Abstract
Organic dust exposure in agricultural animal environments results in airway diseases. Dendritic cells (DCs) orchestrate inflammatory immune response in the airways, but little is known about how organic dust affects differentiation and maturation of monocyte-derived immature and mature DCs (iDCs, mDCs). Peripheral blood monocytes were differentiated in vitro into iDCs with granulocyte-macrophage colony stimulating factor + IL-4 (6 days) with and without swine facility organic dust extract (ODE, 0.1%). Unlike control iDCs, ODE-conditioned iDCs maintained key monocyte properties (increased mCD14, increased phagocytic ability) while expressing DC features [increased mCD83, HLA-DR, CD80, CD86, diminished cytokine (TNF-α, IL-6) responsiveness]. At day 6, iDCs were cultured for an additional 48 h (days 7 and 8) with lipopolysaccharide (LPS) to induce mDCs. ODE-conditioned mDCs maintained high expression of mCD14+ and elevated phagocytosis while their DC features weakened as evidenced by decreased CD11c, CD83, HLA-DR, CD86, and CCR7 expression and reduced lymphocyte-stimulating capacity. Similar results were observed when monocytes were exposed to ODE for only the first 48 h and with ODE depleted of endotoxin. Control iDCs exposed to ODE during the final 2 days of iDC maturation (days 7 and 8) did not differ from control (no ODE) iDCs in surface marker expression and phagocytic ability, but exhibited enhanced lymphocyte-stimulating capacity. Dust exposure alters monocyte differentiation to iDCs and prevents maturation of iDC to mDCs. The first 48 h of monocyte differentiation appears to be the susceptible period to exposure. Environmental exposures present during early monocyte differentiation may impact the critical balance of DCs in the lung.
Keywords: phagocytosis, lymphocyte proliferation, innate cell surface molecules, cytokines, swine
dendritic cells (DCs) are highly specialized antigen-presenting cells (APC) that regulate innate and adaptive immune responses (3, 4). DCs are present in lung epithelial layers, mostly within interalveolar septa, where they are among the cells that present a first line of defense against inhaled foreign particles (31). In chronic obstructive pulmonary diseases it has been hypothesized that an imbalance of DC immunity occurs and may increase susceptibility of patients to recurrent respiratory infections (29). Most studies focus on the role of cigarette smoke and tend to show impairment in the normal maturation processes of DCs with subsequent alterations in DC function (16, 22, 29, 30). Whereas cigarette smoke is well recognized as the important etiologic agent in obstructive lung disease and chronic bronchitis, other occupational and environmental inhalational exposures can result in significant airway disease (32). Agricultural workers, particularly swine farmers, exhibit a high prevalence of chronic airway disease, which is thought to be due, in part, to chronic exposure to inhaled organic dust (33).
Organic dust is a complex environmental material containing particulate matter and gram-positive and gram-negative microbial-associated components, which can induce immune responses. It is increasingly recognized that although endotoxin is present in the dust, the endotoxin component does not completely explain the inflammatory response in cultured monocytes, epithelial cells, and whole blood (15, 17, 20, 24) nor the in vivo inflammatory outcomes in swine farmers (8). Our prior studies suggest that gram-positive bacterial components and/or combinations of gram-positive and gram-negative bacteria, as found in organic dust, might exert more powerful effects on host defense cells than that of any individual bacterial component (19, 20).
In culture, DCs derived from monocytes exist in two functionally and phenotypically distinct states: immature and mature (13, 26). Immature DCs (iDCs) are effective at phagocytosis and express relatively low levels of surface major histocompatibility complex (MHC) class II products and costimulatory molecules (CD80, CD86) but high surface levels of CD206 (25). Immature DCs are also proficient in inflammatory cytokine secretion but are not effective at antigen presentation. In contrast, mature DCs (mDCs) in culture exhibit reduced capacity for antigen uptake but rather increased ability for antigen presentation, chemotaxis, and stimulation of T cells. This phenotype is marked by expression of high levels of MHC class II and costimulatory molecules. However, this view may be an oversimplification for understanding the DC system in vivo since differing inflammatory cell patterns and DC phenotypes are present in the airways of subjects with chronic respiratory diseases and whether this reflects differences in maturation status or separate sublineages is not clear (29).
Monocytes can be recruited to sites of inflammation and, depending on which maturation and differentiation factors are present in the airways milieu, differentiate into DC phenotypes or macrophages (12, 31). We have previously found that the presence of organic dust alters monocytes' ability to differentiate into macrophages as evidenced by impaired host defense function in the macrophages (19). In this study, we hypothesized that dust exposure from swine facilities would impair human monocyte differentiation into iDCs and iDC maturation to mDCs. Given the complexity of DCs, we tested this hypothesis by differentiating monocytes into iDCs and mDCs in the presence (or absence) of organic dust. Standard DC culture conditions (26) were applied and the effect of timing of dust exposure was also assessed by separately examining exposure at both the early (first 48 h) and late (last 48 h) phases of monocyte differentiation. Cell phenotype and function were measured and included immune cell surface phenotypes, phagocytosis, and cytokine responsiveness. Also evaluated was the ability of mDCs to induce lymphocyte proliferation. We demonstrate here that early and continuous organic dust exposure, independent of endotoxin, maintains DCs in a functionally and phenotypically immature state, thereby limiting their ability to achieve full DC maturation status. This in vitro model provides novel insight into how inhaled environmental agents such as dust may modulate and impact the phenotype and function of monocyte-derived DCs in the lungs.
METHODS
Preparation of Organic Dust Extract
Organic dust was obtained from settled surface dust from local swine confinement animal feeding operation facilities that housed ∼500–700 animals. The dust was placed into solution and sterile filtered (organic dust extract; ODE) by a standard published procedure (24). Complete analysis of this dust has been previously published (19) Briefly, analysis of the dust prior to placing into extract form revealed trace metals, gram-positive bacteria (98%), gram-negative bacteria (2%), and high muramic acid (predominant biomarker of gram-positive bacteria, but to a lesser extent gram-negative bacteria). The total protein concentration in the dust extract (100% concentration) was ∼2–4 mg/ml, by Bradford protein assay (Bio-Rad, Hercules, CA).
