Abstract
Recently, we reported that reactive oxygen species (ROS) generated by NADPH oxidase (NOX) contribute to aberrant responses in pulmonary resistance arteries (PRAs) of piglets exposed to 3 days of hypoxia (Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008). An objective of the present study was to determine whether NOX-derived ROS also contribute to altered PRA responses at a more advanced stage of pulmonary hypertension, after 10 days of hypoxia. We further wished to advance knowledge about the specific NOX and antioxidant enzymes that are altered at early and later stages of pulmonary hypertension. Piglets were raised in room air (control) or hypoxia for 3 or 10 days. Using a cannulated artery technique, we found that treatments with agents that inhibit NOX (apocynin) or remove ROS [an SOD mimetic (M40403) + polyethylene glycol-catalase] diminished responses to ACh in PRAs from piglets exposed to 10 days of hypoxia. Western blot analysis showed an increase in expression of NOX1 and the membrane fraction of p67phox. Expression of NOX4, SOD2, and catalase were unchanged, whereas expression of SOD1 was reduced, in arteries from piglets raised in hypoxia for 3 or 10 days. Markers of oxidant stress, F2-isoprostanes, measured by gas chromatography-mass spectrometry, were increased in PRAs from piglets raised in hypoxia for 3 days, but not 10 days. We conclude that ROS derived from some, but not all, NOX family members, as well as alterations in the antioxidant enzyme SOD1, contribute to aberrant PRA responses at an early and a more progressive stage of chronic hypoxia-induced pulmonary hypertension in newborn piglets.
Keywords: superoxide dismutase enzymes, SOD1, SOD2, NOX4, NOX1, p67phox, catalase, F2-isoprostanes, M40403
scientific and clinical advances implicate disruption of enzymatic systems that produce and regulate reactive oxygen species (ROS) in the pathogenesis of a number of vascular diseases, including pulmonary hypertension (11, 37, 40, 53, 64, 65). In particular, there is growing evidence that NADPH oxidase (NOX) family members are important sources for ROS generation in vascular diseases. Yet, compared with the systemic circulation, little is known about the role of the various NOX family members in the pulmonary circulation (10, 22, 28, 39, 45, 62). Even less is known about the role of NOX family members in regulating vascular tone and reactivity in the neonatal compared with the adult pulmonary circulation. Fundamental differences in the regulation of pulmonary vascular tone in newborns and adults caution against extrapolation of findings regarding NOX signaling in adult lungs to the newborn (52, 74). Regulation of vascular tone is known to differ between larger (conduit-level) pulmonary arteries and smaller (resistance-level) pulmonary arteries (PRAs) (2, 3, 60); however, little attention has been given to the potential contribution of ROS and NOX to abnormal reactivity in the resistance-level vessels of the adult or newborn lung. With this in mind, we sought to examine the role of ROS and NOX family members in the development of pulmonary hypertension in newborns, as well as within the vessels most clearly associated with regulating vascular tone, i.e., PRAs.
The signaling abnormalities involved in the pathogenesis of chronic forms of pulmonary hypertension are likely to vary with duration of the disorder. For example, the functional and structural changes observed in chronic hypoxia-induced pulmonary hypertension have been shown to depend, at least in part, on the duration of hypoxic exposure (31, 32, 54). We previously showed that pulmonary hypertension develops when newborn piglets are exposed to 3 days of hypoxia and that the degree of pulmonary hypertension worsens when hypoxic exposure is extended from 3 to 10 days (20). Moreover, we have provided evidence that aberrations in pulmonary vascular responsiveness evolve over the 10 days of exposure to chronic hypoxia. For example, 3 days of exposure to chronic hypoxia enhances pulmonary vascular constriction to subsequent acute hypoxic challenges (20). However, after 10 days of exposure to chronic hypoxia, the exaggerated constrictor response to acute hypoxia is no longer present (20). We also found that pulmonary vascular constriction to nitric oxide synthase inhibitors remains intact after 3 days of exposure to hypoxia but is lost with extension of chronic hypoxia from 3 to 10 days (18, 19). This latter finding suggests that pulmonary vascular nitric oxide signaling becomes impaired between 3 and 10 days of exposure to chronic hypoxia. These findings emphasize the progressive nature of pulmonary hypertension in this neonatal model of chronic hypoxic exposure and demonstrate that these two time points reflect distinct stages of disease progression characterized by different functional abnormalities. This raises the probability that the signaling abnormalities involved at the two stages differ, at least quantitatively, if not qualitatively. Of interest, early and later time periods of exposure to chronic hypoxia are associated with loss of the pulmonary vascular dilation response to the agonist ACh (18, 19).
Recently, we showed that ROS, derived at least in part from NOX, are involved with the aberrant responses to ACh that develop in PRAs at the early stage (i.e., 3 days of hypoxic exposure) of pulmonary hypertension in newborn piglets (17). One purpose of the present study was to determine whether ROS derived from NOX are also involved with the aberrant vascular responses that are found in PRAs of piglets at a more progressive stage of pulmonary hypertension (i.e., 10 days of hypoxic exposure). In addition, we wanted to expand our knowledge regarding the specific NOX family members that are involved in the pathogenesis of pulmonary hypertension at either stage of the disorder. We were particularly interested in evaluating NOX1 and NOX4, because they have been implicated in the pathogenesis of a variety of vascular diseases and because they have putative roles as O2 sensors (24, 28, 45). Furthermore, we wanted to investigate the relationship between ROS signaling and oxidative stress in pulmonary vascular tissue at early and later stages of chronic hypoxia-induced pulmonary hypertension.
METHODS
Animals.
Newborn (2-day-old) pigs (York-Landrace mixed breed) were placed in a hypoxic normobaric environment for 3 or 10 days. O2 content was regulated at 10–12% O2 (64–78 Torr Po2). CO2 was absorbed with soda lime, and Pco2 was maintained at 3–6 Torr. The chamber was opened twice each day: for cleaning of the chamber and for weighing of the animals. The piglets were fed artificial sow milk ad libitum. We previously found no differences in vascular responses between piglets raised in a room-air environment and piglets raised by the vendor (19, 20). Therefore, we used control piglets on the day of arrival from the vendor, at postnatal ages of 5–6 days or 12 days, i.e., postnatal ages comparable to those of the hypoxic piglets on the day of study. At the time of study, all piglets were preanesthetized with ketamine (30 mg/kg im) and acepromazine (2 mg/kg im) and then anesthetized with pentobarbital sodium (10 mg/kg iv). All animals were given heparin (1,000 IU/kg iv) and then exsanguinated. The thorax was opened, and the lungs were removed and placed in cold (4°C) Krebs solution (in mM: 141 Na+, 4.7 K+, 125 Cl−, 2.5 Ca2+, 0.72 Mg2+, 1.7 H2PO4−, 25 HCO3−, and 11 glucose) until use. All experimental protocols adhered to the National Institutes of Health guidelines for the use of experimental animals and were approved by the Animal Care and Use Committee of Vanderbilt University Medical Center, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Use.
Cannulated artery preparation.
Piglet PRAs (80–300 μm diameter) were isolated, cannulated, and pressurized for continuous measurement of diameter using our previously published methods (17, 18). Briefly, an arterial segment was threaded onto a proximal cannula and tied in place with a 22-μm nylon suture. The distal end of the artery was then tied onto the distal cannula, the artery was filled with Krebs solution, and large side branches were tied off. The distance between the cannula tips was adjusted with a micrometer connected to the proximal cannula so that the slack was taken out of the artery. The exterior of the artery was suffused with Krebs solution from a reservoir at 37°C and aerated with a gas mixture containing O2, CO2, and N2, giving a Po2 of 140 Torr, a Pco2 of 38 Torr, and pH of 7.37. The arterial lumen was gravity filled from a syringe containing Krebs solution and connected to the cannula with polyethylene tubing.
