Abstract
Extravascular lung water includes all of the fluid within the lung but outside of the vasculature. Lung water increases as a result of increased hydrostatic vascular pressure or from an increase in lung endothelial and epithelial permeability or both. Experimentally, extravascular lung water has been measured gravimetrically. Clinically, the chest radiograph is used to determine whether extravascular lung water is present but is an insensitive instrument for determining the quantity of lung water. Bedside measurement of extravascular lung water in patients is now possible using a single indicator thermodilution method. This review critically evaluates the experimental and clinical evidence supporting the potential value of measuring extravascular lung water in patients using the single indicator method.
Keywords: acute lung injury, acute respiratory distress syndrome, pulmonary edema
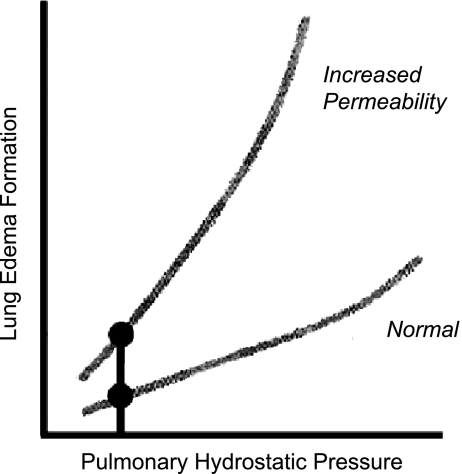
the amount of extravascular lung water (EVLW) present in the lung may be an important determinant of clinical outcome. EVLW encompasses all of the fluid within the lung but outside of the vascular compartment. This includes extravasated plasma as well as intracellular water, lymphatic fluid, and surfactant. In patients with respiratory failure, the ability of the alveoli to clear fluid from the air spaces is unable to keep up with the extravasation of fluid into this space. This may occur as a result of increased hydrostatic pressure, as occurs in cardiogenic pulmonary edema, or from an increase in lung endothelial and epithelial permeability, clinically recognized as acute lung injury (ALI) or the acute respiratory distress syndrome (ARDS). An increase in intravascular volume and pressure may amplify the quantity of EVLW in the presence of increased lung vascular permeability (Fig. 1; Ref. 5). Mild degrees of EVLW are often restricted to the interstitial space, but more extensive lung water reaches the distal air spaces of the lung.
Fig. 1.
Relationship between pulmonary hydrostatic pressure and pulmonary edema formation under normal conditions and in the presence of increased vascular permeability. Under normal conditions, increased hydrostatic pressure leads to pulmonary edema formation. However, in the presence of increased pulmonary vascular permeability, an increase in hydrostatic pressure amplifies the formation of pulmonary edema. Adapted from manuscript with permission from the American College of Chest Physicians (5).
Until recently, there were no methods available to accurately quantify the amount of EVLW in patients. Most of the clinical indices currently used for detecting lung water are neither sensitive nor specific enough to provide useful information about the extravasation and clearance of EVLW. The most commonly used method to estimate the amount of lung water present is the chest radiograph. However, there are several reports of poor interobserver agreement for interpretation of chest radiographs, even among experts. One report by Rubenfeld and coworkers (43) found the interobserver agreement among 21 expert pulmonary and critical care physicians to be moderate (κ = 0.55) in determining whether chest radiographs from intubated, hypoxemic patients met the American-European consensus Conference (AECC) radiographic criteria for ALI/ARDS. Furthermore, the chest radiograph is limited by a lack of sensitivity to small changes in the quantity of EVLW (13). Experimental studies in animals have indicated that evidence of EVLW on the chest radiograph only appears when lung water increases by >35% (51). Advanced imaging such as magnetic resonance imaging allows for a more quantitative assessment of lung water, however, it is expensive and impractical for clinical use (10).
An accurate method of quantifying EVLW could be valuable to more accurately delineate the extent of lung injury for diagnostic and prognostic purposes as well as to monitor response to therapy. The first report of using EVLW measurements to successfully guide the clinical management of critically ill patients is from Eisenberg and coworkers (11) over 20 yr ago. This prospective, randomized study compared a fluid management strategy guided by EVLW measurements with routine management. In this pre-low tidal volume era, the mortality rate for patients with ALI was 60–70%. Eisenberg and coworkers (11) demonstrated that of the patients with an initially elevated EVLW (>14 ml/kg) and a pulmonary capillary wedge pressure <18 mmHg, there was 100% mortality in the routine management group but only 33% mortality in the EVLW management group. A later trial by this same group of investigators reported that a fluid management approach guided by EVLW measurements resulted in fewer ventilator and intensive care unit (ICU) days than a strategy guided by pulmonary capillary wedge pressure measurements (28). Both of these studies used the thermal-indocyanine green (ICG) method to measure lung water. However, the double indicator method is cumbersome and impractical for use at the bedside. Recently, a new method to quantify EVLW has been established (46). With the introduction of the single indicator transpulmonary thermodilution method, bedside measurement of EVLW in the ICU is more feasible.
This review will critically evaluate the experimental and clinical evidence supporting the potential value of measuring EVLW in patients. The first objective is to describe the different methods used to measure EVLW as well as to discuss the limitations of these methods. The second objective is to review the experimental studies comparing EVLW measurement using the single indicator method with the gold standard gravimetric method. The third objective is to review patient outcomes in clinical studies that utilized lung water measurements in critically ill patients to monitor response to therapy. The final objective is to identify research issues that need to be addressed to test the clinical value of measuring lung water.
MEASUREMENT OF EVLW
Gravimetric method.
The gravimetric method of estimating EVLW is an experimental method and is the gold standard against which in vivo methods must be evaluated (52). This method compares the measured wet and dry weights of the lungs in animals or in postmortem subjects, permitting only one measurement. In 1965, Pearce and coworkers (34) improved on the original gravimetric method by introducing a technique in which the water in the pulmonary blood and pulmonary extravascular space are estimated separately. After bilateral pneumonectomy, a measured amount of water is added to the lungs followed by homogenization of the lungs in a blender. The homogenate is then weighed, evaporated in a heated sand bath, and reweighed to determine dry weight. The amount of hemoglobin in the homogenate and in the blood is measured to determine the weight of blood in the lungs. EVLW is the difference between the lung water and the blood water. The gravimetric method is an accurate measure of EVLW. There is no consensus on what qualifies as an “abnormal” value of EVLW. Some authors have defined elevated EVLW as >7 ml/kg (10, 11, 19, 28), whereas others have defined it as >10 ml/kg (3, 25, 45).
Double indicator method.
The double indicator dilution method involves the simultaneous injection of both a diffusible indicator (cold saline) as well as an intravascular indicator (serum protein-bound dye) for the measurement of EVLW. The assumption is that the thermal indicator, cold saline, will rapidly distribute throughout the entire intrathoracic volume of fluid and that the serum protein-bound indicator will remain within the intravascular space. The difference between the volumes of distribution of these two indicators is equal to the EVLW.
In 1954, Chinard and Enns (8) were the first investigators to inject boluses containing a serum protein-bound dye (Evans blue) and labeled water to study exchange between the blood and tissues in canine lungs. However, it was not until the 1980s that this method was used to measure EVLW in patients (22, 53). The bedside method employed cold saline as the diffusible indicator and ICG as the intravascular indicator. Soon after the introduction of the double indicator method into clinical practice, Eisenberg and coworkers (11) reported that using a fluid management protocol guided by EVLW measurements may expedite the clearance of EVLW and lead to improved outcomes in critically ill patients. The follow-up study by Mitchell and coworkers (28) confirmed these findings. Despite the potential value of the double indicator method, it was time-consuming and expensive, and for these reasons its use fell out of favor. However, the double indicator method was an important advance in the quantification of EVLW and laid the foundation for the development of the single indicator method.
Single indicator method.
Clinical measurements of EVLW are possible using a single indicator transpulmonary thermodilution method. The PiCCO and PiCCO2 systems (Pulsion Medical Systems, Munich, Germany) use a thermal indicator to determine EVLW, cardiac output (CO), and volumetric measures. Insertion of a central venous catheter and a femoral arterial catheter with a thermistor tip are required to make the measurements. Cold saline (8°C) is injected into the central venous catheter; the volume of cold saline injected is between 10 and 20 ml, depending on the body surface area of the patient. Following this, the thermistor tip on the femoral arterial catheter measures the downstream temperature change within the abdominal aorta. EVLW reflects all fluid that is outside of the pulmonary vasculature during transit of the thermal indicator. This includes interstitial and alveolar fluid.
