Introduction
The matrix metalloproteinases (MMPs) function as regulators of the dynamic tissue remodeling that occurs in the endometrial lining of the uterus during the normal human menstrual cycle; dysregulation of the MMPs is thought to contribute to the development of both endometriosis and endometrial cancer (1). MMP-1 expression was found to be significantly higher in endometriotic lesions than in surrounding endometrium (2;3), while MMP-3 levels have been reported to be both lower (4) and higher (5) in women with endometriosis compared to women without. MMP-7 expression was found to be significantly higher in endometrial hyperplasia and adenocarcinomas than normal endometrium (6) and further, was associated with higher grade endometrial tumors (7), and both myometrial (6) and lymph node invasion (8). These three MMP genes are located on the negative strand of chromosome 11, and functional polymorphisms that influence their respective transcription levels have been identified for each (9–12). However, previous studies on MMP SNPs and endometrial cancer are sparse; two studies were found to have evaluated a single MMP-1 SNP, and results were inconsistent. Therefore, this comprehensive study of individual genetic variation across MMP-1, MMP-3, and MMP-7 was undertaken to evaluate associations with endometrial cancer susceptibility.
Materials and Methods
The Shanghai Endometrial Cancer Study (SECS) is a large, population-based case-control study that has been previously described (13;14). Briefly, cases were women diagnosed with endometrial cancer between January 1997 and December 2003, aged 30–69, identified from the Shanghai Cancer Registry. Controls were randomly selected from the Shanghai Resident Registry and frequency matched to cases in 5-year intervals. Of 1,458 identified eligible cases, in-person interviews were completed for 1,204 (82.6%). Reasons for nonparticipation included refusal (N=137, 9.4%), death before interview (N=66, 4.5%), inability to be located (N=37, 2.5%), and health or communication problems (N=14, 1.0%). Of eligible controls identified (1,629), in-person interviews were completed for 1,212 (74.4%). Reasons for nonparticipation included refusal (N=340, 20.9%), absence during the study period (N=61, 3.7%), and health or communication problems (N=16, 1.1%). Institutional review board approval was granted by relevant institutions in both China and the United States. Informed consent was obtained from each included participant. DNA samples were provided and available for 87.3% of cases (N=1,052) and 87.3% (N=1,058) of controls.
Haplotype tagging SNPs (htSNPs) were selected from Han Chinese data from the HapMap Project (15) using the Tagger program (16) to capture SNPs with a minimum minor allele frequency (MAF) of 0.05 in either MMP-1, MMP-3, or MMP-7 (± 5kb) with an r2 of 0.90 or greater. Known or potentially functional SNPs were forced into the htSNP selection process. For MMP-1, 17 SNPs were selected, with 14 successfully genotyped. For MMP-3, seven SNPs were selected, with six successfully genotyped. For MMP-7, 12 SNPs were selected, with 11 successfully genotyped. Genotyping was conducted using the Affymetrix Targeted Genotyping System (Affymetrix, Santa Clara, CA) (17) for 1,037 cases (98.6%) and 1,018 controls (96.2%).
Hardy-Weinberg equilibrium (HWE) was applied to test the observed and expected genotype frequencies for cases and controls (χ2-test). Associations between SNPs and covariates were evaluated with the χ2 test or t-test when appropriate. Covariates considered included age at diagnosis, education, age at menarche, age at menopause among postmenopausal women, menopausal status, number of pregnancies, oral contraceptive use, BMI, WHR, physical activity in the preceding decade, and first-degree family history of breast, colorectal, or endometrial cancer. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were determined by logistic regression using additive models that included adjustment for age and education. Dominant and recessive models were additionally employed when appropriate. Linkage disequilibrium was assessed by Haploview (18). All statistical tests were two-tailed, and p-values were considered to be statistically significant when ≤ 0.05.