Inflow pressure was adjusted by a change in the height of the infusion syringe. Pressure transducers were placed on the inflow side between the syringe and the artery and at the outflow end of the system. The artery was discarded if the pressures were not equal (indicating a leak in the vessel). The diameter of the artery was observed continuously with a video system consisting of a color camera (model VCC-151, Hitachi) and a television monitor. Vessel diameters were measured with a video scaler (model IV 550, FOR A, Gainesville, FL) that was calibrated with a micrometer scale.
Cannulated artery protocols.
Each artery was allowed to equilibrate for 30 min to establish basal tone. The control arteries were equilibrated at a transmural pressure of 15 cmH2O, and the hypoxic arteries were equilibrated at a transmural pressure of 25 cmH2O. These pressures were used because they represent in vivo pressure (19, 20). We previously showed no effect from these transmural pressures on pulmonary arterial responses to ACh (21). After basal tone was established, all arteries were tested for viability by contraction to the thromboxane A2 mimetic U46619 (10−8 M). To check for a functional endothelium in control arteries, responses to ACh (10−6 M) were evaluated. We previously found that hypoxic arteries constricted to ACh but dilated to another endothelium-dependent agent, the Ca2+ ionophore A23187 (21). Therefore, responses to A23187 were used to check for a functional endothelium in hypoxic arteries.
In one series of studies, we evaluated the contribution of endogenous O2•− and H2O2 on ACh responses in control and hypoxic arteries from the 10-day group. For these studies, changes in vessel diameter induced by ACh (10−8–10−5 M) were measured before and after addition of a cell-permeable SOD mimetic, M40403 (3 μg/ml), which dismutates O2•− to H2O2, and an H2O2-decomposing enzyme, polyethylene glycol-catalase (PEG-CAT, 250 U/ml), which converts H2O2 to H2O. For all these studies, after assessment for viability and a functional endothelium, changes in vessel diameter were measured in response to cumulative doses (10−8–10−5 M) of ACh. Next, the vessels were washed with Krebs solution, and M40403 and PEG-CAT were added to the reservoir. At 20 min after addition of the ROS-decomposing enzymes, dose responses to ACh (10−8–10−5 M) were repeated.
In another series of studies, we evaluated the contribution of NADPH oxidase to changes in vessel diameter in response to ACh in control and hypoxic arteries from the 10-day group. For these studies, changes in vessel diameter were continuously monitored, while cumulative doses of ACh (10−8–10−5 M) were added before and then 20 min after the addition of an NADPH oxidase inhibitor, apocynin (APO, 10−6 M).
For all the above-described studies, vessel responses to the vehicle used for solubilization of the ROS-decomposing enzymes or NADPH oxidase inhibitors were evaluated.
Immunoblot analyses of NOX1, NOX4, the NADPH oxidase subunit p67phox, SOD1, SOD2, and catalase.
Pulmonary arteries (≤300 μm diameter) were dissected from lungs of both groups of control piglets and both groups of hypoxic piglets, frozen in liquid nitrogen, and stored at −80°C until use for immunoblot analysis.
We performed preliminary studies with different amounts of total protein to determine the dynamic range of the immunoblot analysis. An amount of protein that was within the dynamic range of the immunoblot analysis (30 μg for NOX1, 20 μg for NOX4, 5 μg for total p67phox, 15 μg for the membrane fraction of p67phox, 2.5 μg for SOD1, 2.5 μg for SOD2, and 2.5 μg for catalase) was then used to compare protein abundances between homogenates of small pulmonary arteries from each group of hypoxic piglets and their comparably aged group of control piglets (see below).
Frozen samples of small pulmonary arteries from all groups of piglets (n = 5 for each group) were crushed under liquid N2 in a prechilled mortar and pestle into a fine powder, transferred to a tube containing homogenization buffer with protease inhibitors, and then sonicated using three 15-s pulses, with care taken not to foam the sample. Vessel homogenates used for the p67phox determinations were then centrifuged at 9,000 g for 10 min at 4°C, and some of the supernatant was stored at −80°C as “total homogenate.” The remainder of the supernatant was centrifuged at 100,000 g for 2 h at 4°C, and the pellet was resuspended in the homogenization buffer and stored at −80°C as the “membrane fraction.” Protein concentrations for all homogenates were determined by protein assay (Bradford). All homogenates were diluted with PBS to obtain a protein concentration of 1 mg/ml. Aliquots of the protein solutions were solubilized in an equal volume of denaturing, reducing sample buffer, heated to 80°C for 15 min, and centrifuged for 3 min at 5,600 g in a microfuge. Equal volumes of these supernatants were applied to Tris-glycine precast 4–20% polyacrylamide gels (Novex) so that equal amounts of protein were loaded. Electrophoresis was carried out in 25 mM Tris, 192 mM glycine, and 0.1% SDS (pH 8.3) at 125 V for 1.7 h. The proteins were transferred from the gel to a nitrocellulose membrane (Novex) using a Bio-Rad transfer box at 100 V for 1 h in 25 mM Tris, 192 mM glycine, and 20% methanol (pH 8.3). The membrane was incubated overnight at 4°C in PBS containing 10% nonfat dry milk and 0.1% Tween 20 to block nonspecific protein binding. For detection of the protein of interest, the nitrocellulose membrane was incubated overnight at 4°C with the primary antibody [1:500 dilution for NOX1 (Santa Cruz Biotechnology); 1:500 dilution for NOX4 (Abcam); 1:500 dilution for total p67phox and 1:1,000 dilution for the membrane fraction of p67phox (BD Transduction Laboratories); 1:1,000 dilution for SOD1 and 1:3,000 dilution for SOD2 (Stressgen Biotechnology); and 1:2,000 dilution for catalase (Chemicon International)] diluted in PBS containing 0.1% Tween 20 and 1% nonfat dry milk (carrier buffer) and then incubation for 1 h at room temperature with a horseradish peroxidase-conjugated secondary antibody (Zymed) diluted 1:5,000 in the carrier buffer. The nitrocellulose membrane was washed three times between the first two incubations with the carrier buffer and three times with the carrier buffer plus one time with PBS containing 0.1% Tween 20 after the final incubation. For visualization of the antibody, the membranes were developed using enhanced chemiluminescence reagents (ECL, Amersham), and the chemiluminescent signal was captured on X-ray film (ECL Hyperfilm, Kodak). Similar procedures were followed to reprobe the membranes for β-actin (Sigma; 1:5,000 dilution to reprobe for β-actin on membranes used for SOD1, SOD2, and catalase and 1:50,000 dilution to reprobe for β-actin on membranes used for p67phox, NOX1, and NOX4). The bands for each protein were quantified using densitometry.
To determine the influence of postnatal age, studies similar to those described above were performed to compare protein abundances of NOX1, NOX4, total and membrane fractions of the NADPH oxidase subunit p67phox, SOD1, SOD2, and catalase between homogenates of small pulmonary arteries from each group of control [5- to 6-day-old (n = 5) and 12-day-old (n = 5)] piglets.
SOD activity assays.
Pulmonary arteries (≤300 μm diameter) were dissected from lungs of both groups of control and hypoxic piglets and stored at −80°C until use. SOD activity was determined using an SOD assay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's protocols. For all vessels, the assay was performed in the absence and presence of potassium cyanide to allow for the determination of the total SOD and SOD2 activity, respectively. The SOD1 activity was calculated as the difference between total SOD activity and SOD2 activity. Protein concentrations (Bradford assay) were determined for all vessel samples and used to normalize the SOD activities.
Lucigenin-enhanced chemiluminescence.
Pulmonary arteries (≤300 μm diameter) were dissected from lungs of both groups of control and hypoxic piglets and placed into wells containing PBS. APO (10−6 M) was added to some wells, and then all wells were incubated for 20 min at 37°C. Then NADPH (10−4 M) was added to some wells, and the plate was placed into a luminometer (BMG Fluostar Optima) and allowed to equilibrate for 20 min at 37°C. For each well, after injection of lucigenin [9,9′-bis(N-methylacridinium nitrate), 5 μmol/l], scintillation counts, i.e., relative light units (RLUs), were obtained for 500 s. Some wells contained no vessels so that the RLUs could be background corrected. In addition, at the end of the assay, the vessels were dried so that the RLUs could be normalized to vessel dry weight.