The mean endotoxin concentration in a 100% ODE concentration (Limulus amebocyte lysate gel clot assay; Cambrex, Walkersville, MD) was 0.0048 mg/ml (range: 0.0026–0.0070 mg/ml). In the majority of experimental conditions, an ODE concentration of 0.1% was utilized. With endotoxin used as a biomarker of exposure, a 0.1% concentration of ODE equates to ∼4.8 ng/ml of endotoxin, consistent with a “low” swine barn exposure condition (9). ODE was applied to polymyxin B columns (Pierce, Rockford, IL) to assess the response of endotoxin-depleted dust (mean endotoxin concentration after depletion was <0.2 ng/ml).
Monocyte-Derived iDC and mDC Preparation
Human monocytes were obtained from the University of Nebraska Medical Center (UNMC) Elutriation Core Facility. Monocytes were isolated by countercurrent centrifugal elutriation of mononuclear leukocyte-rich fractions of blood cells from healthy donors undergoing leukapheresis as previously published (34). As measured by flow cytometry, elutriated monocytes were >99% pure as determined by surface antigen expression of mCD14.
Immature monocyte-derived DCs.
To induce the in vitro differentiation of monocytes to iDCs and to determine the immunomodulatory effect of organic dust on this differentiation process, monocytes were cultured in complete RPMI with or without ODE (0.1%) in the presence of 1,000 U/ml of recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN) and human recombinant IL-4 (20 ng/ml; R&D Systems) for 6 days. Medium was changed every 48–72 h. Complete RPMI was supplemented with 10% heat-inactivated fetal bovine serum (Biofluids), 2-mercaptoethanol (5 × 10−5 M), 50 μg/ml of streptomycin (Invitrogen, Carlsbad, CA), and 80 U/ml of amphotericin B (Invitrogen). At the end of day 6, control iDCs (no ODE) were defined as monocytes cultured with GM-CSF + IL-4, whereas ODE-conditioned iDCs were defined as monocytes cultured with GM-CSF + IL-4 + ODE (0.1%). To investigate the effect of timing of the ODE exposure in the differentiation cycle of monocytes to iDCs, monocytes were cultured with ODE (0.1%) and GM-CSF + IL-4 for the initial 48 h only, and then cells were washed and maintained in culture with GM-CSF + IL-4 for the remainder of the week; these cells are referred to as “early ODE-conditioned” iDCs. The outline of the experimental design is depicted in Table 1.
Table 1.
Experimental in vitro culture conditions for the differentiation of human peripheral blood monocytes into immature dendritic cells and mature dendritic cells in the presence or absence of organic dust extract (0.1%) at differing time intervals
| Condition | GM-CSF + IL-4 (days 1–6) | Dust Tx (days 1 and 2) | Dust Tx (days 3–6) | LPS (days 7 and 8) | Dust Tx (days 7 and 8) |
|---|---|---|---|---|---|
| Control iDC | + | − | − | − | − |
| Continuous ODE iDC | + | + | + | − | − |
| Early ODE iDC | + | + | − | − | − |
| Endotoxin-depleted ODE iDC | + | + | + | − | − |
| Control mDC | + | − | − | + | |
| Continuous ODE mDC | + | + | + | + | − |
| Early ODE mDC | + | + | − | + | − |
| Late ODE mDC | + | − | − | + | + |
Tx, treatment condition; GM-CSF, granulocyte-macrophage colony stimulating factor; IL-4, interleukin-4; LPS, lipopolysaccharide; iDC, immature dendritic cells; mDC, mature dendritic cells; ODE, organic dust extract.
Mature monocyte-derived dendritic cells (mDCs).
To determine how organic dust exposure effects full maturation of DCs, at day 6, control and ODE (early and continuous)-conditioned iDCs were washed and then cultured for an additional 48 h with a standard maturation agent, LPS (25) [100 ng/ml from Escherichia coli (O55:B5); Sigma, St. Louis, MO]. In other experiments, to determine the effect of ODE exposure on maturation from iDC to mDCs, control iDCs were exposed to ODE (0.1%) + LPS for the final 48 h (days 7 and 8). These cells are referred to as “late ODE-conditioned mDCs.” In all experimental studies, control and conditioned DCs were investigated in side-by-side experiments. Because we and others have previously found that ODE-induced inflammation is not dependent on endotoxin (15, 19, 20, 24), iDCs and mDCs were also cultured in the presence of dust extract (0.1%) that was depleted of endotoxin as an additional control. Cell morphology was also observed with an inverted microscope (Nikon TMS).
Flow Cytometry
Control and ODE-conditioned iDCs (day 6) and control and ODE-conditioned mDCs (day 8) were evaluated for cell surface marker expression by flow cytometry for MHC II [human leukocyte antigen (HLA)-DR], B7 costimulatory molecules (CD80, CD86), and CCR7 (chemotactic receptor). Cells were also stained for mCD14 (monocyte marker), CD11c (monocyte/dendritic cell marker), mCD83 (dendritic cell marker), mCD16 (FcγRIII, macrophage marker), and CD206 (human mannose receptor; iDC marker) to ensure appropriate cell differentiation. DCs (5 × 105 cells) were stained in a standard procedure with saturating concentrations of antibodies (BD Biosciences Pharmingen) against mCD14 (FITC-conjugated), CD83 [phycoerythrin (PE)], CD80 (PE-Cy5), CD86 (PE), HLA-DR (FITC), CD11c (PE-Cy5), CCR7 (PE-Cy7), and CD206 (FITC) in PBS containing 0.1% bovine albumin. To account for nonspecific binding, cells were also incubated with isotype conjugated control antibodies. Flow-cytometric analyses were performed with the FACSCalibur dual-laser cytometers (Becton-Dickinson, Lincoln Park, NJ) that is housed at the Cell Analysis Core Facility at UNMC. Cell surface molecule expression was reported as mean fluorescence intensity (MFI) minus isotype background MFI. In all experiments, control cells from the same donor (cells incubated with GM-CSF + IL-4 alone) were run and compared in side-by-side experiments with ODE-conditioned cells.
