Abstract
Understanding the transmission patterns of West Nile and St. Louis encephalitis viruses (family Flaviviridae, genus Flavivirus, WNV and SLEV) could result in an increased ability to predict transmission risk to humans. To examine transmission patterns between vector and host, we trapped mosquitoes in three Florida counties from June to November 2005 by using chicken-baited lard can mosquito traps. These traps were used to monitor for presence of WNV and SLEV in mosquitoes and subsequent transmission of these viruses to chickens. In total, 166,615 female mosquitoes were sorted into 4,009 pools based on species and bloodfed status, and they were tested for presence of WNV and SLEV. Sera from 209 chickens were tested for WNV and SLEV antibodies. We detected eight WNV-positive Culex nigripalpus Theobald mosquito pools; SLEV was not detected in any pools. Six positive pools were collected in August and September from Duval County, one pool in September from Manatee County, and one pool in November from Indian River County. Of the eight chickens potentially exposed to WNV, antibodies were detected in only one chicken, indicating a low rate of transmission relative to the observed mosquito infection rates. Low virus transmission rates relative to infection rates would suggest that using sentinel chicken seroconversion data as a means of arbovirus surveillance may underestimate the prevalence of WNV in the mosquito population. However, using mosquito infection rates may overestimate the risk of arboviral transmission. A variety of factors might account for the observed low level of transmission including a lack of viral dissemination in mosquito vectors.
Keywords: West Nile virus, St. Louis encephalitis virus, surveillance, infection, vector–host transmission
West Nile virus (family Flaviviridae, genus Flavivirus, WNV) and St. Louis encephalitis virus (family Flaviviridae, genus Flavivirus, SLEV) are members of the Japanese encephalitis virus complex (Hayes 1989). Both viruses are maintained through an enzootic transmission cycle involving a variety of avian and mosquito species (Hayes 1989, Komar et al. 2003). West Nile virus was first introduced in the United States in 1999 (CDC 1999, Lanciotti et al. 1999, Marfin and Gubler 2001), and it has rapidly spread throughout the country, causing numerous outbreaks of human disease. West Nile virus was first detected in Florida in 2001 when 12 humans, 492 horses, and 194 sentinel chickens were reported WNV-positive (Blackmore et al. 2003, Godsey et al. 2005). From 2001 to 2005, Florida had 196 human cases of WNV, including two focal outbreaks (Dade County in 2004 and Pinellas County in 2005). The highest level of WNV activity in Florida occurred in 2003, when 1,247 sentinel chicken seroconversions and 94 human cases were reported (Florida Department of Health 2003). In 2005, 385 WNV seroconversions occurred in sentinel chickens in 27 counties, with 21 human cases reported (Florida Department of Health 2005). The most recent epidemic of SLEV was in 1990, lasting from July through December (Shroyer 1991; Day and Curtis 1993, 1999; Day and Stark 1996). In 2005, five sentinel chicken seroconversions for SLEV in total were reported in three counties, and no human cases of SLEV were reported (Florida Department of Health 2005).
Arbovirus surveillance in Florida relies on sentinel chicken serosurveillance and passive detection of human and horse cases (Blackmore et al. 2003). Monitoring disease activity by using sentinel chickens can provide critical information regarding periods of increased transmission and the increased probability of potential outbreaks. However, sentinel chicken surveillance does not provide information regarding the species of mosquito that are transmitting the virus under natural conditions, in part because of the large diversity of mosquitoes that may feed on sentinel chickens. In addition, such monitoring does not provide precise information about the frequency of successful transmission events from mosquito to vertebrate host. Understanding factors that can influence arbovirus transmission as opposed to simply assessing infection rates can lead to more effective risk prediction models. Standard arbovirus surveillance and some experimental studies have examined virus-positive mosquito pools and sentinel chicken seroconversions (Day and Stark 1996, Blackmore et al. 2003, Godsey et al. 2005) but the specific factors that determine the transmission of these viruses remain poorly described.
Arbovirus transmission by infected mosquitoes has been examined under laboratory conditions and, to a lesser degree, in the field. In the laboratory, transmission ability is often assumed after a disseminated infection(Dohmet al. 2002, Girard et al. 2004), although virus dissemination does not always allow successful transmission (Turell et al. 2000, 2005, 2006a). Laboratories generally measure transmission by allowing the vector to feed on a naïve host (Turell et al. 2000, 2005) or use the capillary tube transmission assay (Colton and Nasci 2006); however, studies examining field transmission events are less common. Transmission rates for SLEV and Western equine encephalitis virus (family Togaviridae, genus Alphavirus, WEEV) by Culex tarsalis Coquillett have been examined in California, and they were less than infection rates (Reeves et al. 1961). field transmission of WNV by Cx. nigripalpus has been documented in Florida (Rutledge et al. 2003), and transmission rates were found to be lower than infection rates. The research of Rutledge et al. (2003) was carried out during a localized outbreak of WNV in northern Florida in August 2001. Examination of WNV in saliva after intrathoracic inoculation has shown that there are geographic and temporal differences in saliva titers even within the same species of mosquito (Colton and Nasci 2006), although the use of the capillary tube transmission assay may cause fluctuations in the amount of WNV collected from saliva. However, under natural conditions, arbovirus transmission rates also may vary. Transmission rates of infected mosquitoes may vary at different times of the year or in different locations due to changes in the environment or mosquito populations.
Culex spp. mosquitoes are considered the primary vectors of WNV and SLEV (Hayes 1989, CDC 2000), and the predominant species of concern in Florida is Cx. nigripalpus (Chamberlain et al. 1964, Dow et al. 1964, Shroyer 1991, Godsey et al. 2005). Cx. nigripalpus is abundant in southern Florida, and northern Florida is considered a transition zone for Cx. nigripalpus and focal Cx. salinarius populations (Provost 1969; Mitchell et al. 1980; Day and Curtis 1989, 1993; Zyzak et al. 2002). Behavioral and life history characteristics of Cx. nigripalpus such as synchronized host seeking and blood feeding, as well as shifts in their feeding behavior from primarily ornithophilic to generalist, likely make it a significant vector of encephalitis viruses (Chamberlain et al. 1964, Dow et al. 1964, Edman and Taylor 1968, Day and Curtis 1993, Gomes et al. 2003). Cx. nigripalpus has been demonstrated to be a vector of SLEV (Tsai and Mitchell 1988, Godsey et al. 2005), and field evidence had implicated this species as a predominant vector in SLEV epidemics in Florida (Chamberlain et al. 1964, Dow et al. 1964, Shroyer 1991, Day and Curtis 1993, Day and Stark 1996). Laboratory testing of Cx. nigripalpus and other Culex spp. has demonstrated vector competence for WNV (Sardelis et al. 2001; Turell et al. 2001, 2005, 2006a; Colton and Nasci 2006). Furthermore, field evidence also implicates Cx. nigripalpus in WNV transmission (Rutledge et al. 2003). Rainfall and drought patterns that influence bionomics of Cx. nigripalpus and thereby outbreaks of SLEV favor similar epidemic potential for WNV in Florida (Shroyer 1991, Day and Stark 1996, Shaman et al. 2005).