The method of using only a single cold saline indicator to accurately measure EVLW was derived from the measurements and calculations originally used to measure lung water with the double indicator method (46). This derivation is summarized here and described in detail with diagrams below. The double indicator method directly measures the intrathoracic thermal volume (ITTV) via cold saline and the intrathoracic blood volume (ITBV) via ICG. EVLW is the difference between the ITTV and the ITBV (EVLW = ITTV − ITBV). Using the single indicator method, ITTV is directly measured using a cold saline indicator, and ITBV is calculated. In a landmark study, Sakka and coworkers (46) examined the relationship between ITBV and global end-diastolic volume (GEDV; the sum of the end-diastolic volumes of all 4 cardiac chambers) and derived an equation using linear regression to describe this relationship. The equation is ITBV = (1.25 × GEDV) − 28.4 ml. Since ITBV no longer needs to be directly measured, a single cold saline indicator is now all that is needed to measure EVLW. The detailed descriptions and diagrams are adapted from the study by Sakka and coworkers (46). Mean transit time (Mtt) and exponential downslope decay time (Dst) are determined based on the thermodilution curve generated while making the EVLW measurement.
ITTV.
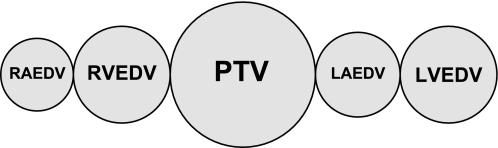
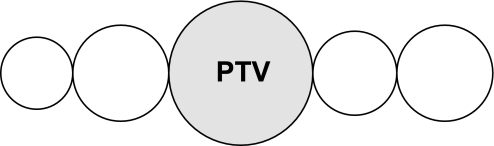
ITTV can be calculated by multiplying the CO by the Mtt of the cold saline (Mtt-cold saline), the freely diffusible indicator. The thermal indicator mixes with the largest possible volume of distribution (Fig. 2). ITTV = CO × Mtt-cold saline.
Fig. 2.
Intrathoracic thermal volume (ITTV) = cardiac output (CO) × mean transit time (Mtt)-cold saline. RAEDV, right atrial end-diastolic volume; RVEDV, right ventricular end-diastolic volume; PTV, pulmonary thermal volume; LAEDV, left atrial end-diastolic volume; LVEDV, left ventricular end-diastolic volume.
ITBV.
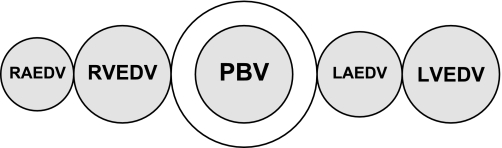
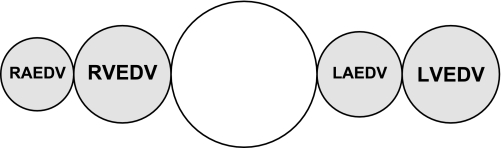
Using the double indicator method, the ITBV is determined by multiplying the CO by the Mtt of ICG (Mtt-ICG), the indicator that remains within the vasculature (Fig. 3). ITBV = CO × Mtt-ICG.
Fig. 3.
Intrathoracic blood volume (ITBV) = CO × Mtt-indocyanine green (ICG). PBV, pulmonary blood volume; RAEDV, right atrial end-diastolic volume.
EVLW.
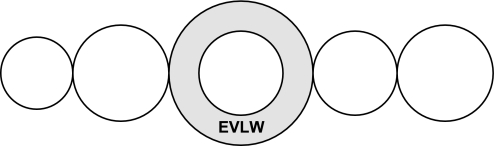
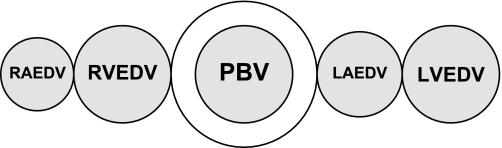
EVLW is the difference between the ITTV and the ITBV (Fig. 4). EVLW = ITTV − ITBV.
Fig. 4.
Extravascular lung water (EVLW) = ITTV − ITBV.
Pulmonary thermal volume.
Using the single indicator method, ITBV can be calculated from the GEDV. The ITTV is composed of the PTV and the GEDV. The pulmonary thermal volume (PTV) can be determined as the product of CO and the exponential Dst of the cold saline. The exponential Dst is derived from the thermodilution curve (Fig. 5). PTV = CO × DSt-cold saline.
Fig. 5.
PTV = CO × downslope decay time (Dst)-cold saline.
This calculation assumes that the decay of the dilution curve is primarily determined by the largest compartment in a series of several mixing chambers with identical flow (46). In this case, the pulmonary vasculature is the largest compartment in series with all four cardiac chambers.
GEDV.
The GEDV, the sum of the right and left heart end-diastolic volumes, is then calculated as (Fig. 6) : GEDV = ITTV − PTV (ml).
Fig. 6.
Global end-diastolic volume (GEDV) = ITTV − PTV (ml).
As mentioned earlier, Sakka and coworkers (46) demonstrated a linear relationship between ITBV and GEDV and derived the following equation to calculate ITBV (Fig. 7) : ITBV = (1.25 × GEDV) − 28.4 (ml).
Fig. 7.
ITBV = (1.25 × GEDV) − 28.4 (ml).
EVLW.
As with the double indicator method, EVLW is the difference between the ITTV and the ITBV (Fig. 8). EVLW (ml/kg) = ITTV − ITBV (ml).
Fig. 8.
EVLW (ml/kg) = ITTV − ITBV (ml).
After deriving the method for determining EVLW using the single indicator thermodilution method, the authors (46) then validated the equation in a group of 209 critically ill patients. Both methods were used to measure EVLW in all patients, and the single indicator measurements correlated well with the double indicator measurements (mean difference: EVLW-DI − EVLW-SI = −0.2 ml/kg; SD: 1.4 ml/kg; Ref. 46).
Indexing EVLW to predicted body weight.
Accurate measurements of lung water are important because they may be used for diagnostic and prognostic purposes and to guide therapy. EVLW measurements were originally indexed to the actual body weight of the patient. Recently, investigators have learned that indexing lung water to actual body weight diminishes its sensitivity as a measure of lung injury severity in obese patients (36). Height and sex are the most important factors determining lung volumes; adult lung size does not increase with body weight (3). Therefore, indexing EVLW to actual body weight will underestimate lung water in obese patients (36). Predicted body weight (PBW) is also referred to as ideal body weight and is determined by the height and sex of a patient. The calculations for PBW are as follows:
Females: 45.5 + 0.91 [height (in cm) − 152.4].
Males: 50.0 + 0.91 [height (in cm) − 152.4].
Indexing EVLW to actual body weight may not accurately depict the presence or quantity of EVLW. In addition, this technical error will affect the association between EVLW and other clinical markers of lung injury and may decrease the predictive value of lung water for survival. In fact, when using the low tidal volume strategy of ventilation for ALI/ARDS, the tidal volume is determined using the patient's PBW (5).
A recent study by Berkowitz and coworkers (3) found that indexing EVLW to PBW increased the number of ARDS patients with elevated EVLW while decreasing the number of non-ARDS sepsis patients with elevated EVLW. In response to this finding, Phillips and Smith (37) reexamined their data on EVLW measurements in patients with sepsis-associated ARDS. They too found that indexing EVLW to PBW increased the proportion of patients with sepsis-associated ARDS who had an EVLW >10 ml/kg. Berkowitz and coworkers (3) also reported that indexing EVLW to PBW improved the correlation of lung water measurements with oxygenation and the lung injury score. In another study, EVLW >16 ml/kg predicted death with 100% specificity and 86% sensitivity when indexed to PBW (36). Indexing EVLW to PBW appears to improve the predictive value of EVLW for survival and the correlation of EVLW with markers of lung injury severity.
SINGLE INDICATOR METHOD COMPARED WITH GRAVIMETRIC METHOD
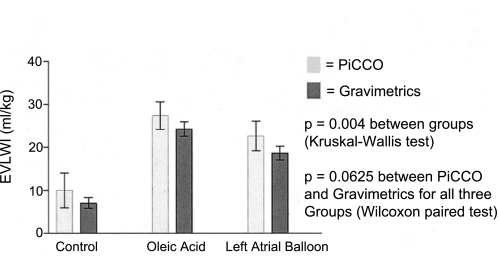
The accuracy of the single indicator method for measuring lung water has been confirmed by comparison with the gold standard gravimetric method in experimental animal studies (Table 1). The first comparison of the single indicator method with the gravimetric method was by Katzenelson and coworkers in 2004 (15). They found a close correlation between the single indicator and gravimetric lung water measurements under conditions of both hydrostatic EVLW (left atrial balloon-induced hydrostatic EVLW) and increased permeability EVLW (oleic acid-induced ALI) in anesthetized, mechanically ventilated dogs (Fig. 9). They concluded that the single indicator method is reliable and allows for the detection of EVLW regardless of the etiology of lung water formation (15).
