Abstract
Pulmonary vascular endothelial cells express a variety of ion channels that mediate Ca2+ influx in response to diverse environmental stimuli. However, it is not clear whether Ca2+ influx from discrete ion channels is functionally coupled to specific outcomes. Thus we conducted a systematic study in mouse lung to address whether the α1G T-type Ca2+ channel and the transient receptor potential channel TRPV4 have discrete functional roles in pulmonary capillary endothelium. We used real-time fluorescence imaging for endothelial cytosolic Ca2+, immunohistochemistry to probe for surface expression of P-selectin, and the filtration coefficient to specifically measure lung endothelial permeability. We demonstrate that membrane depolarization via exposure of pulmonary vascular endothelium to a high-K+ perfusate induces Ca2+ entry into alveolar septal endothelial cells and exclusively leads to the surface expression of P-selectin. In contrast, Ca2+ entry in septal endothelium evoked by the selective TRPV4 activator 4α-phorbol-12,13-didecanoate (4α-PDD) specifically increases lung endothelial permeability without effect on P-selectin expression. Pharmacological blockade or knockout of α1G abolishes depolarization-induced Ca2+ entry and surface expression of P-selectin but does not prevent 4α-PDD-activated Ca2+ entry and the resultant increase in permeability. Conversely, blockade or knockout of TRPV4 specifically abolishes 4α-PDD-activated Ca2+ entry and the increase in permeability, while not impacting depolarization-induced Ca2+ entry and surface expression of P-selectin. We conclude that in alveolar septal capillaries Ca2+ entry through α1G and TRPV4 channels differentially and specifically regulates the transition of endothelial procoagulant phenotype and barrier integrity, respectively.
Keywords: T-type calcium channel, transient receptor potential vanilloid 4, vascular permeability
vascular endothelium serves a wide variety of physiological functions and is an active participant in vascular pathology (1, 2). In many circumstances, endothelial cell functions ranging from maintenance of endothelial barrier integrity, endo- and exocytosis, and hormone metabolism to cell proliferation, migration, and angiogenesis can be regulated by physiological transitions in cytosolic Ca2+ concentration ([Ca2+]i) (20, 30). Within the same cell, Ca2+ entry across the plasma membrane through Ca2+-permeable channels provides a specific link between a large array of extracellular stimuli and an equally diverse collection of cellular responses. While the intimate association between these channels and Ca2+-regulated targets is thought to provide one mechanism for selective targeting of Ca2+ entry, resulting in specific cellular responses (27), this scenario has not been explored rigorously in lung endothelium.
Endothelial cells exhibit a collection of assorted Ca2+ channels, including receptor- and store-operated Ca2+ channels and various Ca2+-permeable nonselective cation channels including mechanosensitive and cyclic nucleotide-gated channels (20). The transient receptor potential (TRP) vanilloid 4 (TRPV4) and α1G (CaV3.1) T-type Ca2+ channels represent two novel endothelial cell Ca2+ entry pathways in the alveolar septal capillary network (28, 35). TRPV4, a member of the vanilloid subfamily of TRP channels, has been characterized as a cation channel functioning in transduction of membrane stretch, shear stress, and direct mechanical activation (14, 16, 21, 23). Pharmacological activation of TRPV4 causes Ca2+ entry-dependent acute lung injury in an isolated, perfused lung model (4). Furthermore, TRPV4-mediated Ca2+ entry is an essential determinant for high vascular or airway pressure-induced injury to the lung (8, 10, 33). A functional T-type Ca2+ channel containing the α1G-subunit was recently documented in pulmonary microvascular endothelial cells (32, 35). In physiological settings, activation of Gq-linked signaling cascades and resultant depolarization of the plasma membrane potential can elicit Ca2+ entry through the α1G T-type Ca2+ channel. In lung microvascular endothelium, such Ca2+ entry triggers release of von Willebrand factor (34) and surface expression of P-selectin (S. Wu, unpublished observation). Despite these observations, we do not know whether Ca2+ entry via T-type Ca2+ channels is able to disrupt endothelial barrier integrity or, conversely, whether TRPV4-mediated Ca2+ entry is capable of triggering surface expression of P-selectin. Dedication of Ca2+ signaling to specific targets is not an inconsequential problem in alveolar septal endothelium, since septal endothelial cells are markedly attenuated (13).
In this study we systemically evaluated the discrete functional role of α1G T-type Ca2+ and TRPV4 channels, two endothelial Ca2+-permeable channels expressed in the alveolar capillary endothelium. In intact mouse lung, we examined whether Ca2+ entry through these two channels is selectively targeted to the surface expression of P-selectin or to regulation of endothelial cell barrier function. We resolved that the response to Ca2+ entry via α1G T-type Ca2+ channels selectively regulates the surface expression of P-selectin in alveolar septal capillary endothelium, whereas Ca2+ entry through TRPV4 channels results in selective impairment of endothelial barrier integrity. Our results suggest that the capacity to selectively regulate different Ca2+ entry pathways is of great importance for discrete and directed regulation of endothelial cell function in the alveolar septal wall.