Phagocytosis Assay
Phagocytic ability of iDCs and mDCs was assessed by flow cytometry utilizing previously published methods (2, 19). Briefly, Saccharomyces cerevisiae zymosan A BioParticles (Molecular Probes, Eugene, OR) conjugated to FITC were opsonized with opsonizing reagent (IgG) for 45 min. After being washed, DCs at 4 × 106 cells/ml were incubated with FITC-labeled zymosan (Molecular Probes) at 4 × 107 particles/ml (1:10 ratio) for 0 and 60 min in the presence of 10% human AB serum. Cells were fixed with 1% paraformaldehyde and analyzed on the same day of particle exposure by flow cytometry. Particle uptake was identified as a rightward shift in fluorescence on histogram analysis, and phagocytic ability was determined by assessing the average MFI from the proportion of cells in the zymosan-exposed population at 60 min compared with cells exposed for 0 min (expressed as fold change in MFI).
Cytokine/Chemokine Assays
At day 6, iDCs (5 × 105 cells/ml) in duplicate were subsequently challenged with a high concentration of ODE (1%) and media (control) to determine cytokine responsiveness of control and ODE-conditioned iDCs. After 5 h, cell-free supernatant was subsequently harvested and stored at −20°C until assayed for TNF-α, IL-6, and IL-10 secretion. In all experiments, cell counts were made and cell viability after the 5-h culture condition was assured by Trypan blue exclusion method.
TNF-α, IL-6, and IL-10 protein were assayed by sandwich ELISA as previously published (20). Cytokine secretion was reported as concentration (pg/ml) per 5 × 105 viable cells.
One-Way Mixed Lymphocyte Reaction
To determine the ability of control and conditioned-mature DCs (day 8) to stimulate lymphocyte proliferation, a one-way mixed lymphocyte reaction assay was performed with allogeneic peripheral blood lymphocytes (PBL) from healthy volunteers. Peripheral blood was taken with written, informed consent as approved by the Institutional Review Board. Peripheral blood mononuclear cells were isolated from heparinized blood samples by density gradient centrifugation over Ficoll-Paque (Pharmacia, Uppsala, Sweden). Cells were washed twice and monocytes were allowed to adhere. Nonadherent PBLs were collected and cultured for 3 days in 96-well round-bottom microplates in the presence of irradiated (6,000 rads) control and ODE-conditioned mDCs at 1:4, 1:8, 1:16, or 1:32 ratio in side-by-side experiments. After 72 h, 1 μCI of [methyl-3H] thymidine was added for the last 16 h of culture. PBL proliferation was measured by thymidine uptake by scintillation spectroscopy and read as counts per minute (CPM). Data between experimental groups were expressed as a stimulation index (SI). SI was calculated as a ratio: DC-induced PBL proliferation divided by PBL proliferation alone (no DC stimulus) as determined by CPM.
Statistical Analysis
Data are presented as means ± SE. Statistics were performed using two-tailed, nonpaired, or paired t-tests and nonparametric Mann-Whitney U-test where appropriate to determine significant changes among treatment groups by use of SPSS (16.0) software.
RESULTS
Organic Dust Enhances Cell Surface Marker Expression on Immature Dcs
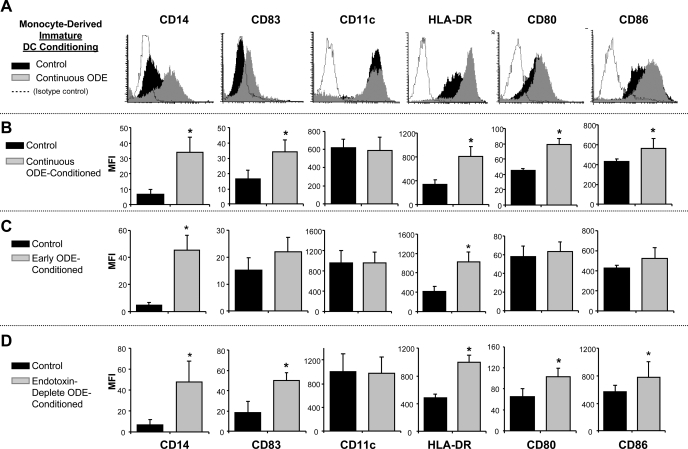
To examine the effect of ODE on differentiation and cell surface phenotype expression of iDCs derived from monocytes, human monocytes were incubated with and without ODE (0.1%; continuous or early exposure conditioning) in the presence of GM-CSF + IL-4 for 6 days as detailed in methods. After 6 days, control iDCs demonstrated surface marker expression consistent with an iDC phenotype (low mCD14+, low mCD83+, high mCD206+). However, ODE-exposed monocytes retained high mCD14+ expression along with increased expression of mCD83, MHC class II molecule (HLA-DR), and the B7 costimulatory molecules (CD80, CD86; Fig. 1, A and B; n = 4 separate experiments; P < 0.05). Cell surface expression was also modulated in cells conditioned with ODE for only the initial 48 h (early ODE-conditioned iDC). Early ODE-conditioned iDCs demonstrated high mCD14 and HLA-DR expression (Fig. 1C; N = 5; P < 0.05), but no significant change in mCD83 and costimulatory molecule(s) expression. Regarded as a differentiation hallmark of iDCs (25), cell surface expression of the human mannose receptor, CD206, was significantly decreased on continuous ODE-conditioned iDCs compared with control iDCs (MFI: control vs. ODE-conditioned 361.1 ± 25.6 vs. 91.7 ± 14.2, N = 3, P = 0.008). Similar results were observed with early ODE-conditioned iDCs (data not shown). Together these results suggest that organic dust exposure affects the ability of monocytes to fully differentiate into iDCs by maintaining high mCD14 and low CD206 expression and that this effect may, in part, be due to events occurring within the first 48 h of dust exposure.
Fig. 1.