We monitored arbovirus transmission by using chicken-baited traps to capture mosquitoes for virus testing and sought to determine transmission events from vector to vertebrate host. We used a trap that allowed host-seeking mosquitoes to bloodfeed but prevented the mosquitoes from escaping. This allowed us to measure transmission rates to sentinel chickens in addition to infection rates in mosquitoes. Standard arbovirus surveillance methods using sentinel chicken seroconversions do not allow for the examination of infection rates in mosquitoes, nor do they allow for the testing of the specific mosquitoes that may have fed upon the chicken. In the current study, we identified the species of mosquito that was responsible for any observed transmission events. finally, we evaluated associations between virus-positive mosquitoes and viral antibody status of bait chickens. Based on previous research (Rutledge et al. 2003), we expected a low rate of field transmission relative to mosquito infection rates, but the study by Rutledge et al. (2003) lacked information on potential geographic and temporal variation that is needed for accurate risk assessment.
Materials and Methods
Study Sites
Three field sites in Florida were selected for monitoring during the 2005 season based on WNV and SLEV activity during the preceding two years. Manatee County (27° 34′25″ N, 82° 28′30″ W), Indian River County (27° 34′27″ N, 80° 26′11″ W), and Duval County (30° 20′50″ N, 81° 52′37″ W) each contained one field site consisting of four traps (Fig. 1). There were sentinel chicken seroconversions for WNV in 2004 in all three counties, and, based on historic sentinel chicken seroconversion rates, all three counties were predicted to have arbovirus activity in 2005. The transmission periods for SLEV and presumably WNV in Florida are broken down into four periods: maintenance, January through March; amplification, April through June; early transmission, July through September; and late transmission, October through December (Day and Curtis 1993; Shaman et al. 2002, 2004). Mosquito trapping was conducted from late June to mid-November in 2005, encompassing the expected transmission periods of WNV and SLEV in Florida. The three field sites encompassed a variety of Florida ecosystems. The Duval County site is considered a Florida scrub ecosystem with variety of pine trees and saw palmetto [Serenoa repens (Bartr.)] (Myers 1990), whereas the Indian River County and Manatee county sites are both considered temperate hardwood forests (Platt and Schwartz 1990). The Indian River County site is located in a small cabbage palm [Sabal palmetto (Walter) Loddiges ex Schultes & Schultes] hammock surrounded by cultivated orange and palm groves and has extensive ground cover not commonly found in hardwood hammocks. The Manatee County site is a hardwood forest frequently inundated with standing water after rainfall events, although it is not wet enough to be considered a hydric hammock swamp (Ewel 1990). In the Manatee County and Indian River County sites, local mosquito control districts had sentinel chicken coops located nearby (<100 m), which are tested routinely by the Florida Department of Health as part of their arbovirus surveillance effort. This provided additional serosurveillance data concurrent with our trapping efforts. The field site in Duval County was located on a private Navy military air field, and it was further away from the nearest sentinel chicken coop (≥20 km).
Fig. 1.
Field sites in Florida during June 2005 through November 2005. Each specific location contained four traps. At each field site, traps were set weekly at dusk and collected at dawn the following morning.
Research was conducted in compliance with the Animal Welfare Act and other Federal statues and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The facility where this research was conducted is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Specimen Collection
Weekly mosquito collections were made from each site using chicken-baited lard can traps. Lard can traps are designed to capture bird-feeding mosquitoes, and they will capture all mosquitoes attracted to the bait animal. These traps have been shown to predominantly capture Culex spp., including Cx. nigripalpus (Bellamy and Reeves 1952, Davies 1971, Rutledge et al. 2003). Lard can traps allow for the detection of mosquito species-specific arbovirus transmission events. Each lard can trap was baited with a 4–6-wk-old chicken identified with a numbered leg band and restrained in a mesh stocking which prevented movement but still allowed mosquitoes to feed. Before field exposure, chickens were reared in mosquito-proof cages, and each chicken was used in the field only once. Four lard can traps were placed at sunset at predetermined locations 20–30 m apart. Traps were retrieved the following morning at sunrise. At sunrise, chickens were removed from the traps, transported back to the laboratory, and held in a cage with the other chickens from the same trap night. One unexposed control chicken was also placed in each cage to control for the possibility of bird-to-bird virus transmission.
Mosquitoes were anesthetized using triethylamine (Kramer et al. 1990) and then removed from each trap. They were transported back to the laboratory, and sorted into pools of ≤50 mosquitoes based on date, trap, species, and bloodfed status. The bloodfed status was determined by visual inspection of each mosquito, which would not detect mosquitoes that had ingested trace amount of blood from the chicken. Sorted mosquitoes were then stored at −80°C for later processing. Chickens were bled 11–14 d after field exposure and ≥0.5 ml of sera was separated from the blood using a centrifuge and stored at −80°C for later testing.
Plaque Assay for Detection of WNV and SLEV in Mosquitoes
Before virus assays, two 4.5-mm zinc-plated beads (BB-caliber air gun shot) and 0.9 ml of BA-1 diluent (medium 199 with Earle’s salts, 10% bovine serum albumin, 10% penicillin/streptomycin, 4% Fungizone, 0.6% sodium bicarbonate, 6 g of Tris, pH 7.6, titrated with 1 N HCl to a pH of 7.4 in a total volume of 1 liter with Milli-Q sterile distilled water) were added to each mosquito pool. Samples were homogenized at 25 Hz for 3 min (TissueLyser, QIAGEN, Valencia, CA) and centrifuged at 4°C and 3,148 × g for 4 min. Mosquito pools were tested for virus presence by plaque assay in 12-well tissue culture plates (Costar, Corning, NY) containing monolayers of African green monkey kidney (Vero) cells. Plaque assays were carried out by inoculating monolayers with 0.1 ml of samples from the homogenized pool and incubating for 30 min at room temperature to allow virus to attach to cells. Subsequently, cells were over-laid with a 0.8% agarose solution and plates were incubated in a 5% CO2 environment at 35°C. After 48 or 120 h, samples tested for WNV or SLEV, respectively, were subjected to a second overlay containing 0.8% agarose solution with 0.0006% neutral red stain. The timing of the second overlay is specific to the virus being tested. Samples were scored as virus-positive or-negative 24 h after the second overlay based on presence or absence of plaques. Original samples were returned to a −80°C freezer for storage until later confirmation of virus presence by real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR).