MATERIALS AND METHODS
Animals.
Experimental protocols for mice were approved by the Institutional Animal Care and Use Committee of the University of South Alabama College of Medicine. The α1G- and TRPV4-null mutations were originally described by Kim et al. (12) and Liedtke and Friedman (15), respectively. Homozygous α1G-deficient (α1G−/−) and TRPV4-deficient (TRPV4−/−) mice and their wild-type littermates (α1G+/+ and TRPV4+/+) were generated by heterozygote mating. Mice of either sex ranging from 8 to 12 wk in age and from 18 to 35 g in weight were used in the study.
Isolated lung preparation.
Mouse lungs were isolated for perfusion as previously described (4, 8, 10). Briefly, mice were anesthetized intraperitoneally with pentobarbital sodium (50 mg/kg body wt). After tracheotomy the trachea was cannulated, and the mice were ventilated with a gas mixture of 20% O2-5% CO2-75% N2 (MiniVent, type 845, Hugo Sachs Elektronik). Tidal volume was adjusted to obtain a peak inspiratory pressure of 9 cmH2O at a respiratory rate of 60 breaths/min, with a positive end-expiratory pressure of 1.5 cmH2O. The chest was opened, 100 IU of heparin sodium was injected into the right ventricle, and a suture was placed around the pulmonary artery and aorta. A plastic cannula (0.86-mm internal diameter, 1.27-mm outside diameter) connected to a reservoir was advanced into the pulmonary artery via an incision in the right ventricular free wall, and the cannula was secured with suture. Next, a second plastic cannula of the same size was advanced into the left atrium via an incision in the apex of the left ventricle. Both cannulas were secured by a cotton thread tied around the ventricles. All lungs were perfused with Earle's buffered salt solution (EBSS) containing 4% bovine serum albumin (BSA) (4% BSA/EBSS) with the use of a roller pump (Reglo Digital compact cassette pump, ISMATEC SA, Labortechnik-Analytik) at a constant flow rate of 1.5 ml/min in a recirculating system with a total volume of 25 ml. The venous outflow was collected in a reservoir the height of which was adjusted to a fixed venous pressure (5 cmH2O, zeroed at the midlung level).
Experimental protocols.
The perfusate was preheated to 37°C before the experiments were started. Pharmacological agents, as well as the antibody used for probing for endothelial surface P-selectin expression, were added to the perfusate in the reservoir. Three separate strategies were used. 1) Lungs from wild-type mice were challenged with high-K+ (25 mmol/l) perfusate to activate the α1G T-type channel or with 4α-phorbol-12,13-didecanoate (4α-PDD, final concentration 10 μmol/l) to activate TRPV4. 2) In separate groups of wild-type lungs, pretreatment with mibefradil (10 μmol/l) or ruthenium red (3 μmol/l) led to pharmacological blockade of α1G T-type channels or TRPV4, respectively. 3) Finally, lungs from mice lacking the α1G subunit of the T-type channel and from TRPV4−/− mice were challenged with high K+ or 4α-PDD. In each experimental strategy, separate groups of lungs were evaluated with one of three functional outcomes: endothelial Ca2+ transients, endothelial surface expression of P-selectin, or endothelial permeability.
In situ real-time fluorescence microscopy for endothelial cytosolic Ca2+.
An intravital microscopy technique was utilized to image [Ca2+]i in subpleural endothelium of isolated mouse lungs. Vascular endothelium in isolated lungs was loaded with the calcium-sensitive fluorophore fluo-4 acetoxymethyl ester (fluo-4 AM, 4.5 μmol/l; Molecular Probes, Eugene, OR) via vascular perfusion for 15 min and subsequently perfused with dye-free perfusate for 20 min. The perfusate was not recirculated during this period. Lungs were then placed onto the coverslip window at the bottom of the chamber with the posterior surface of the lung gently touching the coverslip. The chamber was placed on the stage of an epifluorescence microscope (inverted Nikon TE-2000 fluorescence microscope) fitted with a ×20 objective. Excitation of the lung surface was accomplished with a 120-W metal halide lamp (E-cite 120; Exfo Photonics Solutions, Mississauga, ON, Canada), and the filter setting was 494 nm for excitation and 516 nm for emission. Images of fluo-4-loaded endothelial cells in the subpleural capillary network were sequentially acquired every 2 min. Ventilation was suspended during the period of data acquisition. The fluo-4 fluorescence intensity at each time point was acquired and analyzed with MetaMorph imaging software (Universal Imaging, West Chester, PA). Total fluo-4 fluorescence intensity was adjusted by reducing the lamp illumination intensity to minimize photobleaching of the dye, although some degree of photobleaching still occurred during the experiment. Data were corrected both for the calculated rates of photobleaching for each individual experiment and for actual rates of photobleaching obtained from imaging-referenced perfused lungs and displayed as ratio normalized to baseline intensity.