Results
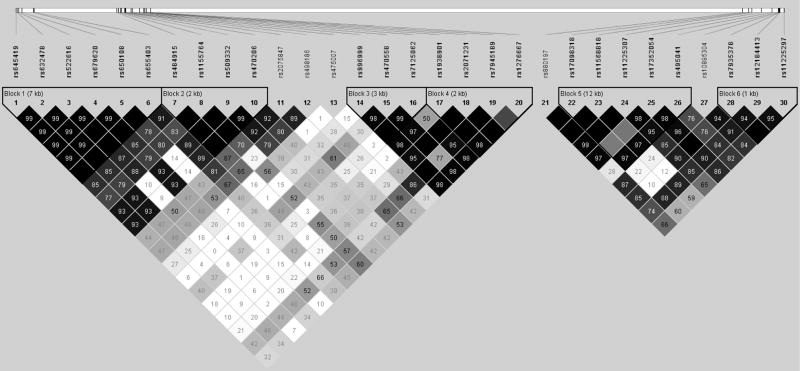
Consistent with previous SECS analyses (13;14) and other epidemiologic studies, cases and controls included in the current study differed in regards to age at menarche, age at menopause, menopausal status, number of pregnancies, use of oral contraceptives, BMI and WHR, physical activity, and first degree family history of cancer (data not shown). SNPs included in this study are listed in Table 1; their order corresponds to the open reading frames of the genes on the negative strand of chromosome 11. Of the 31 polymorphisms genotyped, one was found not to be polymorphic in this study population (MMP-7 rs11568819) and thus not included in our analyses. No SNPs were found to deviate from HWE. Associations with endometrial cancer risk were calculated in additive effect models that included adjustment for age and education; further adjustment for BMI, number of pregnancies, menopausal status, or family history of cancer did not appreciably alter the effect estimates. No significant associations were observed. Two MMP-7 SNPs, rs17098318 and rs11568818, both tended to confer an increased, but non-significant, risk of endometrial cancer for homozygotes, in both additive and recessive models. Further, no SNPs were found to have effects that significantly differed by menopausal status. The linkage disequilibrium (LD) structure of these 30 SNPs is shown in Figure 1, and includes six haplotype blocks. Similar to single SNP analysis, no significant effects were observed in haplotype analysis of these MMP polymorphisms (data not shown).
Table 1.
MMP-3, -1, and -7 SNPs and Endometrial Cancer Risk, evaluated among 1,037 cases and 1,018 controls, The Shanghai Endometrial Cancer Study
| Endometrial Cancer Risk, OR (95% CI) § | |||||||
|---|---|---|---|---|---|---|---|
| Gene, SNP | Region | Alleles 1 | MAF 2 | HWE p-value 3 | AB | BB | p-value |
| MMP-3 | |||||||
| rs645419 | promoter | G/A | 32.6% | 0.573 | 1.1 (0.9–1.3) | 1.0 (0.8–1.4) | 0.628 |
| rs632478 | promoter | C/A | 33.0% | 0.766 | 1.1 (0.9–1.3) | 1.0 (0.8–1.4) | 0.717 |
| rs522616 | promoter | A/G | 36.2% | 0.700 | 1.0 (0.8–1.2) | 1.2 (0.9–1.6) | 0.321 |
| rs679620 | exon 2 | G/A | 32.9% | 0.754 | 1.1 (0.9–1.3) | 1.0 (0.8–1.4) | 0.765 |
| rs650108 | intron 8 | A/G | 40.2% | 0.905 | 1.0 (0.9–1.3) | 1.1 (0.8–1.4) | 0.624 |
| rs655403 | intron 8 | C/T | 7.2% | 0.552 | 1.0 (0.8–1.3) | 1.5 (0.4–5.3) | 0.832 |
| MMP-1 | |||||||
| rs484915 | promoter | A/T | 34.2% | 0.311 | 1.0 (0.8–1.2) | 0.9 (0.7–1.3) | 0.777 |
| rs1155764 | promoter | T/G | 22.0% | 0.765 | 0.9 (0.7–1.0) | 1.1 (0.7–1.7) | 0.397 |