RNA Extraction and Quantitative Real-Time Taq-Man Quantitative (q)RT-PCR Detection of WNV and SLEV in Mosquitoes
Virus-positive samples were confirmed by real-time TaqMan qRT-PCR. Viral RNA was extracted from 0.250 ml of the original mosquito pool and eluted in 0.050 ml of buffer with the MagNA Pure LC System and Total Nucleic Acid Isolation kit (Roche Diagnostics, Mannheim, Germany). Presence of viral RNA in each sample was determined using the LightCycler 480 system (Roche Diagnostics) and Superscript III One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA). qRT-PCR was carried out as described previously (Lanciotti et al. 2000, Lanciotti and Kerst 2001). In a 20-µl total volume, this assay included 5 µl of viral RNA, 20 pmol of each primer: WNV forward 5′-3′: TCAGCGATCTCTCCACCAAAG [nucleotides 1160–1180]; WNV reverse 5′-3′: GGGTCAGCACGTTTGTCATTG [nucleotides 1209–1229]; SLEV forward 5′-3′: CTGGCTGTCGGAGGGATTCT [nucleotides 2420–2439]; SLEV reverse 5′-3′: TAGGTCAATTGCACATCCCG [nucleotides 2487–2468]), and 4 pmol of probe: WNV 5′6-FAM/BHQ-2: TGCCCGACCATGGGAGAAGCTC [nucleotides 1186–1207], SLEV 5′6-FAM/BHQ-2: TCTGGCGACCAGCGTGCAAGCCG (nucleotides 2444–2466). Samples were amplified using theLC480 (Roche Diagnostics) with the following temperature cycling protocol: 48°C for 30 min, 95°C for 2 min, 40 cycles of alternating temperatures of 95°C for 10 s and 60°C for 15 s, followed by 50°C for 30 s. Standard curves were created based on data acquired from 10-fold serial dilutions of WNV and SLEV stocks of known concentration.
Plaque Reduction Neutralization Test for Detection of WNV and SLEV Antibodies in Chicken Serum
Serum samples were diluted 1:10 with medium 199 (with Earle’s salts, 10% fetal bovine serum, penicillin/streptomycin, and Fungizone) and heat inactivated at 56°C for 30 min. Equal volumes of serum samples and either SLEV (strain TBH28-SM11) or WNV (strain WN-FL03p2–3) were mixed for a final expected concentration of 100 plaque-forming units (PFU)/0.1 ml and incubated at 35°C for 1 h. Then, 0.1 ml of the mixture was added to six-well tissue culture plates (Costar) containing monolayers of Vero cells and incubated for 30 min at room temperature to allow virus to attach to cells. Subsequently, cells were overlaid with a 0.8% agarose solution and plates were incubated in a 5%CO2 environment at 35°C. After 48 h or 120 h, samples tested for WNV or SLEV, respectively, were subjected to a second overlay containing 0.8% agarose solution with 0.0006% neutral red stain. Plates were scored 24 h after the second overlay and samples exhibiting >75% plaque reductions were tested further. Such samples were serially diluted two-fold from 1:20 to 1:1,280 and virus specific first and second overlays of the plaque reduction neutralization test were carried out as previously described. Serum titers were determined for samples that caused ≥90% plaque reduction as compared with controls lacking serum.
Statistical Analysis of Trap Collections
Trap count data were compared using a multiple analysis of variance (ANOVA) analysis using JMP version 4 (SAS Institute 2001). The data were analyzed against site, trapping week, and trap location with the number of female Cx. nigripalpus collected as the dependent variable. Trap location was specific to each site, and the trap location term was nested within the site term for the statistical analysis. Trapping week was coded using the epidemiological week value for each week trapping was conducted because trapping was not conducted on the same night at all sites. When a positive mosquito pool was identified, the minimum infection rate (MIR) per 1,000 mosquitoes was calculated using all mosquitoes of the same species captured the same night from all traps combined at the site. A multiple ANOVA analysis using JMP version 4 (SAS Institute 2001) was used to compare the MIR and transmission rates against site and trapping week.
Results
We captured 20 different mosquito species from all three sites, totaling 166,615 female mosquitoes. The Indian River County collection site yielded the greatest number of species (16). Of the 20 species captured, six species were reported from all three sites (Table 1), and the exact species composition varied between sites. The most common species collected from all three sites were Cx. nigripalpus (98.18%), Ochlerotatus atlanticus (Dyar & Knab) (0.82%), and Culex erraticus (Dyar & Knab) (0.42%). Other species collected included Aedes albopictus (Skuse), Psorophora ciliata (F.), Aedes vexans (Meigen), Ochlerotatus fulvus pallens (Ross); Ochlerotatus taeniorhynchus (Weidemann), Culex quinquefasciatus Say, Ochlerotatus triseriatus (Say), Anopheles crucians Weidemann, Culex pilosus (Dyar & Knab), Psorophora ferox (von Humboldt), and Culex territans Walker.
Table 1.
Mosquito species captured at each of the three field sites
| Species | Duval County | Indian River County | Manatee County | |||
|---|---|---|---|---|---|---|
| No. captured |
% from site |
No. captured |
% from site |
No. captured |
% from site |
|
| Cx. nigripalpus | 42,973 | 96.17 | 103,552 | 99.52 | 17,062 | 95.45 |
| Oc. atlanticus | 1,349 | 3.02 | 11 | 0.01 | 1 | <0.01 |
| Cx. erraticus | 55 | 0.12 | 229 | 0.22 | 415 | 2.32 |
| Mansonia dyari Belkin, Heinemann & Page | 17 | 0.02 | 239 | 1.34 | ||
| Ochlerotatus infirmatus (Dyar & Knab) | 68 | 0.15 | 106 | 0.10 | 13 | 0.07 |
| Psorophora columbiae (Dyar & Knab) | 109 | 0.24 | 77 | 0.07 | ||
| Mansonia tittilans (Walker) | 1 | <0.01 | 27 | 0.03 | 77 | 0.43 |
| Cx. salinarius | 92 | 0.21 | 1 | <0.01 | - | |
| Coquillettidia perturbans (Walker) | 16 | 0.04 | 1 | <0.01 | 65 | 0.36 |
| Othera | 21 | 0.05 | 34 | 0.03 | 4 | 0.02 |
| Total | 44,684 | 104,055 | 17,876 | |||
Other species include Ae. albopictus, Ps. ciliata, Ae. vexans, Oc. fulvus pallens (Ross), Oc. taeniorhynchus (Weidemann), Cx. quinquefasciatus Say, Oc. triseriatus (Say), An. crucians, Cx. pilosus, Ps. ferox, and Cx. territans.