Table 1.
Single indicator method vs. gravimetric method of measuring extravascular lung water
| First Author (Ref.) | Model | Etiology of Extravascular Lung Water | n | PiCCO, ml/kg | Gravimetric, ml/kg | |
|---|---|---|---|---|---|---|
| (mean±SD) | ||||||
| Katzenelson (15) | Canine | Control | 5 | 9.4±3.5 | 7.08±2.8 | |
| Oleic acid | 5 | 27.4±3.2 | 24.2±3.7 | |||
| Left atrial balloon-induced hydrostatic EVLW | 4 | 22.6±3.4 | 19.04±3.9 | |||
| Kirov (17) | Sheep | Control | 4 | 8.9±0.6 | 6.2±0.3 | |
| Oleic acid | 7 | 11.8±1.0 | 7.1±0.6 | |||
| Escherichia coli endotoxin | 7 | 18.2±0.9 | 11.8±0.7 | |||
| Rossi (42) | Porcine | Control | 6 | 11.7±0.7 | 6.6±0.6 | |
| E. coli endotoxin | 5 | 16.8±0.8 | 11.1±0.7 | |||
EVLW, extravascular lung water; PiCCO, advanced hemodynamic monitoring device (Pulsion Medical Systems, Munich, Germany).
Fig. 9.
EVLW index (EVLWI) measured using the gravimetric method and the single indicator method (PiCCO) (means ± SD). Adapted from manuscript with permission from the Society of Critical Care Medicine (15).
In this same study, the investigators noted a consistent overestimation of 3.01 ± 1.34 ml/kg using the single indicator method compared with the gravimetric method (15). There are several possible explanations for this finding. The thermal indicator also equilibrates with the myocardium and vessel walls, possibly leading to a larger volume of distribution and a small increase in the EVLW measurement (15). Similarly, recirculation of the indicator can lead to an overestimation of lung water. Also, the calculation of ITBV using the single indicator method is derived from the GEDV. This calculation requires the use of a linear regression equation that relies on coefficients derived from animal and patient models (15). The PiCCO system uses coefficients that are accurate for human use, and since this study was done in dogs, this may have influenced the results. Overall, this is not a significant limitation because it is a small, consistent difference.
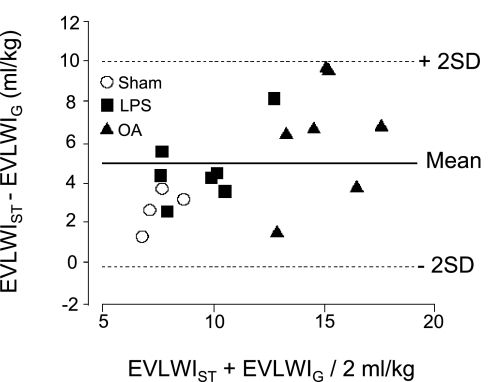
The next study comparing the single indicator method to the gravimetric method reproduced the findings of Katzenelson and coworkers (15) using a sepsis model of ALI in awake sheep. Kirov and coworkers (17) measured EVLW in the settings of oleic acid injury and LPS infusion. They found that single indicator and gravimetric EVLW measurements were closely correlated (Fig. 10). Similar to the previous study, measurements using the single indicator method overestimated the quantity of lung water (mean difference 4.9 ml/kg). The relationship between ITBV and GEDV differs by species (16). The linear regression equation for humans is ITBV = (1.25 × GEDV) − 28.4 ml. Determining the linear regression equation for the species under investigation and using it to replace the default equation (for humans) employed by the PiCCO device reduces the overestimation of the lung water measurement (16).
Fig. 10.
Bland-Altman plot for the EVLWI measured using the single indicator method (EVLWIST) and the gravimetric method (EVLWIG) in sheep. The x-axis shows the mean of EVLWI measurements by transpulmonary thermodilution and gravimetry. The y-axis shows the difference between the 2 methods. The bold line indicates the value for the mean difference between EVLWIST and EVLWIG, and each dashed line indicates 2 SDs. Mean difference = EVLWIST − EVLWIG = 4.91 ml/kg (SD 2.54 ml/kg). Adapted from manuscript with permission from Critical Care (17).
Two years later, Rossi and coworkers (42) compared the single indicator method with the gravimetric method in a porcine model of endotoxemia. In addition to determining the measurement agreement between these two methods, they also tested the relationship between ITBV and GEDV in the porcine model and determined the effects of changes in pulmonary ventilation and perfusion on EVLW measurement. Similar to the prior studies, the single indicator method consistently overestimated EVLW compared with the gravimetric method (mean bias 5.4 ml/kg). To determine the linear regression relationship between ITBV and GEDV in a porcine model, Rossi and coworkers (42) used the double indicator method to directly measure ITBV along with the single indicator method. The linear regression relationship they determined was ITBV = 1.52 × GEDV + 49.7 ml. When this equation was used rather than the original linear regression relationship determined by Sakka and coworkers (ITBV = 1.25 × GEDV − 28.4 ml; Ref. 46), the accuracy of the single indicator method improved. Furthermore, they determined that changes in ventilation (bronchial plugging with distal lung deflation) and perfusion (pulmonary artery occlusion) significantly reduced EVLW as measured by the single indicator method.
LIMITATIONS OF EVLW MEASUREMENT
Effros and coworkers (10) recently published a review in this journal detailing the methodology of both original and current lung water measurements. Their review provided an in-depth critique of these methods and described some of the limitations of measuring EVLW. We have briefly reviewed some of the key assumptions of the single indicator method as well as clinical situations in which this method may be less accurate.
Assumptions of the single indicator method.
The estimation of EVLW using the single indicator thermodilution method relies on two assumptions. The first is that GEDV has a constant and predictable relationship with ITBV. Using the single indicator method, ITBV is estimated, in contrast to the double indicator method in which it is measured (46). It is assumed that the ratio between the volume of blood in the heart and in the pulmonary circulation remains consistently equal to 4:1 (27). Thus any factor that can affect the pulmonary blood volume (PBV) or cardiac dimensions may also affect the ratio of cardiac to PBV. This would alter the estimation of EVLW using the single indicator method. The second assumption is that the product of the CO and the exponential Dst of the thermal indicator (PTV = CO × DSt-cold saline) accurately represents the PTV allowing for an accurate estimation of GEDV (GEDV = ITTV − PTV; Ref. 27).
To test these hypotheses, Michard and coworkers (27) measured several parameters including anatomic (height, weight), mechanical [tidal volume, positive end-expiratory pressure (PEEP)], physiological (pulmonary edema, hypoxic vasoconstriction), and pharmacological (vasoactive drugs) factors to determine which factors may influence the accuracy of EVLW estimation by the single indicator method compared with the double indicator method in a cohort of surgical ICU patients. They found that the amount of EVLW, tidal volume, PEEP, and arterial Po2 (PaO2)/fraction of inspired oxygen (FiO2) significantly affected the estimation of EVLW using the single indicator method. Compared with the double indicator measurements, the single indicator measurements underestimated the EVLW. However, the difference was <10% and was considered clinically acceptable (27).
Decreased pulmonary perfusion.
Since the inception of thermodilution methods to measure EVLW, it has been assumed that lung water will be underestimated in regions with decreased pulmonary vascular perfusion. The thermodilution method for measuring EVLW relies on heat exchange across the alveolar epithelial and endothelial barriers. The cold saline thermal indicator must have access to all lung tissue because the ITTV is determined by measuring the quantity of heat transferred between the cooled blood and the surrounding tissues during transit through the intrathoracic vessels (48). If the thermal indicator does not have access to all lung tissue because of decreased perfusion, then EVLW will be consistently underestimated because the thermal indicator cannot detect lung water in these regions. Large pulmonary vessel obstruction (as in acute pulmonary embolism) or pulmonary vascular microembolism (as in cases of ALI) can result in decreased pulmonary perfusion. A third cause of decreased perfusion is hypoxic vasoconstriction that can be associated with EVLW (see Pulmonary edema). A number of studies (detailed below) have tested the impact of decreased pulmonary perfusion on EVLW measurements via these mechanisms.
