Abstract
Infection of neonatal lung by respiratory syncytial virus (RSV) is a common cause of respiratory dysfunction. Lung alveolar type II and bronchiolar epithelial (Clara) cells secrete surfactant protein A (SP-A), a collectin that is an important component of the pulmonary innate immune system. SP-A binds to the virus, targeting the infectious agent for clearance by host defense mechanisms. We have previously shown that while the steady-state level of SP-A mRNA increases approximately threefold after RSV infection, steady-state levels of cellular and secreted SP-A protein decrease 40–60% in human type II cells in primary culture, suggesting a mechanism where the virus alters components of the innate immune response in infected cells. In these studies, we find that changes in SP-A mRNA and protein levels in RSV-infected NCI-H441 cells (a bronchiolar epithelial cell line) recapitulate the results in SP-A expression observed in primary lung cells. While SP-A protein is normally ubiquitinated, there is no change in the level of SP-A protein ubiquitination or proteasome activity during RSV infection, suggesting that the reduced levels of SP-A protein are not due to degradation by activated proteasomes. SP-A mRNA is appropriately processed and exported from the nucleus to the cytoplasm during RSV infection. As evidenced by polysome analysis of SP-A mRNA and pulse-chase analysis of newly synthesized SP-A protein, we find a decrease in translational efficiency that is specific for SP-A mRNA. Therefore, the decrease in SP-A protein levels observed after RSV infection of pulmonary bronchiolar epithelial cells results from a mechanism that affects SP-A mRNA translation efficiency.
Keywords: posttranscriptional regulation, collectin, ubiquitination
respiratory syncytial virus (RSV) is a negative-sense, single-stranded RNA virus that belongs to the paramyxovirus family of viruses (14, 22). In humans, RSV infects epithelial cells lining the nose and the lung (22). Two virally derived glycoproteins important for infection, the G protein and the fusion protein (F), are located within the envelope that surrounds the virus (14, 22). The G protein is a transmembrane protein that aids in attachment of the virus to host cells (37). The F protein is an integral membrane protein important for fusion of the viral particle with the host cell surface during infection (64). The F protein is also implicated in the formation of the characteristic syncytia that occur when the virus leaves one cell to infect adjacent cells (64).
In humans, RSV is the most important respiratory pathogen of infants and children (23, 52, 63). RSV can cause lower respiratory tract infection (LRI), resulting in bronchiolitis and pneumonia with up to 2.5% of infected children in the United States requiring hospitalization (13, 17, 44, 45, 52, 63). During the first year of life, ∼50% of children become infected with RSV, and by the age of 3, everyone has experienced at least one RSV infection (14, 25). Of growing concern to the medical establishment are other classes of individuals considered at high risk for LRIs resulting from RSV infections. Due to incomplete immunity to RSV infection at a young age, those who are immune-compromised and the growing elderly population are at increased risk for developing LRI from repeat RSV infections (15, 20). Recent estimates suggest that, in the United States, ∼10,000 deaths annually can be attributed to RSV infection in the elderly (61). In addition to LRI, experimental evidence has implicated a link between viral infections in infants and small children, including RSV, to allergic sensitization and increased susceptibility to superinfections (1, 2, 13, 17, 34, 35, 45, 47, 53).
During normal breathing, the lung mediates the exchange of oxygen and CO2 between epithelial cells and capillaries in the lung alveolus. Consequently, pathogenic microorganisms and other irritants gain access to the lung epithelium during inhalation of oxygen. Regulation of fluid balance during gas exchange and neutralization of foreign particles requires the presence of the lipoprotein complex known as surfactant, that is expressed by pulmonary alveolar type II epithelial cells (21). Four proteins, each with different functions within the lung, constitute the protein portion of surfactant. Surfactant protein B (SP-B) and SP-C are hydrophobic cell surface proteins that serve to reduce the surface tension at the alveolar air-liquid interface and thereby prevent alveolar collapse during normal breathing (10). SP-A and SP-D are hydrophilic proteins that belong to the collectin family of proteins (24), which function in the innate host defense of the lung by binding to glycoconjugates and lipids present on many pathogens (12, 26). Binding of SP-A and SP-D to pathogens enhances phagocytosis and killing of the pathogens by resident macrophages (50, 62, 66). They also stimulate chemotaxis and generation of reactive oxidants, particularly in macrophages (50, 66). The importance of SP-A and SP-D in the innate immune response is further demonstrated by increased susceptibility to infection by viral and bacterial pathogens in transgenic mice with targeted disruption of these two genes (11, 36).
Human alveolar type II cells grown in primary culture express all four surfactant proteins (3). Previous studies have shown that infection of human alveolar type II cells in primary culture with RSV alters the expression of SP-A mRNA and SP-A protein (4); expression of SP-A mRNA increases approximately threefold. However, the level of cellular and secreted SP-A protein decreases (4). In human infants with severe RSV infections, bronchoalveolar lavage fluid was shown to have reduced levels of SP-A protein (33). These data suggest that viral infections can alter the innate protective ability of the lung. To begin an investigation of the mechanism by which SP-A protein homeostasis is altered during RSV infection, we infected the human NCI-H441 cells, a human bronchiolar epithelial cell line that expresses SP-A (6, 18), with RSV. Similar to observations in primary human alveolar type II cells, NCI-H441 cells show an increase in SP-A mRNA during RSV infection, but a decrease in the cellular and secreted SP-A protein. We undertook a systematic approach to examine SP-A mRNA processing and SP-A protein biosynthesis following RSV infection. While SP-A mRNA processing is unaffected by RSV infection, de novo SP-A protein levels are inhibited by a process involving reduced efficiency of SP-A mRNA translation.
MATERIALS AND METHODS
Cell culture.
The lung adenocarcinoma cell line NCI-H441 (18, 49) and HEp-2 (8, 43) were obtained from American Type Culture Collection (ATCC; HTB-174 and CCL-23, respectively). NCI-H441 cells produce SP-A protein, contain cytoplasmic structures that resemble those found in Clara cell granules, and have been used as a model system to study surfactant protein. Cells were maintained in RPMI-1640 media or MEM (MediaTech, Manassas, VA) containing 10% (vol/vol) fetal bovine serum (Atlas Biologicals, Fort Collins, CO) and 1% (vol/vol) penicillin/streptomycin (Invitrogen, Carlsbad, CA). All cell culture incubations were performed in a humidified incubator at 37°C with 5% CO2.
RSV infection and propagation.
The human RSV strain A2 (#VR-1540; ATCC, Manassas, VA) was used in all experiments. RSV was propagated in HEp-2 cells, and the RSV virions were sucrose purified as previously described (32). Titers for the purified RSV virions were determined by serial dilution and plaque assays using HEp-2 cells. The infectivity of NCI-H441 cells by RSV was also determined by immunostaining with an antibody to the fusion protein of RSV (Fitzgerald, Concord, MA). Regardless of the amount of RSV used, ∼30% of NCI-H441 cells showed positive staining. For infection experiments, the virus stock of RSV was diluted in serum-free culture medium to a multiplicity of infection (MOI) = 1.0, which is defined as the number of plaque-forming units (pfu) per number of HEp-2 cells. Before infection, NCI-H441 cells were plated at ∼80% confluency and allowed to grow overnight. Cells were exposed to RSV for 2 h, washed, and incubated for 24 h in serum-free RPMI medium. After incubation, cells and serum-free media were harvested for either RNA or protein and stored at −80°C until assayed. Control samples were prepared from uninfected NCI-H441 cultures that were processed in the same manner.