Cell surface marker expression in immature dendritic cells (DCs) with and without organic dust extract (ODE) 0.1% conditioning. At 6 days, continuous conditioning of cells with ODE (A and B) enhanced human leukocyte antigen (HLA)-DR, CD80, CD86, mCD83, and mCD14 expression compared with control immature DCs. A: representative histogram of 1 of 4 separate studies. B: means ± SE of the mean fluorescence intensity (MFI). C: early ODE-conditioning vs. control immature DCs. D: endotoxin-depleted ODE-conditioned vs. control immature DCs depicting means ± SE of the MFI. N = 4 separate studies. *Statistically significant (P < 0.05).
To determine whether these observations were dependent on endotoxin, iDCs were exposed to endotoxin-depleted ODE (0.1%) and compared with control iDCs. Endotoxin-depleted ODE modulated innate cell surface molecule expression similar to non-endotoxin-depleted ODE (Fig. 1D; N = 3). These results suggest that nonendotoxin components in ODE are important in modulating dust-induced innate immune cell surface marker expression on APCs.
Organic Dust Weakens Innate Cell Surface Marker Expression Associated With Fully Mature DCs
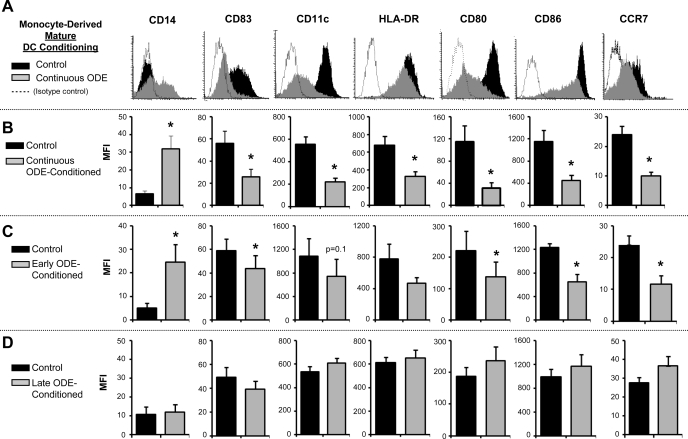
At day 6, control and ODE-conditioned iDCs were cultured with LPS for an additional 48 h to induce full DC maturation. By light microscopy, control mDCs were loosely adherent with formed, multiple fine cytoplasmic protrusions typical of mDC development. Although morphology of ODE-conditioned mDCs was similar to control mDCs, dust-conditioned cells were observed to be slightly more adherent and elongated (data not shown). All DCs were strikingly different from that of macrophages, and mCD16, a macrophage marker, was not detected on the cell surface (data not shown). Consistent with appropriate maturation, expression of the CD206 was downregulated in control mDCs compared with control iDCs (MFI: control iDCs 361.1 ± 25.6 vs. control mDCs 54.26 ± 9.1; N = 3; P < 0.001). Expression of CD206 in ODE-conditioned mDCs was nonsignificantly increased compared with control mDCs (MFI: control mDCs 45.8 ± 7.0 vs. ODE mDCs 102.0 ± 16.5; N = 3; P = 0.1).
Compared with control mDCs, continuous ODE conditioning resulted in significantly increased expression of mCD14+ (Fig. 2) but significantly decreased expression of CD11c, mCD83, HLA-DR, CD80, and CD86 (Fig. 2, A and B, N = 6 separate experiments; P < 0.05). Similar findings were demonstrated with endotoxin-depleted ODE-conditioned mDCs (data not shown). Exposure of cells to ODE for only the first 48 h of differentiation (early ODE exposure), demonstrated similar results to continuous exposure, namely reduced expression of CD11c, CD80, and CD86, and increased expression of mCD14 compared with control mDCs (Fig. 2C, N = 6; P < 0.05). For iDCs undergoing late ODE exposure with LPS, there was no difference mCD14, mCD83, CD11c, HLA-DR, CD80, and CD86 expression compared with control mDCs (Fig. 2D; N = 4). These findings suggest that exposure to organic dust during early DC development prevents full expression of cell surface molecules associated with DC maturation. Moreover, it suggests that the monocyte differentiation cycle has a susceptible period whereby exogenous stimuli can influence its development.
Fig. 2.
Cell surface marker expression in mature DCs (day 8) with and without ODE 0.1% conditioning (A and B). Continuous ODE-conditioning resulted in diminished HLA-DR, CD80, CD86, mCD83, CD11c, CCR7, but upregulated mCD14 compared with control mature DCs in side-by-side experiments. A: representative histogram of 1 of 3–6 separate studies. B: means ± SE of the MFI, N = 3–6. C: early ODE conditioning resulted in diminished CD11c, CD86, and CCR7 but enhanced mCD14 expression, means ± SE of the MFI, N = 3–6. D: late ODE-conditioned mature DCs vs. control mature DCs demonstrated no difference in cell surface molecule expression (N = 3). *Statistically significant (P < 0.05).
Organic Dust Exposure Diminishes CCR7 Expression of Mature DCs
In these studies, we investigated the cell surface expression of the potent leukocyte chemotactic receptor, CCR7, which is also recognized to be associated with a mDC phenotype (27). We demonstrated that control mDCs had upregulated CCR7 expression, as CCR7 expression was not detected on iDCs. Compared with control mDCs, continuous and early ODE conditioning resulted in significantly decreased expression of CCR7 (Fig. 2, A–C; N = 3; P < 0.05). For iDCs undergoing late ODE exposure with LPS, there was no significant difference in CCR7 expression compared with control mDCs (Fig. 2D; N = 3; P = 0.20). These data suggest that exposure of organic dust early (first 48 h) in the monocyte-to-DC differentiation cycle may result in dampening of chemotactic ability. Since CCR7 is also regarded as a marker of mDC phenotype, these data are also consistent with above studies demonstrating that organic dust exposure impairs full DC maturation.
