experiments were performed at least three times. The means were analyzed using ANOVA for multiple comparisons; when ANOVA indicated significance, the Tukey-Kramer test was used to compare groups with an internal control. For all other experiments in which two conditions were being compared, a two-tailed Student's t-test assuming equal variance was used. All data were analyzed using GraphPad Prism 3.0 (GraphPad Software, San Diego, CA). Results were expressed as the average and SE. Asterisks are used in the figures to denote significant differences compared with control P < 0.05 (*) and P < 0.01 (**).
RESULTS
TNFα blocks the clearance of apoptotic cells by alveolar macrophages.
To investigate the effect of TNFα on clearance of apoptotic cells from the lungs, we first established an animal model in which TNFα (or PBS control) was instilled directly into the lungs of recipient mice followed immediately by a second instillation containing PKH-labeled apoptotic murine thymocytes. Ninety minutes later, the animals were euthanized, and whole lung lavage was performed. Apoptotic cell clearance was assessed using flow cytometry (Fig. 1) and light microscopy. Lavage fluid was supplemented with EDTA to minimize nonspecific binding between the phagocytes and apoptotic cells.
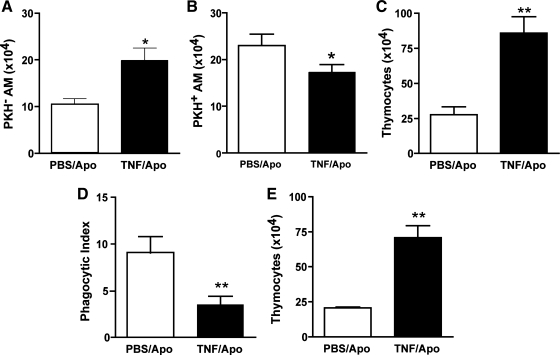
The total number of alveolar macrophages per milliliter of BAL fluid was similar between mice that received TNFα plus apoptotic thymocytes (35.9 ± 0.4 × 104) and mice treated with apoptotic thymocytes alone (33.9 ± 0.5 × 104). However, the number of PKH-negative macrophages (i.e., macrophages not associated with apoptotic thymocytes) was increased in mice treated with TNFα plus apoptotic cells (Fig. 2A). In concordance, the number of PKH-positive macrophages (i.e., macrophages that had bound or ingested the apoptotic cells) was decreased in TNFα-treated animals (Fig. 2B). This effect was mirrored by a reciprocal increase in the number of free thymocytes in lavage fluid (Fig. 2C) suggesting decreased engulfment of apoptotic cells in TNFα-treated mice.
Fig. 2.
TNFα inhibits apoptotic cell clearance by alveolar macrophages in vivo at 90 min. PKH26-labeled apoptotic thymocytes (107 per mouse) were directly instilled into the tracheas of recipient mice simultaneously with TNFα (50 ng) or PBS as a control. Flow cytometry (A–C) and light microscopy (D and E) were used to assess efferocytosis. Macrophages were identified as F4/80+ events. The number of macrophages that were not associated with apoptotic cells (PKH− AM) was increased in TNFα-treated mice (A), whereas alveolar macrophages that were associated with apoptotic cells (PKH+ AM) were concomitantly decreased (B). This led to greater recovery of PKH-labeled thymocytes (C). The phagocytic index (number of apoptotic cells ingested per macrophage × 100) was reduced in mice treated with TNFα (D). Impaired removal of apoptotic cells from the lungs of TNFα-treated animals was further supported by an increased recovery of noningested apoptotic thymocytes (E). *P < 0.05 vs. control; **P < 0.01, n = 8 per group.
To confirm our findings from flow cytometry, we examined Wright-Giemsa-stained samples of BAL fluid with light microscopy. In line with our results from flow cytometry, alveolar macrophages from TNFα-treated animals demonstrated a 50% decrease in apoptotic cell phagocytosis (Fig. 2D). Moreover, direct counting of thymocytes from lavage fluid confirmed an increase in the number of thymocytes recovered from mice treated with TNFα (Fig. 2E). Together, these results suggest impaired removal of apoptotic cells by alveolar macrophages from mice treated with TNFα.