Isolation of cellular RNA.
Cytoplasmic and nuclear RNA was prepared by the guanidinium isothiocyanate procedure (7, 38). Briefly, cytoplasmic RNA was isolated by lysing cells in Nonidet P-40 lysis buffer (0.6% NP-40, 0.15 M NaCl, 10 mM Tris, pH 8.0, 0.1 mM EDTA). The lysed cells were centrifuged, the supernatant was layered over a 5.7 M CsCl gradient, and the CsCl gradient was subjected to ultracentrifugation at 42,000 rpm overnight. Following centrifugation, the pellet was resuspended in 1 mM sodium citrate, extracted two times with chloroform:butanol (4:1), and stored in ethanol. To prepare nuclear RNA, the pellet from the cytoplasmic RNA lysis was washed in DOC wash solution (0.5% deoxycholate, 0.6% NP-40, 0.15 M NaCl, 10 mM Tris, pH 8.0, 0.1 mM EDTA) followed by lysis of the pellet in guanidinium isothiocyanate buffer. Nuclear RNA was purified by ultracentrifugation and extraction as for the cytoplasmic fraction. Nuclear and cytoplasmic RNA was stored at −80°C until assayed.
Isolation of cellular proteins.
Proteins were isolated from cells as described previously (2). For total cellular protein, the cells were scraped into ice-cold lysis buffer [10 mM Tris, pH 7.6, 3 mM MgCl2, 40 mM KCl, 2 mM DTT, 5% glycerol, and 0.5% NP-40 containing a protease inhibitor cocktail tablet (11-836-170001; Roche Applied Science, Indianapolis, IN) per 10 ml]. After a freeze-thaw cycle, the cells were centrifuged at 600 g for 10 min to remove cellular debris, and the supernatant containing the cytosolic proteins was aliquoted. The protein concentration was determined using the BCA Protein Assay kit (#23235; Pierce Biotechnology, Rockford, IL) per instructions, and the protein aliquots were stored at −80°C. To prepare secreted protein, the serum-free RPMI media was harvested from the cells and centrifuged at 600 g for 10 min to remove cellular debris, and the supernatant containing the secreted proteins was aliquoted and stored at −80°C.
Immunoblot analysis of proteins.
Prepared culture medium (20 μl) and cellular protein (20 μg) was solubilized in an equal volume loading buffer (0.1 M Tris, pH 7.4, 50 μM DTT, 0.01% bromphenol blue, 2% SDS, and 10% glycerol) and boiled for 5 min. Proteins were resolved on denaturing 4–20% Tris-glycine polyacrylamide gels (#EC6025, Invitrogen). After protein resolution, gels were transferred to Trans-blot nitrocellulose paper (#162–0112; BioRad Laboratories, Hercules, CA) and incubated with rabbit anti-human SP-A antibody (#sc-13977 1:300 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) followed by peroxidase-conjugated goat anti-rabbit IgG (#A6154 1:16,000 dilution; Sigma Chemical, St. Louis, MO). The immune complexes were detected with the ECL Plus Western Blotting Detection System (#RPN2135; Amersham Biosciences, Piscataway, NJ) and quantified on a Storm 840 Phosphorimager (GE Healthcare, Piscataway, NJ). β-actin was detected similarly using mouse anti-xenopus antibody (#ab6276 1:5,000 dilution; Abcam, Cambridge, MA) followed by peroxidase-conjugated rabbit anti-mouse IgG (#ab6728 1:160,000 dilution, Abcam).
Northern analysis.
Cesium chloride-purified nuclear and cytoplasmic RNA was electrophoresed, transferred to nitrocellulose, and probed using a 32P-labeled rabbit SP-A cDNA, rabbit cyclophilin cDNA, or U6 snRNA cDNA. The radioactive signal was quantified on a Storm 840 Phosphorimager (GE Healthcare).
Polysome preparations.
Control and RSV-infected cells were harvested 24 h after infection by trypsin digestion and washed two times with cold PBS. The cells were then lysed for 5 min on ice in 1 ml of special NP-40 lysis buffer (50 mM Tris·HCl, pH 8.0, 100 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 200 units of RNAsin). The lysed cells were spun at 3,000 g for 2 min at 4°C, and the lysate was transferred to new tubes containing 13.3 μl 50 mg/ml heparin, 15 μl 10 mg/ml cyclohexamide, 20 μl 1 M DTT, and 10 μl 0.1 M PMSF. The lysates were spun at 11,000 rpm for 5 min, and the supernatant was loaded on top of a 15–40% sucrose gradient. The polysomes were separated by ultracentrifugation for 2 h at 38,000 rpm and 4°C followed by slow deceleration. Immediately following ultracentrifugation, 0.5-ml aliquots were harvested from the top of the gradient, in a cold room. To each aliquot, 5 μl of 10 mg/ml proteinase K and 15 μl of 20% SDS was added, and the aliquots incubated for 15 min at 37°C. Each aliquot was extracted once with phenol:chloroform, once with chloroform:isoamyl alcohol, and stored in ethanol at −80°C.
Pulse-chase labeling of cellular proteins.
At 18 h post-RSV infection, NCI-H441 cells were placed in methionine and cysteine-free RPMI media (R7513, Sigma Chemical) for 2 h to starve the cells. Next, the cells were pulsed for 2 h with 35S methionine and cysteine (NEG772002MC; Perkin-Elmer, Waltham, MA) followed by a chase with serum-free media. The cells were then harvested for protein at 2, 4, and 6 h.
Immunoprecipitation of proteins.
Equal amounts of the harvested proteins were immunoprecipitated with protein A-sepharose (PA50-00-0002; Rockland Immunochemicals, Gilbertsville, PA) bound antibody against SP-A protein, and the immunoprecipitated proteins were resolved on 4–20% Tris-glycine polyacrylamide gels (EC6025; Invitrogen, Carlsbad, CA). The gels containing radiolabeled protein were dried, and the immunoprecipitated proteins were visualized by autoradiography. The gels containing unlabeled protein were transferred to Trans-blot nitrocellulose paper (162-0112; BioRad Laboratories, Hercules, CA) and incubated with rabbit anti-human ubiquitin antibody (A-100 1:1,000 dilution; Boston Biochem, Cambridge, MA) followed by peroxidase-conjugated goat anti-rabbit IgG (A6154, 1:16,000 dilution, Sigma).
Determination of proteasome activity.