Dihydroethidium fluorescence imaging.
Lung tissue samples obtained from terminal lung lobules of both groups of control piglets and both groups of hypoxic piglets (n = 5 for each group) were snap frozen in liquid nitrogen, embedded in optimal tissue cutting medium, cryosectioned into 30-μm slices, mounted on Fisherbrand ProbeOn Plus microscope slides, and kept frozen at 20°C until use. In preparation for imaging, slides were removed from 20°C, treated with equal volumes of M40403 (2 μg/ml) or HEPES buffer, and then placed in a dark box in an incubator at 37°C for 20 min. Next, the slides were incubated with dihydroethidium (DHE, 2 μM) for an additional 20 min at 37°C in the dark. The DHE was drained, the slides were mounted in FluoroG (Southern Biotech, Birmingham, AL), and coverslips were applied for image analysis. M40403-treated and untreated slides were immediately sequentially imaged with a Zeiss LSM 510 META inverted confocal microscope using a 543-nm laser and a 585-nm long-pass filter. For M40403-treated and untreated slides, the microscope was focused on ∼150-μm-diameter pulmonary vessels. All images were obtained within 20 min. Metamorph (version 7.6) analysis software (Molecular Devices, Downingtown, PA) was used to quantitate the average value of the integrated sum (to correct for variability in vessel size) of fluorescence for each M40403-treated and untreated vessel.
Amplex Red assay.
The Amplex Red assay (Molecular Probes, Eugene, OR) is a fluorometric horseradish peroxidase-linked assay that can be used to detect H2O2. The Amplex Red reagent 10-acetyl-3,7-dihydroxyphenoxazine, in the presence of horseradish peroxidase, reacts with H2O2 in samples and generates a fluorescent product. With minor modifications, the assay was carried out according to the manufacturer's protocol. Pulmonary arteries (≤300 μm diameter) were dissected from lungs of both groups of control and hypoxic piglets and placed into wells containing PBS. Some vessels were treated with PEG-CAT (250 U/ml). All arteries were incubated for 30 min with Amplex Red (20 μM) and horseradish peroxidase (0.1 U/ml) at 37°C in the dark. Fluorescence was then measured for 60 min at 595 nm with an excitation wavelength of 530 nm using a fluorescent plate reader (BMG Fluostar Optima). The values are given as the average fluorescence at 595 nm. Background fluorescence, determined in a control reaction well without sample, was subtracted from each value. In addition, at the end of the assay, the vessels were dried, and the fluorescence values were normalized to vessel dry weight.
F2-isoprostane analyses.
F2-isoprostanes were determined using a stable isotope-dilution method with detection by gas chromatography-mass spectrometry and selective ion monitoring, as previously described (43, 44). Briefly, small (≤300-μm-diameter) pulmonary arteries were dissected from each group of piglets, frozen in liquid N2, and stored at −80°C until use. On the day of use, vessels were weighed and homogenized in Folch solution, the chloroform layer was evaporated, lipids were chemically hydrolyzed using KOH, and a stable isotope, 8-isoprostaglandin F2α-d4, was added as an internal standard. After extraction using C-18 and silica Sep-Pac cartridges, purification by thin-layer chromatography, and conversion to O-methyloxime pentafluorobenzyl ester trimethylsilyl derivatives, the compound was dissolved in undecane that is dried over a bed of calcium hydride. Negative ion chemical ionization mass spectrometry was performed with instruments (Hewlett-Packard model HP5989A and Agilent Technologies model 5973) interfaced with monitoring ions for F2-isoprostanes [mass-to-charge ratio (m/z) 569] and the [2H4]15-F2α-isoprostane internal standard (m/z 573). The ion source temperature was 250°C, and electron energy was 70 eV. All measurements were corrected for the sample weight.
Statistics.
Values are means ± SE. For cannulated artery studies, the change from baseline diameter in response to each agent or each dose of ACh was calculated for all vessels and then compared between treated and untreated vessels for control and hypoxic groups using ANOVA with Fisher's protected least significant difference post hoc comparison test (42).
An unpaired t-test was used to compare NOX1, NOX4, total p67phox, the membrane fraction of p67phox, SOD1, SOD2, and catalase amounts between control and hypoxic arteries and between the two different ages of control arteries. An unpaired t-test was used to compare SOD1 and SOD2 activities and F2-isoprostanes between control and hypoxic arteries. For the lucigenin, DHE, and Amplex Red assays, measurements were compared between treated and untreated vessels for control and hypoxic groups by ANOVA with Fisher's protected least significant difference post hoc comparison test (42).
Materials.
ACh, A23187, and APO were obtained from Sigma Chemical. M40403 was a generous gift from Activbiotics (Lexington, MA). PEG-CAT was solubilized in distilled H2O. M40403 was solubilized in 26 mM NaHCO3 buffer. ACh was solubilized in saline. APO was solubilized in DMSO. Concentrations for each drug listed in cannulated artery protocols, lucigenin-derived chemiluminescence, and Amplex Red assays were expressed as final molar concentrations in the vessel bath or wells.
RESULTS
The mean diameter of vessels used for all cannulated artery studies was 192 ± 6 μm for control arteries and 185 ± 6 μm for hypoxic arteries. Arterial diameter was not significantly changed by any of the vehicles in the concentrations used for solubilization of any of the agents.
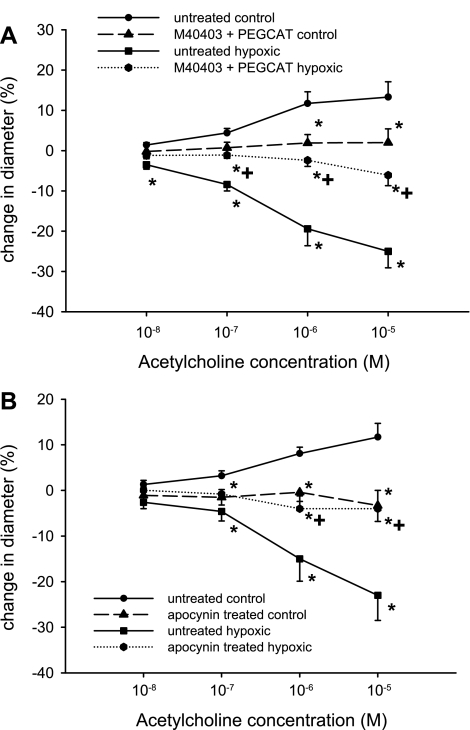
In 12-day-old piglets, untreated control arteries (i.e., arteries from piglets raised in normoxic conditions for 10 days) dilated to ACh (Fig. 1), whereas untreated arteries from piglets raised under hypoxic conditions for 10 days constricted to ACh (Fig. 1). Combined treatment with an SOD mimetic, M40403, and PEG-CAT to decompose O2•− and H2O2 reduced dilation to ACh in control arteries (Fig. 1A) and diminished constriction to ACh in hypoxic arteries (Fig. 1A). Results for arteries treated with the NADPH oxidase inhibitor APO were similar to those for arteries treated with the combination of agents to decompose O2•− and H2O2. That is, compared with their respective group of untreated arteries (Fig. 1B), control arteries treated with APO exhibited less dilation to ACh (Fig. 1B), whereas constriction to ACh was reduced in hypoxic arteries treated with APO (Fig. 1B). The effect of APO or M40403 + PEG-CAT on baseline diameter was minimal and did not differ between the control and hypoxic arteries (data not shown).
Fig. 1.
A: ACh-induced changes in diameter of untreated arteries and arteries treated with the SOD mimetic M40403 + the H2O2-decomposing agent polyethylene glycol-catalase (PEG-CAT) from piglets raised in normoxic (control; n = 12 untreated and 11 treated arteries) and hypoxic (n = 5 untreated and 5 treated arteries) conditions for 10 days. Values are means ± SE. *Different from untreated control; +different from untreated hypoxia (P < 0.05, by ANOVA with Fisher's protected least significant difference post hoc comparison test). B: ACh-induced changes in diameter of untreated arteries and arteries treated with the NADPH oxidase inhibitor apocynin from piglets raised in normoxic (control; n = 8 untreated and 8 treated arteries) and hypoxic (n = 8 untreated and 8 treated arteries) conditions for 10 days. Values are means ± SE. *Different from untreated control; +different from untreated hypoxia (P < 0.05, by ANOVA with Fisher's protected least significant difference post hoc comparison test).