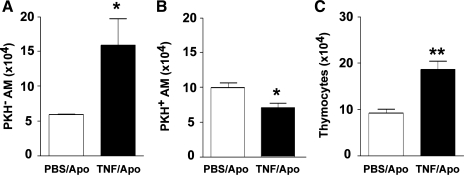
We next sought to determine if the coadministration of TNFα and apoptotic cells would result in sustained impairment of apoptotic cell clearance. As with previous experiments, fluorescently labeled apoptotic thymocytes were instilled into the lungs immediately following the administration of TNFα or PBS control. Whole lung lavage was performed 24 h later. Results were similar to those from the 90-min time point in that lavage fluid from TNFα-treated animals contained fewer alveolar macrophages with ingested or bound thymocytes (Fig. 3, A and B) and increased numbers of recovered thymocytes (Fig. 3C).
Fig. 3.
TNFα inhibits apoptotic cell clearance by alveolar macrophages in vivo at 24 h. Whole lung lavage was performed 24 h after coinstillation of apoptotic thymocytes with TNFα or PBS control. In animals treated with TNFα, there was an increase in the number of macrophages not associated with thymocytes (PKH− AM) (A) and a corresponding decrease in the number of macrophages with bound or ingested apoptotic cells (PKH+ AM) (B). Greater numbers of apoptotic thymocytes were contained in BAL from TNFα-treated mice (C). *P < 0.05 vs. control; **P < 0.01, n = 6 per group.
Inflammatory cells are recruited to the lungs of mice treated with TNFα and apoptotic cells.
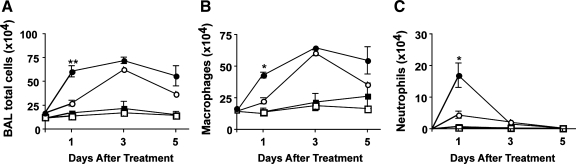
Impaired clearance of apoptotic cells results in the accumulation of late apoptotic and secondarily necrotic cells. Since the release of toxic intracellular contents from dying cells can exacerbate proinflammatory responses (10, 20, 24), we sought to determine if mice treated with TNFα plus apoptotic cells would exhibit enhanced recruitment of inflammatory cells to the lungs. Mice were treated with intratracheal TNFα (or PBS control) plus apoptotic cells (or PBS control) and then euthanized 1, 3, or 5 days later. Total leukocyte recovery was determined using a hemacytometer, and differential cell counts were determined by examining Wright-Giemsa-stained specimens under light microscopy. BAL cellular composition was determined by multiplying differential leukocyte counts by the total number of recovered cells. At 24 h, there were more total cells present in BAL fluid from mice treated with TNFα plus apoptotic cells than from mice treated with TNFα alone or PBS control (Fig. 4A). Leukocytes accounted for a significant portion of this increase. In fact, at 24 h, mice treated with a combination of TNFα and apoptotic thymocytes had significantly more monocytes/macrophages (Fig. 4B) and neutrophils (Fig. 4C) in their BAL fluid than mice treated with TNFα alone. By day 3, BAL leukocyte counts from mice treated with TNFα plus apoptotic cells approximated those from mice treated with TNFα alone. No inflammatory cells were evident in BAL fluid from mice treated with apoptotic cells alone.
Fig. 4.
Apoptotic cells augment TNFα-induced recruitment of leukocytes to the lungs. To determine the effect of apoptotic cells on TNF-induced inflammation in the lungs, mice were treated in 4 groups: intratracheal TNFα + apoptotic cells (●), TNFα alone (○), apoptotic cells alone (■), or PBS control (□). Animals were killed 1, 3, or 5 days after treatment. BAL fluid was analyzed for total cells (A), macrophages (B), and neutrophils (C). Values are presented as cell counts per milliliter of BAL fluid ± SD. *P < 0.05 vs. control, **P < 0.01 vs. control.
In concordance with our first set of experiments, at 24 h there were significantly more apoptotic thymocytes present in lavage fluid from mice treated with a combination of TNFα plus apoptotic cells compared with mice treated with apoptotic cells alone (data not shown). However, by day 3, the number of apoptotic cells was very low in both treatment groups. These findings suggest that TNFα prevents timely removal of apoptotic cells from the air spaces, and more importantly, that delayed clearance of apoptotic cells exacerbates the proinflammatory effects of TNFα.
TNFα-mediated defects in apoptotic cell clearance occur through TNFα receptors-1 and -2.