Activity of the 20S proteasome was determined using the 20S Proteasome Assay Kit (K-900, Boston Biochem). Briefly, varying concentrations of cellular protein from NCI-H441 cells treated in the absence or presence of RSV were incubated with the fluorogenic substrate Suc-Leu-Leu-Val-Try-aminomethylcoumarin (AMC) at 37°C (A9891, Sigma). The hydrolytic release of free AMC by the cellular 20S proteasome was measured over time using a Fluoroskan Ascent fluorimeter (Ex380 nm; Em460 nm) (Thermo Scientific). To quantitate 20S proteasome activity, a standard curve was generated using AMC and purified 20S proteasome.
Interferon-γ treatment.
NCI-H441 cells were plated at ∼80% confluency and allowed to grow overnight. Each plate was washed three times with Hanks' buffered salt solution. RPMI media without serum and antibiotic was added to each plate. Interferon-γ (285-1F; R&D Systems, Minneapolis, MN) was added to the treated plates at a concentration of 30 ng/ml. After 24-h incubation, cellular lysates and media were harvested.
Statistical analysis.
Statistical analysis of infected samples compared with uninfected samples was performed by t-test or paired t-test after normalization.
RESULTS
RSV infection alters mRNA steady-state levels.
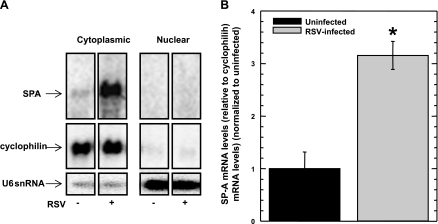
We have previously shown that RSV infection of human type II epithelial cells in primary culture from isolated fetal lung explants increases the SP-A mRNA steady-state levels approximately threefold (4). While isolated human type II epithelial cells from fetal lung explants are necessary for many studies, the isolation of primary type II cells is technically challenging. Therefore, a lung adenocarcinoma cell line, NCI-H441, which expresses both SP-A genes endogenously [specifically, the 6A4 isoform of SP-A1 and the 1A5 isoform of SP-A2 (27)], was used as a convenient in vitro system to study the effect that RSV infection exerts on the SP-A mRNA level and the SP-A protein level. NCI-H441 cells were exposed to RSV at a MOI of 1 for 2 h, and the cells harvested 24 h later. Cytoplasmic mRNA fractions were isolated, and the SP-A mRNA levels were determined by Northern blot analysis using a probe directed against the SP-A coding sequence. To control for mRNA loading, the level of the endogenous cyclophilin A mRNA was determined by Northern blot analysis. The results of a representative blot are shown in Fig. 1A. Shown in Fig. 1B is a graph that represents the normalized SP-A mRNA levels in uninfected and RSV-infected NCI-H441 cells. Similar to what was previously reported with isolated human type II epithelial cells from fetal lung explants, at 24 h post-RSV infection, the steady-state level of SP-A mRNA from infected NCI-H441 cells increases approximately threefold compared with SP-A mRNA steady-state levels from uninfected NCI-H441 cells. These results indicate that NCI-H441 cells can be infected by RSV, which alters the steady-state level of SP-A mRNA in a similar way to that observed with isolated human type II epithelial cells.
Fig. 1.
Steady-state SP-A mRNA levels in NCI-H441 cells increase in the presence of respiratory syncytial virus (RSV) infection, but SP-A mRNA processing or nuclear export is not altered. NCI-H441 cells were treated in the absence (−) or presence (+) of RSV (MOI = 1) for 2 h. At 24 h post-RSV infection, the nuclear and cytoplasmic fractions of the cells were isolated, after which RNA was harvested from the fractions and equal amounts were subjected to Northern analysis. A: representative autoradiographs showing steady-state levels of SP-A mRNA, cyclophilin A mRNA, and nucleus-specific U6 snRNA. Shown are individual lanes from a single gel. B: graphical representation of steady-state levels of cytoplasmic SP-A mRNA (relative to cyclophilin A mRNA levels) with levels from uninfected cells normalized as 1. Experiments were performed in triplicate (n = 6). *Significance by t-test (P < 0.01).
SP-A mRNA is appropriately processed and transported from the nucleus.
To determine whether SP-A mRNA transcribed during RSV infection is appropriately spliced and polyadenylated, Northern blot analysis was performed to characterize the size of the SP-A mRNA. From the results shown in Fig. 1A, only one species of SP-A mRNA from both uninfected and RSV-infected NCI-H441 cells is observed, suggesting that SP-A mRNA is appropriately spliced and polyadenylated, and is therefore capable of being translated. Although the SP-A mRNA is appropriately processed during RSV infection, export of the SP-A mRNA from the nucleus to the cytoplasm may be inhibited. Nuclear RNA from both uninfected and RSV-infected NCI-H441 cells were prepared and subjected to Northern analysis to determine whether the SP-A mRNA is sequestered within the nucleus. As seen in Fig. 1A, very little SP-A mRNA was observed within the nuclear fraction of NCI-H441 cells in the absence or presence of RSV infection. Similarly, the cyclophilin housekeeping gene used as a control was not observed within the nuclear fraction. To control for the possibility that the lack of SP-A mRNA in the nuclear fraction was a result of nuclear RNA degradation, the blot was stripped and probed for the nucleus-specific U6 snRNA. As shown in Fig. 1A, the U6 snRNA was robustly expressed within the nuclear fraction, suggesting a lack of RSV-induced nonspecific RNA degradation in the nucleus. These results indicate that RSV has no effect on processing, maturation, or transport of SP-A mRNA to the cytoplasm.
RSV infection decreases steady-state SP-A protein levels.
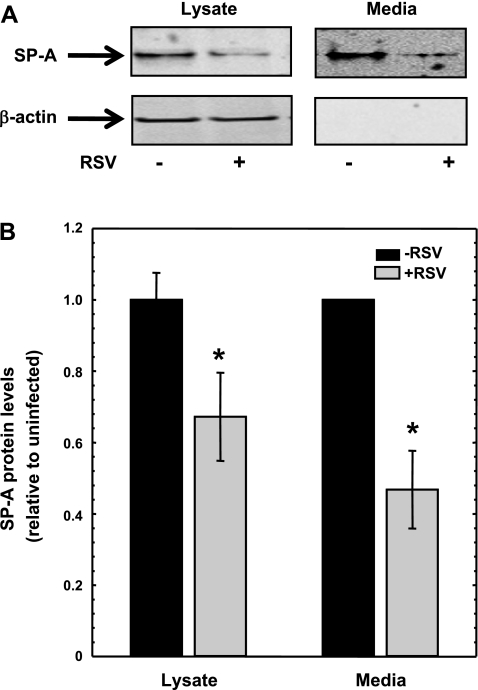
Using isolated human type II epithelial cells from fetal lung explants, we have previously shown that RSV infection increased the SP-A mRNA levels, whereas the SP-A protein levels decreased. The decrease in SP-A protein levels was observed in the cellular lysates and in the media from RSV-infected human type II epithelial cells. To further confirm whether NCI-H441 cells could be used as an in vitro model system, the steady-state level of SP-A protein from NCI-H441 cells incubated in the absence and presence of RSV was determined. NCI-H441 cells were infected for 2 h with RSV (MOI = 1), and the media and cell lysates were harvested for protein at 24 h. Figure 2A shows a typical immunoblot probed with an antibody specific for SP-A protein. To control for equal protein loading, the blots were subsequently stripped and incubated with an antibody against β-actin. At 24 h post-RSV infection, the levels of SP-A protein within the NCI-H441 cells were decreased ∼40% compared with uninfected NCI-H441 cells (Fig. 2, A and B). Because SP-A is a secreted protein, the steady-state level of SP-A protein residing in the media was determined. At 24 h postinfection, SP-A protein steady-state levels were ∼60% less in the media from RSV-infected NCI-H441 cells compared with the media from uninfected NCI-H441 cells (Fig. 2B). Since the NCI-H441 cell line recapitulates the effect of RSV infection on isolated human type II epithelial cells with regards to SP-A mRNA and protein levels, this cell line was used to address the effect of RSV infection on SP-A protein biosynthesis.