| rs509332 | promoter | A/G | 13.4% | 0.124 | 1.1 (0.9–1.4) | 0.8 (0.4–1.5) | 0.639 |
| rs470206 | promoter | G/A | 13.4% | 0.114 | 1.1 (0.9–1.4) | 0.8 (0.5–1.5) | 0.672 |
| rs2075847 | promoter | T/C | 24.0% | 0.253 | 1.1 (0.9–1.3) | 1.2 (0.8–1.6) | 0.369 |
| rs498186 | promoter | A/C | 44.0% | 0.966 | 1.0 (0.8–1.2) | 0.9 (0.7–1.2) | 0.507 |
| rs475007 | promoter | T/A | 34.0% | 0.702 | 0.9 (0.8–1.1) | 1.1 (0.9–1.5) | 0.626 |
| rs996999 | intron 4 | C/T | 49.1% | 0.291 | 1.1 (0.9–1.3) | 1.0 (0.7–1.2) | 0.731 |
| rs470558 | exon 5 | G/A | 12.4% | 0.642 | 0.9 (0.7–1.1) | 1.0 (0.5–2.2) | 0.421 |
| rs7125062 | intron 6 | C/T | 30.6% | 0.478 | 1.0 (0.8–1.2) | 0.8 (0.6–1.1) | 0.472 |
| rs1938901 | intron 8 | T/C | 42.4% | 0.691 | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) | 0.440 |
| rs2071231 | intron 9 | T/G | 20.4% | 0.607 | 1.1 (0.9–1.3) | 1.2 (0.8–1.8) | 0.173 |
| rs7945189 | 3′ FR * | C/T | 8.8% | 0.250 | 0.9 (0.7–1.1) | 1.3 (0.4–4.3) | 0.411 |
| rs1470504 | 3′ FR * | G/A | 13.9% | 0.663 | 0.9 (0.7–1.1) | 0.9 (0.4–1.7) | 0.328 |
| MMP-7 | |||||||
| rs880197 | promoter | A/T | 38.7% | 0.755 | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 0.230 |
| rs17098318 | promoter | G/A | 7.9% | 0.309 | 1.0 (0.8–1.2) | 2.2 (0.7–7.2) | 0.782 |
| rs11568818 | promoter | A/G | 8.0% | 0.281 | 1.0 (0.7–1.2) | 2.0 (0.6–6.5) | 0.970 |
| rs11225307 | intron 3 | A/G | 26.5% | 0.461 | 1.1 (0.9–1.3) | 1.1 (0.8–1.6) | 0.325 |
| rs17352054 | intron 5 | A/C | 12.0% | 0.682 | 1.2 (1.0–1.5) | 1.1 (0.6–2.3) | 0.112 |
| rs495041 | 3′ FR * | C/T | 49.5% | 0.508 | 0.9 (0.8–1.2) | 1.0 (0.8–1.2) | 0.741 |
| rs10895304 | 3′ FR * | A/G | 24.5% | 0.853 | 1.0 (0.8–1.2) | 1.0 (0.7–1.5) | 0.865 |
| rs7935378 | 3′ FR * | T/C | 23.0% | 0.941 | 0.9 (0.8–1.1) | 1.1 (0.7–1.6) | 0.655 |
| rs12184413 | 3′ FR * | C/T | 29.5% | 0.577 | 0.9 (0.8–1.1) | 1.0 (0.7–1.4) | 0.636 |
| rs11225297 | 3′ FR * | A/T | 20.7% | 0.629 | 1.0 (0.8–1.2) | 0.9 (0.5–1.4) | 0.572 |
Major and minor alleles as determined by the distribution among SECS controls
Minor allele frequency among SECS controls
Hardy-Weinberg equilibrium test, p-value among SECS controls
Odds Ratio and 95% Confidence Interval for the risk of endometrial cancer, age and education adjusted; AA major allele homozygous, BB minor allele homozygous, AB heterozygous; p-value for trend
3′ Flanking region, downstream of the coding region
Figure 1. LD Structure of 30 MMP-3, -1, and -7 SNPs, The Shanghai Endometrial Cancer Study.
Linkage Disequilibrium (LD) of MMP-3, -1, and -7 on chromosome 11 among 1,018 controls from the Shanghai Endometrial Cancer Study; value shown in D′.