Organic Dust Exposure Enhances Phagocytic Ability of Immature and Mature DCs
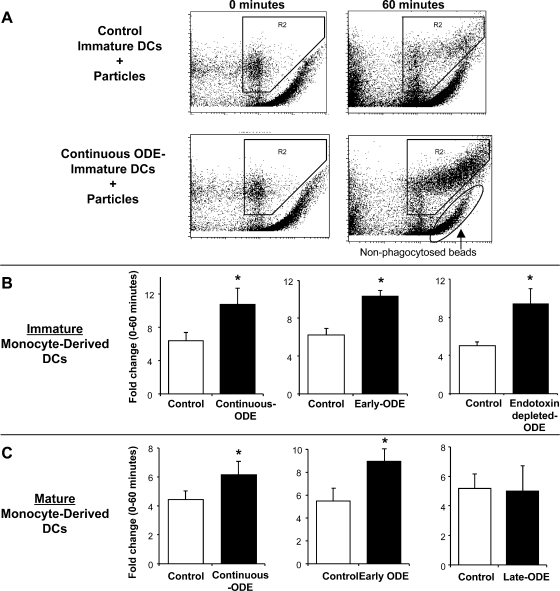
After 6 days, phagocytosis of IgG-opsonized zymosan particles was measured at 0 and 60 min in control and ODE-conditioned iDCs by flow cytometry utilizing previously published techniques (19). Compared with control iDCs, phagocytosis was significantly enhanced in continuous ODE- conditioned iDCs (fold change: 6.4 ± 1.67 vs. 10.77 ± 1.97, N = 3; P = 0.048; Fig. 3, A and B). Early exposure to ODE also significantly enhanced phagocytosis at 6 days compared with control iDCs (fold change: control vs. early ODE-conditioned 6.25 ± 0.69 vs. 10.38 ± 0.55, N = 4; P = 0.037; Fig. 3B). This enhancement in phagocytic ability was also demonstrated in endotoxin-depleted ODE-conditioned iDCs (fold change: 5.1 ± 0.42 vs. 9.4 ± 1.58, P = 0.038, N = 3, Fig. 3B).
Fig. 3.
Phagocytosis of IgG-opsonized Saccharomyces cerevisiae zymosan bioparticles in immature and mature DCs conditioned with and without ODE 0.1%. A: representative dot plot of rightward shift in fluorescence from separate studies of control immature DCs and continuous ODE-conditioned immature DCs at 0 and 60 min. B: continuous ODE-conditioning (N = 3), early ODE-conditioning (N = 4), and endotoxin-depleted ODE-conditioning monocyte-derived immature DCs (N = 3) compared with same donor control immature DCs. C: continuous ODE-conditioning (N = 5), early-ODE conditioning (N = 6), and late ODE-conditioning monocyte-derived mature DCs (N = 4) compared with same-donor control mature DCs in side-by-side experiments. Phagocytic ability expressed as fold change in MFI (proportion of cells in the zymosan-exposed population at 60 min compared with cells exposed for 0 min). *Statistically significant (P < 0.05).
To investigate whether the enhancement of phagocytic ability would persist after maturation with LPS, ODE-conditioned iDCs (continuous and early-exposure iDCs) were cultured for an additional 48 h with LPS and then analyzed for phagocytic ability. Phagocytic ability was significantly increased in continuous ODE-conditioned and early ODE-conditioned mDCs compared with control mDCs (Fig. 3C). Enhanced phagocytic ability was also observed with endotoxin-depleted ODE-conditioned mDCs (fold change: control vs. endotoxin-depleted ODE-conditioned 3.93 ± 0.72 vs. 6.86 ± 0.72; N = 3; P = 0.03). There was no significant difference in late ODE-conditioned mDCs compared with control mDCs (Fig. 3C). These studies showing increased phagocytic ability of iDCs and mDCs with early and continuous ODE-conditioning suggest the preservation of a key professional phagocyte-like function and hence promotion of innate immune responses, rather than increased interaction between innate and acquired immunity via enhanced DC behavior.
Organic Dust Exposure Reduces Cytokine Responsiveness of Immature DCs
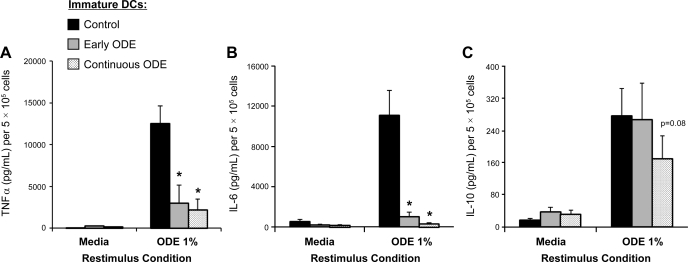
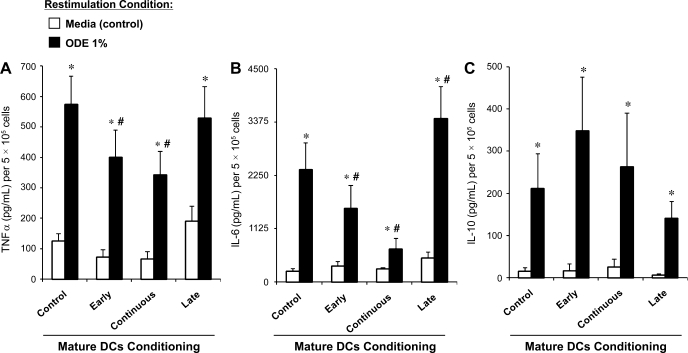
In these studies, the effect of ODE on TNF-α, IL-6, and IL-10 cytokine responsiveness of iDCs was investigated. As described in methods, after 6 days in culture, control and ODE-conditioned iDCs were subsequently rechallenged with high-concentration ODE (1%) for 5 h. Control iDCs and ODE-conditioned iDCs secreted TNF-α, IL-6, and IL-10 when restimulated with ODE (1%) compared with no restimulation (media control, Fig. 4, A–C, N = 4, P < 0.05). However, we found significantly reduced concentrations of TNF-α and IL-6, but not IL-10, following restimulation of ODE-conditioned iDCs (early and continuous) vs. controls (Fig. 4, A–C, N = 4, P < 0.05). There was no difference in cell count or cell viability as determined by the Trypan blue exclusion method between treatment conditions to explain these results. These data suggest that organic dust conditioning impairs inflammatory cytokine responsiveness of immature monocyte-derived DCs but that the anti-inflammatory cytokine IL-10 may be important in mediating the modulated iDC phenotype.