Fig. 2.
Steady-state cellular and secreted SP-A levels in NCI-H441 cells decrease in the presence of RSV infection. NCI-H441 cells were treated in the absence (−) or presence (+) of RSV (MOI = 1) for 2 h. At 24 h post-RSV infection, cellular protein and media were isolated and equal amounts were subjected to Western analysis. A: representative immunoblots showing steady-state levels of SP-A protein and β-actin protein from lysates and media. The lysates and media were run on separate gels. B: graphical representation of steady-state levels of the SP-A protein from lysates and media. Equal amounts of lysates were normalized to β-actin protein. The average of SP-A protein in the absence of RSV is set to 1. The normalized average of SP-A protein in the presence of RSV is shown as a ratio relative to normalized SP-A protein level in the absence of RSV. Equal volumes of media from H441 cells incubated in the absence or presence of RSV were quantified for SP-A protein. For each experiment, the level of SP-A protein in the absence of RSV is set to 1. The level of SP-A protein in the presence of RSV is shown as a ratio relative to SP-A protein level in the absence of RSV. Experiments were performed in triplicate (n = 10). *Significance by t-test (P < 0.01) for cell lysates and by the paired t-test (P < 0.01) for media.
SP-A protein synthesis rate is not saturated in NCI-H441 cells.
The lack of increased SP-A protein synthesis in the presence of an increase in the steady-state level of SP-A mRNA may result from a saturation in the SP-A protein synthesis rate. Previously published results using the same NCI-H441 cells have shown that the herbal medicine Baicalin can increase the SP-A mRNA and protein level ∼1.7-fold (9). In a separate report, the level of the SP-A mRNA was shown to increase approximately twofold when NCI-H441 cells were treated with IFN-γ (57). However, in this case, the SP-A protein level was not reported. We therefore treated NCI-H441 cells with IFN-γ and measured the level of the SP-A protein in the lysate and in the media. As shown in Fig. 3, IFN-γ is able to increase the intracellular and secreted SP-A protein greater than twofold. These results would suggest that SP-A protein synthesis is not limiting in the NCI-H441 cell line and that the decrease in SP-A protein synthesis observed during RSV infection is specific to the infection.
Fig. 3.
IFN-γ increases SP-A protein levels in NCI-H441 cells. NCI-H441 cells were treated in the absence (−) or presence (+) of IFN-γ for 20 h. Media and cellular lysates were harvested and subjected to Western blot analysis for SP-A protein. For normalization of the cellular SP-A protein, the blots were reprobed with an antibody against β-actin. A representative blot is shown indicating that IFN-γ treatment increases the level of SP-A protein both intracellularly and extracellularly. The lysates and media were run on separate gels.
SP-A mRNA is located in lighter polysome fractions during RSV infection.
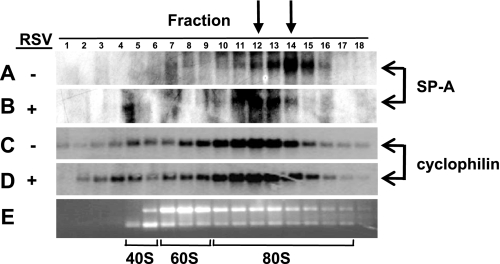
Having observed that the SP-A mRNA is appropriately processed and exported to the cytoplasm during RSV infection, the possibility that RSV infection inhibits translation of the SP-A mRNA was determined. The amount of ribosome loading, or the polysome profile, onto the SP-A mRNA in the absence and presence of RSV infection was assessed. Total cellular mRNA from uninfected and RSV-infected NCI-H441 cells was harvested and passed over a sucrose gradient by ultrahigh centrifugation, and aliquots of mRNA were collected from the top of the gradient. Species of mRNA that contain fewer translating ribosomes would be observed in the lower-numbered fractions, and species of mRNA contain higher numbers of translating ribosomes would be observed in the higher-numbered fractions. A portion of the mRNA from each aliquot was subject to Northern blot analysis and indicates that the SP-A mRNA from RSV-infected NCI-H441 cells is consistently observed in lighter polysome fractions, with a shift of approximately two fractions (Fig. 4, A and B). Since host cell proteins are often sequestered by the virus for viral replication and gene expression, the shift in ribosome loading seen with the SP-A mRNA during RSV infection may be a global effect that would be observed with other host cell mRNAs. To address whether RSV infection results in a global inhibition of mRNA translation, the polysome profile of the cyclophilin A mRNA was also determined. A representative example of the cyclophilin A mRNA polysome profile is shown in Fig. 4, C and D. In contrast to what was observed with the SP-A mRNA, RSV infection does not lead to a shift in ribosome loading of the cyclophilin A mRNA and suggests that the decrease in ribosome loading during RSV infection is specific to a subset of host mRNAs.
Fig. 4.
RSV infection alters the polysome profile of the SP-A mRNA. NCI-H441 cells were treated in the absence (−) or presence (+) of RSV (MOI = 1) for 2 h. At 24 h post-RSV infection, RNA was harvested from the cells and equilibrated over a sucrose gradient as described in materials and methods, and aliquots were removed from the top of the gradient. Equal volumes of RNA were subjected to Northern analysis. A: SP-A mRNA levels in the fractions isolated from uninfected cells. B: SP-A mRNA levels in the fractions isolated from RSV-infected cells. C: cyclophilin A mRNA levels in the fractions isolated from uninfected cells. D: cyclophilin A mRNA levels in the fractions isolated from RSV-infected cells. E: agarose gel electrophoresis and ethidium bromide stain of total RNA from each fraction to show the 40S, 60S, and 80S ribosomal bands. Arrows at top indicate fraction 14 of uninfected cells and fraction 12 of RSV-infected cells containing the highest levels of SP-A mRNA.
RSV infection does not alter the amount of newly synthesized SP-A protein.