Discussion
Promoter polymorphisms in the matrix metalloproteinase (MMP) -1, -3, and -7 genes have been associated with altered susceptibility to cancer in human populations (17; 19–30), although studies on endometrial cancer risk and MMP SNPs have been lacking. Two small studies evaluating MMP-1 -1607 1G/2G (rs1799750) and MMP-3 -1171 5A/6A (rs35068180 and rs3025059) and endometriosis found mixed results. No association for either SNP was seen among 56 cases and 71 controls (31), while the MMP-1 2G allele was found to confer an increased risk of endometriosis among 100 cases and 150 controls (32). Similarly, the MMP-1 -1607 2G allele was found to confer an increased risk of endometrial adenocarcinoma among 100 cases and 150 controls (33), while no difference was seen between 107 cases and 213 controls (34). Unfortunately, neither of these functional SNPs were genotyped in the current study. However, MMP-3 rs679620 was genotyped and shares moderate linkage disequilibrium with MMP-1 rs1799750 (D′=0.79, r2=0.60) (35); no association with endometrial cancer risk was observed. To our knowledge, no previous studies of MMP-3 or MMP-7 SNPs and endometrial cancer risk have been conducted. In this study, both of the functional promoter MMP-7 SNPs were genotyped. While MMP-7 -153 C/T (rs11568819) was not found to be polymorphic in this population, MMP-7 -181 A/G (rs11568818) and another promoter SNP in high LD (rs17098318, D′=1.0, r2=0.99) both seemed to confer an increased risk of endometrial cancer in homozygote carriers of the rare allele. This is similar to our findings for breast cancer risk among pre-menopausal women (17), and may indicate a real, but low prevalence association that the current study lacked adequate power to detect under recessive models. Given the size of our study population, this analysis had only 31% power to detect a recessive effect of an OR of 2.0 for a gene with a MAF of only 8%. However, for additive associations, we had greater than 92% power to detect an OR of 1.4 for a SNP with a MAF of 10%, greater than 93% power to detect an OR of 1.3 for a SNP with a MAF of 20%, and greater than 77% power to detect an OR of 1.2 for a SNP with a MAF of 30%. In summary, 30 haplotype tagging polymorphisms in MMP-1, -3, and -7 were evaluated among 1,037 endometrial cancer cases and 1,018 controls; none were found to be significantly associated with endometrial cancer risk.
Acknowledgments
We would like to thank Dr. Fan Jin for her contributions to implementing the study in Shanghai, Regina Courtney, Qing Wang, Dr. Shawn Levy, and the Vanderbilt Microarray Shared Resource for their contributions to the genotyping, and Brandy Venuti for her assistance in the preparation of this manuscript. The Vanderbilt Microarray Shared Resource is supported by the Vanderbilt-Ingram Cancer Center (P30 CA68485), the Vanderbilt Diabetes Research and Training Center (P60 DK20593), the Vanderbilt Digestive Disease Center (P30 DK58404), and the Vanderbilt Vision Center (P30 EY08126). This study would not have been possible without the support of the study participants and research staff of the Shanghai Endometrial Cancer Study. This work was supported by USPHS grant R01 CA92585 from the National Cancer Institute.
References
- 1.Curry TE, Jr, Osteen KG. The Matrix Metalloproteinase System: Changes, Regulation, and Impact throughout the Ovarian and Uterine Reproductive Cycle. Endocr Rev. 2003;24:428–65. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- 2.Hudelist G, Lass H, Keckstein J, et al. Interleukin 1{alpha} and tissue-lytic matrix metalloproteinase-1 are elevated in ectopic endometrium of patients with endometriosis. Hum Reprod. 2005;20:1695–1701. doi: 10.1093/humrep/deh794. [DOI] [PubMed] [Google Scholar]