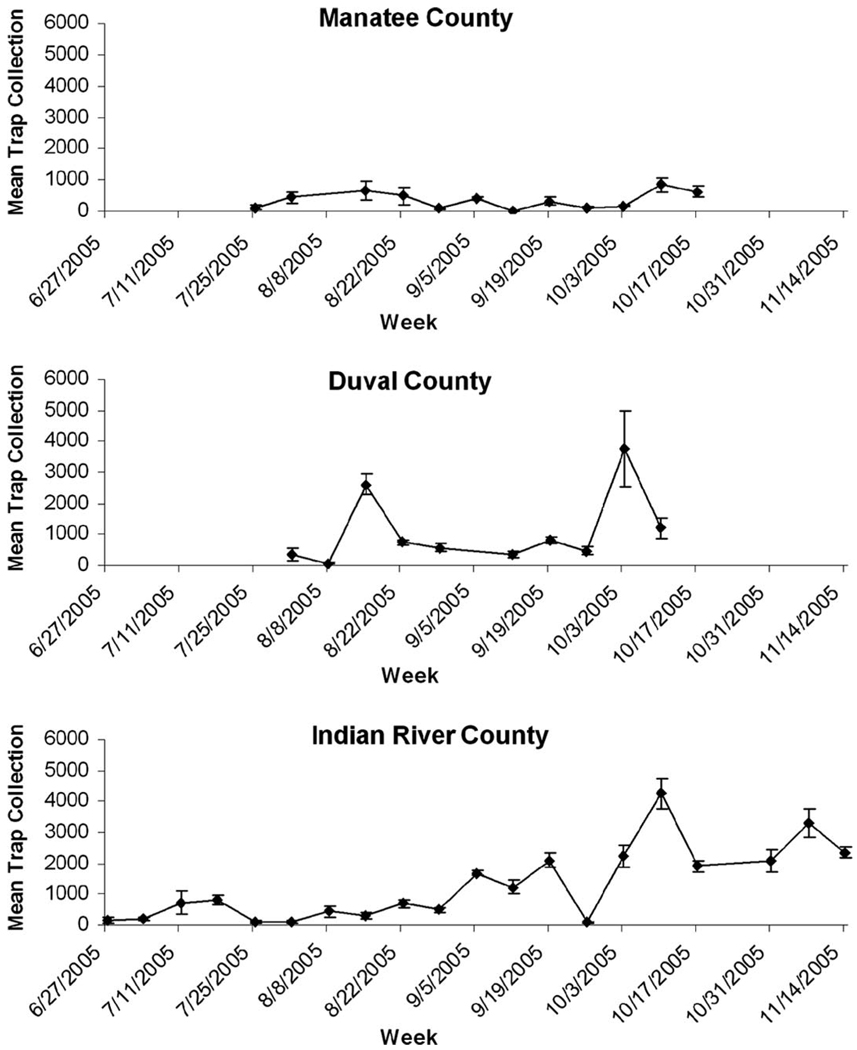
Cx. nigripalpus abundance varied temporally, with the Duval County and Indian River County sites showing a distinct peak in the number captured in the lard can traps in October (Fig. 2). The Manatee County site showed a much smaller increase in the abundance of Cx. nigripalpus in October. Due to the high proportion of Cx. nigripalpus in our collections, only this species was used for the statistical analysis. Our analysis indicated that trap location within each site did not influence the number of Cx. nigripalpus collected (Table 2). Both week and site had a significant effect on the number of Cx. nigripalpus collected as well as a significant interaction term (Table 2). This interaction indicates that the population changes differed between the sites, potentially due to Manatee County having a smaller increase in Cx. nigripalpus abundance in the fall than the other two field sites.
Fig. 2.
The mean number ± SE of adult female Culex nigripalpus caught in 2005. Duval County and Indian River County show an increased number of Cx. nigripalpus caught starting in October 2005. Manatee County shows a slight increase in October, but abundance is not significantly different from the previous weeks.
Table 2.
Effects of week site, and trap location on the no. of Cx. nigripalpus collected, results of multiple ANOVA
| df | Sum of squares |
F ratio | P | |
|---|---|---|---|---|
| Week | 1 | 17,403,389 | 20.1324 | <0.0001 |
| Trap location (site) | 9 | 5,144,027 | 0.6612 | 0.7428 |
| Site | 2 | 28,031,023 | 16.2133 | <0.0001 |
| Week × site | 2 | 8,694,594 | 5.0290 | 0.0077 |
| Week × trap | 9 | 2,834,987 | 0.3644 | 0.9502 |
| location (site) |
The trap location variable is nested within the site variable.
All 166,615 mosquitoes were sorted into a total of 4,009 single-species pools for testing, with a range of 1–50 mosquitoes per pool and a mean value of 42 mosquitoes per pool. The majority of these pools consisted of Cx. nigripalpus (3,489 pools, or >87%), although all mosquitoes were pooled and tested. We tested 553 in total pools from Manatee County (399 Cx. nigripalpus pools), 2,357 pools from Indian River County (2,188 Cx. nigripalpus pools), and 1,099 pools from Duval County (902 Cx. nigripalpus pools).
We found eight WNV-positive mosquito pools that were geographically and temporally distinct, with at least one positive pool from each field site (Table 3). Of the eight mosquito pools found positive, six of them came from Duval County over a 5-wk period. All positive pools consisted of Cx. nigripalpus mosquitoes. All but one of the positive pools consisted only of bloodfed mosquitoes, indicating that in seven of these cases a virus-positive mosquito fed on a chicken. The remaining positive pool consisted entirely of unfed mosquitoes, suggesting that the chicken may not have been exposed although it is possible that an infected mosquito from the positive pool probed the chicken or ingested a trace amount of blood. There were no SLEV-positive pools identified. Minimum infection rates were calculated for each night a positive mosquito sample was found (Table 3). MIR values did not differ significantly geographically or temporally, nor was there a significant interaction between these variables (Table 4).
Table 3.