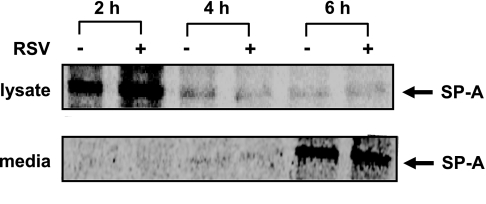
The small but reproducible shift of the SP-A mRNA to lighter polysome fractions during RSV infection would be predicted to result in a decrease in the amount of newly synthesized SP-A protein. To determine the rate of SP-A protein synthesis in the absence and presence of RSV, a pulse-chase analysis followed by immunoprecipitation, as described in materials and methods, was performed. Cellular SP-A protein can be easily observed at 2 h post-chase with decreasing levels of newly synthesized SP-A proteins at later time points (Fig. 5). Visually and by quantitation of the radioactive protein bands, no measurable change in the amount of newly synthesized SP-A protein in the absence or presence of RSV infection was observed. Simultaneously, the amount of newly synthesized protein that was exported to the media was analyzed and quantitated as for the protein lysates. Newly synthesized SP-A protein was not observed in the media until 4 h post-chase with an increase in the amount of newly synthesized SP-A protein secreted into the media at longer time points (Fig. 5). Similar to the results obtained with the cellular lysates, there was no measurable change in the amount of newly synthesized SP-A protein secreted into the media in the absence or presence of RSV infection. Surprisingly, no change in de novo SP-A protein synthesis was observed during RSV infection; however, the fact that RSV infection leads to a threefold increase in SP-A mRNA levels and a decrease in polysome loading suggests that SP-A mRNA translation is affected.
Fig. 5.
De novo SP-A protein synthesis and secretion is not affected by RSV infection. Eighteen hours post-RSV infection, NCI-H441 cells were incubated in the presence of [35S]methionine for 2 h. The cells were washed, and cellular and secreted protein was harvested at various time points. Equal amounts of protein were immunoprecipitated with an antibody specific for SP-A, and the immunoprecipitated protein was separated by SDS-PAGE. Shown are representative autoradiographs of the labeled proteins. Newly synthesized SP-A is not significantly increased in RSV-infected cells despite a 3-fold increase in SP-A mRNA.
SP-A protein is ubiquitinated, suggesting that it is a target for the proteasome.
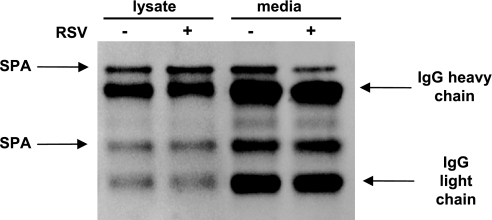
Since RSV infection leads to a decrease in the steady-state level of SP-A protein and no measurable increase in de novo SP-A protein synthesis despite a threefold increase in SP-A mRNA, the SP-A protein may be an RSV-induced target for degradation. The principal mechanism for protein degradation within the cell is the ubiquitin proteasome pathway. The proteasome is a multimeric enzyme that selectively degrades proteins that contain polyubiquitin chains (46). To determine whether the SP-A protein could be a target for catabolism by the proteasome, cellular lysates and media harvested from NCI-H441 cells incubated in the absence or presence of RSV were immunoprecipitated with an antibody against SP-A. The immunoprecipitated proteins were separated on a denaturing gel and immunoblotted with an antibody against ubiquitin. As shown in Fig. 6, a portion of the SP-A protein is ubiquitinated. Although both cellular and secreted SP-A protein is ubiquitinated, in neither case does RSV infection result in a measurable increase in the amount of ubiquitinated SP-A protein.
Fig. 6.
Cellular and secreted SP-A is normally ubiquitinated, and RSV infection does not alter the level of ubiquitination. NCI-H441 cells were treated in the absence (−) or presence (+) of RSV (MOI = 1) for 2 h. At 24 h post-RSV infection, cellular protein and media were isolated, and SP-A protein from equal volumes of lysates or media were immunoprecipitated with an antibody to SP-A protein. The immunoprecipitated SP-A protein was subjected to Western analysis using an antibody specific for ubiquitin. Shown is the resulting immunoblot with the positions of ubiquitinated SP-A, IgG heavy chain, and IgG light chain indicated by arrows.
RSV infection does not increase proteasome activity within the cell.
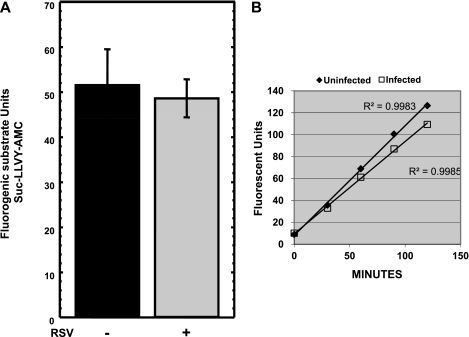
While RSV infection does not alter the amount of SP-A protein that is ubiquitinated, RSV infection may increase the amount or the activity of the proteasome within the cell, leading to a decrease in the steady-state levels of SP-A protein. The proteolytic activity of the endogenous proteosome from NCI-H441 cells on the fluorogenic substrate Suc-LLVY-AMC (7-amino-4-methylcoumarin) was determined. The proteosome is able to cleave the tyrosine residue releasing the AMC fluorophore, and the amount of fluorescent units released over time is measured on a fluorometer. Equal amounts of protein from NCI-H441 cells incubated in the absence or presence of RSV was incubated for 60 min at 37°C with the fluorogenic substrate. In repeat experiments, the amount of fluorescent units released by the proteasome was unchanged in the absence or presence of RSV infection (Fig. 7A). Measuring proteasome activity at a single time point might represent complete proteolysis of the substrate (end point), although the rate of proteolysis by the proteasome from uninfected and RSV-infected NCI-H441 cells may be different. Therefore, the kinetic activity of the proteosome in the lysates from NCI-H441 cells incubated in the absence and presence of RSV was determined. The increase in fluorescent units was measured at 30-min intervals over a 120-min time period (Fig. 7B). When measured over time, RSV infection does not increase the activity of the cellular proteasome and may cause a slight decrease in proteasome activity. Thus, SP-A protein is subject to ubiquitination and proteolysis by the proteasome; however, the RSV-induced decrease in steady-state SP-A protein levels is likely to result from degradation of the SP-A protein by a nonproteasome-dependent pathway.
Fig. 7.
RSV infection does not increase proteasome activity or the kinetics of proteasome activation. A: NCI-H441 cells were treated in the absence or presence of RSV, and the cells were harvested for protein 24 h post-RSV infection. Equal amounts of protein from uninfected and infected cells were incubated with the fluorogenic substrate Suc-LLVY-AMC for 60 min, and changes in fluorescence were measured. Shown is a graph of at least 3 independent experiments. B: NCI-H441 cells were treated in the absence or presence of RSV, and the cells were harvested for protein 24 h post-RSV infection. Equal amounts of protein from uninfected and infected cells were incubated with the fluorogenic substrate Suc-LLVY-AMC, and the increase in fluorescent units was measured at 30-min intervals. Shown is a representative graph measuring the kinetics of proteasome activity over time.