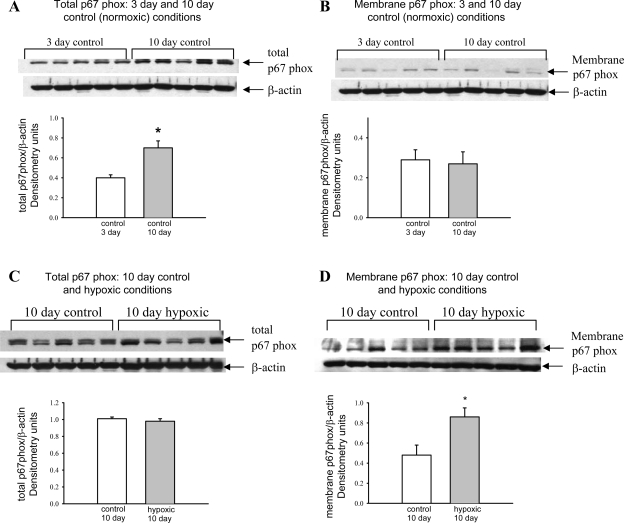
Immunoblot analyses with corresponding densitometry of total and membrane fractions of p67phox in small pulmonary artery homogenates from both groups of control piglets are shown in Fig. 2, A and B. During this relatively short developmental window of time between 5–6 and 12 days of age, there is an increase in total, but not membrane-associated, p67phox. In Fig. 2, C and D, total p67phox and the membrane fraction of p67phox in pulmonary artery homogenates from piglets raised for 10 days in hypoxic conditions and their age-matched controls are compared. Total p67phox protein abundance was not changed by 10 days of hypoxia (Fig. 2C); however, the membrane fraction of p67phox was increased in pulmonary artery homogenates from hypoxic compared with control piglets (Fig. 2D).
Fig. 2.
Immunoblot results and corresponding densitometry for total p67phox and the membrane fraction of p67phox relative to β-actin. A and B: total p67phox and the membrane fraction of p67phox in pulmonary resistance artery (PRA) homogenates from piglets in 3- and 10-day control groups (n = 5 piglets in each control group). C and D: total p67phox and the membrane fraction of p67phox in PRA homogenates from piglets in the 10-day normoxic (control) and 10-day hypoxic (n = 5 piglets in each group) groups. Values are means ± SE. *P < 0.05, by unpaired t-test.
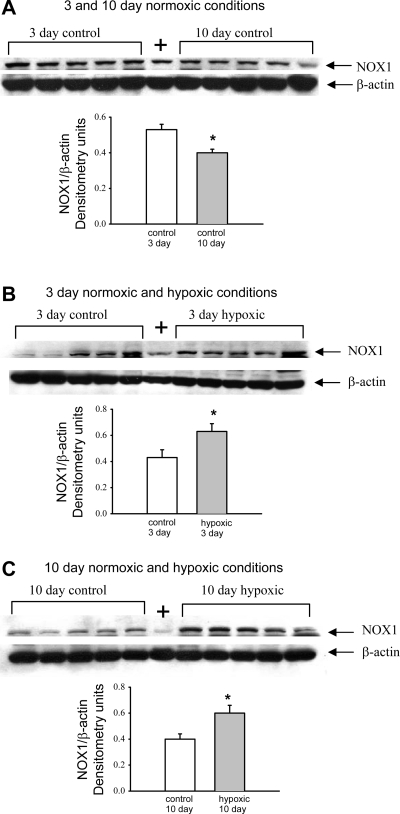
Immunoblot analyses of NOX1 in pulmonary artery homogenates from piglets raised in control (normoxic) conditions for 3 and 10 days reveal a maturation-dependent decrease in NOX1 abundance (Fig. 3A). At 3 and 10 days, NOX1 abundance was increased in pulmonary artery homogenates from hypoxic piglets compared with their comparably aged control groups (Fig. 3, B and C).
Fig. 3.
Immunoblot results and corresponding densitometry for NADPH oxidase (NOX1) relative to β-actin. A: NOX1 in PRA homogenates from piglets in the 3- and 10-day control groups (n = 5 piglets in each group). B: NOX1 in PRA homogenates from piglets raised in normoxic (control) and hypoxic conditions for 3 days (n = 5 piglets in each group). C: NOX1 in PRA homogenates from piglets raised in normoxic (control) and hypoxic conditions for 10 days (n = 5 piglets in each group). +, Positive control (Jurkat whole cell lysates obtained from Santa Cruz Biotechnology). Values are means ± SE. *Different from control (P < 0.05, by unpaired t-test).
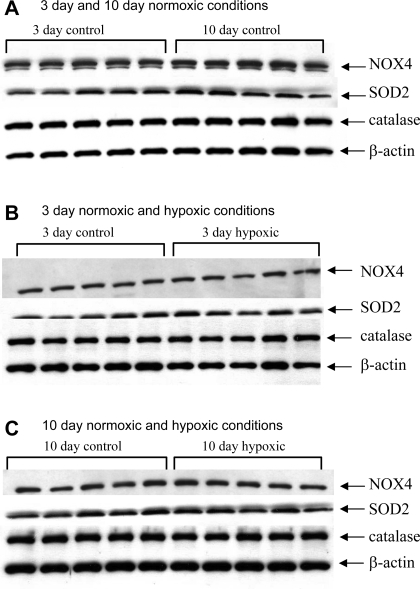
In contrast to NOX1, the abundance of NOX4 in pulmonary artery homogenates was similar in the two control groups (Fig. 4A); NOX4 abundance was also similar in hypoxic and normoxic piglets in the 3- and 10-day exposure groups (Fig. 4, B and C; summary densitometry not shown). Similarly, no differences in the amounts of SOD2 or catalase were found when pulmonary artery homogenates from piglets raised in control conditions were compared with those from piglets raised in hypoxic conditions for comparable time periods (Fig. 4, B and C; densitometry not shown). Similar to NOX4, there was no maturation-dependent change in SOD2 or catalase between the two groups of control (normoxic) piglets (Fig. 4A).
Fig. 4.
Immunoblot results for NOX4, SOD2, catalase, and β-actin. A: NOX4, SOD2, catalase, and β-actin protein abundances in PRA homogenates from piglets in 3- and 10-day control groups (n = 5 piglets in each group). B: protein abundances in PRA homogenates from piglets raised in normoxic (control) and hypoxic conditions for 3 days (n = 5 piglets in each group). C: protein abundances in PRA homogenates from piglets raised in normoxic (control) and hypoxic conditions for 10 days (n = 5 piglets in each group). Densitometry values relative to β-actin (not shown) do not differ for any of the proteins between any of the groups.
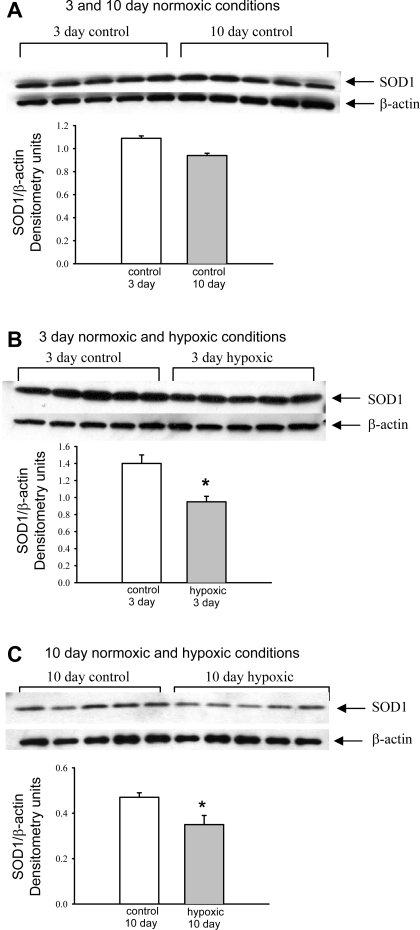
Immunoblot analyses of SOD1 abundance are shown in Fig. 5. There was no significant difference in the abundance of SOD1 in pulmonary artery homogenates from the 3- and 10-day control groups (Fig. 5A). In contrast, the amount of SOD1 in homogenates of pulmonary arteries was significantly less in both groups of hypoxic piglets than in their comparably aged control groups (Fig. 5, B and C).
