Abstract
Activating EGFR mutations are common in many cancers including glioblastoma. However, clinical responses to EGFR inhibitors are infrequent and short-lived. We demonstrate that the Src family kinases (SFKs) Fyn and Src are effectors of oncogenic EGFR signaling, enhancing invasion and tumor cell survival in vivo. Expression of a constitutively active EGFR mutant, EGFRvIII, resulted in activating phosphorylation and physical association with Src and Fyn, promoting tumor growth and motility. Gene silencing of Fyn and Src limited EGFR and EGFRvIII-dependent tumor cell motility. The SFK inhibitor dasatinib inhibited invasion, promoted tumor regression and induced apoptosis in vivo, significantly prolonging survival of an orthotopic glioblastoma model expressing endogenous EGFRvIII. Dasatinib enhanced the efficacy of an anti-EGFR monoclonal antibody (mAb 806) in vivo, further limiting tumor growth and extending survival. Examination of a large cohort of clinical samples demonstrated frequent coactivation of EGFR and SFKs in glioblastoma patients. These results establish a mechanism linking EGFR signaling with Fyn and Src activation to promote tumor progression and invasion in vivo and provide rationale for combined anti-EGFR and anti-SFK targeted therapies.
Keywords: Fyn, Src, EGFR, glioblastoma, targeted therapy
Introduction
EGFR is mutated in many cancers, including up to 45% of glioblastoma patients (1-4). EGFR signaling promotes PI3K pathway activation and tumor growth and survival (5). EGFR has also been linked to the invasive behavior of glioblastomas (6, 7). Glioblastoma cells diffusely infiltrate the surrounding brain, including into the contralateral hemisphere, limiting the efficacy of local therapies and rendering complete surgical excision impossible. The anti-EGFR antibody Cetuximab limited glioblastoma invasion in an orthotopic xenograft model (8). However, the mechanisms by which EGFR promotes glioma cell invasion are not fully understood.
The non-receptor tyrosine kinase Src was one of the first oncogenes identified, and the Src family of kinases (SFKs) collectively regulates a variety of cellular functions in many cancer types including proliferation, invasion, motility, survival, differentiation, and angiogenesis (9-11). Src greatly enhances EGFR-mediated transformation (12-15), raising the possibility that Src and/or its related family members may be effectors of mutant EGFR signaling in cancer, including in glioblastoma. Recent work indicates that Src is frequently activated in glioblastoma cell lines and patients, suggesting that this family of kinases may be important targets for therapy in glioblastoma patients (16). However, the mechanism by which Src family kinases become activated in glioblastoma, and their role in potentially modifying response to targeted therapies has yet to be elucidated. Here we uncover a molecular mechanism by which persistent EGFR signaling activates Fyn and Src to enhance glioblastoma invasion and tumor survival in vivo. We show that Fyn and Src inhibition by either genetic or pharmacologic means greatly limits tumor invasion and promotes tumor cell apoptosis, and we demonstrate the efficacy of combining the SFK inhibitor dasatinib with the targeted anti-EGFR antibody mAb 806. Demonstrating clinical relevance, we show EGFR and SFK coactivation in a large cohort of glioblastoma patients.
Materials and Methods
Microarray analysis
Gene expression profiling with Affymetrix high density oligonucleotide microarrays was performed and analyzed as previously described (17, 18).
Global phosphotyrosine profiling
Approximately 1 × 108 U87MG, U87-EGFR, and U87-EGFRvIII cells cultured in 10% FBS were stimulated with 10 ng/ml EGF (Sigma) for 5 minutes, harvested, and processed for phosphotyrosine profiling as previously described (19). Details are described in Supplementary Materials and Methods.
Cell culture
The human glioblastoma cell lines LN229, T98G, U87MG, and U138MG were purchased from the American Type Culture Collection (ATCC). U373MG was purchased from the European Collection of Cell Cultures. The primary human glioblastoma lines GM1600 and GM2345 were derived from patient tumors as described previously (20). All cells were routinely maintained in MEM containing 10% fetal bovine serum (Omega Scientific), 1x penicillin-streptomycin-glutamine (Gibco-Invitrogen), 1x non-essential amino acids, 1 mM sodium pyruvate, and 0.15% sodium bicarbonate and grown at 37°C in 5% CO2.