Immunohistochemistry for endothelial surface P-selectin.
For immunohistochemistry, the lungs were rinsed with 4% BSA/EBSS, fixed in 4% paraformaldehyde in PBS by vascular perfusion, immersed in fixative, and embedded in paraffin. Five-micrometer paraffin sections were deparaffinized in xylene and rehydrated in a graded alcohol series. Endogenous peroxidase was quenched with 3% H2O2 in PBS. The sections were then washed in distilled water and heated in 95°C in antigen retrieval buffer (Dako, Glostrup, Denmark). Nonspecific staining was blocked with 2% normal serum from the species from which the secondary antibodies were raised, plus 1% BSA, 0.05% sodium azide, and 0.1% gelatin from cold fish skin for 1 h. Endogenous biotin was inhibited with a streptavidin/biotin blocking kit (Vector Laboratories, Servion, Switzerland). The sections were then incubated with goat anti-mouse polyclonal antibody (5 μg/ml) for P-selectin (R&D Systems, Minneapolis, MN) overnight at 4°C. Next, the sections were incubated with biotinylated rabbit anti-goat antibody (1:1,000) (Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h. Sections were then washed and incubated with StreptABComplex/HRP (Dako) for 60 min. Antibody binding was revealed by using H2O2 as a substrate and diaminobenzidine (Sigma) as chromogen. Negative controls were obtained by omission of the primary antibody. Counterstaining was performed with Mayer's hematoxylin (Sigma). An upright optical microscope (E600W, Nikon, Melville, NY) equipped with a charge-coupled device color camera and image-capture software interface (QImaging, Surrey, BC, Canada) was used for image acquisition (×40 objective).
Morphometric assessment of P-selectin expression.
The extent of P-selectin expression in the alveolar septal network was assessed with a morphometric approach. Images were visualized in Adobe Photoshop with a grid overlay. The P-selectin volume fraction in the alveolar septal wall for each experiment was determined with a point counting method. A total of 2–7 images per lung from 3 lungs in each treatment group were analyzed, yielding 162 total images and an average of 155 total septal points per image. The P-selectin volume fraction for each lung was calculated as the ratio of P-selectin positive points relative to total points landing on the alveolar septal wall. The volume fraction data were then averaged for each treatment group (4).
Measurement of endothelial permeability.
The filtration coefficient (Kf) was measured as an index of pulmonary endothelial permeability (22) by relating the increase in the rate of lung weight gain to the increase in capillary pressure, the latter assessed with double vascular occlusion (29). Kf was measured at baseline and again after challenge with high K+ or 4α-PDD in lungs from wild-type mice, mice lacking the α1G-subunit of the T-type channel, and TRPV4−/− mice. Lungs from additional wild-type mice were pretreated with the T-channel antagonist mibefradil (10 μmol/l) at the beginning of the baseline measurements before subsequent administration of 4α-PDD or high K+. In each group, responses from four or five lungs were analyzed.
Data analysis.
Numerical data are reported as means ± SE. One-way ANOVA was used to evaluate differences between experimental groups, with a Student Newman-Keuls post hoc test as appropriate. Significance was considered P < 0.05.
RESULTS
Ca2+ transients result from activation of either α1G or TRPV4 in alveolar septal endothelium.
We induced plasma membrane depolarization by exposing endothelium in the isolated lung to high-K+ perfusate. The steady-state membrane potential with 25 mmol/l K+ is predicted to range between −49 and −45 mV, according to the Nernst equation. Membrane potentials in this range lie within the window current range of voltages for activation of the endothelial α1G T-type channel (−60 to −30 mV) (32, 35). Depolarization induced a slowly developing and sustained increase in [Ca2+]i in septal capillary endothelium. This depolarization-induced [Ca2+]i transient was diminished by pharmacological blockade of the T-type channel with mibefradil and abolished in α1G-deficient lungs (Fig. 1, A–C). Depolarization also induced a intracellular [Ca2+]i transient in alveolar capillary endothelium in TRPV4-deficient lungs, which was virtually identical to that in wild-type lungs and completely blocked by mibefradil (Fig. 1D). These observations demonstrate that depolarization evokes Ca2+ entry predominantly through the α1G T-type channel expressed in alveolar septal endothelium. Similarly, we found that 4α-PDD induced a rather rapidly progressing and sustained [Ca2+]i rise in septal capillary endothelium. The 4α-PDD-induced [Ca2+]i transient was diminished by pharmacological blockade of TRPV4 with ruthenium red and abolished in TRPV4-deficient lungs (Fig. 2, A and B). 4α-PDD also induced a time course and amplitude of [Ca2+]i transient in alveolar capillary endothelium in α1G-deficient lungs similar to that in wild-type lungs, an effect completely blocked by ruthenium red (Fig. 2C). In no instance did activation of TRPV4 or the α1G T-type channel change lung capillary pressure (data not shown), thus ruling out any secondary contribution to endothelial Ca2+ transients via mechanical stress.