DISCUSSION
When viruses invade mammalian cells, there is a race between the virus and the mammalian immune system. The virus must work quickly to replicate before the host's immune system can either shut down viral replication or destroy the virally infected cells. The innate immune response, which is thought to be an ancient defense mechanism, is our nonspecific first line of defense against invading pathogens and functions to recruit immune cells to the site of inflammation, to activate the complement cascade, and to activate the more specific adaptive immune response. SP-A protein, a member of the collectin family of proteins involved in the innate immune response, is an important player in both normal breathing and the prevention of disease by invading pathogens. Consistent with its function in the innate immune response, SP-A protein can target some gram-positive and gram-negative bacteria for clearance by phagocytic cells through opsonization of the pathogen via the SP-A carbohydrate recognition domain (CRD). In addition, SP-A can bind to alveolar macrophages with high affinity and promote chemotaxis and phagocytosis of microbial species (41, 42). Importantly, SP-A has been shown to bind through its CRD to the glycoprotein of RSV thereby inhibiting entry of the virus into lung epithelial cells (19).
Although SP-A and other surfactant proteins aid in normal respiration, respiratory infections due to colonization of the lung epithelium by pathogenic organisms still occur. In children, RSV is the most common cause of viral lower respiratory tract infections, and, in the U.S., results in the hospitalization of more than 120,000 young children yearly (59, 63). The mortality rate of those hospitalized with RSV infection is ∼2% (60). While essentially all children are infected with RSV by 2 years of age, less than 2% of children infected with RSV require hospitalization (59). Since 98% of children are able to clear RSV infections on their own, a decreased or defective innate immune response in some children could explain the more severe RSV infections.
Because of the importance SP-A plays in the innate immune defense of the lung, pathogens may attempt to evade the innate immune response by altering SP-A homeostasis. Indeed, evidence by Alcorn et al. (4) has shown that the level of the SP-A mRNA increases approximately threefold during RSV infection of isolated human type II epithelial cells. However, the level of secreted and cellular SP-A protein decreases during RSV infection (4). These data would suggest that, in some cases, RSV can alter or inhibit SP-A homeostasis.
We have systematically investigated the effect of RSV infection on each step of RNA processing and protein translation. In this study, we chose to use the NCI-H441 lung epithelial cell line rather than isolated primary human type II epithelial cells, which are more expensive and more difficult to maintain in culture. Using the NCI-H441 cell line, we were able to recapitulate the increase in SP-A mRNA and corresponding decrease in SP-A protein that was previously reported using pulmonary alveolar epithelial type II cells in primary culture. We first investigated whether RSV infection could alter processing of the SP-A pre-mRNA, as some viral proteins have been shown to alter processing of host pre-mRNA. For example, the non-structural protein (NS-1) of influenza virus can inhibit the splicing of host pre-mRNA by binding to a region within the spliceosomal U6 snRNA (16, 55). This same viral protein also inhibits the export of poly(A)-containing host mRNA from the nucleus by binding to the poly(A) tail (5, 16, 54). The blockage of host mRNA export could increase the availability of ribosomes for translation of viral mRNA while simultaneously inhibiting protein expression of host mRNAs. Consequently, the virus could downregulate proteins involved in the immune response to provide a more favorable environment for viral replication. However, in the case of RSV infection, we found that neither splicing nor polyadenylation of the SP-A pre-mRNA is inhibited. Furthermore, export of the SP-A mRNA from the nucleus to the cytoplasm is not altered by RSV infection, and the SP-A mRNA appears to be suitable for translation.
Viruses not only alter host mRNA processing, but in some cases, virally encoded proteins can interfere with translation. Indeed, the capsid protein of rubella virus has been shown to bind to the host cell poly(A)-binding protein (PABP) (29). PABP interacts with other translation initiation factors forming a complex that promotes circularization of mRNAs (3, 30, 31). The rubella capsid protein binds to a region of PABP known to interact with translational regulators (29) blocking circularization of the mRNA. By sequestering PABP, translation of cellular mRNAs could be inhibited, resulting in decreased protein production. Therefore, we investigated whether RSV infection could inhibit translation of the SP-A mRNA and thus account for the decrease in SP-A protein that we observed. By polysome analysis, we observed a slight but reproducible shift to lighter polysome fractions of the SP-A mRNA in the presence of RSV. Additionally, pulse/chase analysis indicates that newly synthesized SP-A protein is similar in the absence or presence of RSV infection despite the approximate threefold increase in SP-A mRNA. Our treatment of NCI-H441 cells with IFN-γ resulted in an increase in SP-A protein. These results along with the previously reported ability of IFN-γ to increase SP-A mRNA indicates that these cells are not endogenously saturated in their ability to produce SP-A protein. The presence of increased mRNA but no measurable increase in de novo SP-A protein synthesis suggests that RSV infection may alter the cell's ability to maximally translate the SP-A mRNA. An alternative explanation for the similar de novo SP-A protein synthesis in the absence or presence of RSV is that a SP-A protein-specific protease is induced during RSV infection. In this scenario, RSV infection induces a protease that degrades a portion of the newly synthesized SP-A protein.
Here we show that RSV may attempt to evade the immune response by altering expression levels of the innate immune protein SP-A. We provide evidence that after infection with RSV, although there is a threefold increase in SP-A mRNA, there is no increase in the amount of de novo SP-A protein and that ribosome loading onto the SP-A mRNA is not as efficient. One explanation for these results is that microRNAs, induced after RSV infection, may bind to the SP-A mRNA and inhibit SP-A mRNA translation. Indeed, in Caenorhabditis elegans miRNA directed against the lin-14 and lin-28 mRNA have been shown to repress protein production after translation initiation (51, 58). Additionally, in mammalian cells, the c-Myc transcription factor was shown to upregulate the transcription of E2F1 mRNA and the expression of two miRNAs directed against the E2F1 mRNA (48). In parallel with the results we observe after RSV infection where SP-A mRNA is increased but there is no increase in SP-A protein synthesis, the c-Myc protein can both activate transcription and limit translation of the E2F1 mRNA. Although we propose that RSV infection may limit translation of the SP-A mRNA, there are likely to be additional RSV-induced avenues to control SP-A protein production. It is interesting that equal amounts of SP-A protein are produced in the absence or presence of RSV infection, yet the steady-state levels of cellular and secreted SP-A protein are decreased. This decrease in protein level is not likely regulated by the endogenous ubiquitin-proteasome pathway but upon a specific RSV-induced protease located within cellular lysate or secreted into the media. We have addressed the possibility of an RSV-induced secreted protease by incubating media from RSV-infected cells with the media from uninfected cells. Even after 24-h incubation, we observed no additional degradation of the SP-A protein from uninfected media. It is therefore unlikely that a secreted protease is responsible for the decrease in SP-A protein observed during RSV infection. However, we cannot rule out the possibility that a cellular SP-A-specific protease is responsible for a decrease in SP-A protein steady-state levels.