Western blot and immunuoprecipitation
Cells were harvested in ice-cold modified RIPA lysis buffer as previously described (21). Dasatinib (Bristol-Myers Squibb Oncology) treated cells were incubated with drug for 6 hours before lysis. Details of western blotting and immunoprecipitation are described in Supplementary Materials and Methods.
siRNA and plasmid transfection
siRNAs were kindly provided by GNF. Duplex siRNAs specifically targeting Fyn, Src, or scrambled control sequences were transfected into glioblastoma cells using 25 nM TransIT-TKO Transfection reagent (Mirus Bio Corp.) according to the manufacturer's protocol. Cells were then lysed for analysis of protein knockdown by western blot or used in invasion assays 72 hours after siRNA transfection. Fyn and Src cDNAs were generated by RT-PCR using total RNA from U87MG cells, cloned into pcDNA3.1D/TOPO (Invitrogen,) and transfected into glioblastoma cells using FuGene 6 Reagent (Roche). Transfected cells were selected in 0.5 mg/ml G418 for two weeks and surviving clones pooled for analysis.
Cell invasion assay
Six-well Transwell polycarbonate membrane inserts with 8.0 μm pores (CoStar) were coated from the bottom with 50 μg/ml growth factor-reduced Matrigel (BD Biosciences) or 50 μg/ml BSA as described previously (22). Full details are provided in Supplementary Materials and Methods.
Mouse tumor models
Generation and intracranial implantation of transformed mouse astrocytes has been described previously (23, 24). Dasatinib (17.5 mg/kg twice daily) was orally administered 7 days after tumor implantation. Bioluminescence enabled GBM39 human glioblastoma cells were generated, maintained, and orthotopically implanted as previously described (25). Dasatinib therapy (35 mg/kg twice daily) was initiated 14 days after implantation when bioluminescence imaging indicated log growth rate of tumors. U87 derived cell lines were inoculated subcutaneously into both flanks of female nude mice (Animal Research Centre, Perth, Australia) as previously reported (26). Monoclonal antibody mAb 806 (IgG2b) was produced in the Biological Production Facility (Ludwig Institute of Cancer Research, Melbourne) as described (27). Details are provided in Supplementary Materials and Methods.
Immunohistochemistry
Following anesthetization and sacrifice of mice, brains were removed and fixed in zinc-formalin overnight, then immersed in 70% ethanol and embedded into paraffin. Paraffin tissue sections were processed and stained as described in Supplementary Materials and Methods. Quantification of staining was performed using Soft Imaging System software as previously described (28)
Tissue microarray and tumor lysates
Tissue microarrays were generated and immunohistochemically stained as described previously (29). Protein lysates from frozen tumor samples were prepared as described previously (29) and 25 μg of each sample run on 10% SDS-PAGE for immunoblotting as detailed above and in Supplementary Materials and Methods.
Additional methods used in this study are described in Supplementary Materials and Methods.
Results
Multiple SFKs are expressed in glioblastoma clinical samples
To determine the expression of SFK members in glioblastoma patients, the global gene expression profiles of 41 glioblastoma clinical samples and 19 normal brain samples were analyzed using Affymetrix U133 human genome arrays. Fyn, Src, Lyn, Yes, Hck and Blk were all overexpressed in GBM relative to normal brain with varying patterns of expression (Fig. 1A). Fyn, Src, and Yes were widely expressed across all GBMs. Lyn and Hck were largely restricted to a subgroup of GBMs designated type 2B “mesenchymal”(17, 30). Fyn was most significantly correlated with EGFR gene expression, particularly in the type 2B subset (r2= 0.62, p<0.001); Hck and Blk were negatively correlated with EGFR expression. Thus, multiple SFKs are expressed in non-overlapping distrubutions, and Fyn is most tightly correlated with EGFR expression.