Historically, during the initial development of a thermodilution method to measure EVLW, there was concern that regions of the lung with poor perfusion would not be reached by certain indicators. Chinard and Enns (8) introduced the use of isotopically labeled water as an indicator for lung water measurement. Subsequently, heat was selected as an alternative indicator to heavy water because heat diffuses through water 50 times faster than labeled water and easily crosses tissue membranes (9). It was thought that since heat diffuses through water quickly and efficiently that this would permit thermal equilibration within underperfused regions via surrounding well-perfused regions (26). Furthermore, using a thermal indicator is safer than using a radioactive indicator.
Pulmonary artery obstruction can occur in patients with pulmonary embolism. Experimentally, Schreiber and coworkers (48) interrupted perfusion to the middle and lower lobe of the right lung in a porcine model and then measured EVLW using the double indicator method. They reported large, reversible (with reopening of the vessels) decreases in EVLW, ITBV, and GEDV.
More commonly, critically ill patients can experience pulmonary microvascular obstruction secondary to ALI from a variety of etiologies (26). Oppenheimer and coworkers (33) experimentally tested the accuracy of the double indicator method compared with the gravimetric method under conditions of increased permeability (induced by oleic acid) and decreased perfusion (induced by 500-μm glass beads). The infusion of glass beads (0.25 g/kg) into the pulmonary vasculature of dogs led to a decrease in pulmonary blood flow of ∼50% as well as an underestimation of the lung water measurement by 45% in the presence of a significant amount of lung water. A follow-up experimental study by Beckett and Gray (2) using a dog model and smaller glass beads of 175-μm diameter at low dose (0.32 g/kg) and high dose (0.65 g/kg) found that the double indicator method underestimated the EVLW by only 16% compared with the gravimetric method. The size of the glass beads in this experiment was expected to occlude pulmonary blood flow to areas at least as large as a pulmonary lobule. These studies suggest that when pulmonary vessels of 500 μm or greater diameter are occluded, diffusion of the thermal indicator is insufficient to measure lung water in regions of underperfusion. However, when pulmonary vessels of 175 μm or smaller are embolized, the thermal indicator is able to measure the underperfused region by diffusing locally through well-perfused regions, and the underestimation of EVLW becomes much less (9). Thus one might anticipate that, in contrast to pulmonary embolism, patients with ALI would not be expected to have occlusion of pulmonary vessels >175 μm.
An experimental study by Roch and coworkers (41) compared lung water measurements using the double indicator method with the gravimetric method in porcine models of direct lung injury [hydrochloric acid (HCl) instilled into airway] and indirect lung injury (oleic acid infusion into superior vena cava via pulmonary artery catheter). Their aim was to determine the accuracy of the double indicator method in two different models of ALI, direct and indirect. Overall, the double indicator method consistently underestimated EVLW by ∼30% compared with gravimetric measurements. In the oleic acid injury group, there was a significant correlation between the two methods, but EVLW was consistently underestimated using the double indicator method (mean bias −5.2 ml/kg). In the HCl injury group, there was poor agreement between the two methods. These findings confirmed the observations of a prior study in which there was poor accuracy of the double indicator method in a canine HCl injury model (6). HCl produces a characteristic focal lung injury and is accompanied by a redistribution of pulmonary blood flow away from injured lung (6). However, other experimental lung injury etiologies such as oleic acid or volume overload produce a more generalized lung injury and may allow for a more accurate measurement of EVLW (6). Roch and coworkers (41) hypothesized that the variation in EVLW measured by the double indicator method can reflect changes in the amount of EVLW present as well as changes in the pulmonary perfusion pattern.
In an extension of these studies, Nirmalan and coworkers (32) examined the accuracy of the double indicator method compared with the gravimetric method in a porcine model subjected to oleic acid injury and mild anemia. The authors infused oleic acid directly into the right atrium and then removed 10% of the blood volume. EVLW measurements were made at baseline, 30 min (after lung injury and hemorrhage), and 120 min. The animals were then volume-resuscitated over a period of 60 min, and a final EVLW measurement was made at 180 min. Linear regression was used to determine an accurate estimate of ITBV obtained through fixed transformation of GEDV. This approach, which is the basis for the single indicator method, had not yet been validated in subjects with ALI and mild deficits in total blood volume (32). These conditions frequently coexist in many patients and may affect the relationship between ITBV and GEDV. The data showed that measurement errors were within clinically tolerable limits (mean error in deriving ITBV from GEDV was 4.5%, SD 4.2%; range 0.05–19%) and were not significantly altered by small changes in total blood volume (32).
Pulmonary edema.
Gas exchange in the lung is ultimately determined by how well perfusion is matched to ventilation. There is limited information regarding how regional lung perfusion is affected in patients with elevated EVLW due to ALI. However, in animal models of ALI, perfusion to edematous lung regions is almost always decreased (49). The potential dependence on homogeneous pulmonary perfusion has been cited as a barrier to the widespread acceptance of the dilution methods of measuring EVLW.
Fernández-Mondéjar and coworkers (13) determined the accuracy of the single indicator method under normal conditions compared with ALI. In a porcine model, they measured EVLW before and after the intratracheal administration of either 250 or 500 ml of saline solution. The before and after EVLW measurements were made first in a normal lung (control group) and then under conditions of moderate lung injury (after 3–4 bronchoalveolar lavages) and severe lung injury (after 7–9 bronchoalveolar lavages). The percentage of EVLW detected was significantly higher in the normal lung control group (72 ± 12%) than in the lung injury group (31 ± 16%) (13). The accuracy of the EVLW measurements in the normal lung is important because no other clinical index is capable of detecting early increases in EVLW (13). However, the single indicator method was less accurate in edematous lungs, and the inaccuracy increased with the severity of EVLW in these studies (13).
Measurement of EVLW is sensitive enough to detect clinically important changes in the amount of lung water present (36). Accurate detection of small increases in lung water using EVLW measurements may precede detection by conventional clinical parameters (14). The ability to detect small changes in EVLW may be of greater clinical value and allow for more precise monitoring of responses to therapeutic measures. In addition to detecting early increases in EVLW, the optimal method of measuring lung injury should also reflect resolution of the condition following therapeutic intervention. A later experimental study by Fernández-Mondéjar and coworkers (14) was designed to detect small (10–20%) increases in EVLW. They measured EVLW before and after intratracheal administration of 50 ml of saline in a porcine model under normal conditions and in the presence of moderate lung injury (after 3–4 bronchoalveolar lavages) and severe lung injury (after 7–9 bronchoalveolar lavages). Under normal conditions the single indicator method detected 84% of the saline and in the presence of elevated lung water 77% of the saline was detected. The authors (14) concluded that small changes in EVLW can be detected using the single indicator method.
Using a novel approach, Schuster and coworkers (49) used PET scanning to define the relationship between regional pulmonary perfusion and the amount of lung water in ICU patients with both ALI and non-ALI etiologies of elevated lung water. The authors examined 16 patients with elevated EVLW and 7 healthy volunteers. In contrast to prior experimental animal studies, they found that the regional pulmonary blood flow in patients with moderate to severe increases in lung water was not significantly different from healthy subjects. They hypothesized that mechanisms such as hypoxic vasoconstriction that maintain more normal ventilation-perfusion matching appear to be blunted early on. This result applied to patients with either increased hydrostatic pressure or increased pulmonary vascular permeability (49).
PEEP.
PEEP is commonly used in mechanically ventilated patients to prevent atelectasis and promote lung expansion and may affect the value of EVLW measured in critically ill patients. Some experimental models have shown an increase in lung water in response to PEEP, whereas others have demonstrated a protective effect of PEEP leading to decreased lung water (44).
There has been debate over the mechanisms of PEEP that may lead to either increased or decreased EVLW. PEEP may affect the measured value of EVLW and also directly impact the amount of lung water present, thereby indirectly affecting the measured value of EVLW. High levels of PEEP may be responsible for pulmonary vascular defects by applying pressure to the capillary endothelium, which leads to pulmonary capillary collapse (26), thus potentially resulting in an underestimation of EVLW. Alternatively, the use of PEEP may cause a redistribution of pulmonary blood flow toward previously underperfused lung regions. This effect would allow the thermal indicator to more readily distribute throughout the lung, leading to a more accurate EVLW measurement. An example of this is an experimental study that demonstrated that PEEP caused a reversible increase in the amount of EVLW measured in a canine model of HCl injury and oleic acid injury (7). PEEP may also directly decrease EVLW by decreasing pulmonary capillary pressure (mediated through a reduction in CO) or may directly increase EVLW by reducing lymph flow (one mechanism of EVLW clearance) (26).