- 3.Hudelist G, Keckstein J, Czerwenka K, et al. Estrogen receptor [beta] and matrix metalloproteinase 1 are coexpressed in uterine endometrium and endometriotic lesions of patients with endometriosis. Fertility and Sterility. 2005;84:1249–56. doi: 10.1016/j.fertnstert.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Uzan C, Cortez A, Dufournet C, Fauvet R, Siffroi JP, Darai E. Eutopic endometrium and peritoneal, ovarian and bowel endometriotic tissues express a different profile of matrix metalloproteinases-2, -3 and -11, and of tissue inhibitor metalloproteinases-1 and -2. Virchows Arch. 2004;445:603–9. doi: 10.1007/s00428-004-1117-y. [DOI] [PubMed] [Google Scholar]
- 5.Gilabert-Estelles J, Estelles A, Gilabert J, et al. Expression of several components of the plasminogen activator and matrix metalloproteinase systems in endometriosis. Hum Reprod. 2003;18:1516–22. doi: 10.1093/humrep/deg300. [DOI] [PubMed] [Google Scholar]
- 6.Obokata A, Watanabe J, Nishimura Y, Arai T, Kawaguchi M, Kuramoto H. Significance of matrix metalloproteinase-7 [correction of matrix metalloproteinase-2], -11 and tissue inhibitor of metalloproteinase-1 expression in normal, hyperplastic and neoplastic endometrium. Anticancer Res. 2007;27:95–105. [PubMed] [Google Scholar]
- 7.Misugi F, Sumi T, Okamoto E, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in uterine endometrial carcinoma and a correlation between expression of matrix metalloproteinase-7 and prognosis. Int J Mol Med. 2005;16:541–6. [PubMed] [Google Scholar]
- 8.Ueno H, Yamashita K, Azumano I, Inoue M, Okada Y. Enhanced production and activation of matrix metalloproteinase-7 (matrilysin) in human endometrial carcinomas. Int J Cancer. 1999;84:470–7. doi: 10.1002/(sici)1097-0215(19991022)84:5<470::aid-ijc4>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Ye S, Watts GF, Mandalia S, Humphries SE, Henney AM. Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis. Br Heart J. 1995;73:209–15. doi: 10.1136/hrt.73.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM. Progression of Coronary Atherosclerosis Is Associated with a Common Genetic Variant of the Human Stromelysin-1 Promoter Which Results in Reduced Gene Expression. J Biol Chem. 1996;271:13055–60. doi: 10.1074/jbc.271.22.13055. [DOI] [PubMed] [Google Scholar]
- 11.Rutter JL, Mitchell TI, Buttice G, et al. A Single Nucleotide Polymorphism in the Matrix Metalloproteinase-1 Promoter Creates an Ets Binding Site and Augments Transcription. Cancer Res. 1998;58:5321–5. [PubMed] [Google Scholar]
- 12.Jormsjo S, Whatling C, Walter DH, Zeiher AM, Hamsten A, Eriksson P. Allele-Specific Regulation of Matrix Metalloproteinase-7 Promoter Activity Is Associated With Coronary Artery Luminal Dimensions Among Hypercholesterolemic Patients. Arterioscler Thromb Vasc Biol. 2001;21:1834–9. doi: 10.1161/hq1101.098229. [DOI] [PubMed] [Google Scholar]
- 13.Xu WH, Dai Q, Xiang YB, et al. Interaction of Soy Food and Tea Consumption with CYP19A1 Genetic Polymorphisms in the Development of Endometrial Cancer. Am J Epidemiol. 2007;166:1420–30. doi: 10.1093/aje/kwm242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deming SL, Zheng W, Xu WH, et al. UGT1A1 Genetic Polymorphisms, Endogenous Estrogen Exposure, Soy Food Intake, and Endometrial Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2008;17:563–70. doi: 10.1158/1055-9965.EPI-07-0752. [DOI] [PubMed] [Google Scholar]
- 15.The International HapMap Project. Nature. 2003;426:789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 16.de Bakker PIW, McVean G, Sabeti PC, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38:1166–72. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beeghly-Fadiel A, Long JR, Gao YT, et al. Common MMP-7 Polymorphisms and Breast Cancer Susceptibility: A Multistage Study of Association and Functionality. Cancer Res. 2008;68:6453–9. doi: 10.1158/0008-5472.CAN-08-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T, Uchida K, Okayama N, et al. Association of matrix metalloproteinase (MMP)-1 promoter polymorphism with head and neck squamous cell carcinoma. Cancer Lett. 2004;211:19–24. doi: 10.1016/j.canlet.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 20.Hirata H, Okayama N, Naito K, et al. Association of a haplotype of matrix metalloproteinase (MMP)-1 and MMP-3 polymorphisms with renal cell carcinoma. Carcinogenesis. 2004;25:2379–84. doi: 10.1093/carcin/bgh254. [DOI] [PubMed] [Google Scholar]