Using the RegRNA program (28), we probed both human SP-A genes for putative translational regulatory elements within the 3′UTR. Numerous miRNA binding sites were identified. In addition, both SP-A mRNAs contain several gamma-interferon-activated inhibitor of ceruloplasmin mRNA translation (GAIT) elements. GAIT elements form specific secondary structures and are bound by a trans-acting factor. IFN-γ induces expression of ceruloplasmin mRNA and protein, which contains ferroxidase activity, in activated macrophages/monocytes. However, this induced expression is dramatically inhibited ∼16 h after induction, presumably as a means to control the immune response (39, 40, 56). It is possible that the putative GAIT elements within the 3′UTR of the SP-A mRNAs may also serve to regulate expression of the SP-A protein during the immune response. Although we observed no increase in IFN-γ production during RSV infection (unpublished results), other cytokines or signal transduction pathways may activate expression of trans-acting factors that would bind to the SP-A mRNA and modulate translation. In addition, sequences within the 5′UTR may also regulate SP-A mRNA translation during RSV infection. The 5′UTR of the SP-A mRNA has been shown to undergo alternative splicing of untranslated exons. Furthermore, the 5′UTR splicing pattern observed with the SP-A2 gene resulted in a higher translational efficiency (65). It is therefore possible that RSV infection changes the splicing pattern within the SP-A 5′UTR to a less efficient pattern resulting in a decrease in SP-A protein. Future experiments will be directed towards understanding whether elements within the SP-A mRNA 5′UTR, 3′UTR, and/or coding region may alter translation during RSV infection and towards the identification of putative SP-A protein-specific proteases.
NOTE ADDED IN PROOF
The presentation of Figs. 1, 2, and 3 has been modified from the original version posted in Articles in Press. In Fig. 1A, individual lanes from a single gel have been separated into individual panels to indicate derivation from multiple images. In Figs. 2A and 3, individual lysates and media from separate gels have been separated into individual panels to indicate derivation from multiple images. The results and conclusions of this study stand. These revised figures appear here in the final published version of the manuscript.
GRANTS
This study was supported by National Institutes of Health Grants HL-68116 (J. L. Alcorn) and ES-012927 (G. N. Colasurdo and J. L. Alcorn).
Supplementary Material
REFERENCES
- 1.Abramson JS, Wheeler JG. Virus-induced neutrophil dysfunction: role in the pathogenesis of bacterial infections. Pediatr Infect Dis J 13: 643–652, 1994 [PubMed] [Google Scholar]
- 2.Al-Darraji AM, Cutlip RC, Lehmkuhl HD, Graham DL, Kluge JP, Frank GH. Experimental infection of lambs with bovine respiratory syncytial virus and Pasteurella haemolytica: clinical and microbiologic studies. Am J Vet Res 43: 236–240, 1982 [PubMed] [Google Scholar]
- 3.Alcorn JL, Smith ME, Smith JF, Margraf LR, Mendelson CR. Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am J Respir Cell Mol Biol 17: 672–682, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Alcorn JL, Stark JM, Chiappetta CL, Jenkins G, Colasurdo GN. Effects of RSV infection on pulmonary surfactant protein SP-A in cultured human type II cells: contrasting consequences on SP-A mRNA and protein. Am J Physiol Lung Cell Mol Physiol 289: L1113–L1122, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Caplen FV, Nemeroff ME, Qiu Y, Krug RM. Nucleocytoplasmic transport: the influenza virus NS1 protein regulates the transport of spliced NS2 mRNA and its precursor NS1 mRNA. Genes Dev 6: 255–267, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Brower M, Carney DN, Oie HK, Gazdar AF, Minna JD. Growth of cell lines and clinical specimens of human non-small cell lung cancer in a serum-free defined medium. Cancer Res 46: 798–806, 1986 [PubMed] [Google Scholar]
- 7.Carter MS, Li S, Wilkinson MF. A splicing-dependent regulatory mechanism that detects translation signals. EMBO J 15: 5965–5975, 1996 [PMC free article] [PubMed] [Google Scholar]
- 8.Chen TR. Re-evaluation of HeLa, HeLa S3, and HEp-2 karyotypes. Cytogenet Cell Genet 48: 19–24, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Cheng KT, Huang YC, Lin YS, Su B, Chen CM. Effects of baicalin on the gene expression of surfactant protein A (SP-A) in lung adenocarcinoma cell line H441. Planta Med 69: 300–304, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Clements J, King R. The Biochemical Basis of Pulmonary Function. New York: Dekker, 1976 [Google Scholar]
- 11.Crouch E, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol 63: 521–554, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Crouch EC. Collectins and pulmonary host defense. Am J Respir Cell Mol Biol 19: 177–201, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Denny FW, Clyde WA., Jr Acute lower respiratory tract infections in nonhospitalized children. J Pediatr 108: 635–646, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Domachowske JB, Rosenberg HF. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev 12: 298–309, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Englund JA, Sullivan CJ, Jordan MC, Dehner LP, Vercellotti GM, Balfour HH., Jr Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med 109: 203–208, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Fortes P, Beloso A, Ortin J. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J 13: 704–712, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foy HM, Cooney MK, Maletzky AJ, Grayston JT. Incidence and etiology of pneumonia, croup and bronchiolitis in preschool children belonging to a prepaid medical care group over a four-year period. Am J Epidemiol 97: 80–92, 1973 [DOI] [PubMed] [Google Scholar]
- 18.Gazdar AF, Linnoila RI, Kurita Y, Oie HK, Mulshine JL, Clark JC, Whitsett JA. Peripheral airway cell differentiation in human lung cancer cell lines. Cancer Res 50: 5481–5487, 1990 [PubMed] [Google Scholar]
- 19.Ghildyal R, Hartley C, Varrasso A, Meanger J, Voelker DR, Anders EM, Mills J. Surfactant protein A binds to the fusion glycoprotein of respiratory syncytial virus and neutralizes virion infectivity. J Infect Dis 180: 2009–2013, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Griffin MR, Coffey CS, Neuzil KM, Mitchel EF, Jr, Wright PF, Edwards KM. Winter viruses: influenza- and respiratory syncytial virus-related morbidity in chronic lung disease. Arch Intern Med 162: 1229–1236, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Haagsman HP, Diemel RV. Surfactant-associated proteins: functions and structural variation. Comp Biochem Physiol A Mol Integr Physiol 129: 91–108, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Hacking D, Hull J. Respiratory syncytial virus–viral biology and the host response. J Infect 45: 18–24, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Hall CB, Douglas RG, Jr, Geiman JM. Quantitative shedding patterns of respiratory syncytial virus in infants. J Infect Dis 132: 151–156, 1975 [DOI] [PubMed] [Google Scholar]