Fig. 1.
Depolarization-induced endothelial cytosolic Ca2+ concentration ([Ca2+]i) response in alveolar septal capillaries examined by real-time epifluorescence imaging of subpleural fluo-4-loaded alveolar septal capillary endothelium. A: sketch outlines the field of subpleural alveoli and septa where real-time epifluorescence imaging was obtained. B: representative sets of pseudocolor-coded images of alveolar endothelium during 20-min experiments. Note the emerging of a slowly developing and sustained increase in [Ca2+]i following exposure of vascular endothelium to high-K+ perfusion in wild-type lungs (α1G+/+, top) and virtually no change in [Ca2+]i following the same exposure in lungs deficient for α1G (α1G−/−, bottom). Arrow denotes when high-K+ (25 mmol/l) perfusion was started. C and D: time course summary of changes in fractional fluo-4 fluorescence intensity at any given time point, normalized to basal fluorescence intensity measured right before high-K+ perfusion started (F/F0) and compared among groups. C: high K+ alone (□) and high K+ together with mibefradil (Mib, 10 μmol/l) (■) in α1G+/+ lungs and high K+ in α1G−/− lungs (red □) (n = 3 each, *P < 0.05, high K+ vs. high K+ + Mib; #P < 0.05 high K+ in α1G+/+ vs. high K+ in α1G−/−). D: high K+ alone (□) and high K+ together with mibefradil (■) in transient receptor potential vanilloid 4 (TRPV4)−/− lungs (n = 3 each, *P < 0.05 high K+ vs. high K+ + Mib). Black dashed line denotes high K+ in α1G+/+; red dashed line denotes high K+ together with mibefradil in α1G+/+. Data were obtained on an inverted Nikon TE-2000 fluorescence microscope with a ×60 objective; fluo-4 fluorescence intensity was acquired and analyzed with MetaMorph imaging software.
Fig. 2.
Selective TRPV4 activator 4α-phorbol-12,13-didecanoate (4α-PDD) induces an endothelial [Ca2+]i response in alveolar septal capillaries. Ca2+ responses were examined by real-time epifluorescence imaging of subpleural fluo-4-loaded alveolar septal capillary endothelium. A: representative sets of pseudocolor-coded images of alveolar endothelium during 20-min experiments. Note the emerging of a rather rapidly progressing and sustained [Ca2+]i rise following vascular perfusion of 4α-PDD in wild-type lungs (TRPV4+/+, top) and virtually no change in [Ca2+]i following the same treatment in lungs deficient for TRPV4 (TRPV4−/−, bottom). Arrow denotes when 4α-PDD (10 μmol/l) perfusion was started. B and C: time course summary of changes in fractional fluo-4 fluorescence intensity at any given time point normalized to basal fluorescence intensity measured right before 4α-PDD was applied (F/F0) and compared among groups. C: 4α-PDD alone (□), 4α-PDD together with ruthenium red (3 μmol/l) (■) in TRPV4+/+ lungs, and 4α-PDD in TRPV4−/− lungs (red □) (n = 3 each, *P < 0.05, 4α-PDD vs. 4α-PDD + ruthenium red; #P < 0.05, 4α-PDD in TRPV4+/+ vs. 4α-PDD in TRPV4−/−). D: 4α-PDD alone (□) and 4α-PDD together with ruthenium red (■) in α1G−/− lungs (n = 3 each, *P < 0.05 4α-PDD vs. 4α-PDD + ruthenium red). Black dashed line denotes 4α-PDD in TRPV4+/+; red dashed line denotes 4α-PDD together with ruthenium red in TRPV4+/+. Data were obtained on an inverted Nikon TE-2000 fluorescence microscope with a ×60 objective; fluo-4 fluorescence intensity was acquired and analyzed with MetaMorph imaging software.
Ca2+ entry via α1G, but not via TRPV4, triggers P-selectin expression in alveolar septal endothelium.