Fig. 5.
Immunoblot results and corresponding densitometry for SOD1 relative to β-actin. A: SOD1 in PRA homogenates from piglets in the 3- and 10-day control groups (n = 5 piglets in each group). B: SOD1 in PRA homogenates from piglets raised in normoxic (control) and hypoxic conditions for 3 days (n = 5 piglets in each group). C: SOD1 in PRA homogenates from piglets raised in normoxic (control) and hypoxic conditions for 10 days (n = 5 piglets in each group). Values are means ± SE. *Different from control (P < 0.05, by unpaired t-test).
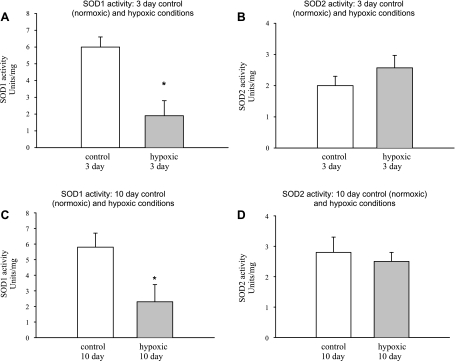
Consistent with immunoblot determinations of SOD1 protein abundance (Fig. 5, B and C), SOD1 activity was diminished in arteries from both groups of hypoxic piglets relative to their comparably aged control groups (Fig. 6, A and C). By comparison, consistent with immunoblot determinations of SOD2 protein abundance, SOD2 activity was similar for pulmonary arteries of hypoxic piglets and their comparably aged group of control piglets (Fig. 6, B and D).
Fig. 6.
SOD1 and SOD2 activities. A and B: SOD1 and SOD2 activity in PRAs from piglets raised in normoxic (control) and hypoxic conditions for 3 days (n = 5 piglets in each group). C and D: SOD1 and SOD2 activity in PRAs from piglets raised in normoxic (control) and hypoxic conditions for 10 days (n = 5 piglets in each group). Values are means ± SE. *Different from control (P < 0.05, by unpaired t-test).
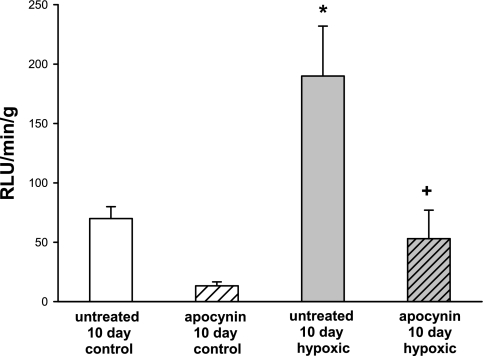
Lucigenin-derived chemiluminescence for small pulmonary arteries from piglets raised in normoxic (control) and hypoxic conditions for 10 days is shown in Fig. 7. In the absence of NADPH, lucigenin-derived chemiluminescence was undetectable in either group of arteries. In contrast, as shown in Fig. 7, in the presence of NADPH, lucigenin-derived chemiluminescence was markedly greater in PRAs from hypoxic piglets than in PRAs from comparably aged control piglets. APO reduced lucigenin-derived chemiluminescence in PRAs from hypoxic piglets to a level similar to that measured in untreated control PRAs.
Fig. 7.
NADPH-stimulated chemiluminescence in untreated PRAs and PRAs treated with the NADPH oxidase inhibitor apocynin from piglets raised in normoxic (control; n = 23 untreated and 13 apocynin-treated PRAs) or hypoxic (n = 35 untreated and 17 apocynin-treated PRAs) conditions for 10 days. RLU, relative light units. Values are means ± SE. *Different from untreated control; +different from untreated hypoxia (P < 0.05, by ANOVA with Fisher's protected least significant difference post hoc comparison test).
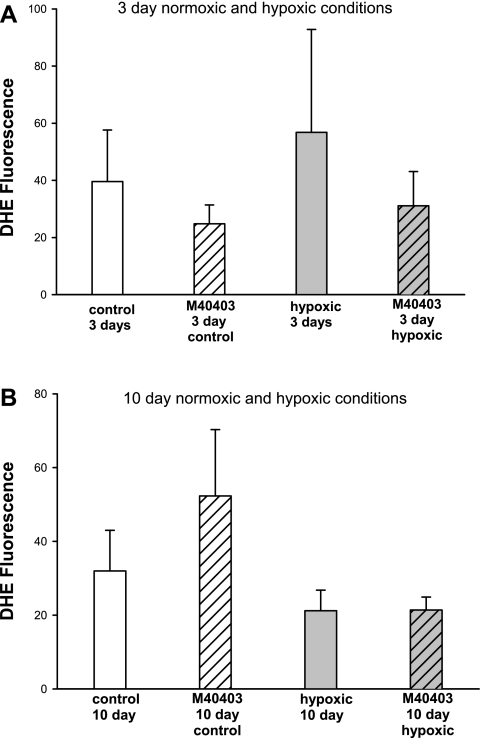
In Fig. 8, results of DHE fluorescence are summarized by confocal microscopy for PRAs in sections of lung obtained from piglets raised in normoxic and hypoxic conditions for 3 and 10 days. Regardless of the presence or absence of M40403, no differences in fluorescence were detected between PRAs from piglets raised under normoxic conditions and PRAs from piglets raised under hypoxic conditions for comparable time periods (Fig. 8).
Fig. 8.
Dihydroethidium (DHE) fluorescence in PRAs in untreated lung sections and lung sections treated with M40403 from piglets raised in normoxic or hypoxic conditions for 3 days (A; n = 5 each in untreated control, M40403-treated control, untreated hypoxic, and M40403-treated hypoxic groups) or 10 days (B; n = 5 each in untreated control, M40403-treated control, hypoxic, and M40403-treated hypoxic groups). Values are means ± SE.
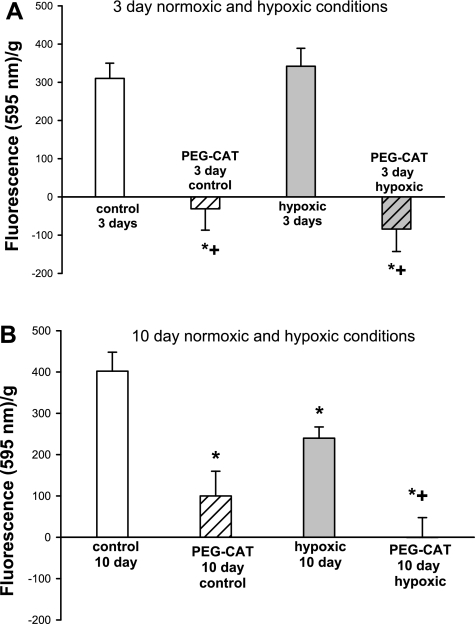
Results of the Amplex Red assay in PRAs from piglets raised in normoxic (control) or hypoxic conditions for 3 and 10 days are summarized in Fig. 9. As determined by the Amplex Red assay, H2O2 was detected and was reduced by treatment with PEG-CAT in PRAs from all groups of piglets (Fig. 9). No difference in amounts of H2O2 were detected between PRAs of piglets raised under normoxic and hypoxic conditions for 3 days (Fig. 9A). In contrast, the amount of H2O2 detected in PRAs from piglets raised in hypoxic conditions for 10 days was one-half of that detected in PRAs from comparably aged control piglets (Fig. 9B).
Fig. 9.