Fig. 4.
Secretion of TNF-α (A), IL-6 (B), and IL-10 (C) in monocyte-derived immature DCs after restimulation with high-concentration ODE 1% for 5 h. Cytokine secretion was reduced in early and continuous ODE (0.1%)-conditioned immature DCs compared with controls (N = 4 separate experiments). Mean results are presented per 5 × 105 cells ± SE. *Statistically significant (P < 0.05).
Further studies examined cytokine responsiveness in control and ODE-conditioned mDCs (Fig. 5, A–C). As expected, mature control DCs, overall, secreted less TNF-α and IL-6 when restimulated with high-concentration ODE (1%) compared with control iDCs, consistent with successful DC maturation properties. Control, early, continuous, and late ODE-conditioned mDCs demonstrated a significant increase in TNF-α, IL-6, and IL-10 after ODE (1%) restimulation compared with no restimulation (media control). A tolerant response was observed with ODE-conditioned (early and continuous) mDCs compared with control mDCs as marked by a significant reduction in TNF-α and IL-6 secretion. In late-ODE-conditioned mDCs (cells cultured with ODE + LPS for the final 48 h), there was enhanced IL-6 but not TNF-α secretion compared with control mDCs. In all ODE-conditioned mDCs, IL-10 secretion remained unchanged from control mDCs after restimulation (Fig. 5C). These studies suggest that timing of dust exposure in DC development is important and that early and continuous dust exposure impairs inflammatory cytokine responsiveness of the monocyte-derived mDC but that IL-10 production persists.
Fig. 5.
Secretion of TNF-α (A). IL-6 (B), and IL-10 (C) in monocyte-derived mature DCs after restimulation with ODE 1% for 5 h. *Significantly different from media (control) restimulation (P < 0.05). #Significantly different from control mature DCs restimulated with ODE 1% (P < 0.05). N = 4 separate experiments. Mean results are presented per 5 × 105 cells ± SE.
ODE Effects Ability of Mature DCs to Induce Lymphocyte Proliferation
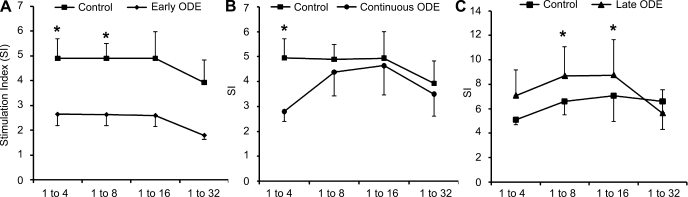
In these studies, control and ODE-conditioned mDCs were investigated for their ability to induce allogenic PBL proliferation. Early and continuous ODE-conditioned mDCs demonstrated a significant reduction in the ability to induce lymphocyte proliferation compared with control mDCs in side-by-side experiments (early, Fig. 6A, DC-to-PBL ratio 1:4, P = 0.03; 1:8 ratio, P = 0.01; 1:16 ratio, P = 0.1; 1:32 ratio, P = 0.08, N = 7 separate experiments; continuous, Fig. 6B, DC-to-PBL ratio 1:4 P = 0.003, N = 8 separate experiments). In contrast, late ODE-conditioned mDCs (DCs incubated with ODE + LPS) showed increased induction of lymphocyte proliferation (Fig. 6C, 1:8 ratio, P = 0.03; 1:16 ratio, P = 0.04, N = 6 separate experiments). These data indicate that exposure of organic dust early (first 48 h) in the monocyte to DC differentiation cycle results in an impairment of mDCs to induce lymphocyte activation, a key DC feature. Late exposure to organic dust results in enhancement of DC function in lymphocyte-stimulating capacity.
Fig. 6.
Peripheral blood lymphocyte (PBL) proliferation induced by ODE (0.1%)-conditioned mature DCs and control mature DCs by 3-day mixed lymphocyte reaction assay. A: early ODE-conditioned mature DCs (⧫). B: continuous ODE-conditioned mature DCs (•). C: late ODE-conditioned mature DCs (▴) with matched control mature DCs (▪) at DC-to-PBL ratio on the x-axis. Symbols represent the mean stimulation index (SI) results ± SE of a minimum of 7 independent experiments. PBL proliferation was measured by thymidine uptake by scintillation spectroscopy and read as counts per minute (CPM). SI was calculated as a ratio: DC-induced PBL proliferation divided by PBL proliferation alone (no DC stimulus) as determined by CPM. *Statistically significant (P < 0.05) between ODE-conditioned mature DCs vs. control mature DC.
DISCUSSION
DCs are professional APCs that orchestrate immune responses with a potential role in the pathogenesis of organic-dust induced chronic airway diseases. Inflammation and tissue damage can trigger activation and differentiation of monocytes to DCs, and the environmental milieu in the airways during monocyte differentiation and DC maturation may modify these processes, particularly in individuals with chronic respiratory disease where variability in DC sublineage and/or maturation status has been observed (29, 31, 12). In this study, healthy volunteer blood monocytes exposed in vitro to DC culture conditions with organic dust exhibited typical iDC phenotype properties such as enhanced expression of costimulatory cell surface molecules (CD80, CD86). However, unlike control cells, organic dust-exposed monocytes, though exhibiting iDC features, also maintained two important features of undifferentiated monocytes, namely elevated expression of mCD14 and elevated phagocytosis. Moreover, continued exposure of iDCs to organic dust further impaired their ability to obtain full DC maturation status. The resulting DC phenotype induced by dust exposure was marked by an overall reduction in antigen presentation capability as evidenced by reduced expression of antigen-presenting and chemotactic receptor cell surface molecules, enhanced phagocytic ability, dampened lymphocyte-stimulating capacity, and reduced cytokine responsiveness.