West Nile virus-positive mosquito pools collected from three field sites in 2005
| Collection date |
Field site | Total no. of mosquitoes from all traps |
Total no. of species in all traps |
Positive pools | MIR per 1,000a |
||||
|---|---|---|---|---|---|---|---|---|---|
| Species positive |
Size of pool |
Bloodfed | Total no. collected |
Chicken seropositive |
|||||
| 23 Aug. 2005 | Duval | 3,142 | 8 | Cx. nigripalpus | 50 | Yes | 2,975 | No | 0.34 |
| 30 Aug. 2005 | Duval | 2,354 | 5 | Cx. nigripalpus | 50 | Yes | 2,302 | Nob | 1.30c |
| 30 Aug. 2005 | Duval | 2,354 | 5 | Cx. nigripalpus | 50 | No | 2,302 | Nob | 1.30c |
| 30 Aug. 2005 | Duval | 2,354 | 5 | Cx. nigripalpus | 50 | Yes | 2,302 | Nob | 1.30c |
| 1 Sept. 2005 | Manatee | 372 | 5 | Cx. nigripalpus | 27 | Yes | 341 | No | 2.93 |
| 20 Sept. 2005 | Duval | 3,780 | 9 | Cx. nigripalpus | 50 | Yes | 3,247 | Yes | 0.31 |
| 30 Sept. 2005 | Duval | 1,979 | 7 | Cx. nigripalpus | 50 | Yes | 1,904 | No | 0.53 |
| 1 Nov. 2005 | Indian River | 8,389 | 6 | Cx. nigripalpus | 50 | Yes | 8,305 | No | 0.12 |
Due to the proximity of each trap to the others, it is likely the traps collected mosquitoes from the same host seeking mosquito pop. Thus, the MIR per 1,000 value is based on the total no. of the same species capture at the trap site on the same night by combining data from the four traps. Analyzing the collections from the individual traps separately would imply that the mosquito populations are distinct from each other.
Each positive pool collected in DUV on 30 August 2005 came from a different trap, so a total of three chickens were potentially exposed to a virus-positive mosquito.
On 30 August 2005 in DUV, three traps each had one positive pool. The MIR is based on all four trap collections combined.
Table 4.
Effects of week and site on the calculated MIR results of multiple ANOVA
| df | Sum of squares | F ratio | P | |
|---|---|---|---|---|
| Week | 1 | 0.019532 | 0.0720 | 0.0790 |
| Site | 2 | 0.738095 | 1.3596 | 0.270 |
| Week × site | 2 | 0.074011 | 0.1363 | 0.873 |
In total, 169 chickens were used for field collections, and 43 chickens were used as controls for testing bird-to-bird transmission. Of the field-exposed chickens, three died while in the field, leaving a total of 209 birds tested for virus antibodies. One of 209 chickens tested positive for WNV antibodies at a dilution 1:640 with a >90% plaque reduction (Table 3). Because eight chickens were potentially exposed to WNV-infected mosquitoes this shows only one instance of arbovirus transmission from mosquito to bird. This transmission event occurred in Duval County on 9 September 2005. The Duval County study site had the highest level of arboviral activity, with six of the eight mosquito pools that were positive for WNV. There was no evidence of chicken-to-chicken transmission of WNV as none of the control chickens were WNV-positive. No chickens tested positive for SLEV antibodies. Transmission rates were not analyzed statistically due to small sample size.
Discussion
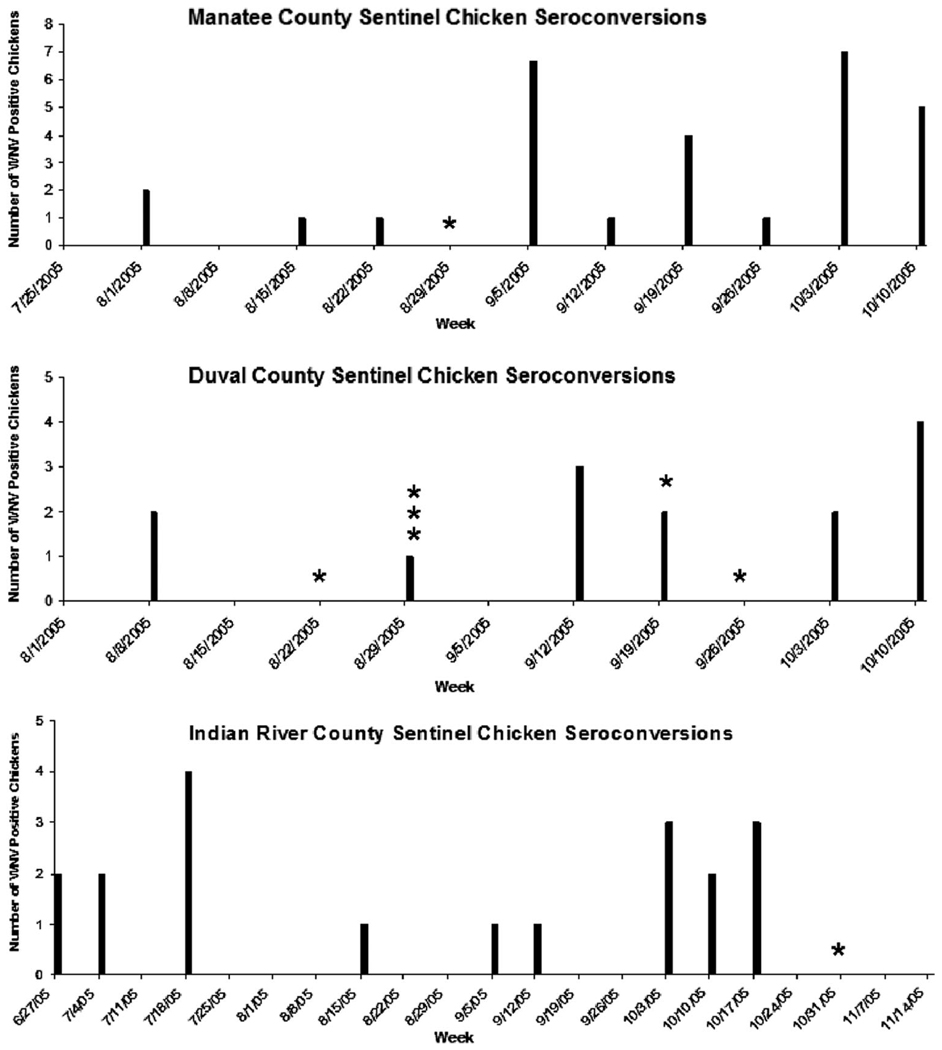
In 2005, 27 counties in Florida reported WNV activity in sentinel or wild birds, mosquito pools, human cases, or horse cases (Florida Department of Health 2005). Statewide surveillance data indicated that the counties we studied had sentinel chicken seroconversions attributed to WNV (Florida Department of Health 2005) concurrent with our trapping effort (Fig. 3). Manatee County Mosquito Control reported a total of 42 WNV sentinel chickens seroconversions in 2005, 29 of which occurred concurrently with our trapping effort. Eight of the WNV sentinel chicken seroconversions reported in Manatee County occurred within 2 wk after the positive mosquito pool we collected on 1 September 2005. This included three sentinel chickens from the sentinel chicken coop located near our trapping site that were identified as seropositive on 9 September 2005 and an additional bird from the same coop identified as seropositive on 12 September 2005. These chickens were likely infected with WNV around the same period of time that we collected our single WNV-positive mosquito pool in Manatee County, indicating that transmission was occurring despite the fact our bait chicken did not seroconvert. Duval County (City of Jacksonville Mosquito Control District) reported a total of 16 WNV sentinel chicken seroconversions in 2005 and 14 were concurrent with our trapping effort. Eight of these 14 seroconversions occurred in August or September, similar to our results. Duval County also reported one human case of WNV in mid-October 2005. Indian River County Mosquito Control reported a total of 19 WNV sentinel chicken seroconversions in 2005, all of which were concurrent with our trapping effort. Two of these 19 seroconversions occurred at the sentinel chicken coop located near our trapping site, on 15 September and 6 October 2005. Indian River County did not have any instances of sentinel chicken seroconversion contemporaneous with the positive mosquito pool from this study on 1 November 2005.