Amplex Red fluorescence in PRAs from piglets raised in normoxic (control) or hypoxic conditions for 3 days (A; n = 14 control, 12 PEG-CAT-treated control, 14 hypoxic, and 12 PEG-CAT-treated hypoxic) or 10 days (B; n = 20 control, 19 PEG-CAT-treated control, 17 hypoxic, and 16 PEG-CAT-treated hypoxic). Values are means ± SE. *Different from control; +different from hypoxic (P < 0.05, by ANOVA with Fisher's protected least significant difference post hoc comparison test).
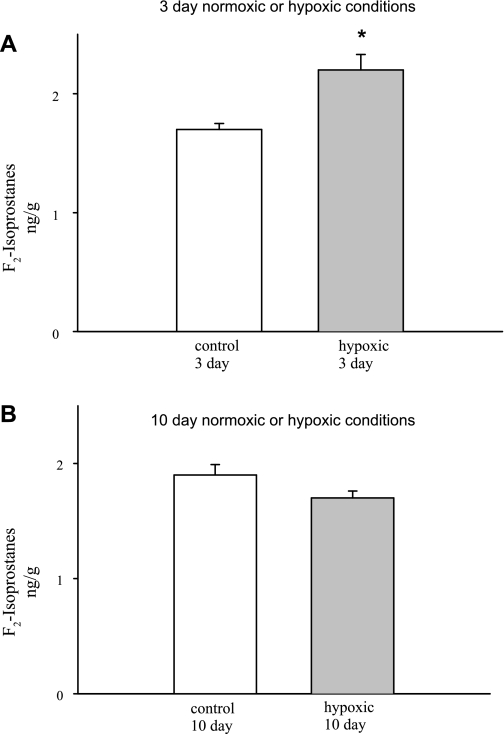
F2-isoprostane generation in PRAs from piglets raised in normoxic (control) or hypoxic conditions for 3 and 10 days is summarized in Fig. 10. F2-isoprostane generation was greater in PRAs from piglets raised in hypoxic conditions for 3 days than in PRAs from piglets raised in normoxic conditions for 3 days (Fig. 10A). In contrast, F2-isoprostane generation did not differ between PRAs from piglets raised in normoxic (control) and hypoxic conditions for 10 days (Fig. 10B).
Fig. 10.
F2-isoprostane generation in PRAs from piglets raised in normoxic or hypoxic conditions for 3 days (A; n = 5 control and 5 hypoxic) or 10 days (B; n = 5 control and 5 hypoxic). Values are means ± SE. *Different from control (P < 0.05, by unpaired t-test).
DISCUSSION
Recently, our laboratory demonstrated that ROS, produced in part by NADPH oxidase, contribute to the altered pulmonary artery responses in piglets exposed to 3 days of hypoxia (17), an early stage of pulmonary hypertension. Findings in the present study extend our previous results, demonstrating that ROS and NADPH oxidase continue to mediate aberrant pulmonary arterial responses in piglets exposed to 10 days of hypoxia, a more advanced stage of pulmonary hypertension. Important new findings in the present study are that the NOX family member NOX1 is increased and the antioxidant enzyme SOD1 is diminished in PRAs of piglets exposed to 3 and 10 days of chronic hypoxia. These data support a growing body of evidence demonstrating a role for NOX-derived ROS signaling in vascular physiology and disruption of this signaling in vasculopathies (4, 25, 37, 45, 55, 62), including pulmonary hypertension (10, 22, 39, 45, 48, 66).
Our conclusion that ROS contribute to aberrant pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension is based in part on our finding that treatment with the cell-permeable SOD mimetic M40403 + an H2O2-decomposing enzyme, PEG-CAT, diminished constriction to ACh in PRAs from piglets exposed to 10 days of hypoxia. We also demonstrated that the NADPH oxidase inhibitor APO diminished constriction to ACh in hypoxic arteries, further supporting a role for ROS derived from the NOX family. These functional findings are similar to our recent findings from studies of PRAs from piglets exposed to 3 days of hypoxia (17). However, it is possible that sources of ROS in addition to NOX family members could contribute to aberrant responses in pulmonary arteries from piglets exposed to 3 or 10 days of hypoxia.
The functional findings in the present study with PRAs from 12-day-old control piglets are similar to our recently reported findings with PRAs from the younger, 5- to 6-day-old, control piglets (17). Consistent with our findings, other investigators also found that ROS-decomposing agents diminish agonist-induced dilation in pulmonary arteries from normal, control animals (22, 36). Moreover, findings from the present study and our recent study (17) show in both groups of control piglets that treatment with the NADPH oxidase inhibitor APO has an effect similar to that of removal of ROS. Taken together, our findings indicate that dilation to ACh in PRAs from both age groups of control piglets is mediated by ROS derived, at least in part, from NOX family members.
Our findings do not necessitate that ROS play direct roles as dilators in control arteries or that ROS play direct roles as constrictors in hypoxic arteries. Rather, one possibility is that ROS stimulate production of dilator arachidonic acid metabolites, including cyclooxygenase-dependent dilators, in control arteries, whereas ROS stimulate production of constrictor arachidonic acid metabolites in hypoxic arteries. This possibility is based on the knowledge that ROS stimulate release of arachidonic acid (8, 51) and is supported by our previous findings that the cyclooxygenase inhibitor indomethacin abolishes ACh-induced dilation in PRAs from 12-day-old control piglets and reduces ACh-induced constriction in PRAs from comparably aged piglets exposed to 10 days of chronic hypoxia (18). However, the precise signaling mechanisms by which ROS mediate disparate responses in control and hypoxic arteries are not known and merit further study.
In the present study, we show that the amount of the NOX subunit p67phox is increased in the membrane fraction of PRAs from piglets exposed to 10 days of hypoxia. This is similar to our previously reported findings from studies of pulmonary arteries from piglets exposed to 3 days of hypoxia (17). Translocation of p67phox from the cytosol to the membrane is required for activation of some NOX family members (49). Thus this finding about p67phox provides additional support to the notion that the NOX family is a source of ROS in the arteries from piglets exposed to 3 and 10 days of hypoxia.
We also explored the involvement of NOX1 and NOX4, because both of these NADPH oxidases have been shown to be expressed in the vasculature of a number of organs and are thought to be involved with vascular pathology (9, 41, 50, 61, 63). To our knowledge, we are the first to report that NOX1 protein is altered in the pulmonary circulation of any species or age of animal with pulmonary hypertension due to hypoxic exposure. Our observations in PRAs are particularly important, because these vessels are a principal site for regulation of pulmonary vascular tone in response to changes in Po2. NADPH oxidases, and NOX1 specifically (24), have been proposed as possible O2 sensors (24, 27, 73). Moreover, NOX1 expression has been shown to be involved with upregulation of cell proliferation and cell growth (12, 35, 63). Therefore, an intriguing possibility that merits future investigation is that an increase in NOX1 expression contributes to the remodeling of the pulmonary circulation that is well known to occur with exposure to chronic hypoxia.
We know of only two other studies in which the effects of chronic hypoxia on NOX1 or NOX4 in the lung have been evaluated (28, 45). In contrast to our findings with newborn piglets, NOX1 was unaltered in lungs of adult mice exposed to 3, 7, or 21 days of chronic hypoxia (45). Also, in contrast to our findings, NOX4 was increased in PRAs of hypoxic mice (45). Moreover, a recent study showed an increase in NOX4 expression in adult human pulmonary arterial smooth muscle cells cultured under hypoxic conditions (28). Disparities in results between these previous studies and our present study could be due to differences in duration of hypoxic exposure, age, species, and sites along the longitudinal axis of the pulmonary circulation.
Our findings do not necessarily mean that basal ROS release is elevated in pulmonary arteries from the hypoxic piglets. This is in part because those NOX family NADPH oxidases that function as multicomponent enzyme systems need complete assembly of all membrane-linked and cytosolic subunits on the plasma membrane for ROS generation (16, 38, 49, 59). Rather than supporting the idea that more activated, completely preassembled NADPH oxidase is present, our assessments of ROS are most consistent with the notion that NADPH oxidase has been primed during chronic hypoxic exposure. That is, we did not detect elevations in basal release of O2•− or H2O2 in PRAs from piglets exposed to either duration of chronic hypoxia. Yet, consistent with the presence of primed enzyme (16, 59), in the presence of the substrate NADPH, O2•− production, as assessed by lucigenin-derived chemiluminescence, was greater in hypoxic than in control arteries (Fig. 9). The presence of primed enzyme could enable the hypoxic arteries to respond to appropriate stimuli with more rapid and vigorous constriction than would occur in the nonprimed state.