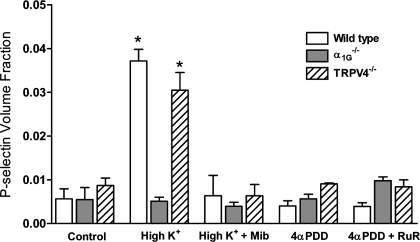
Immunohistochemistry demonstrated extensive focal immunoreactivity to P-selectin in septal capillary endothelium in wild-type lungs exposed to high-K+ perfusion, whereas in control lungs without exposure to high-K+ perfusion the endothelium in alveolar septa occasionally showed sporadic and weak immunoreactivity to P-selectin regardless of their genotypes (Fig. 3). This depolarization-associated surface expression of P-selectin was also detected in septal capillary endothelium from TRPV4-deficient lungs (Fig. 3). However, such depolarization-induced surface expression of P-selectin was not observed when the α1G T-type channel was blocked by mibefradil during high-K+ perfusion (Fig. 3) or in lungs deficient for α1G-subunits (Fig. 3). Conversely, despite development of intracellular [Ca2+]i transients with activation of TRPV4 by 4α-PDD similar to those evoked by membrane depolarization (Figs. 1C and 2B), surface expression of P-selectin was not detected in alveolar septal capillary endothelium in either wild-type or α1G-deficient lungs after 4α-PDD stimulation (Fig. 4). To verify this subjective assessment, we utilized a morphometric approach to determine a volume fraction for P-selectin in the alveolar septal wall in each group. The results of this quantitative assessment (Fig. 5) show clearly that the P-selectin volume fraction is selectively increased in wild-type or TRPV4−/− lungs challenged with high K+ (P < 0.05), an effect abrogated by blockade or knockout of the T-type channels. Activation of TRPV4 had no impact on P-selectin volume fraction. These results decidedly demonstrate that only α1G-mediated Ca2+ entry is functionally linked to regulated surface expression of P-selectin in alveolar septal capillary endothelium.
Fig. 3.
α1G-mediated Ca2+ entry triggers P-selectin surface expression in alveolar septal endothelium. Representative micrographs of immunohistochemistry for surface P-selectin in tissue sections from wild-type, α1G−/−, and TRPV4−/− lungs that had been perfused with regular perfusate [5 mmol/l K+ in 4% bovine serum albumin (BSA)/Earle's buffered salt solution (EBSS)] (Control, top), high (25 mmol/l)-K+ perfusate (High K+, middle), and high-K+ perfusate together with mibefradil (10 μmol/l) (High K+ + Mib, bottom). Note extensive focal immunoreactivity to P-selectin in alveolar septal endothelium (dark brown staining) from wild-type and TRPV4−/− lungs exposed to high-K+ perfusion (middle) and only sporadic and weak immunoreactivity to P-selectin (dark brown staining) in alveolar septa in the rest of the micrographs. Studies were replicated 3 times each. Images were acquired with a Nikon Eclipse E600W upright optical microscope with a ×40, 0.75 numerical aperture objective.
Fig. 4.
TRPV4-mediated Ca2+ entry is not involved in regulated P-selectin surface expression in alveolar septal endothelium. Representative micrographs of immunohistochemistry for surface P-selectin in tissue sections from wild-type, α1G−/−, and TRPV4−/− lungs that had been perfused with 4α-PDD (10 μmol/l) (4α-PDD, top) and 4α-PDD with ruthenium red (3 μmol/l) (4α-PDD + RuR, bottom). Note that throughout the panels only sporadic and weak immunoreactivity to P-selectin (dark brown staining) is detected in alveolar septa. Studies were replicated 3 times each. Images were acquired with a Nikon Eclipse E600W upright optical microscope with a ×40, 0.75 numerical aperture objective.
Fig. 5.
Morphometric assessment of P-selectin expression. P-selectin expression in the alveolar septal wall was quantitatively assessed with a point-counting strategy, yielding a measure of the P-selectin volume fraction (4). P-selectin volume fraction (means ± SE) increased significantly in wild-type or TRPV4−/− lungs treated with high K+. This increase was not observed in lungs from α1G−/− mice or in any high-K+ lung pretreated with mibefradil. Similarly, 4α-PDD had no impact on P-selectin volume fraction in any group. *P < 0.05 compared with all other groups.
Ca2+ entry via TRPV4, but not that via α1G, impacts lung endothelial barrier function.
Ca2+ mediated by depolarization-induced activation of the α1G T-type channel had no impact on lung endothelial permeability. Treatment of lungs with high K+ at 25 mmol/l, a dose that evokes significant Ca2+ entry in septal endothelium and P-selectin expression in the alveolar septal wall, did not alter Kf in wild-type lungs or in those lacking a functional TRPV4 or α1G T-type channel (Fig. 6). In contrast, activation of TRPV4 with 4α-PDD in wild-type lungs increased Kf 3.5-fold (P < 0.05) (Fig. 6). We showed previously (8, 10, 33) that the permeability response to 4α-PDD is specific to Ca2+ entry via TRPV4 and is blocked by use of a low-Ca2+ perfusate or by pretreatment of lungs with ruthenium red. In this study, we confirmed that knockout of the TRPV4 channel abrogated the permeability response to 4α-PDD, whereas the permeability response was retained in wild-type lungs treated with mibefradil and in lungs from α1G−/− mice.