Fig. 3.
Sentinel chicken seroconversions detected by the county mosquito control districts concurrent with our trapping efforts in 2005. The stars represent each positive mosquito pool that we collected from each county. Indian River County had one WNV seroconversion in the sentinel chicken flock located near our trapping site on 12 September 2005 and 3 October 2006. Manatee County had three WNV seroconversions in the sentinel flock located near our trapping site during the week of 5 September 2005, and an additional seroconversion located in the same flock during the week of 12 September 2005. There was no sentinel flock located near the trapping site in Duval County.
As evidenced by the sentinel chicken surveillance data, WNV was found at all three trapping locations during the arbovirus transmission period. The sentinel chicken surveillance data suggest that our trapping methods may be able to identify periods of increased infection and transmission, as seen in the Manatee County sentinel chicken surveillance in early September. However, our trapping methods are less likely to identify sporadic mosquito infection and transmission that may routinely occur during the arbovirus transmission season, as seen in Indian River County. Indian River County reported two seroconversions for SLEV, and Manatee County reported one seroconversion for SLEV, none of which occurred in the sentinel chicken coops near our trapping sites. Duval County did not report any seroconversions for SLEV. We found no infection or transmission of SLEV in our trapping effort.
All virus isolates came from pools of Cx. nigripalpus, which is not surprising given the low frequency of other species in our traps. The relative abundance of Cx. nigripalpus versus other mosquito species was possibly due to an inherent bias in the trap design (J.F.D., unpublished data), although other Culex species, especially Cx. quinquefasciatus, have been shown to enter these traps (Rutledge et al. 2003). The small sample sizes of other species limited our ability to determine their vector status. Previous reports of Florida mosquitoes have indicated the presence of WNV in other genera, although Culex spp. mosquitoes are most often found positive (Blackmore et al. 2003, Godsey et al. 2005). field and laboratory evidence has implicated Cx. nigripalpus as a vector for both SLEV and WNV in Florida (Tsai and Mitchell 1988, Shroyer 1991; Turell et al. 2001, 2005; Rutledge et al. 2003; Godsey et al. 2005; Colton and Nasci 2006), and our trapping methods allow us to focus on that species. Cx. nigripalpus is an opportunistic feeder, and it shifts its feeding behavior from avian to generalist in late summer and early autumn (Edman and Taylor 1968, Gomes et al. 2003, Day 2005). This shift in feeding behavior coincides with a general increase in mosquito population observed at all three sites, although the increase observed in Manatee County was much smaller. As the occurrence and abundance of Cx. nigripalpus increases, this species may be more likely to encounter mammalian hosts, including humans. Thus, in addition to its role in enzootic transmission, Cx. nigripalpus infection and transmission rates may be used as an indicator of the risk of epidemic transmission to humans.
Our data indicate that field transmission of WNV to restrained chickens is much lower than observed mosquito infection rates, although our sample size consisted of only eight positive mosquito pools. We found transmission occurring in only one of seven of the instances when a chicken was definitely exposed to an infected mosquito, which prevented any significant comparison of geographic or temporal influences on transmission rates. In all but one case, we are confident that the infected mosquito fed on the chicken as the WNV-positive mosquito pools consisted of only bloodfed mosquitoes. Analysis of CO2-baited lard trap collections have indicated that <0.5% of the females captures in lard cans are bloodfed when captured (C.J.V., unpublished data). Based on visual inspections, the majority of these previously bloodfed females were only a partial engorged and may be seeking an addition bloodmeal. The eighth virus-positive pools consisted of non-bloodfed mosquitoes. Minimum infection rates did not differ significantly by site or week, although the variable week was close to being significant (Table 4). Our trapping efforts were conducted during the time of year when arbovirus transmission is expected, and if we had trapped continuously throughout the year we may have seen a significant effect of trapping week on the MIR values. Our findings of low transmission rates relative to infection rates correspond with previous work examining WNV transmission in Florida conducted using similar trapping methods (Rutledge et al. 2003), although we were unable to analyze any temporal or geographic variation in transmission rates due to our small sample size. Studies examining field transmission of SLEV and WEEV also reported similar results, with infection rates 4 times greater than transmission rates (Reeves et al. 1961). Low transmission rates may be the result of a number of factors including low titers in the infectious bloodmeal (Tiawsirisup et al. 2005) and salivary gland barriers (Hardy et al. 1983). The most likely explanation is the lack of viral dissemination in mosquitoes. Because mosquito samples were pooled, it was not possible to differentiate between disseminated and non-disseminated infected mosquitoes.
Several vector competence experiments have indicated there is a high probability for transmission to occur when there is viral dissemination, and dissemination rates in virus infected mosquitoes are dependent upon the temperature at which the mosquitoes are held in laboratory conditions (Turell et al. 2006a, 2006b). Dissemination and transmission rates for Cx. nigripalpus and other mosquito species infected with WNV have been studied under laboratory conditions, and vector competence varies between species. Although Cx. nigripalpus seems to disseminate the virus at lower rates than other Culex species (Turell et al. 2005), it is still a competent laboratory vector (Colton and Nasci 2006). Laboratory analysis using an established colony of Cx. quinquefasciatus indicates that dissemination rates at temperatures between 25 and 28°C can be relatively low, although dissemination rates at 30°C significantly increase (Richards et al. 2007; Dohm et al. 2002). Temperature records from the National Climatic Data Center at the National Oceanic and Atmospheric Administration (NOAA) indicate that all three study sites experienced a minimum of 57 d of >30°C temperatures from August through October (NOAA 2006), likely indicating sufficient extrinsic incubation temperatures (EIT) necessary for high levels of dissemination (Richards et al. 2007; Dohm et al. 2002).
A lack of dissemination under natural conditions may simply be due to the lack of sufficient time for infected mosquitoes to complete the extrinsic incubation period (EIP) for WNV. Cx. nigripalpus females are able to complete egg development after a bloodmeal in ≈72 h to 96 h, depending on the EIT (Nayar and Knight 1981, Day and Edman 1988). It is possible that at EITs <30°C and when oviposition sites are plentiful, infected mosquitoes may not have sufficient time complete the EIP necessary for transmission before the mosquito completes a gonotropic cycle and searches for a subsequent bloodmeal. In 2005, there were regular wetting events at all three field locations (NOAA 2006), potentially allowing for a rapid completion of the gonotropic cycle and a subsequent feeding by an infected mosquito. This rapid completion of the gonotropic cycle may have contributed to the lack of transmission observed in our trapping surveys, as the EIP may not have been of adequate length.