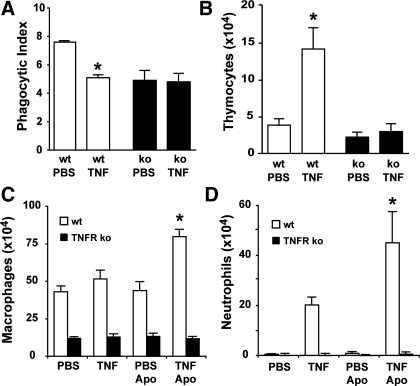
TNFα is a homotrimeric ligand that signals through two distinct cell surface receptors, TNFα receptor-1 (TNFR1, p55) and TNFα receptor-2 (TNFR2, p75) (19). To confirm that TNFα-mediated defects in apoptotic cell clearance occur through these receptors, we used double mutant mice lacking functional TNFR1 and TNFR2 (25). Wild-type and mutant mice were treated with apoptotic thymocytes and either TNFα or PBS. Ninety minutes later, whole lung lavage was performed. Importantly, treatment with intratracheal TNFα reduced the clearance of apoptotic cells by alveolar macrophages in wild-type mice but not in the mutant animals (Fig. 5A). In conjunction, BAL fluid from TNFα-treated wild-type mice contained greater numbers of apoptotic thymocytes than BAL from TNFα-treated mutant mice (Fig. 5B). These findings suggest that TNFα-mediated defects in apoptotic cell clearance require signaling through TNFR1 and TNFR2.
Fig. 5.
TNFα-related defects in apoptotic cell clearance and enhancement of inflammation occur through TNFα receptors p55 and p75. Wild-type mice (white bars) and mice deficient for both TNFα receptor-1 and -2 (p55−/−, p75−/−) (black bars) were instilled with PBS or TNFα followed by apoptotic thymocytes (107 per mouse). Whole lung lavage was performed 90 min later. The alveolar macrophage phagocytic index (A) and apoptotic thymocyte recovery (B) were determined on Wright-Giemsa-stained specimens. Leukocyte recruitment to the lungs in wild-type and TNFα receptor mutant mice was evaluated 24 h after intratracheal administration of TNFα (or PBS control) + apoptotic cells (or PBS control). The number of macrophages/monocytes (C) and neutrophils (D) per milliliter of BAL fluid was determined by light microscopy. *P < 0.05 vs. PBS control, n = 8 mice per group.
We next sought to determine if TNFR1 and TNFR2 were involved in the augmented pulmonary inflammation observed in animals treated with TNFα plus apoptotic cells. As in previous experiments, wild-type mice and TNFα receptor mutant mice were administered intratracheal TNFα (or PBS control) with or without apoptotic thymocytes. Whole lung lavage was performed 24 h later. In wild-type mice, coinstillation of TNFα and apoptotic cells resulted in greater numbers of macrophages (Fig. 5C) and neutrophils (Fig. 5D) in the air spaces than treatment with TNFα alone. This effect was not recapitulated in TNFα receptor mutant animals. In these animals, treatment with TNFα plus apoptotic cells or TNFα alone yielded cell counts that were similar to treatment with PBS.
Impaired clearance of apoptotic cells in the presence of TNFα results in the generation of proinflammatory mediators.
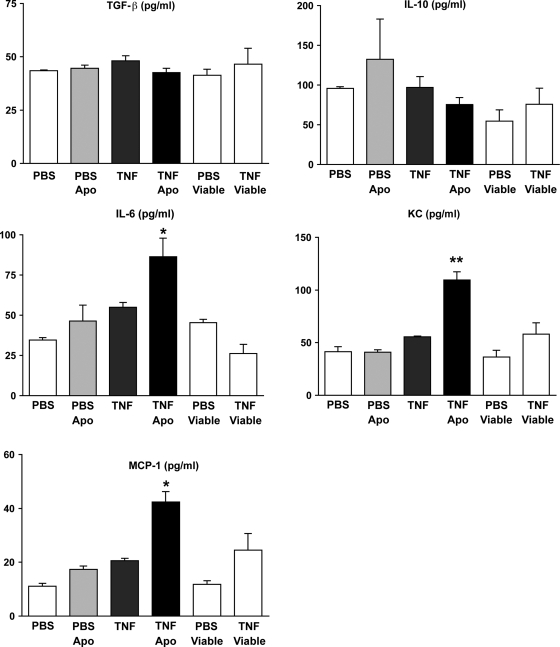
We have previously shown that instillation of apoptotic cells into LPS-injured lungs leads to the generation of anti-inflammatory mediators and suppression of inflammation (16). This effect is dependent on removal of apoptotic cells by alveolar macrophages and is mediated by macrophage release of TGF-β. However, in our model of TNFα-induced lung injury, apoptotic cell clearance is impaired and recruitment of inflammatory cells is enhanced. We therefore questioned whether production of anti-inflammatory cytokines (TGF-β, IL-10) would be reduced in favor of proinflammatory cytokines (IL-6, KC, MCP-1). To investigate, mice were treated with apoptotic cells (or PBS control) in the presence and absence of exogenous TNFα. Whole lung lavage was performed 24 h later. In the absence of TNFα, instillation of apoptotic cells into naïve mouse lungs had no effect on the production of anti-inflammatory or proinflammatory mediators (Fig. 6). On the other hand, when apoptotic cells were coinstilled with TNFα (i.e., under conditions where apoptotic cell clearance was decreased), the levels of the proinflammatory cytokines IL-6, KC, and MCP-1 were elevated significantly. Administration of TNFα alone did not increase proinflammatory cytokine levels above baseline. No difference in cytokine and chemokine production was observed in lungs treated with TNFα in combination with viable thymocytes.