Fig. 6.
TRPV4-mediated, but not α1G-mediated, Ca2+ entry increases lung endothelial permeability. Paired measurements of the filtration coefficient (Kf) were made in isolated mouse lungs at baseline (BL) and after treatment (F) with high K+ (25 mmol/l) or 4α-PDD (10 μmol/l), to activate the T-type Ca2+ channel or TRPV4, respectively. Activation of T-type Ca2+ channels with high K+ did not increase Kf in any group. In contrast, in wild-type mouse lung (WT) 4α-PDD increased Kf ∼3.5-fold, an effect absent in lungs from TRPV4−/− mice (TRPV4−/−). Neither blockade of α1G channels in wild-type lungs with mibefradil (WT + Mib, 10 μmol/l) nor knockout of the α1G subunit of T-type channels (α1G−/−) prevented the permeability response to 4α-PDD. (*P < 0.05 compared with baseline).
DISCUSSION
This study provides compelling evidence for the fidelity, i.e., functional specificity, of Ca2+ signals in the alveolar septal network. Both α1G T-type and TRPV4 channels are expressed in alveolar septal capillaries. Activation of each of these channels provokes a significant rise in cytosolic [Ca2+]i in capillary endothelium. Nonetheless, the signal provided by Ca2+ entry through each of these channels results in a discrete functional outcome for the endothelium. Ca2+ transients through the α1G T-type Ca2+ channel specifically lead to surface expression of the adhesion molecule P-selectin, without impacting barrier function. In contrast, Ca2+ transients through TRPV4 result in impaired barrier function, while having no impact on the surface expression of P-selectin.
We previously established (32, 34, 35) that thrombin-induced transitions in plasmalemmal membrane potential in cultured pulmonary microvascular endothelial cells activate the endothelial T-type Ca2+ channel to promote Ca2+ entry, which contributes to the agonist-induced rise in [Ca2+]i. Our recent finding of a mibefradil-inhibitable, depolarization-activated low-threshold [Ca2+]i transient in rat septal endothelium (S. Wu, unpublished observation), along with the results of the present study in mouse lung, provide evidence that septal capillary endothelium in situ exhibits functional T-type Ca2+ channels. TRPV4 is also expressed in both pulmonary macro- and microvascular endothelial cells and in lung endothelium in situ (4). Recent work by our group and others also revealed TRPV4-dependent endothelial [Ca2+]i transients in the in vivo subpleural capillary network elicited by mechanical stress. Both increased intravascular and airway pressures resulted in an elevation in [Ca2+]i, an effect abolished in TRPV4-deficient lungs (8, 10, 33). Additionally, activation of TRPV4 channels by the selective TRPV4 activator 4α-PDD was confirmed by whole cell patch-clamp recordings in pulmonary microvascular endothelial cells (33). However, the impact of TRPV4 activation on endothelial barrier function is clearly localized to the alveolar septal wall (4). Despite this previous work, the possibility remained that in the attenuated configuration of alveolar septal endothelial cells Ca2+ transients might not be constrained to regulation of specific functional outcomes.
Global Ca2+ transients, such as those induced by Ca2+ ionophores, perturb endothelial function both in cultured endothelial cells and in endothelium in vivo. Endothelial barrier disruption, increased barrier permeability to fluid and/or solutes, and development of a proinflammatory phenotype can result from ionophore-induced Ca2+ influx (3, 11). In a more realistic scenario, endothelial Ca2+ transients in vivo result from specific stimuli, such as selective ligand-receptor interactions or a mechanical stress such as shear. Furthermore, rather than a homogeneous response to such stimulation throughout the pulmonary vasculature, growing evidence supports the notion of phenotypic heterogeneity in endothelial function. Our previous work and that of others demonstrates distinct Ca2+-dependent alterations in endothelial barrier function elicited by activation of TRP channels, for example. Activation of store-operated Ca2+ channels, thought to be heterotetramers of TRPC1 and TRPC4, results specifically in barrier disruption in extra-alveolar vessel endothelium, development of perivascular cuffs without alveolar flooding, and reduced lung compliance (4, 6, 17, 31). In contrast, activation of TRPV4 elicits selective disruption of endothelium in alveolar septal capillaries, alveolar flooding, and consequent hypoxemia (4, 10, 17). Further evidence supporting such heterogeneity is the observation that chronic heart failure specifically results in downregulated expression of TRPC1 and TRPC4 in extra-alveolar endothelium and loss of the permeability response to store depletion, while leaving the intact permeability response to arachidonic acid metabolites that activate TRPV4 (3, 4).