Dissemination may take longer at EITs <30°C, compared with higher EITs, but if the mosquito population is allowed sufficient time for virus incubation, they may still reach high rates of dissemination. A laboratory population of Cx. quinquefasciatus held at 26°C reached >80% dissemination after 20 d, although populations held at lower EITs did not surpass 45% dissemination even after >30 d (Dohm et al. 2002). During drought conditions when suitable oviposition sites are rare, gravid Cx. nigripalpus females are able to retain their eggs for an extended period, up to 5 mo (Day and Edman 1988, Day and Curtis 1989). A drought may enhance future arbovirus transmission by allowing sufficient time for infected mosquitoes to complete the EIP during a single gonotropic cycle.
We observed that infection rates, and minimum field infection rates (MFIR) are not necessarily equivalent to transmission rates. The relationship between infected mosquitoes and infectious mosquitoes (infected mosquitoes capable of transmission) may be affected by factors such as EIT and time. Because an infected mosquito may be incapable of transmitting a virus at a particular point in time, an estimate based on the mosquito infection rates may overestimate risk of transmission to humans. Sentinel chicken seroconversion may be sufficient predictor for human risk, but it is critical to remember that this indicator may underestimate the arboviral infection rate in a mosquito population.
By increasing our understanding of how arbovirus infection and transmission rates in mosquitoes correlate under field conditions and determining what factors influence changes in transmission rates, we may be able to anticipate conditions when there is a greater risk of transmission to humans. Assessing the time periods that involve increased risk to humans should increase the efficacy of education, mosquito control, and personal protection measures, reducing the overall risk of transmission to human populations. Ongoing studies will expand this research over multiple years and allow further analysis of potential factors that may influence infection and transmission rates.
Acknowledgments
We appreciate the assistance of Robert Frommer, Todd Walker, Donald Shroyer, and Marah Clark in site selection. We also thank the United States Navy for allowing us to trap at the Whitehouse Air Field. Roxanne Rutledge and Michael Reiskind provided appreciated comments on the manuscript. Sara Lynn, Hilda Lynn, Carol Thomas, and Dadrie Baptiste provided assistance in the field and in the laboratory. We thank the Manatee County Mosquito Control District and the Jacksonville Mosquito Control Division for the use of facilities. This research was funded by National Institutes of Health grant R01 AI-042164.
References Cited
- Bellamy RE, Reeves WC. A portable mosquito bait-trap. Mosq. News. 1952;12:256–258. [Google Scholar]
- Blackmore CG, Stark LM, Jeters WC, Oliveri RL, Brooks RG, Conti LA, Wiersma ST. Surveillance results from the first West Nile virus transmission season in Florida, 2001. Am. J. Trop. Med. Hyg. 2003;69:141–150. [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Outbreak of West Nile-like viral encephalitis–New York,1999. Morb. Mortal. Wkly. Rep. 1999;48:845–849. [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Update: West Nile virus activity–eastern United States,2000. Morb. Mortal. Wkly. Rep. 2000;49:1044–1047. [PubMed] [Google Scholar]
- Chamberlain RW, Sudia WD, Coleman PH, Beadle LD. Vector studies in the St. Louis encephalitis epidemic, Tampa Bay area, Florida. Am. J. Trop. Med. Hyg. 1964;13:456–461. doi: 10.4269/ajtmh.1964.13.456. [DOI] [PubMed] [Google Scholar]
- Colton L, Nasci RS. Quantification of West Nile virus in the saliva of Culex species collected from the Southern United States. J. Am. Mosq. Control Assoc. 2006;22:57–63. doi: 10.2987/8756-971X(2006)22[57:QOWNVI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Davies JB. A small mosquito trap for use with animal or carbon dioxide baits. Mosq. News. 1971;31:441–443. [Google Scholar]
- Day JF. Host-seeking strategies of mosquito disease vectors. J. Am. Mosq. Control Assoc. 2005;21S:17–22. doi: 10.2987/8756-971X(2005)21[17:HSOMDV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Day JF, Edman JD. Host location, blood-feeding and oviposition behavior of Culex nigripalpus (Diptera: Culicidae): their influence on St. Louis encephalitis virus transmission in southern Florida. In: Scott TW, Grumpstrup-Scott G, editors. Proceedings of a symposium: the role of vector-host interactions in disease transmission. Lanham, MD: Entomological Society of America; 1988. pp. 1–8. Miscellaneous Publication 68. [Google Scholar]
- Day JF, Curtis GA. Influence of rainfall on Culex nigripalpus (Diptera: Culicidae) blood-feeding behavior in Indian River County, Florida. J. Med. Entomol. 1989;82:32–37. doi: 10.1093/jmedent/27.1.43. [DOI] [PubMed] [Google Scholar]
- Day JF, Curtis GA. Annual emergence patterns of Culex nigripalpus females before, during and after a widespread St. Louis encephalitis epidemic in South Florida. J. Am. Mosq. Control Assoc. 1993;9:249–255. [PubMed] [Google Scholar]
- Day JF, Curtis GA. Blood feeding and oviposition by Culex nigripalpus (Diptera: Culicidae) before,during, and after a widespread St. Louis encephalitis virus epidemic in Florida. J. Med. Entomol. 1999;36:176–181. doi: 10.1093/jmedent/36.2.176. [DOI] [PubMed] [Google Scholar]
- Day JF, Stark LM. Transmission patterns of St.Louis encephalitis and Eastern equine encephalitis viruses in Florida: 1978–1993. J. Med. Entomol. 1996;33:132–139. doi: 10.1093/jmedent/33.1.132. [DOI] [PubMed] [Google Scholar]
- Dohm DJ, O’Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Dow RP, Coleman PH, Meadows KE, Work TH. Isolation of St. Louis encephalitis viruses from mosquitoes in the Tampa Bay area of Florida during the epidemic of 1962. Am. J. Trop. Med. Hyg. 1964;13:462–474. doi: 10.4269/ajtmh.1964.13.462. [DOI] [PubMed] [Google Scholar]
- Edman JD, Taylor DJ. Culex nigripalpus: seasonal shift in the bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science (Wash., D.C.) 1968;161:67–68. doi: 10.1126/science.161.3836.67. [DOI] [PubMed] [Google Scholar]
- Ewel KC. In: Swamps. Myers R, Ewel J, editors. Orlando, FL: Ecosystems of Florida University of Central Florida Press; 1990. pp. 281–317. [Google Scholar]