DCs orchestrate innate inflammatory responses and adaptive immunity through activation of T cells (13) MHC class II molecules represent the first classical signal in the process of antigen presentation, and costimulatory molecules (CD80, CD86) represent the second signal. In appropriate culture conditions (GM-CSF + IL-4 for 6 days), CD14+ monocytes differentiate into iDC (17), which compared with CD14+ monocytes have higher expression of MHC class II and costimulatory molecules but lower relative expression compared with mDCs. This standard DC culture system does not account for environmental agents that may be present in vivo during the maturation of monocytes to DCs. Environmental agents and the effects they impart in the airways likely play a significant role in the fate of the maturing APC or DCs. The effect of one such environmental agent, cigarette smoke and/or nicotine, during in vitro monocyte-derived DC development has led to conflicting reports. Enhancement of DC activation (1) and a suppression of DC function (30) have both been demonstrated. In this study, when organic dust extract was given during the initial 48 h of monocyte differentiation to an iDC, a strong upregulation of HLA-DR was observed, and further dust incubation also resulted in enhanced expression of costimulatory molecules (CD80 and CD86). These results at first glance suggest that organic dust, when present early and during monocyte-derived DC differentiation, may drive cells toward an APC function. However, iDCs following dust exposure also maintained elevated phagocytosis and enhanced expression of CD14, two features that define undifferentiated, professional monocytes. Moreover, we observed that iDCs exposed to organic dust followed by incubation with LPS ultimately prevented the ability of iDCs to achieve full DC maturation status, as demonstrated by impairment of complete upregulation of CD11c, mCD83, HLA-DR, CD80, CD86, and CCR7.
Timing of organic dust exposure appeared to be very important. Exposure to dust only during the first 48 h of monocyte differentiation resulted in muted expression of several innate cell surface molecules, and these results were more striking when exposure continued beyond the first 48 h. In contrast, when the dust was administered during the last 48 h of exposure, there were no differences in the APC surface marker expression compared with control mDCs. It is noted that organic dust alone (without LPS) induced full maturation of DCs from normal iDCs (data not shown). We speculate that timing of environmental inflammatory insults during differentiation of DCs may account for the wide variability observed in pulmonary DC phenotypes in chronic airway diseases (29). For example, subjects with chronic obstructive pulmonary disease have been shown to have increased iDCs in the lung (6, 7, 28) but also a reduction of mDCs (23). Whether these observations are due to smoking itself are unclear. An impaired or reduced population of mDCs may weaken the ability of the lung to defend against repeated microbial and viral invasions that can contribute to worsening of airway disease over time (29). Since DCs and/or APCs are complex innate immune cells, it is possible that environmental factors (organic dusts, cigarette smoke, occupational agents) present in the airway during maturation are, in part, driving the variability in lung DC phenotypes.
The finding that monocytes differentiated with GM-CSF + IL-4 plus organic dust coexpress CD14 and CD83 and have enhanced phagocytic capability is significant. Expression of cell surface CD83, a prototypical DC maturation marker, is normally matched by complete downregulation of mCD14 and weak phagocytosis in fully mature DCs. However, there are reports that interferon (IFN)-α alone or in the absence of GM-CSF + IL-4 induced a monocyte-derived DC-like cell, coined a “novel nondendritic mature APC,” which coexpresses mCD83 and mCD14 (10). These cells also exhibited an increase in phagocytic ability compared with control mDCs, consistent with our data. In this organic dust model, IFN-α protein levels were not detected (data not shown). Another study showed that high-dose LPS (100 ng/ml) plus GM-CSF + IL-4 resulted in a monocyte-derived high-mCD14+ DC-like cell with enhanced phagocytic ability and preserved lymphocyte-stimulating capacity; mCD83 was not investigated (18). The mechanisms for this novel mixed function APC are not known, but others have speculated that CD14+CD83+ cells induced by IFN-α may represent a fast, long-lasting response to pathogens, perhaps mediated through STAT-1 (10). Our data support the finding of the existence of a CD83+CD14+ APC with enhanced phagocytic ability. Our results also support that the first 48 h of monocyte differentiation may be critical in the regulation of mCD14 expression, and future studies should focus on the early sequence of events in a monocyte's maturation cycle. However, it is also recognized that mCD14 is an important cell surface molecule aiding in Toll-like receptor signaling (11) and its persistent upregulation may mark its role in this innate immune response.
Phagocytosis is a process by which cellular debris and pathogens are removed, but also the process by which APCs can take up antigen and process it for presentation to T cells. Immature DCs are efficient at antigen uptake, but during DC maturation antigen uptake ability decreases as antigen presenting ability is enhanced (13). Organic dust conditioning significantly increased phagocytic ability in both immature and mature DC cell culture models, and this observation was not dependent on endotoxin. These findings differ from the effect of organic dust on monocyte-derived macrophages, whereby the dust exposure significantly impaired macrophage phagocytic ability (19). The present results could be interpreted two ways. An increase in phagocytic ability would promote antigen surveillance of the airways and pathogen removal. However, failure to switch from a high phagocytic ability to a low phagocytic ability may indicate an impairment of antigen presenting ability indicative of a fully functional mDC.
Indeed, we observed that organic dust exposure during monocyte-derived DC maturation impaired lymphocyte activation as organic dust exposure, particularly in the first 48 h, significantly dampened the ability of DCs to induce lymphocyte proliferation compared with control DCs. The converse was seen when exposure to the organic dust was late. Compared with controls, cells matured from iDCs with LPS + ODE demonstrated enhanced ability to induce lymphocyte proliferation, even though cell surface molecule expression and phagocytic ability were similar to those of controls. This later observation is consistent with the well-recognized finding that gram-positive and gram-negative components enhance maturation of DCs from iDCs (21). Similarly, the leukocyte chemotactic receptor, CCR7, was decreased with early dust exposure, but no significant difference when exposure to organic dust was late. Differential expression of CCR7 suggests that chemotaxis of mDCs may be regulated with organic dust exposure. Together, these studies support the notion that the maturation cycle of DCs has temporal vulnerability, hence the timing of inflammatory insults in modulating immune responses is important.