Our present study extends the notion of endothelial heterogeneity in the intact lung to include heterogeneity in regulation of endothelial function within the alveolar septal network. We have documented clear-cut dedication of Ca2+ signals elicited by activation of TRPV4 and α1G T-type Ca2+ channels expressed in alveolar capillary endothelium to specific functional outcomes, increased barrier permeability and P-selectin surface expression, respectively. Such specificity in targeting in septal capillary endothelium implies both discrete colocalization of plasmalemmal Ca2+ channels and their functional targets. Unfortunately, there is no information at hand that documents the subcellular localization of either TRPV4 or the α1G T-type Ca2+ channel in endothelium. Several of the TRPC channels have been reported to be tethered in a caveolar compartment, which serves as a nidus for signaling complexes. In human umbilical vein endothelial cells TRPV4 coimmunoprecipitates with caveolin-1, and TRPV4-mediated relaxation of mesenteric arteries was attenuated in caveolin-1-knockout mice (25). An intracellular P-selectin pool appears to be the proximate target of Ca2+ transients elicited by activation of α1G T-type Ca2+ channels. Interestingly, in alveolar capillary endothelium, this P-selectin pool is not stored in Weibel-Palade bodies (S. Wu, unpublished observation). We have documented the presence of Weibel-Palade bodies in endothelium of extra-alveolar microvessels in mouse, rat, and human lung, but not in alveolar capillary endothelium in any of these species (S. Wu, unpublished observation). The specific molecular target of Ca2+ transients elicited by activation of TRPV4, leading to barrier disruption, is unknown. Endothelial junctional complexes are not a likely target, since junctions appear intact even in the face of substantial ultrastructural derangement (4).
Despite clear-cut evidence for discrete roles of TRPV4 and the α1G channel, we cannot conclude that endothelial barrier dysfunction and P-selectin surface expression are always elicited by distinct stimuli. As a point in fact, increased intravascular pressure evokes a TRPV4-dependent Ca2+ transient in both septal capillary and venular capillary endothelium (10, 33). When pressure is increased from basal (5 cmH2O) to 10 or 20 cmH2O, nitric oxide synthesis and P-selectin expression result (33), although endothelial permeability remains normal (9, 10). Permeability only increases when pressures exceed ∼28 cmH2O in mouse lung (10), an effect on barrier function that requires Ca2+ entry via TRPV4. Since Ca2+ is a small, diffusible molecule, these studies suggest that additional mechanisms are required to limit dispersion of intracellular Ca2+ signals (5, 7). Ischemia-reperfusion injury to lung provides additional compelling evidence of the complex mechanisms by which Ca2+ transients regulate endothelial function. Even brief periods of lung ischemia elicit increases in intracellular Ca2+ in lung endothelium in situ (26), yet Ca2+ entry may not play a role in the permeability response to ischemia-reperfusion in rat lung (6). Furthermore, the signaling mechanisms underlying the permeability response to ischemia-reperfusion per se and administration of A-23187, a Ca2+ ionophore, are distinct (11). Nonetheless, increased intracellular Ca2+ has been clearly linked to P-selectin expression (Ref. 9; S. Wu, unpublished observations), and blockade of P-selectin expression induced with ischemia-reperfusion significantly attenuates the permeability response to this stimulus (18) and improves survival (19).
In conclusion, results of this study clearly document that Ca2+ transients in lung septal endothelium are targeted to specific functional outcomes, depending on the Ca2+ channel source. Ca2+ signaling initiated by activation of TRPV4 results in impairment of endothelial barrier integrity, while that initiated by activation of the α1G T-type Ca2+ channel results in only surface expression of P-selectin. Although endothelial cells in general are known to express a number of Ca2+ channels (20), the diversity in Ca2+ channel expression in alveolar septal endothelium has not been extensively explored (28). Understanding the complexity of Ca2+-dependent regulation of function in septal endothelium will require a more complete catalog of Ca2+ channel expression as well as more in-depth understanding of the mechanisms for regulated compartmentalization of Ca2+ entry. Possible candidate mechanisms include 1) buffering of Ca2+ in the cytosol by sequestration into endoplasmic reticulum stores or other subcellular organelles, 2) buffering of free Ca2+ resulting from its binding to regulatory proteins such as calmodulin, 3) extrusion mediated by plasmalemmal membrane Ca2+-ATPases, 4) extrusion by Na+/Ca2+ exchangers, and/or 5) limitation of Ca2+ entry via Ca2+-mediated closure of the channel. This is a problem similar to that presented by synthesis and targeting of cAMP in endothelium (24). Finally, and perhaps more importantly, we will need tools to investigate discrete signaling within Ca2+ microdomains in septal endothelium elicited by activation of specific Ca2+ channels in the endothelial plasmalemmal membrane.