Myers and coworkers (30) examined a porcine model under conditions of ALI induced by Pseudomonas aeruginosa. They exposed the animals to varying rates of fluid resuscitation and applied increasing levels of PEEP and compared them with a control group with no PEEP. EVLW in the pigs who received PEEP was less than or equal to the EVLW in the control group independent of how much fluid resuscitation the animals had received (30). Thus PEEP may provide a protective effect, limiting the amount of lung water in the setting of ALI and fluid resuscitation. An alternative possibility is that PEEP may be causing pulmonary vascular defects leading to a falsely low EVLW measurement.
Ruiz-Bailen and coworkers (44) examined the effect of early application of PEEP on the amount of lung water in a porcine model of ALI (oleic acid). The animals were divided into three groups: 10 cmH2O PEEP immediately after oleic acid infusion, 10 cmH2O PEEP 120 min after oleic acid infusion, and a control group without PEEP. After 6 h, the gravimetric method was used to determine the amount of EVLW present. The amount of EVLW in the first group was significantly less than in the second and third groups (11.5 ± 2.0 vs. 19.1 ± 2.6 and 25.8 ± 1.6 ml/kg). The authors concluded that a PEEP of 10 cmH2O reduced the amount of EVLW, and this protective effect was most pronounced early on in the course of injury.
Fernández Mondéjar and coworkers (12) examined the effect of different levels of PEEP on EVLW and lymphatic drainage in a canine model of hydrostatic lung water (left atrial balloon). The amount of EVLW significantly increased after inflation of the left atrial balloon in all animals. The animals were then divided into three groups: 1) 20 cmH2O PEEP applied at 120 min, 2) 10 cmH2O PEEP applied at 60 and 90 min, and 3) 20 cmH2O PEEP applied at 60 and 90 min. After 90 min, the EVLW as measured by the double indicator method was significantly greater in the first group compared with the second and third groups (21.2 ± 5.1 vs. 12.8 ± 2.0 and 14.8 ± 4.8 ml/kg). Although the authors noted an increase in lymphatic drainage with PEEP set at 10 cmH2O, they believed that the lower EVLW in the animals with PEEP was due to a reduction in the capillary transmural pressure as this is known to be a more dominant force in the formation and resolution of EVLW.
Major pulmonary resection.
ALI in patients following lung resection is a major cause of postoperative mortality (23). Postoperative ALI is similar to ARDS in terms of its clinical, radiographic, and histopathological features, however, the identification of patients with postoperative ALI is complicated given the lack of universally implemented diagnostic criteria (23). Furthermore, similar to ARDS, postoperative ALI is not clinically evident until a significant amount of lung water is already present. A method to identify patients at risk for postoperative ALI or diagnose patients early in the disease course may improve clinical outcomes.
As detailed above, a primary assumption of the single indicator method is that GEDV has a constant and predictable relationship with ITBV. To determine EVLW using the single indicator method, the ITBV is determined based on its linear relationship with the measured GEDV. ITBV comprises the PBV and the GEDV. Following lung resection, the PBV changes, and therefore the relationship between ITBV and GEDV may be altered as well.
The effect of lung resection on the accuracy of EVLW measurements was first studied in a porcine pneumonectomy model by Roch and coworkers (40). They determined the accuracy of both the single and double indicator methods in single and double lung porcine models of ALI (oleic acid). In the double lung group, the PiCCO measurements slightly overestimated the gravimetric measurements [mean bias +1.5 ml/kg, 95% confidence interval (CI), −1.5 to +4.3 ml/kg]. After pneumonectomy, the PiCCO measurements further overestimated the gravimetric measurements (mean bias +5.0 ml/kg, 95% CI, +3.4 to +6.8 ml/kg). Furthermore, the overestimation of EVLW as measured with the PiCCO system increased as the amount of lung water increased. Using the double indicator method, ITBV was measured before and after pneumonectomy. After pneumonectomy, the ITBV decreased by 10%, and there was a less reliable linear relationship between ITBV and GEDV. Therefore, it is possible that after pneumonectomy, the PiCCO system may overestimate ITBV, leading to an underestimation of EVLW. However, in this experiment, the single indicator method overestimated EVLW after pneumonectomy. The explanation for this finding is unclear. Similar to prior studies, EVLW is overestimated using the single indicator method compared with gravimetric method because 1) there is equilibration of the thermal indicator with myocardium and vessel walls, and 2) PiCCO uses coefficients for the calculation of ITBV that are appropriate for humans (15, 17). This explains the overestimation using the single indicator method in a double lung model, however, in a single lung model, the EVLW should be “less overestimated” given the alteration of the relationship between ITBV and GEDV that is unrecognized by the PiCCO system.
The first study to examine the relationship between ITBV and GEDV after pulmonary resection in patients is currently in press. Naidu and coworkers (31) measured EVLW using both the single and double indicator methods in three patients before and after pulmonary resection (left lower lobectomy, left pneumonectomy, and left upper lobectomy). In all three patients, the relationship between ITBV and GEDV changed immediately after surgery and remained at the new state up to 12 h postoperatively. The single indicator method underestimated EVLW compared with the double indicator method in two patients (left lower lobectomy and left pneumonectomy) and overestimated EVLW in one patient (left upper lobectomy). Overall, the single indicator method consistently overestimated or underestimated EVLW in each patient. Larger clinical studies are needed to determine the clinical value of EVLW measurements in patients after pulmonary resection. Routine clinical use to determine the absolute quantity of EVLW cannot be recommended in these patients at this time. Nonetheless, measuring lung water may be useful for following trends within the same patient.
Anatomic and physiological abnormalities that can alter EVLW measurement.
There are certain anatomic and physiological abnormalities that can lead to inaccurate measures of EVLW. The presence of large aortic aneurysms will lead to an overestimation of EVLW (46). Arterial catheters placed too far peripherally will also lead to overestimation due to a prolonged Mtt of the thermal indicator (46). Finally, measurement of EVLW may be inaccurate in patients with intracardiac shunts (46).
CLINICAL TRIALS
Potential significance of EVLW measurement in patients with ALI/ARDS.
Recently, several studies have examined the value of measuring EVLW in critically ill patients with sepsis. The hallmark of sepsis is increased capillary permeability; in the lungs, this leads to increased EVLW (25). A prospective study by Martin and coworkers (25) investigated whether the initial subclinical increase in capillary permeability could be detected by measuring EVLW with the single indicator method. They enrolled 29 critically ill patients with severe sepsis and followed them for 28 days to determine the occurrence of ARDS and death. Patients were considered to have elevated EVLW if any of the measurements were >10 ml/kg. Interestingly, over half of the patients with severe sepsis who did not meet the clinical criteria for ARDS had an elevated EVLW that correlated with measures of lung injury including the PaO2/FiO2, the lung injury score, and the chest radiograph findings. However, the EVLW measurements were indexed to actual body weight, confounding these findings. Half of these patients were adequately hypoxemic to diagnose ARDS by the AECC criteria (4), but they did not have the required bilateral radiographic infiltrates. These patients may have had radiographically unrecognized ALI early in the lung injury severity spectrum.
Another study by Kuzkov and coworkers (19) examined 38 patients with septic shock and ALI to determine whether EVLW as measured by the single indicator method is associated with physiological indices of lung injury, plasma endothelin, and clinical outcomes. In their study, EVLW was normal (defined as <7 ml/kg) in 26% of the patients. However, as in the prior study, the EVLW was indexed to actual body weight, leading to lower EVLW measurements than if the measurements were indexed to PBW. They concluded that increased EVLW occurs in patients with ALI/ARDS but is not a necessary feature, especially in patients with sepsis of nonpulmonary origin. However, 95% of patients with pulmonary sepsis-induced ALI had an elevated EVLW.
The findings of these studies have implications regarding the detection of ALI/ARDS in patients with sepsis. The criteria for defining ALI/ARDS were standardized in 1994 by the AECC (4). These criteria include bilateral lung infiltrates on the chest radiograph, PaO2/FiO2 < 200 for ARDS and < 300 for ALI, and a pulmonary capillary wedge pressure < 18 mmHg or no clinical evidence of left atrial hypertension. There has been concern regarding the sensitivity and specificity of the definition for ALI/ARDS. There is a need for an early accurate diagnostic marker of ALI/ARDS so that therapeutic interventions may be initiated without delay. Furthermore, it is important to identify patients with lung injury early on in the disease process so that they can be enrolled in clinical trials (21). The AECC criteria are usually met once significant hypoxemia is already well established. Measurement of EVLW may be useful in identifying some patients with early lung injury who do not yet satisfy the criteria for ALI/ARDS. Although the pathophysiological changes in ALI/ARDS may lead to an elevated EVLW, it is not part of the diagnostic criteria. An expanded definition of ALI/ARDS in some research or clinical trials might include measures of elevated EVLW, especially in patients with sepsis due to a pulmonary etiology.