DCs can also elicit innate immune and inflammatory responses through production and secretion of cytokines. Although iDCs and mDCs differentiated in the presence of organic dust can still release TNF-α and IL-6 when restimulated with high-dose ODE, the quantitative secretion of TNF-α and IL-6 was significantly decreased in dust-conditioned cells compared with control. A blunting of cytokine responsiveness is consistent with a tolerant state and could perpetuate bacterial colonization and recurrent infections, a hallmark of chronic bronchitis. In contrast, release of IL-10 persisted in the organic dust-conditioned iDCs and mDCs. IL-10 is an anti-inflammatory cytokine that is recognized to downmodulate other cytokines and cell surface receptors (14) and is recognized to play a role in the induction and maintenance of tolerance to allergens and other benign bioaerosols in the respiratory tract (5). Thus it is possible that IL-10 is, in part, mediating the blunted DC phenotype.
In conclusion, organic dust, a complex inflammatory environmental factor, significantly modulated host defense function of DCs derived from peripheral blood monocytes of healthy volunteers. Organic dust exposure, independent of endotoxin, appears to drive immature APC properties on iDCs, while concurrently maintaining certain monocyte phenotypes. Dust exposure, however, ultimately interfered with the ability of iDCs to achieve full DC maturation status. Inflammatory environmental exposures present during DC maturation may account for the variations in DC subtypes observed in the lung. The in vitro model utilized in this study may be important when investigating possible therapeutic interventions aimed at understanding and preventing impaired host defense function in subjects with chronic airway diseases secondary to organic dust exposure.
GRANTS
This study was supported by grants from the National Institute of Environmental Health Sciences (K08: ES015522-01; J. A. Poole), American Academy of Allergy, Asthma Immunology (AAAAI). Interest Section Grant Award (J. A. Poole), and National Institute of Occupational Safety Health (1R01OH008539-01; D. J. Romberger).
Some of the results of these studies have been presented in abstract form (AAAAI meeting, 2009).
ACKNOWLEDGMENTS
The authors thank Victoria Smith and Charles Kuzinski, PhD, in the UNMC Cell Analysis Facility for assistance with flow cytometry measurements. We also thank Myhahn Che and Howard E. Gendelman, MD, in the UNMC Elutriation Facility for isolating monocytes by centrifugal elutriation. We thank Michael Duryee for assistance with experimental studies and Lisa Chudomelka for manuscript preparation assistance.
REFERENCES
- 1.Aicher A, Heeschen C, Mohaupt M, Cooke JP, Zeiher AM, Dimmeler S. Nicotine strongly activates dendritic cell-mediated adaptive immunity: potential role for progression of atherosclerotic lesions. Circulation 107: 604–611, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol 117: 1396–1403, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 18: 767–811, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 392: 245–252, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol 111: S460–S475, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Casolaro MA, Bernaudin JF, Saltini C, Ferrans VJ, Crystal RG. Accumulation of Langerhans' cells on the epithelial surface of the lower respiratory tract in normal subjects in association with cigarette smoking. Am Rev Respir Dis 137: 406–411, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, Joos GF, Brusselle GG. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175: 998–1005, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Donham KJ, Reynolds SJ, Whitten P, Merchant JA, Burmeister L, Popendorf WJ. Respiratory dysfunction in swine production facility workers: dose-response relationships of environmental exposures and pulmonary function. Am J Ind Med 27: 405–418, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Dosman JA, Fukushima Y, Senthilselvan A, Kirychuk SP, Lawson JA, Pahwa P, Cormier Y, Hurst T, Barber EM, Rhodes CS. Respiratory response to endotoxin and dust predicts evidence of inflammatory response in volunteers in a swine barn. Am J Ind Med 49: 761–766, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gerlini G, Mariotti G, Chiarugi A, Di Gennaro P, Caporale R, Parenti A, Cavone L, Tun-Kyi A, Prignano F, Saccardi R, Borgognoni L, Pimpinelli N. Induction of CD83+CD14+ nondendritic antigen-presenting cells by exposure of monocytes to IFN-alpha. J Immunol 181: 2999–3008, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity 29: 182–191, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med 11: 653–660, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell 106: 255–258, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Muller-Suur C, Larsson K, Grunewald J. Organic dust-induced interleukin-12 production activates T- and natural killer cells. Eur Respir J 20: 686–690, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology 109: 365–373, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmberg L, Larsson BM, Malmberg P, Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax 53: 260–264, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Lipopolysaccharide can block the potential of monocytes to differentiate into dendritic cells. J Leukoc Biol 65: 232–240, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Poole JA, Alexis NE, Parks C, MacInnes AK, Gentry-Nielsen MJ, Fey PD, Larsson L, Allen-Gipson D, Von Essen SG, Romberger DJ. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol 122: 375–382, 382 e371–e374, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, Romberger DJ. Repeat organic dust exposure-induced monocyte inflammation is associated with protein kinase C activity. J Allergy Clin Immunol 120: 366–373, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Rescigno M, Granucci F, Ricciardi-Castagnoli P. Molecular events of bacterial-induced maturation of dendritic cells. J Clin Immunol 20: 161–166, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Robbins CS, Franco F, Mouded M, Cernadas M, Shapiro SD. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J Immunol 180: 6623–6628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers AV, Adelroth E, Hattotuwa K, Dewar A, Jeffery PK. Bronchial mucosal dendritic cells in smokers and ex-smokers with COPD: an electron microscopic study. Thorax 63: 108–114, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 93: 289–296, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 182: 389–400, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179: 1109–1118, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Sanchez N, Riol-Blanco L, Rodriguez-Fernandez JL. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol 176: 5153–5159, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Soler P, Moreau A, Basset F, Hance AJ. Cigarette smoking-induced changes in the number and differentiated state of pulmonary dendritic cells/langerhans cells. Am Rev Respir Dis 139: 1112–1117, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Tsoumakidou M, Demedts IK, Brusselle GG, Jeffery PK. Dendritic cells in chronic obstructive pulmonary disease: new players in an old game. Am J Respir Crit Care Med 177: 1180–1186, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol 175: 2684–2691, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med 172: 530–551, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J 30: 993–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 9: 185–196, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Wahl SM, Katona IM, Stadler BM, Wilder RL, Helsel WE, Wahl LM. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). II. Functional properties of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions. Cell Immunol 85: 384–395, 1984 [DOI] [PubMed] [Google Scholar]