- 21.Zinzindohoue F, Blons H, Hans S, et al. Single nucleotide polymorphisms in MMP1 and MMP3 gene promoters as risk factor in head and neck squamous cell carcinoma. Anticancer Res. 2004;24:2021–6. [PubMed] [Google Scholar]
- 22.Lievre A, Milet J, Carayol J, et al. Genetic polymorphisms of MMP1, MMP3 and MMP7 gene promoter and risk of colorectal adenoma. BMC Cancer. 2006;6:270. doi: 10.1186/1471-2407-6-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O-charoenrat P, Leksrisakul P, Sangruchi S. A functional polymorphism in the matrix metalloproteinase-1 gene promoter is associated with susceptibility and aggressiveness of head and neck cancer. Int J Cancer. 2006;118:2548–53. doi: 10.1002/ijc.21644. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Cao Y, Wang Y, et al. Polymorphisms in the matrix metalloproteinase-1, 3, and 9 promoters and susceptibility to adult astrocytoma in northern China. J Neurooncol. 2007;85:65–73. doi: 10.1007/s11060-007-9392-5. [DOI] [PubMed] [Google Scholar]
- 25.Nasr HB, Mestiri S, Chahed K, et al. Matrix metalloproteinase-1 (-1607) 1G/2G and -9 (-1562) C/T promoter polymorphisms: Susceptibility and prognostic implications in nasopharyngeal carcinomas. Clinica Chimica Acta. 2007;384:57–63. doi: 10.1016/j.cca.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Nishizawa R, Nagata M, Noman A, et al. The 2G allele of promoter region of Matrix metalloproteinase-1 as an essential pre-condition for the early onset of oral squamous cell carcinoma. BMC Cancer. 2007;7:187. doi: 10.1186/1471-2407-7-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo M, Park K, Nam J, Kim JC. Clinical implications of matrix metalloproteinase-1, -3, -7, -9, -12, and plasminogen activator inhibitor-1 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol. 2007;22:1064–70. doi: 10.1111/j.1440-1746.2006.04424.x. [DOI] [PubMed] [Google Scholar]
- 28.Sauter W, Rosenberger A, Beckmann L, et al. Matrix Metalloproteinase 1 (MMP1) Is Associated with Early-Onset Lung Cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:1127–35. doi: 10.1158/1055-9965.EPI-07-2840. [DOI] [PubMed] [Google Scholar]
- 29.Singh H, Jain M, Mittal B. MMP-7 (-181A>G) promoter polymorphisms and risk for cervical cancer. Gynecologic Oncology. 2008;110:71–5. doi: 10.1016/j.ygyno.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto M, Furuta T, Kodaira C, et al. Polymorphisms of matrix metalloproteinase-7 and chymase are associated with susceptibility to and progression of gastric cancer in Japan. J Gastroenterol. 2008;43:751–61. doi: 10.1007/s00535-008-2221-6. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari MM, Biondi ML, Rossi G, et al. Analysis of two polymorphisms in the promoter region of matrix metalloproteinase 1 and 3 genes in women with endometriosis. Acta Obstet Gynecol Scand. 2006;85:212–7. doi: 10.1080/00016340500345287. [DOI] [PubMed] [Google Scholar]
- 32.Shan K, Ying W, Jian-Hui Z, Wei G, Na W, Yan L. The function of the SNP in the MMP1 and MMP3 promoter in susceptibility to endometriosis in China. Mol Hum Reprod. 2005;11:423–7. doi: 10.1093/molehr/gah177. [DOI] [PubMed] [Google Scholar]
- 33.Nishioka Y, Kobayashi K, Sagae S, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter in endometrial carcinomas. Jpn J Cancer Res. 2000;91:612–5. doi: 10.1111/j.1349-7006.2000.tb00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto M, Yoshida S, Kennedy S, Deguchi M, Ohara N, Maruo T. Matrix metalloproteinase-1 and -9 promoter polymorphisms and endometrial carcinoma risk in a Japanese population. J Soc Gynecol Investig. 2006;13:523–9. doi: 10.1016/j.jsgi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Beeghly-Fadiel A, Cai Q, Lu W, et al. No Association between MMP-1 or MMP-3 Polymorphisms and Breast Cancer Susceptibility: A report from the Shanghai Breast Cancer Study. Cancer Epidemiology Biomarkers Prev. 2009 doi: 10.1158/1055-9965.EPI-09-0046. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]