Fig. 6.
Coinstillation of apoptotic thymocytes with TNFα results in proinflammatory cytokine and chemokine production in the lungs. Wild-type mice were treated with TNFα (or PBS control) followed immediately by apoptotic murine thymocytes (or an equal volume of PBS as control). Viable thymocytes were administered with PBS or TNFα as an additional control. Whole lung lavage was performed 24 h later. ELISA was performed on cell-free supernatants. Coadministration of apoptotic thymocytes with TNFα had no effect on TGF-β or IL-10 levels (P > 0.05), but led to increased IL-6, KC, and MCP-1 proinflammatory mediator levels. *P < 0.05 vs. TNFα alone; **P < 0.01 vs. TNFα alone, n = 8 mice per group.
Inhibition of apoptotic cell clearance by TNFα during lung injury exacerbates inflammation.
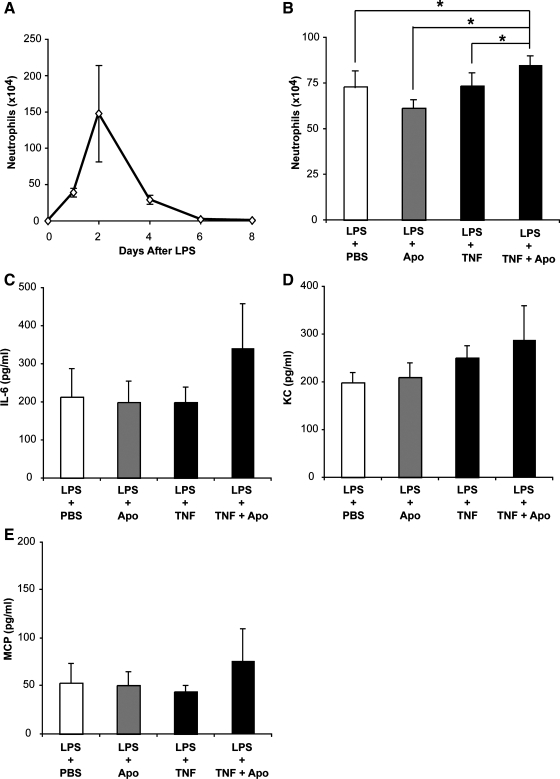
During acute lung injury, high levels of TNFα are present in the air spaces. At the same time, massive neutrophil accumulation occurs. Since neutrophils undergo apoptosis in situ and are cleared locally, we wanted to determine if administration of exogenous TNFα during an inflammatory response would impair clearance of apoptotic cells and exacerbate inflammation. As a first step, we established the kinetics of a self-limited inflammatory response by administering LPS to wild-type mice via direct intratracheal instillation (Fig. 7A). Neutrophil numbers peaked 48 h after LPS treatment and returned to baseline within 6 days. We reasoned that clearance of apoptotic neutrophils would be greatest in the period immediately following their peak accumulation and thus chose to study the effects of TNFα and apoptotic cells in a 24-h window after the neutrophil peak (i.e., 48–72 h after LPS). Consequently, we performed a second set of experiments in which we administered apoptotic cells, TNFα alone, or TNFα plus apoptotic cells 48 h after LPS. Lung lavage was performed 24 h later. In line with our previous observations (16), treatment with apoptotic cells alone abrogated neutrophil counts (Fig. 7B). Conversely, when apoptotic cells were instilled in the presence of exogenous TNFα, inflammation was enhanced. Interestingly, administration of TNFα alone had no effect on neutrophil counts. Concentrations of the proinflammatory cytokines IL-6, KC, and MCP followed a similar pattern and were elevated in mice that received the combination of TNFα and apoptotic cells during inflammation (Fig. 7, C–E).