- 24.Hawgood S, Poulain FR. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol 63: 495–519, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol 133: 1135–1151, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol 21: 547–578, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Hoover RR, Floros J. SP-A 3′-UTR is involved in the glucocorticoid inhibition of human SP-A gene expression. Am J Physiol Lung Cell Mol Physiol 276: L917–L924, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Huang HY, Chien CH, Jen KH, Huang HD. RegRNA: an integrated web server for identifying regulatory RNA motifs and elements. Nucleic Acids Res 34: W429–W434, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilkow CS, Mancinelli V, Beatch MD, Hobman TC. Rubella virus capsid protein interacts with poly(A)-binding protein and inhibits translation. J Virol 82: 4284–4294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imataka H, Gradi A, Sonenberg N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J 17: 7480–7489, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahvejian A, Svitkin YV, Sukarieh R, M'Boutchou MN, Sonenberg N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev 19: 104–113, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao YJ, Piedra PA, Larsen GL, Colasurdo GN. Induction and regulation of nitric oxide synthase in airway epithelial cells by respiratory syncytial virus. Am J Respir Crit Care Med 163: 532–539, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Kerr MH, Paton JY. Surfactant protein levels in severe respiratory syncytial virus infection. Am J Respir Crit Care Med 159: 1115–1118, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Korppi M, Koskela M, Jalonen E, Leinonen M. Serologically indicated pneumococcal respiratory infection in children. Scand J Infect Dis 24: 437–443, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Korppi M, Leinonen M, Koskela M, Makela PH, Launiala K. Bacterial coinfection in children hospitalized with respiratory syncytial virus infections. Pediatr Infect Dis J 8: 687–692, 1989 [DOI] [PubMed] [Google Scholar]
- 36.LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, Korfhagen TR. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol 165: 3934–3940, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Levine S, Klaiber-Franco R, Paradiso PR. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol 68: 2521–2524, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Li S, Leonard D, Wilkinson MF. T cell receptor (TCR) mini-gene mRNA expression regulated by nonsense codons: a nuclear-associated translation-like mechanism. J Exp Med 185: 985–992, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazumder B, Fox PL. Delayed translational silencing of ceruloplasmin transcript in gamma interferon-activated U937 monocytic cells: role of the 3′ untranslated region. Mol Cell Biol 19: 6898–6905, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazumder B, Mukhopadhyay CK, Prok A, Cathcart MK, Fox PL. Induction of ceruloplasmin synthesis by IFN-gamma in human monocytic cells. J Immunol 159: 1938–1944, 1997 [PubMed] [Google Scholar]
- 41.Mikerov AN, Umstead TM, Huang W, Liu W, Phelps DS, Floros J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 288: L150–L158, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Mikerov AN, Wang G, Umstead TM, Zacharatos M, Thomas NJ, Phelps DS, Floros J. Surfactant protein A2 (SP-A2) variants expressed in CHO cells stimulate phagocytosis of Pseudomonas aeruginosa more than do SP-A1 variants. Infect Immun 75: 1403–1412, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore AE, Sabachewsky L, Toolan HW. Culture characteristics of four permanent lines of human cancer cells. Cancer Res 15: 598–602, 1955 [PubMed] [Google Scholar]
- 44.Mufson MA, Krause HE, Mocega HE, Dawson FW. Viruses, Mycoplasma pneumoniae and bacteria associated with lower respiratory tract disease among infants. Am J Epidemiol 91: 192–202, 1970 [DOI] [PubMed] [Google Scholar]
- 45.Mufson MA, Levine HD, Wasil RE, Mocega-Gonzalez HE, Krause HE. Epidemiology of respiratory syncytial virus infection among infants and children in Chicago. Am J Epidemiol 98: 88–95, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteasome system. J Biosci 31: 137–155, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Nohynek H, Eskola J, Laine E, Halonen P, Ruutu P, Saikku P, Kleemola M, Leinonen M. The causes of hospital-treated acute lower respiratory tract infection in children. Am J Dis Child 145: 618–622, 1991 [DOI] [PubMed] [Google Scholar]
- 48.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 435: 839–843, 2005 [DOI] [PubMed] [Google Scholar]
- 49.O'Reilly MA, Gazdar AF, Clark JC, Pilot-Matias TJ, Wert SE, Hull WM, Whitsett JA. Glucocorticoids regulate surfactant protein synthesis in a pulmonary adenocarcinoma cell line. Am J Physiol Lung Cell Mol Physiol 257: L385–L392, 1989 [DOI] [PubMed] [Google Scholar]
- 50.O'Reilly P, Hickman-Davis JM, McArdle P, Young KR, Matalon S. The role of nitric oxide in lung innate immunity: modulation by surfactant protein-A. Mol Cell Biochem 234–235: 39–48, 2002 [PubMed] [Google Scholar]
- 51.Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol 216: 671–680, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Parrott RH, Kim HW, Arrobio JO, Hodes DS, Murphy BR, Brandt CD, Camargo E, Chanock RM. Epidemiology of respiratory syncytial virus infection in Washington, DC. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol 98: 289–300, 1973 [DOI] [PubMed] [Google Scholar]
- 53.Patel J, Faden H, Sharma S, Ogra PL. Effect of respiratory syncytial virus on adherence, colonization and immunity of non-typable Haemophilus influenzae: implications for otitis media. Int J Pediatr Otorhinolaryngol 23: 15–23, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Qiu Y, Krug RM. The influenza virus NS1 protein is a poly(A)-binding protein that inhibits nuclear export of mRNAs containing poly(A). J Virol 68: 2425–2432, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiu Y, Nemeroff M, Krug RM. The influenza virus NS1 protein binds to a specific region in human U6 snRNA and inhibits U6-U2 and U6-U4 snRNA interactions during splicing. RNA 1: 304–316, 1995 [PMC free article] [PubMed] [Google Scholar]
- 56.Sampath P, Mazumder B, Seshadri V, Fox PL. Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3′ untranslated region. Mol Cell Biol 23: 1509–1519, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scavo LM, Ertsey R, Gao BQ. Human surfactant proteins A1 and A2 are differentially regulated during development and by soluble factors. Am J Physiol Lung Cell Mol Physiol 275: L653–L669, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Seggerson K, Tang L, Moss EG. Two genetic circuits repress the Caenorhabditis elegans heterochronic gene lin-28 after translation initiation. Dev Biol 243: 215–225, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282: 1440–1446, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J Infect Dis 183: 16–22, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289: 179–186, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Tino MJ, Wright JR. Surfactant protein A stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 270: L677–L688, 1996 [DOI] [PubMed] [Google Scholar]
- 63.Tripp RA. Pathogenesis of respiratory syncytial virus infection. Viral Immunol 17: 165–181, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Walsh EE, Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol 47: 171–177, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang G, Guo X, Floros J. Differences in the translation efficiency and mRNA stability mediated by 5′-UTR splice variants of human SP-A1 and SP-A2 genes. Am J Physiol Lung Cell Mol Physiol 289: L497–L508, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Wright JR, Youmans DC. Pulmonary surfactant protein A stimulates chemotaxis of alveolar macrophage. Am J Physiol Lung Cell Mol Physiol 264: L338–L344, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.