EVLW measurements and response to therapy.
Only a few clinical trials have tested the potential clinical value of measuring EVLW to guide therapy in critically ill patients. Fluid management affects the development and resolution of EVLW, whether the primary cause is increased hydrostatic pressure or increased lung vascular permeability (28). When vascular permeability is increased, the quantity of EVLW is highly sensitive to elevated hydrostatic pressure (5). Despite this, clinicians are often reluctant to use diuretics or restrict fluid intake in patients with evidence of increased EVLW. The classic dilemma is an attempt to balance the potential benefits of intravascular volume expansion on cardiac and renal function against the potentially negative effect of causing or worsening EVLW in hypotensive patients (28). The FACTT trial (57), a recent randomized controlled trial of the ARDS Network, compared a conservative strategy with a liberal strategy of fluid management. The findings of this trial did not determine the optimal fluid management strategy in hypotensive patients because the fluid conservative intervention was held until 12 h after hypotension resolved (defined as 12 h without the need for vasopressors).
The first study to evaluate the clinical utility of measuring EVLW sought to determine whether EVLW is a better parameter than pulmonary capillary wedge pressure to guide fluid resuscitation for shock and diuretic therapy for increased EVLW (11). Resolution of EVLW was more rapid when EVLW was used as an end point. The investigators also noted that outcomes might be improved by restricting excessive volume expansion in hypotensive patients with ARDS. Overall, this study indicated that a strategy that emphasized intravascular volume restriction in patients with elevated EVLW was safe and well-tolerated despite deviation from routine management (11).
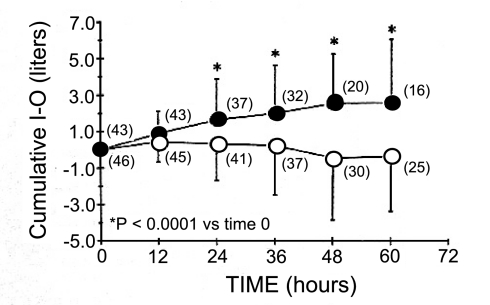
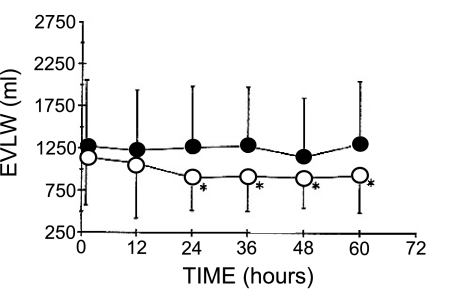
A follow-up study by this same group evaluated whether an alternative protocol to routine fluid management would affect the development or resolution of lung water in patients with elevated EVLW due to either increased hydrostatic pressure or increased permeability (28). The main comparison of this study was the use of EVLW measurements, using the double indicator method, vs. the pulmonary capillary wedge pressure to guide fluid management. Patients in the EVLW group had fewer ventilator days and ICU days than patients in the pulmonary capillary wedge pressure group. For patients with elevated lung water, a lower positive fluid balance was followed by improved resolution of EVLW (Figs. 11 and 12; Ref. 28).
Fig. 11.
A study by Mitchell and coworkers (28) randomized 101 patients, 89 of whom had an initial EVLW > 7 ml/kg. This figure depicts the cumulative I−O (intake minus output) (y-axis) after each time interval (x-axis) for the 89 patients in both management groups [EVLW (○) and pulmonary capillary wedge pressure (●)] with an initial EVLW > 7 ml/kg. Numbers in parentheses indicate number of patients at the time interval still in the study. Adapted from manuscript with permission from the American Thoracic Society (28).
Fig. 12.
Mean EVLW (y-axis) after each time interval (x-axis) for the same 89 patients described in Fig. 11. *P < 0.05 compared with baseline measurement of EVLW. Open circles = EVLW management group; closed circles = pulmonary capillary wedge pressure management group. Adapted from manuscript with permission from the American Thoracic Society (28).
More recently, Perkins and coworkers (35) investigated the efficacy of intravenous salbutamol in accelerating alveolar fluid clearance in patients with ALI/ARDS. In this randomized, double-blind, placebo-controlled clinical trial, 19 patients were assigned to salbutamol and 21 patients to placebo. Patients in the salbutamol group had significantly less EVLW on day 7 than patients in the placebo group (9.2 ± 6 vs. 13.2 ± 3 ml/kg; P = 0.04) as measured by the single indicator method. There was no improvement in oxygenation as indicated by the PaO2/FiO2 in patients treated with salbutamol, although the plateau pressure significantly declined in the salbutamol-treated patients. This study was not powered to detect a difference in mortality (35).
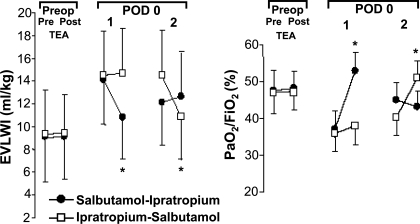
In an elegant crossover study, Licker and coworkers (24) investigated the effect of aerosolized salbutamol on the incidence of ALI following thoracic surgery in high-risk patients. ALI following thoracic surgery, although uncommon, is responsible for the majority of perioperative deaths. Within the first 36 h postoperatively, all patients were treated with aerosolized salbutamol and ipratropium; the drugs were given in a crossover design. EVLW measurements were made before and 50 min after each drug administration using the single indicator method. On postoperative day 0, there was a significant reduction in EVLW (−18 ml/kg ± 10%; P = 0.019) as well as a corresponding increase in the PaO2/FiO2 (+25 ± 13%; P = 0.028) after treatment with salbutamol (Fig. 13). These findings were accompanied by resolution of EVLW on the chest radiograph. These effects were not observed after treatment with ipratropium. This is the first clinical study to show that inhalation of a β2-agonist decreases lung water as measured by the single indicator method (24). These two recent studies (24, 35) indicate that measurement of EVLW may allow clinicians to monitor changes in lung water in response to therapeutic interventions.
Fig. 13.
Time course of EVLWI and arterial Po2 (PaO2)/fraction of inspired oxygen (FiO2). Measurements were made preoperatively (Preop) before (Pre) and 30 min after (Post) initiation of thoracic epidural anesthesia (TEA) and on postoperative day (POD) 0 before and 50 min after drug administration. In this crossover design, subjects underwent inhalation of salbutamol followed by ipratropium bromide (●) or ipratropium bromide followed by salbutamol (□). *P < 0.05 vs. preintervention value. Adapted from manuscript with permission from the American College of Chest Physicians (24).
Another recent study by Mutoh and coworkers (29) monitored cardiac index, GEDV index, and EVLW index using the single indicator method to guide fluid management in 46 patients with subarachnoid hemorrhage (SAH). The primary management goal for patients with SAH is to maintain a high CO via hypervolemia and/or hypertension to prevent secondary brain injury due to hypoperfusion. This study was designed to maximize cardiac index (CI > 3.0 l/min/m2) to prevent secondary brain injury while taking precaution to not cause volume overload (EVLW ≤ 14 ml/kg) in attempt to avoid increasing EVLW. In these patients, it is critical to minimize lung water as patients with pulmonary complications after SAH are more likely to experience vasospasm, which can further increase their morbidity and mortality (29). Patients underwent intravascular volume expansion if cardiac index was <3.0 l/min/m2 and diuresis if EVLW was >15 ml/kg or if there were any signs of increased EVLW or congestive heart failure. In 43 patients (93%), these goal values were met using the fluid management protocol guided by EVLW measurements, and there were no patients who developed increased EVLW or congestive heart failure (29). Hemodynamic and fluid management are critical in these patients, and therefore the use of EVLW measurements to guide therapy may be useful in patients with SAH.
Although quantifying EVLW seems to have clinical value based on these studies, in actuality, these measurements may not improve the clinical care of patients. A recent example of this paradox has been demonstrated with the use of pulmonary artery catheters. Initially, it appeared as though the detailed physiological measurements provided by the pulmonary artery catheter would allow for more precise clinical care of critically ill patients. The hope was that this diagnostic information would then lead to improved patient outcomes. However, several randomized controlled trials demonstrated similar mortality rates regardless of whether a pulmonary artery catheter was used to guide therapy (39, 47, 57). Furthermore, in one trial, patients with a pulmonary artery catheter were more likely to have a pulmonary embolism than patients without a catheter (47). Although measurements of EVLW afforded by the single indictor method might be beneficial in guiding therapy in critically ill patients, well-designed clinical trials are necessary to determine whether this holds true.