Fig. 7.
Instillation of TNFα with apoptotic cells during lung injury exacerbates inflammation. Neutrophil counts were assessed in BAL from wild-type mice challenged with 20 μg of intratracheal LPS to determine the time point associated with maximum inflammation (A). For subsequent experiments, mice were treated with intratracheal LPS followed 48 h later by a second intratracheal administration containing PBS, TNFα, apoptotic thymocytes, or TNFα + thymocytes. BAL was performed 24 h later and analyzed for neutrophils (B) and inflammatory cytokines (C–E). Neutrophil counts were elevated in mice treated with TNFα + apoptotic cells vs. all other groups. *P < 0.05, n = 12 mice per group.
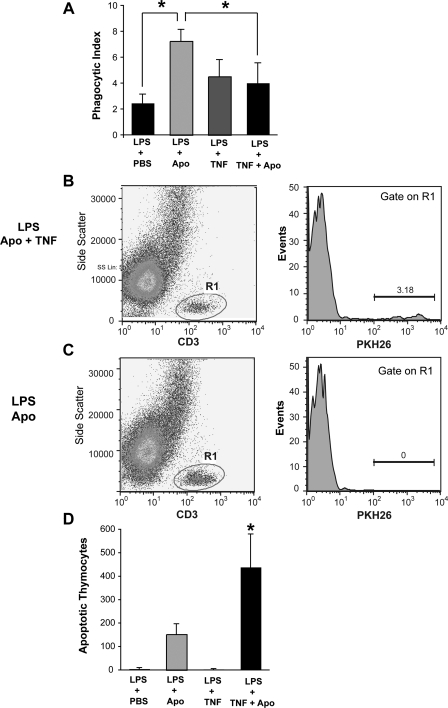
To determine if the proinflammatory effects of TNFα plus apoptotic cells resulted from impairment of apoptotic cell clearance, we quantified phagocytic ingestions in alveolar macrophages obtained from LPS-injured animals using light microscopy. As expected, alveolar macrophages from mice treated with apoptotic cells exhibited the highest phagocytic index (Fig. 8A). The coadministration of TNFα with apoptotic cells resulted in a significant reduction in the phagocytic index compared with treatment with apoptotic cells alone. There was no significant difference in the phagocytic index between PBS-treated mice and mice treated with TNFα (P = 0.35) or TNFα plus apoptotic cells (P = 0.12). To determine if exogenously administered apoptotic cells were still present in the airways at the time of lavage, we performed flow cytometry on BAL specimens (Fig. 8, B–D). Significantly more thymocytes were present in BAL from mice treated with TNFα and apoptotic cells compared with mice treated with apoptotic cells alone, suggesting decreased apoptotic cell clearance.
Fig. 8.
TNFα impairs phagocytosis of apoptotic cells during inflammation. Balb/c mice were treated with intratracheal LPS (20 μg). Forty-eight hours later, PKH26-labeled apoptotic thymocytes (107 per mouse) were directly instilled into the tracheas of recipient mice simultaneously with TNFα (50 ng) or PBS as a control. Lung lavage was performed 24 h later. The phagocytic index was determined by counting the number of phagocytic ingestions on Wright-Giemsa-stained cytospin samples (A). PKH-labeled apoptotic thymocytes were quantified from BAL using flow cytometry. Specimens were stained with an anti-CD3 antibody to identify T cells (gate R1). Cells positive for CD3 were then displayed vs. PKH to determine the percentage of T cells stained with PKH (B and C). The number of apoptotic thymocytes was determined by multiplying the percentage of PKH-positive events by the total hemacytometer cell count (D). P <0.05, n = 6–8 mice per group.