- Florida Department of Health. Florida mosquito-borne disease summary for 2003. 2003 ( http://www.doh.state.fl.us/Environment/community/arboviral/pdfs/2003/Summary_2003.pdf)
- Florida Department of Health. Florida 2005 arbovirus activity by County. 2005 ( http://www.doh.state.fl.us/Environment/community/arboviral/pdf_files/2005_Final_Arboviral_Summary.pdf)
- Girard TA, Klingler KA, Higgs S. West Nile virus dissemination and tissue tropism in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoonotic Dis. 2004;4:109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- Godsey MS, Blackmore MS, Panella NA, Burkhalter K, Gottfried K, Halsey LA, Rutledge R, Langevin SA, Gates R, Lamonte KM, et al. West Nile virus epizootiology in the Southeastern United States, 2001. Vector-Borne Zoonotic Dis. 2005;5:82–89. doi: 10.1089/vbz.2005.5.82. [DOI] [PubMed] [Google Scholar]
- Gomes AC, Silva NN, Marques GRAM, Brito M. Host-feeding patterns of potential human disease vectors in the Paraíba Valley Region, State of São Paolo, Brazil. J. Vector Ecol. 2003;28:74–78. [PubMed] [Google Scholar]
- Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- Hayes C. West Nile fever. In: Monath T, editor. Arboviruses: epidemiology and ecology. vol. V. Boca Raton, FL: CRC; 1989. pp. 59–88. [Google Scholar]
- Komar NS, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Inf. Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD, Presser SB, Houk EJ, Hardy JL. Effect of the anesthetizing agent triethylamine of western equine encephalomyelitis and St. Louis encephalitis viral titers in mosquitoes (Diptera: Culicidae) J. Med. Entomol. 1990;27:1008–1010. doi: 10.1093/jmedent/27.6.1008. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J. Clin. Microbiol. 2001;39:4506–4513. doi: 10.1128/JCM.39.12.4506-4513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the north-eastern United States. Science (Wash., D.C.) 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfin AA, Gubler DJ. West Nile encephalitis: an emerging disease in the United States. Clin. Infect. Dis. 2001;3:1713–1719. doi: 10.1086/322700. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, Francy DB, Monath TP. Arthropod vectors. In: Monath TP, editor. St. Louis encephalitis. Washington, DC: American Public Health Association; 1980. pp. 313–379. [Google Scholar]
- Myers RL. Scrub and High Pine. In: Myers R, Ewel J, editors. Ecosystems of Florida. Orlando, FL: University of Central Florida Press; 1990. pp. 150–229. [Google Scholar]
- Nayar JK, Knight JW. Ovarian development in Culex nigripalpus and its implication for disease transmission. Entomol. Exp. Appl. 1981;29:49–59. [Google Scholar]
- [NOAA] National Oceanic and Atmospheric Administration. Quality controlled local climatological data for Florida. 2006 ( http://cdo.ncdc.noaa.gov/ulcdsw/ULCD?prior=N)
- Platt WJ, Schwartz MW. Temperate hardwood forests. In: Myers R, Ewel J, editors. Ecosystems of Florida. Orlando, FL: University of Central Florida Press; 1990. pp. 194–229. [Google Scholar]
- Provost MW. St. Louis encephalitis in Florida: ten years of research, surveillance and control programs. Jacksonville, FL: Florida State Board of Health; 1969. The natural history of Culex nigripalpus; pp. 42–62. Monograph Series No. 12. [Google Scholar]
- Reeves WC, Bellamy RE, Scrivani RP. Differentiation of encephalitis virus infection rates from transmission rates in mosquito vector populations. Am. J. Hyg. 1961;73:303–315. doi: 10.1093/oxfordjournals.aje.a120190. [DOI] [PubMed] [Google Scholar]
- Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic. Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ. West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J. Med. Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- SAS Institute. JMP statistical discovery software, version 4. Cary, NC: SAS Institute; 2001. [Google Scholar]
- Sardelis MR, Turell MJ, Dohm DJ, O’Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Inf. Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Day JF, Stieglitz M. Drought-induced amplification of Saint Louis encephalitis virus, Florida. Emerg. Inf. Dis. 2002;8:575–580. doi: 10.3201/eid0806.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Day JF, Stieglitz M, Zebiak S, Cane M. Seasonal forecast of St. Louis encephalitis virus transmission, Florida. Emerg. Inf. Dis. 2004;10:802–809. doi: 10.3201/eid1005.030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Day JF, Stieglitz M. Drought-induced amplification and epidemic transmission of West Nile virus in southern Florida. J. Med. Entomol. 2005;42:134–141. doi: 10.1093/jmedent/42.2.134. [DOI] [PubMed] [Google Scholar]
- Shroyer DA. The 1990 Florida epidemic of St. Louis encephalitis: virus infection rates in Culex nigripalpus. J. Fla. Mosq. Control Assoc. 1991;62:69–71. [Google Scholar]
- Tiawsirisup S, Platt KB, Evans RB, Rowley WA. A comparison of West Nile virus transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse) Vector Borne Zoonotic Dis. 2005;5:40–47. doi: 10.1089/vbz.2005.5.40. [DOI] [PubMed] [Google Scholar]
- Tsai TF, Mitchell CJ. St. Louis encephalitis. In: Monath TP, editor. The arboviruses: epidemiology and ecology. vol, 4. Boca Raton, FL: CRC Press; 1988. pp. 113–143. [Google Scholar]
- Turell MJ, O’Guinn ML, Oliver J. Potential for New York mosquitoes to transmit West Nile virus. Am. J. Trop. Med. Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update of the potential of North American mosquitoes (Diptera: Culicidae)to transmit West Nile virus. J. Med. Entomol. 2005;32:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Mores CN, Dohm DJ, Lee W, Kim H, Klein T. Laboratory transmission of Japanese encephalitis, West Nile, and Getah viruses by mosquitoes (Diptera: Culicidae) collected near Camp Greaves, Gyeonggi Province, Republic of Korea, 2003. J. Med. Entomol. 2006a;43:1076–1081. doi: 10.1603/0022-2585(2006)43[1076:ltojew]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Mores CN, Dohm DH, Komilov N, Paragas J, Lee JS, Shermuhemedova D, Endy TP, Kodirov A, Khodjaev S. Laboratory transmission of Japanese encephalitis and West Nile viruses by molestus form of Culex pipiens (Diptera: Culicidae) collected in Uzbekistan in 2004. J. Med. Entomol. 2006b;43:296–300. doi: 10.1603/0022-2585(2006)043[0296:ltojea]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Zyzak M, Loyless T, Cope S, Wooster M, Day JF. Seasonal abundance of Culex nigripalpus Theobald and Culex salinarius Coquillett in north Florida. J. Vector Ecol. 2002;27:155–162. [PubMed] [Google Scholar]