Potential prognostic value of EVLW.
EVLW may be an independent predictor of survival for ICU patients (45). Several clinical studies have categorized patients as having elevated EVLW if the value is >10 ml/kg (3, 25). Sakka and coworkers (45) reported a mortality rate of 65% when EVLW was >15 ml/kg. More recently, Phillips and coworkers (36) found that EVLW index (indexed to PBW) >16 ml/kg predicted mortality with 100% specificity and 86% sensitivity.
EVLW on admission to the ICU may be less accurate than more complex scoring systems (APACHE II, SAPS II, and SOFA; Refs. 17a, 20a, 54a) with respect to prognostic ability. This can be explained by the fact that EVLW measurements represent only lung injury, whereas more complex scoring systems include the function of several organ systems. Most scoring systems have only been validated for the first 24 h after admission to the ICU. However, EVLW as an organ function parameter may have predictive value during the entire clinical course because maximum EVLW has been shown to be a predictor of mortality (36, 45). Furthermore, if the quantity of EVLW increases, despite interventions, then this scenario would provide invaluable clinical information regarding responsiveness to therapy.
Although there are no proven methods to prevent ALI/ARDS in high-risk patients, early therapeutic intervention may limit progression of the lung injury and decrease mortality. Limiting the extent and duration of ALI may also reduce the risk of complications, such as ventilator-induced pneumonia. ALI is characterized by an initial exudative phase clinically recognized as a rapid onset respiratory failure in a patient with risk factors for ALI (55). Some patients recover after the acute phase, whereas others may develop fibrosing alveolitis characterized by continued hypoxemia (usually refractory to oxygen therapy), elevated dead space, worsening pulmonary compliance, and pulmonary hypertension secondary to pulmonary capillary injury (55). Early therapeutic intervention may prevent progression of ALI/ARDS from the acute phase to fibrosing alveolitis or simply stimulate lung water clearance. However, to assess the efficacy of such therapeutic interventions, it would be valuable to have a reliable marker or index of lung injury severity. It may also be possible to detect increases in EVLW earlier in the patient's clinical course using the single indicator method to measure EVLW compared with conventional measures of EVLW, such as the chest radiograph. The earliest pathological finding in ALI/ARDS is increased permeability of the vascular endothelium (1, 55). Although there is currently no reliable clinical indicator to detect this increase in lung vascular permeability, increases in EVLW may be used as a marker for alterations in permeability, signaling the early phase of the syndrome (1).
Furthermore, the clinical progression of acute respiratory failure may not be linearly related to the absolute value of EVLW (28). Small increases in EVLW toward the upper end of the EVLW spectrum may have greater clinical impact than similar changes toward the lower end of the spectrum, perhaps because of the extent of alveolar lung water (28). Ideally, measurement of EVLW would be valuable in detecting lung injury before standard clinical indices as well as before clinical deterioration for patients at the upper end of the EVLW spectrum. Based on experimental studies, it may be possible to detect small changes in EVLW even in the later stages of ALI (14).
Alternative methods of measuring EVLW.
Investigators have tried to use a variety of radiographic modalities in attempt to quantify EVLW. As mentioned briefly, the chest radiograph can be used to determine whether EVLW is present but can only determine the amount of lung water in a semiquantitative fashion. For example, certain characteristic findings are present only when there are mild increases in EVLW. Radiographic densities comprise a greater proportion of the total lung field as the amount of EVLW increases, and the density of the infiltrates worsens as well (20). Computed tomography (CT) is another useful modality. Using CT, the density of infiltrates can be measured in a quantitative fashion, and the regions of the lung with increased EVLW can be determined as well. However, the use of CT is impractical, as it requires transport of a critically ill patient to the radiology suite and exposure to ionizing radiation. Nuclear magnetic resonance (NMR) is a potentially valuable modality for quantifying the amount of EVLW. Several experimental studies have reported a good correlation between NMR measurements of EVLW and the gold standard gravimetric measurements (38, 56). NMR is expensive and technically difficult as the measurements are sensitive to respiratory and cardiac motion, but it does not involve exposure to ionizing radiation (20). The above modalities measure either lung density or total lung water. Using PET, EVLW can be measured directly. PET estimates of EVLW correlate well with gravimetric measures of lung water (50, 54), although PET consistently underestimates gravimetric measures by approximately 10–15%. PET estimates of lung water are reproducible and, more importantly, are sensitive to changes in lung water with the ability to detect a 1-ml increase in EVLW (20). Another technique that has been used to quantify EVLW is electrical impedance tomography (EIT). By measuring thoracic electrical impedance in response to an electrical current passed through the body, a value for resistance is obtained and can be correlated to EVLW (20). One clinical study demonstrated that EVLW estimates using EIT correlated well with the double indicator method (18). A main advantage to EIT is that it is portable, and therefore lung water can be estimated at the bedside.
FUTURE DIRECTIONS
Despite having been developed over 20 yr ago, the thermodilution method of measuring EVLW has not achieved widespread acceptance. One of the main reasons for this lack of use is uncertainty regarding the accuracy of EVLW measurements in patients with perfusion defects and lung edema. Since the majority of critically ill patients have some degree of heterogeneous lung perfusion, clinicians have been reluctant to rely on lung water measurements as a parameter to aid in diagnosis, prognosis, and guiding therapy. However, few studies to date have correlated single indicator lung water measurements with physiological and radiographic findings or used these measurements to monitor response to therapy. The measurement of EVLW in addition to other indices of lung injury severity such as PaO2/FiO2, oxygenation index, and lung compliance may be of value because it allows for the direct quantification of lung water, whereas other indices reflect different aspects of lung injury such as ventilation-perfusion mismatch and chest and abdominal wall contributions to compliance. Alternatively, measuring EVLW may not add any additional clinically relevant information. Only prospective studies will make it possible to determine whether measuring EVLW has clinical value.
Since the single indicator method correlates reasonably well with the gold standard gravimetric method, the foundation for future clinical use is established. The next steps involve more prospective studies in which lung water measurements using this method are compared with other physiological and biochemical indices of ALI. If these studies reveal a close correlation between EVLW measurements and currently used clinical indices of lung dysfunction, then lung water measurements may eventually be of value in the clinical evaluation of some critically ill patients. Finally, the ultimate validation of the single indicator method will require demonstrating that EVLW measurements can accurately determine the onset, progression, and resolution of lung water and that measurement of EVLW is superior to currently used clinical assessments at improving patient outcomes. Thus the single indicator method is promising, however, further clinical studies are needed to determine the clinical value of measuring EVLW in critically ill patients.
GRANTS
M. A. Matthay was supported by National Institutes of Health (NIH) Grant HL-51856, K. D. Liu was supported by NIH/National Center for Research Resources (NCRR) Grant KL2-RR-024130, and L. M. Brown was supported by NIH Grant T32-GM-008258-21.