GRANTS
This work was supported by a grant from the National Heart, Lung, and Blood Institute (HL-066299, S. Wu and M. I. Townsley).
ACKNOWLEDGMENTS
The authors thank Darla J. Reed, Mita Patel, and Sue Barnes for excellent technical assistance.
REFERENCES
- 1.Aird WC. Phenotypic heterogeneity of the endothelium. I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Phenotypic heterogeneity of the endothelium. II. Representative vascular beds. Circ Res 100: 174–190, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Alvarez DF, King JA, Townsley MI. Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. Am J Respir Crit Care Med 172: 1153–1160, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron 57: 536–545, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chetham PM, Babal P, Bridges JP, Moore TM, Stevens T. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 276: L41–L50, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Fakler B, Adelman JP. Control of KCa channels by calcium nano/microdomains. Neuron 59: 873–881, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 293: L923–L932, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ichimura H, Parthasarathi K, Quadri S, Issekutz AC, Bhattacharya J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J Clin Invest 111: 691–699, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jian MY, King JA, Al-Mehdi AB, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol 38: 386–392, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khimenko PL, Moore TM, Wilson PS, Taylor AE. Role of calmodulin and myosin light-chain kinase in lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 271: L121–L125, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking α1G T-type Ca2+ channels. Neuron 31: 35–45, 2001 [DOI] [PubMed] [Google Scholar]
- 13.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67: 139–151, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Liedtke W. Role of TRPV ion channels in sensory transduction of osmotic stimuli in mammals. Exp Physiol 92: 507–512, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA 100: 13698–13703, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liedtke W, Kim C. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell Mol Life Sci 62: 2985–3001, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe K, Alvarez D, King J, Stevens T. Phenotypic heterogeneity in lung capillary and extra-alveolar endothelial cells. Increased extra-alveolar endothelial permeability is sufficient to decrease compliance. J Surg Res 143: 70–77, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore TM, Khimenko P, Adkins WK, Miyasaka M, Taylor AE. Adhesion molecules contribute to ischemia and reperfusion-induced injury in the isolated rat lung. J Appl Physiol 78: 2245–2252, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Naka Y, Toda K, Kayano K, Oz MC, Pinsky DJ. Failure to express the P-selectin gene or P-selectin blockade confers early pulmonary protection after lung ischemia or transplantation. Proc Natl Acad Sci USA 94: 757–761, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001 [DOI] [PubMed] [Google Scholar]
- 21.O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflügers Arch 451: 193–203, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 286: L231–L246, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol 179: 189–205, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci USA 98: 13049–13054, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Song C, Al-Mehdi AB, Fisher AB. An immediate endothelial cell signaling response to lung ischemia. Am J Physiol Lung Cell Mol Physiol 281: L993–L1000, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Taylor CW. Controlling calcium entry. Cell 111: 767–769, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Townsley MI, King JA, Alvarez DF. Ca2+ channels and pulmonary endothelial permeability: insights from study of intact lung and chronic pulmonary hypertension. Microcirculation 13: 725–739, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Townsley MI, Korthuis RJ, Rippe B, Parker JC, Taylor AE. Validation of double vascular occlusion method for Pc,i in lung and skeletal muscle. J Appl Physiol 61: 127–132, 1986 [DOI] [PubMed] [Google Scholar]
- 30.Tran QK, Watanabe H. Calcium signalling in the endothelium. Handb Exp Pharmacol 176: 145–187, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Wu S, Cioffi EA, Alvarez D, Sayner SL, Chen H, Cioffi DL, King J, Creighton JR, Townsley M, Goodman SR, Stevens T. Essential role of a Ca2+-selective, store-operated current (ISOC) in endothelial cell permeability: determinants of the vascular leak site. Circ Res 96: 856–863, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Wu S, Haynes J, Jr, Taylor JT, Obiako BO, Stubbs JR, Li M, Stevens T. CaV3.1 (α1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ Res 93: 346–353, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Yin J, Hoffmann J, Kaestle SM, Neye N, Wang L, Baeurle J, Liedtke W, Wu S, Kuppe H, Pries AR, Kuebler WM. Negative-feedback loop attenuates hydrostatic lung edema via a cGMP-dependent regulation of transient receptor potential vanilloid 4. Circ Res 102: 966–974, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Zhou C, Chen H, Lu F, Sellak H, Daigle JA, Alexeyev MF, Xi Y, Ju J, van Mourik JA, Wu S. CaV3.1 (α1G) controls von Willebrand factor secretion in rat pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 292: L833–L844, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou C, Wu S. T-type calcium channels in pulmonary vascular endothelium. Microcirculation 13: 645–656, 2006 [DOI] [PubMed] [Google Scholar]