It is possible that adequate antioxidant defense mechanisms explain why changes in NOX1 and p67phox might not have yielded detectable elevations in basal ROS production in PRAs of hypoxic newborn piglets. To pursue this possibility, we evaluated the antioxidants SOD1 and SOD2, which convert O2•− to H2O2, and catalase, which breaks H2O2 down to O2 and H2O. We found that for both periods of hypoxic exposure, SOD1 was diminished, while SOD2 and catalase remained unaltered. Diminished expression and activity of SOD1 might contribute to the smaller amounts of H2O2 that we measured in the PRAs of piglets exposed to the longer, 10-day, period of hypoxia. However, at the same time, this change in SOD1 would be anticipated to increase O2•−, which we did not detect. Moreover, although a similar decrease in SOD1 was found in the pulmonary arteries of piglets exposed to the shorter (3-day) period, of hypoxia, no change in H2O2 was detected. There are a number of possible explanations for the apparent lack of concordance between our assessments of ROS and antioxidant enzymes. One is that other antioxidant systems, which could be contributing to the removal of the ROS, are at play (7, 13, 33, 69, 72). Furthermore, there are many enzymatic sources of ROS, in addition to NOXs, that could be concurrently altered (7, 72), perhaps in directions opposite to NOXs. Moreover, detection of ROS is fraught with problems, and all methods have come under close scrutiny (15, 29, 68).
In part on the basis of concerns about reliable detection of ROS, we reasoned that if the balance between ROS-generating enzymes and antioxidant defense systems is such that basal ROS generation is increased with chronic hypoxia, evidence of oxidative stress might be present. F2-isoprostanes are generated as a result of ROS-mediated peroxidation of arachidonic acid and are considered to be reliable markers of oxidative stress (14, 46, 47). These compounds are formed in situ at sites of ROS generation and are noted for their chemical stability (14, 46, 47). Our finding of increased F2-isoprostanes in PRAs of piglets exposed to 3 days of hypoxia is consistent with the hypothesis that ROS production in excess of local antioxidant defense systems has occurred in these tissues at this duration of hypoxic exposure. Moreover, our finding that F2-isoprostanes were similar in PRAs of piglets raised in normoxic or hypoxic conditions for 10 days is consistent with the possibility that basal ROS production and antioxidant defense mechanisms are in balance after this duration of hypoxic exposure. The suggested restoration of balance could occur if the sources of ROS production were reduced and/or antioxidant defenses increased between 3 and 10 days of exposure to hypoxia. Again, our finding of less, not more, potential antioxidant defense via SOD1 at 10 days of hypoxia indicates that the explanation is likely to be complex (26, 34, 58, 67, 70, 71).
In addition to serving as markers of oxidative stress, F2-isoprostanes have been proposed to mediate vasoconstriction in different vascular beds and species (5, 6, 23, 30). However, whether changes in vascular responsiveness that occur during exposure to chronic hypoxia in newborn piglets are due to F2-isoprostanes is unclear and requires further study.
Our findings regarding a possible change in expression of the NOX family enzymes and/or antioxidant defense enzymes between the two groups of control piglets merit comment. Of the enzymes evaluated, only NOX1 and the total amount of p67phox differed between the two age groups. Notably, even though the total amount of p67phox was increased in PRAs from the older, 12-day-old, piglets, the amount of membrane-associated p67phox, which reflects activation of the NOX isoform containing this subunit, did not differ between the two age groups. Of more interest, NOX1 was diminished in PRAs from the 12-day-old control piglets. Taken together with the finding that NOX1 was greater in PRAs from hypoxic piglets than from the comparably aged 12-day-old control piglets, it is possible that exposure to chronic hypoxia prevents the normal postnatal decline in NOX1 expression.
Limitations of our study should be mentioned. We have not evaluated the influence of postnatal age or chronic hypoxia on all known NOX family members. Nor have we evaluated all the antioxidant defense mechanisms that could be present. Another limitation is that APO, which we used to inhibit NOX, has been shown to have nonspecific actions as an ROS scavenger, to increase oxidative stress, and to have effects not related to NOX inhibition, including inhibition of Rho kinase (1, 56, 57). Unfortunately, no specific NOX inhibitors are available.
Despite limitations, our findings extend our previous observations and indicate that ROS derived in part from NADPH oxidase family members play an important role in the aberrant PRA responses to ACh in piglets exposed to 3 and 10 days of hypoxia. Moreover, we now identify NOX1 as an NOX family member involved in the pathogenesis of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Furthermore, we speculate that changes in NOX could contribute to the vascular remodeling that we have found in the hypoxic arteries (20), possibly by preventing normal postnatal changes in NOX1 and concomitant postnatal decreases in vascular wall thickness. In addition, despite our inability to detect elevations in ROS, our finding of a decrease in SOD1 points to a probable imbalance in ROS and antioxidant defense systems that leads to an elevation of F2-isoprostanes, a biomarker of oxidant stress in vivo, after 3 days of hypoxia. Hence, our findings continue to support the development and evaluation of therapies targeting ROS for the treatment of pulmonary hypertension in conditions associated with chronic hypoxia, particularly at early stages of the disorder. Furthermore, the finding that some, but not all, NOX family members appear to be involved suggests that targeting specific NADPH oxidases is a therapeutic challenge worthy of pursuit.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1 HL-68572 (C. D. Fike) and RO1 HL-075511 (J. L. Aschner). Imaging and imaging analysis were performed in part through the use of the Vanderbilt University Medical Center Imaging Shared Resource, which is supported by National Institutes of Health Grants CA-68485, DK-20593, DK-58404, HD-15052, DK-59637, and EY-08126.