REFERENCES
- 1.Baudendistel LJ, Kaminski DL, Dahms TE. Evaluation of extravascular lung water by single thermal indicator. Crit Care Med 14: 52–56, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Beckett RC, Gray BA. Effect of atelectasis and embolization on extravascular thermal volume of the lung. J Appl Physiol 53: 1614–1619, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz DM, Danai PA, Eaton S, Moss M, Martin GS. Accurate characterization of extravascular lung water in acute respiratory distress syndrome. Crit Care Med 36: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest 131: 913–920, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlile PV, Gray BA. Type of lung injury influences the thermal-dye estimation of extravascular lung water. J Appl Physiol 57: 680–685, 1984 [DOI] [PubMed] [Google Scholar]
- 7.Carlile PV, Lowery DD, Gray BA. Effect of PEEP and type of injury on thermal-dye estimation of pulmonary edema. J Appl Physiol 60: 22–31, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Chinard FP, Enns T. Transcapillary pulmonary exchange of water in the dog. Am J Physiol 178: 197–202, 1954 [DOI] [PubMed] [Google Scholar]
- 9.Effros RM. Lung water measurements with the mean transit time approach. J Appl Physiol 59: 673–683, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Effros RM, Pornsuriyasak P, Porszasz J, Casaburi R. Indicator dilution measurements of extravascular lung water: basic assumptions and observations. Am J Physiol Lung Cell Mol Physiol 294: L1023–L1031, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg PR, Hansbrough JR, Anderson D, Schuster DP. A prospective study of lung water measurements during patient management in an intensive care unit. Am Rev Respir Dis 136: 662–668, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Fernández Mondéjar E, Vazquez Mata G, Cárdenas A, Mansilla A, Cantalejo F, Rivera R. Ventilation with positive end-expiratory pressure reduces extravascular lung water and increases lymphatic flow in hydrostatic pulmonary edema. Crit Care Med 24: 1562–1567, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Mondéjar E, Castaño-Pérez J, Rivera-Fernández R, Colmenero-Ruiz M, Manzano F, Pérez-Villares J, de la Chica R. Quantification of lung water by transpulmonary thermodilution in normal and edematous lung. J Crit Care 18: 253–258, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Mondéjar E, Rivera-Fernández R, García-Delgado M, Touma A, Machado J, Chavero J. Small increases in extravascular lung water are accurately detected by transpulmonary thermodilution. J Trauma 59: 1420–1423; discussion 1424, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Katzenelson R, Perel A, Berkenstadt H, Preisman S, Kogan S, Sternik L, Segal E. Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit Care Med 32: 1550–1554, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kirov MY, Kuzkov VV, Fernandez-Mondejar E, Bjertnaes LJ. Measuring extravascular lung water: animals and humans are not the same. Crit Care 10: 415, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirov MY, Kuzkov VV, Kuklin VN, Waerhaug K, Bjertnaes LJ. Extravascular lung water assessed by transpulmonary single thermodilution and postmortem gravimetry in sheep. Crit Care 8: R451–R458, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100: 1619–1636, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Kunst PW, Vonk Noordegraaf A, Raaijmakers E, Bakker J, Groeneveld AB, Postmus PE, de Vries PM. Electrical impedance tomography in the assessment of extravascular lung water in noncardiogenic acute respiratory failure. Chest 116: 1695–1702, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Kuzkov VV, Kirov MY, Sovershaev MA, Kuklin VN, Suborov EV, Waerhaug K, Bjertnaes LJ. Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury. Crit Care Med 34: 1647–1653, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lange NR, Schuster DP. The measurement of lung water. Crit Care 3: R19–R24, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270: 2957–2963, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest 35: 936–943, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis FR, Elings VB, Hill SL, Christensen JM. The measurement of extravascular lung water by thermal-green dye indicator dilution. Ann NY Acad Sci 384: 394–410, 1982 [DOI] [PubMed] [Google Scholar]
- 23.Licker M, de Perrot M, Spiliopoulos A, Robert J, Diaper J, Chevalley C, Tschopp JM. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 97: 1558–1565, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Licker M, Tschopp JM, Robert J, Frey JG, Diaper J, Ellenberger C. Aerosolized salbutamol accelerates the resolution of pulmonary edema after lung resection. Chest 133: 845–852, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Martin GS, Eaton S, Mealer M, Moss M. Extravascular lung water in patients with severe sepsis: a prospective cohort study. Crit Care 9: R74–R82, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michard F. Bedside assessment of extravascular lung water by dilution methods: temptations and pitfalls. Crit Care Med 35: 1186–1192, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Michard F, Schachtrupp A, Toens C. Factors influencing the estimation of extravascular lung water by transpulmonary thermodilution in critically ill patients. Crit Care Med 33: 1243–1247, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JP, Schuller D, Calandrino FS, Schuster DP. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis 145: 990–998, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Mutoh T, Kazumata K, Ajiki M, Ushikoshi S, Terasaka S. Goal-directed fluid management by bedside transpulmonary hemodynamic monitoring after subarachnoid hemorrhage. Stroke 38: 3218–3224, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Myers JC, Reilley TE, Cloutier CT. Effect of positive end-expiratory pressure on extravascular lung water in porcine acute respiratory failure. Crit Care Med 16: 52–54, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Naidu BV, Dronavalli VB, Rajesh PB. Measuring lung water following major lung resection. Interact Cardiovasc Thorac Surg 8: 503–506, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Nirmalan M, Niranjan M, Willard T, Edwards JD, Little RA, Dark PM. Estimation of errors in determining intrathoracic blood volume using thermal dilution in pigs with acute lung injury and haemorrhage. Br J Anaesth 93: 546–551, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Oppenheimer L, Elings VB, Lewis FR. Thermal-dye lung water measurements: effects of edema and embolization. J Surg Res 26: 504–512, 1979 [DOI] [PubMed] [Google Scholar]
- 34.Pearce ML, Yamashita J, Beazell J. Measurement of pulmonary edema. Circ Res 16: 482–488, 1965 [DOI] [PubMed] [Google Scholar]
- 35.Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 173: 281–287, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Phillips CR, Chesnutt MS, Smith SM. Extravascular lung water in sepsis-associated acute respiratory distress syndrome: indexing with predicted body weight improves correlation with severity of illness and survival. Crit Care Med 36: 69–73, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Phillips CR, Smith SM. Predicted body weight-indexed extravascular lung water is elevated in acute respiratory distress syndrome. Crit Care Med 37: 377–378, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Phillips DM, Allen PS, Man SF. Assessment of temporal changes in pulmonary edema with NMR imaging. J Appl Physiol 66: 1197–1208, 1989 [DOI] [PubMed] [Google Scholar]
- 39.Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, Boyer A, Brochard L, Teboul JL. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA 290: 2713–2720, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Roch A, Michelet P, D'Journo B, Brousse D, Blayac D, Lambert D, Auffray JP. Accuracy and limits of transpulmonary dilution methods in estimating extravascular lung water after pneumonectomy. Chest 128: 927–933, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Roch A, Michelet P, Lambert D, Delliaux S, Saby C, Perrin G, Ghez O, Bregeon F, Thomas P, Carpentier JP, Papazian L, Auffray JP. Accuracy of the double indicator method for measurement of extravascular lung water depends on the type of acute lung injury. Crit Care Med 32: 811–817, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Rossi P, Wanecek M, Rudehill A, Konrad D, Weitzberg E, Oldner A. Comparison of a single indicator and gravimetric technique for estimation of extravascular lung water in endotoxemic pigs. Crit Care Med 34: 1437–1443, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest 116: 1347–1353, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Bailen M, Fernandez-Mondejar E, Hurtado-Ruiz B, Colmenero-Ruiz M, Rivera-Fernandez R, Guerrero-Lopez F, Vazquez-Mata G. Immediate application of positive-end expiratory pressure is more effective than delayed positive-end expiratory pressure to reduce extravascular lung water. Crit Care Med 27: 380–384, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Sakka SG, Klein M, Reinhart K, Meier-Hellmann A. Prognostic value of extravascular lung water in critically ill patients. Chest 122: 2080–2086, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Sakka SG, Ruhl CC, Pfeiffer UJ, Beale R, McLuckie A, Reinhart K, Meier-Hellmann A. Assessment of cardiac preload and extravascular lung water by single transpulmonary thermodilution. Intensive Care Med 26: 180–187, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Kirby A, Jacka M. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 348: 5–14, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Schreiber T, Huter L, Schwarzkopf K, Schubert H, Preussler N, Bloos F, Gaser E, Karzai W. Lung perfusion affects preload assessment and lung water calculation with the transpulmonary double indicator method. Intensive Care Med 27: 1814–1818, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Schuster DP, Anderson C, Kozlowski J, Lange N. Regional pulmonary perfusion in patients with acute pulmonary edema. J Nucl Med 43: 863–870, 2002 [PubMed] [Google Scholar]
- 50.Schuster DP, Marklin GF, Mintun MA. Regional changes in extravascular lung water detected by positron emission tomography. J Appl Physiol 60: 1170–1178, 1986 [DOI] [PubMed] [Google Scholar]
- 51.Snashall PD, Keyes SJ, Morgan BM, McAnulty RJ, Mitchell-Heggs PF, McLvor JM, Howlett KA. The radiographic detection of acute pulmonary oedema. A comparison of radiographic appearances, densitometry and lung water in dogs. Br J Radiol 54: 277–288, 1981 [DOI] [PubMed] [Google Scholar]
- 52.Staub NC. Pulmonary edema. Physiol Rev 54: 678–811, 1974 [DOI] [PubMed] [Google Scholar]
- 53.Staub NC, Hogg JC. Conference report of a workshop on the measurement of lung water. Crit Care Med 8: 752–759, 1980 [DOI] [PubMed] [Google Scholar]
- 54.Velazquez M, Haller J, Amundsen T, Schuster DP. Regional lung water measurements with PET: accuracy, reproducibility, and linearity. J Nucl Med 32: 719–725, 1991 [PubMed] [Google Scholar]
- 54a.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Wexler HR, Nicholson RL, Prato FS, Carey LS, Vinitski S, Reese L. Quantitation of lung water by nuclear magnetic resonance imaging. A preliminary study. Invest Radiol 20: 583–590, 1985 [DOI] [PubMed] [Google Scholar]
- 57.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575, 2006 [DOI] [PubMed] [Google Scholar]