DISCUSSION
Numerous studies have shown that recognition and removal of apoptotic cells by phagocytes can suppress the production of proinflammatory mediators including TNFα (8, 10, 16, 20, 27, 28). On the other hand, TNFα has recently been shown in vitro (22), and here in vivo, to impair the ability of macrophages to ingest apoptotic cells. In addition, the normally anti-inflammatory effects of apoptotic cell clearance appear to be abrogated in the presence of TNFα. These observations support the concept that clearance of apoptotic cells represents a balanced response in terms of subsequent inflammation. In other words, phagocytosis of apoptotic cells usually suppresses inflammation; however, in the presence of specific mediators in the local environment [e.g., TNFα (22)] or through differential use of receptors (11, 12), the normally suppressive effect of apoptotic cell clearance may temporarily be overcome. This delicate balance may help explain the observations that have been reported for proinflammatory (rather than the usual anti-inflammatory) consequences of apoptotic cell uptake (20, 29).
Perhaps the simplest explanation for our observation that the inflammatory response to TNFα was exacerbated by coinstillation of apoptotic cells is that TNFα impaired the normally prompt removal of apoptotic cells from the lungs. As a result, the apoptotic cells underwent secondary necrosis, releasing proinflammatory components of their cell membranes and toxic intracellular contents into the air spaces (10, 24). In this context, it is important to note that the neutrophil influx that followed was short-lived and paralleled the disappearance of apoptotic thymocytes. An alternative explanation for the augmented inflammatory response that occurred after coadministration of apoptotic cells with TNFα is that TNFα impacted macrophage phagocytic receptors. In this case, we hypothesize that anti-inflammatory phosphatidylserine recognition mechanisms were reduced (16) and that proinflammatory calreticulin-LRP (LDL receptor-related protein) pathways were enhanced (11).
It is well known that TNFα itself can induce an inflammatory response. Thus our experiments used concentrations of TNFα that caused only mild inflammation, yet suppressed apoptotic cell clearance. Our studies were carried out with apoptotic thymocytes, largely for technical reasons that allowed us to distinguish them from acute inflammatory cells (mostly neutrophils and monocyte/macrophages). In vitro studies have not shown marked differences in the way macrophages recognize and respond to different apoptotic cell types; however, neutrophils that undergo secondary necrosis would be expected to induce far greater tissue damage than thymocytes. With this in mind, we expected that administration of exogenous TNFα at the peak of an inflammatory response would exacerbate lung injury by preventing clearance of apoptotic neutrophils. Surprisingly, this did not occur. Alveolar macrophages obtained from mice treated with LPS and TNFα contained approximately the same number of apoptotic cells as macrophages obtained from mice treated with LPS alone. Moreover, BAL neutrophil counts and proinflammatory cytokine levels were similar in the two groups. Only animals treated with TNFα and apoptotic cells exhibited an exaggerated inflammatory response following LPS.
There are several explanations for why TNFα alone (i.e., without concomitant administration of exogenous apoptotic cells) failed to augment LPS-induced inflammation. One possibility is that neutrophil apoptosis was delayed by the administration of TNFα, a well-recognized neutrophil survival factor (1, 32). Such a delay could potentially provide the alveolar macrophage enough time to recover from the suppressive effects of TNFα before the onset of neutrophil cell death. In this context, it is important to note that in our in vitro systems the suppressive effect of TNFα on macrophage phagocytosis lasted less than 12 h (22). A second possibility for the apparent lack of an augmented inflammatory effect with TNFα and LPS is that TNFα was administered too early in the inflammatory cascade. We chose to administer TNFα at the peak of neutrophil influx; however, we do not know the time at which maximal neutrophil apoptosis and phagocytic clearance normally occur, since under most circumstances apoptotic neutrophils are cleared so quickly that it is difficult to detect their presence (6, 21).
TNFα may both prolong the lifespan of neutrophils and impair their subsequent clearance. It is therefore important to understand how the timing of cell death and the removal of apoptotic cells determine the outcome of an inflammatory response. For example, delaying apoptosis (and subsequent apoptotic cell clearance) by administration of a pan-caspase inhibitor during early inflammation exacerbates inflammation (5). Conversely, caspase administration during the resolution phase of inflammation results in lung fibrosis (5). In models of self-limited inflammation, the clearance of apoptotic cells leads to the production of potent anti-inflammatory mediators including TGF-β and IL-10 (8, 9, 16). It is therefore likely that both timing and amplitude of inflammatory mediator production are critical. Thus, early production of TNFα in an acute inflammatory response may lead to reduced clearance of apoptotic inflammatory cells and enhanced inflammation. Later, as the levels of TNFα decrease and those of TGF-β increase (16), the balance may tip towards the resolution phase of inflammation. The sensitivity of effector cells to inflammatory mediators (e.g., TNFα) and the resultant effect on apoptotic cell clearance may represent an important determinant in the final outcome to tissue injury, final resolution to normal structure and function, persistent inflammation, or if TGF-β is produced in excess and for too long, fibrosis. The presence of increased numbers of apoptotic cells in chronic inflammatory conditions in which TNFα is present (23), such as cystic fibrosis, chronic obstructive pulmonary disease (3, 4), and rheumatoid arthritis (18), might therefore not only reflect increased induction of apoptosis but also decreased clearance of apoptotic cells.