REFERENCES
- 1.Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9: 686–696, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Huang JMC, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res 78: 431–442, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Aschner JL, Smith TK, Kovacs N, Pinheiro JMB, Fuloria M. Mechanisms of bradykinin-mediated dilation in newborn piglet pulmonary conducting and resistance vessels. Am J Physiol Lung Cell Mol Physiol 283: L373–L382, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Belik J, Jankov RP, Pan J, Yi M, Chaudhry I, Tanswell AK. Chronic O2 exposure in the newborn rat results in decreased pulmonary arterial nitric oxide release and altered smooth muscle response to isoprostanes. J Appl Physiol 96: 725–730, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Belik J, Jankov RP, Pan J, Yi M, Pace-Asciak CR, Tanswell AK. Effect of 8-isoprostaglandin F2α on the newborn rat pulmonary arterial muscle and endothelium. J Appl Physiol 95: 1979–1985, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Berg D, Youdim MBH, Riederer P. Redox imbalance. Cell Tissue Res 318: 201–213, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Boyer CS, Bannenberg GL, Neve EPA, Ryrfeldt A, Moldeus P. Evidence for the activation of the signal-responsive phospholipase A2 by exogenous hydrogen peroxide. Biochem Pharmacol 50: 753–761, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Brandes RP. Role of NADPH oxidases in the control of vascular gene expression. Antioxid Redox Signal 5: 803–811, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Buetler TM, Krauskopf A, Ruegg UT. Role of superoxide as a signalling molecule. News Physiol Sci 19: 120–123, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Cano-Dominguez N, Alvarez-Delfin K, Hansberg W, Aguirre J. NADPH oxidases Nox-1 and Nox-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot Cell 7: 1352–1361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comhair SAA, Erzurum SC. The regulation and role of extracellular glutathione peroxidase. Antioxid Redox Signal 7: 72–79, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Cracowski JL. Isoprostanes: an emerging role in vascular physiology and disease? Chem Phys Lipids 128: 75–83, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Diakalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Benna J, Dang PM, Gougerot-Pocidalo M. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and Nox2 mobilization to the plasma membrane. Semin Immunopathol 30: 279–289, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Fike C, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fike CD, Aschner JL, Zhang Y, Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn piglets. Am J Physiol Lung Cell Mol Physiol 286: L1244–L1254, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Fike CD, Kaplowitz MR. Chronic hypoxia alters nitric oxide-dependent pulmonary vascular responses in lungs of newborn pigs. J Appl Physiol 81: 2078–2087, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Fike CD, Kaplowitz MR. Effect of chronic hypoxia on pulmonary vascular pressures in isolated lungs of newborn pigs. J Appl Physiol 77: 2853–2862, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Fike CD, Pfister SL, Kaplowitz MR, Madden JA. Cyclooxygenase contracting factors and altered pulmonary vascular responses in chronically hypoxic newborn piglets. J Appl Physiol 92: 67–74, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, Muller B. Role of reactive oxygen species and gp91 phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol 148: 714–723, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Luis G, Perez-Vizcaino F, Garcia-Munoz F, Mey JGRD, Blanco CE, Villamor E. Age-related differences in vasoconstrictor responses to isoprostanes in piglet pulmonary and mesenteric vascular smooth muscle. Pediatr Res 57: 845–852, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Goyal P, Weissmann N, Grimminger F, Hegel C, Bader L, Rose F, Fink L, Ghofrani HA, Schermuly RT, Schmidt H, Seeger W, Hanze J. Upregulation of NADPH oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic Biol Med 36: 1279–1288, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Griendling KK, Sorescu D, Ushio-Fukai M. NADPH oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Herget J, Wilhelm J, Novotna J, Eckhardt A, Vytasek R, Mrazkova L, Ostadal M. A possible role of the oxidant tissue injury in the development of hypoxic pulmonary hypertension. Physiol Res 49: 493–501, 2000 [PubMed] [Google Scholar]
- 27.Hoidal J, Brar SS, Sturrock AB, Sanders KA, Dinger B, Fidone S, Kennedy TP. The role of NADPH oxidases in airway and pulmonary vascular smooth muscle function. Antioxid Redox Signal 5: 751–758, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor β1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janiszewski M, Souza HP, Liu X, Pedro MA, Zweier JL, Laurindo FRM. Overestimation of NADH-driven vascular oxidase activity due to lucigenin artifacts. Free Radic Biol Med 32: 446–453, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Janssen LJ. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. Am J Physiol Lung Cell Mol Physiol 280: L1067–L1082, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Jeffery TK, Wanstall JC. Comparison of pulmonary vascular function and structure in early and established hypoxic pulmonary hypertension in rats. Can J Physiol Pharmacol 79: 227–237, 2001 [PubMed] [Google Scholar]
- 32.Jeffery TK, Wanstall JC. Phosphodiesterase III and V inhibitors on pulmonary artery from pulmonary hypertensive rats: differences between early and established pulmonary hypertension. J Cardiovasc Pharmacol 32: 213–219, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Jones CI, Zhu H, Martin SF, Han Z, Li Y, Alevriadou BR. Regulation of antioxidants and phase 2 enzymes by shear-induced reactive oxygen species in endothelial cells. Ann Biomed Eng 35: 683–693, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Killilea DW, Hester R, Balczon R, Babal P, Gillespie MN. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 279: L408–L412, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Komatsu D, Kato M, Nakayama J, Miyagawa S, Kamata T. NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene 27: 4724–4732, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Lakshminrusimha S, Wiseman D, Black SM, Russell JA, Gugino SF, Oishi P, Steinhorn RH, Fineman JR. The role of nitric oxide synthase-derived reactive oxygen species in the altered relaxation of pulmonary arteries from lambs with increased pulmonary blood flow. Am J Physiol Heart Circ Physiol 293: H1491–H1497, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 43: 332–347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277: 19952–19960, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Liu JQ, Zelko IN, Erbynn EM, Sham JSK, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91 phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 21: 269–280, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Matsuno K, Yamada H, Iwata K, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved with angiotensin II-mediated hypertension: a study in Nox-1 deficient mice. Circulation 112: 2677–2685, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Meier U. A note on the power of Fisher's least significant difference procedure. Pharm Stat 5: 253–263, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Milatovic D, VanRollins M, Li K, Montine KS, Montine TJ. Suppression of murine cerebral F2-isoprostanes and F4-neuroprostanes from excitotoxicity and innate immune response in vivo by α- or γ-tocopherol. J Chromatogr B Analyt Technol Biomed 827: 88–93, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Milatovic D, Zaja-Milatovic S, Montine KS, Horner PJ, Montine TJ. Pharmacologic suppression of neuronal oxidative damage and dendritic degeneration following direct activation of glial innate immunity in mouse cerebrum. J Neurochem 87: 1518–1526, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MAR, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HHHW, Weissman N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Morrow JD. Quantification of isprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol 25: 279–286, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Morrow JD, Roberts LJ. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am J Respir Crit Care Med 166: S25–30S, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Acute hypoxia simultaneously induces the expression of gp91phox and endothelial nitric oxide synthase in the porcine pulmonary artery. Thorax 60: 305–313, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nauseef WM. Biological roles for the NOX family NADPH oxidases. J Biol Chem 283: 16961–16965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paravicini T, Chrissobolis S, Drummond GR, Sobey CG. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 35: 584–589, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Rao GN, Runge MS, Alexander RW. Hydrogen peroxide activation of cytosolic phospholipase A2 in vascular smooth muscle cells. Biochim Biophys Acta 1265: 67–72, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Reeve HL, Weir EK, Archer SL, Cornfield DN. A maturational shift in pulmonary K+ channels, from Ca2+ sensitive to voltage dependent. Am J Physiol Lung Cell Mol Physiol 275: L1019–L1025, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Rhee SG. H2O2, a necessary evil for cell signalling. Science 312: 1882–1883, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Rodman DM. Chronic hypoxia selectively augments rat pulmonary artery Ca2+ and K+ channel-mediated relaxation. Am J Physiol Lung Cell Mol Physiol 263: L88–L94, 1992 [DOI] [PubMed] [Google Scholar]
- 55.Sanders KA, Hoidal JR. The NOX on pulmonary hypertension. Circ Res 101: 224–226, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Schluter T, Steinbach AC, Steffen A, Rettig R, Grisk O. Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res 80: 271–279, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Selemidis S, Sobey CG, Wingler K, Schmidt HHHW, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther 120: 254–291, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Sham JSK. Hypoxic pulmonary vasoconstriction: ups and downs of reactive oxygen species. Circ Res 91: 649–651, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJD, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol 78: 1025–1042, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Smirnov SV, Beck R, Tammaro P, Ishii T, Aaronson PI. Electrophysiologically distinct smooth muscle cell subtypes in rat conduit and resistance pulmonary arteries. J Physiol 538: 867–878, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor β1 induces Nox4 NADPH oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Suh Y, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase mox1. Nature 401: 79–82, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signalling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Tong M, Chinta S, Raj JU, Gao Y. Hypoxia-induced reactive oxygen species downregulate ETB receptor-mediated contraction of rat pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 290: L570–L578, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Ward JPT, Aaronson PI. Mechanisms of hypoxic pulmonary vasoconstriction: can anyone be right? Respir Physiol 115: 261–271, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med 43: 995–1022, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Wassmann S, Wassman K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 44: 381–386, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol 98: 404–414, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Weir EK, Archer SA. Counterpoint: hypoxic pulmonary vasoconstriction is not mediated by increased production of reactive oxygen species. J Appl Physiol 101: 995–999, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 20: 1430–1442, 2000 [DOI] [PubMed] [Google Scholar]
- 73.Wolin MS, Burke-Wolin TM, Mohazzab HKM. Roles for NADPH oxidases and reactive oxygen species in vascular oxygen sensing mechanisms. Respir Physiol 115: 229–238, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Zellers TM, Vanhoutte PM. Endothelium-dependent relaxations of piglet pulmonary arteries augment with maturation. Pediatr Res 30: 176–180, 1991 [DOI] [PubMed] [Google Scholar]