GRANTS
This work was supported by National Institutes of Health Grants HL-81151, HL-68864, GM-61031, and HL-88138, American Heart Association Grant 0675040N, the PEW Latin American Fellows Program in Biomedical Sciences (V. M. Borges), and the Flight Attendant Medical Research Institute.
ACKNOWLEDGMENTS
We thank Dr. George A. dos Reis and Dr. Carlyne Cool for many useful discussions. We also thank Lindsay Guthrie and Ellen Krug for technical support.
REFERENCES
- 1.Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett 487: 318–322, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Borges VM, Falcao H, Leite-Junior JH, Alvim L, Teixeira GP, Russo M, Nobrega AF, Lopes MF, Rocco PM, Davidson WF, Linden R, Yagita H, Zin WA, DosReis GA. Fas ligand triggers pulmonary silicosis. J Exp Med 194: 155–164, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung KF. Inflammatory mediators in chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy 4: 619–625, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 170: 492–498, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Douglas IS, Diaz del Valle F, Winn RA, Voelkel NF. Beta-catenin in the fibroproliferative response to acute lung injury. Am J Respir Cell Mol Biol 34: 274–285, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Droemann D, Aries SP, Hansen F, Moellers M, Braun J, Katus HA, Dalhoff K. Decreased apoptosis and increased activation of alveolar neutrophils in bacterial pneumonia. Chest 117: 1679–1684, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol 166: 6847–6854, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem 281: 38376–38384, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gaipl US, Sheriff A, Franz S, Munoz LE, Voll RE, Kalden JR, Herrmann M. Inefficient clearance of dying cells and autoreactivity. Curr Top Microbiol Immunol 305: 161–176, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123: 321–334, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115: 13–23, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Henson PM, Cosgrove GP, Vandivier RW. State of the art. Apoptosis and cell homeostasis in chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 512–516, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodge S, Hodge G, Jersmann H, Matthews G, Ahern J, Holmes M, Reynolds PN. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178: 139–148, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 81: 289–296, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 109: 41–50, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, Wenzel SE. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med 172: 972–979, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Ingegnoli F, Fantini F, Favalli EG, Soldi A, Griffini S, Galbiati V, Meroni PL, Cugno M. Inflammatory and prothrombotic biomarkers in patients with rheumatoid arthritis: effects of tumor necrosis factor-alpha blockade. J Autoimmun 31: 175–179, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104: 487–501, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol 171: 2610–2615, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 156: 1969–1977, 1997 [DOI] [PubMed] [Google Scholar]
- 22.McPhillips K, Janssen WJ, Ghosh M, Byrne A, Gardai S, Remigio L, Bratton DL, Kang JL, Henson P. TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J Immunol 178: 8117–8126, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFalpha in pulmonary pathophysiology. Respir Res 7: 125, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz LE, Franz S, Pausch F, Furnrohr B, Sheriff A, Vogt B, Kern PM, Baum W, Stach C, von Laer D, Brachvogel B, Poschl E, Herrmann M, Gaipl US. The influence on the immunomodulatory effects of dying and dead cells of Annexin V. J Leukoc Biol 81: 6–14, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol 160: 943–952, 1998 [PubMed] [Google Scholar]
- 26.Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol 154: 2366–2374, 1995 [PubMed] [Google Scholar]
- 27.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2: 965–975, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 6: 1191–1197, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Uchimura E, Kodaira T, Kurosaka K, Yang D, Watanabe N, Kobayashi Y. Interaction of phagocytes with apoptotic cells leads to production of pro-inflammatory cytokines. Biochem Biophys Res Commun 239: 799–803, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest 109: 661–670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129: 1673–1682, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ward C, Walker A, Dransfield I, Haslett C, Rossi AG. Regulation of granulocyte apoptosis by NF-kappaB. Biochem Soc Trans 32: 465–467, 2004 [DOI] [PubMed] [Google Scholar]