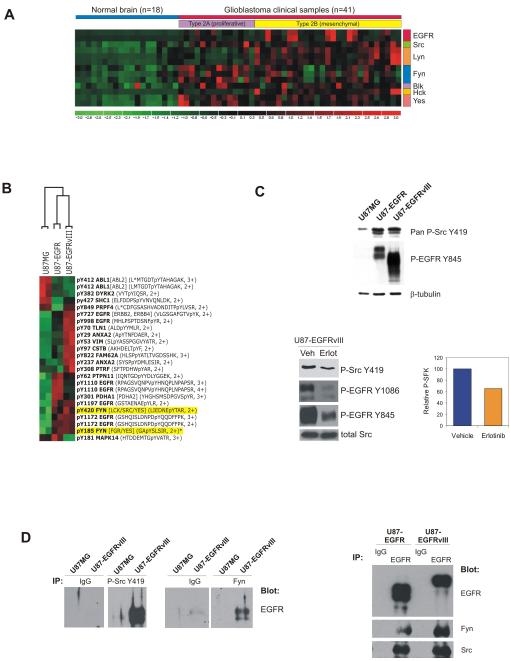
Figure 1.
SFKs are expressed in glioblastomas and are direct effectors of oncogenic EGFR signaling. A, Microarray analysis shows Fyn, Src, and Yes expression across all glioblastomas. Fyn expression was positively correlated with EGFR expression (r2=0.62, p<0.001). B, Global phosphotyrosine profiling identified Y420 and Y185 Fyn phosphorylation in association with EGFR/EGFRvIII overexpression in EGF-stimulated U87 cells. C, Western blot analysis confirmed upregulation of SFK Y419 phosphorylation and EGFR Y845 phosphorylation, a Src substrate site, in EGFR/EGFRvIII-expressing U87 cells (top). Erlotinib inhibits EGFR Y1086 autophosphorylation and mitigates SFK Y419 and EGFR Y845 phosphorylation mediated by EGFRvIII (bottom left). Quantification of P-Src Y419 inhibition due to erlotinib treatment (bottom right). D, U87MG or U87-EGFRvIII lysates immunoprecipitated with pan-P-Src Y419 or Fyn antibodies were immunoblotted to detect EGFR, demonstrating physical interaction between Fyn/phospho-SFKs and EGFRvIII (left). Right, U87-EGFR and U87-EGFRvIII lysates pulled down with EGFR antibodies were immunoblotted against EGFR, Fyn, and Src to demonstrate physical interaction between Fyn /Src and EGFR.
Fyn is a downstream phosphorylation target of EGFR signaling
To determine if any of the SFKs are EGFR targets in an unbiased screen, we performed mass spectrometry-based proteomic phosphotyrosine screening on EGF-stimulated U87MG cells and EGFR or EGFRvIII-expressing U87 cells. Fyn was among the most highly phosphorylated peptides that were differentially induced by EGFRvIII and EGFR signaling, particularly phosphorylation of its a ctivating residue Y420 (Fig. 1B). Immunoblot analysis with a pan phospho-SFK antibody confirmed increased activating SFK phosphorylation in U87-EGFR and U87-EGFRvIII cells compared to their parental counterparts, which was limited by erlotinib treatment (Fig. 1C). Phosphorylation of EGFR Y845, a Src activated site, was likewise reduced by erlotinib. Co-immunoprecipitation analysis demonstrated a physical association between EGFRvIII and Fyn, and between EGFRvIII and the activated form of SFKs (Fig. 1D). Similar results were seen with highly expressed wild-type EGFR. This physical association was diminished but not abrogated by erlotinib treatment (Supplementary Fig. S1).
To directly determine Fyn protein expression and phosphorylation in glioblastoma clinical samples, we performed western blot analyses on 15 GBM autopsy samples from which patient-matched tumor and normal brain lysates were obtained. Immunoprecipitation of Fyn protein followed by immunoblotting revealed elevated Fyn expression and activating phosphorylation in 13/15 tumor samples compared to their normal counterparts (Supplementary Fig. S2).
Fyn promotes motility of EGFR-expressing glioblastoma cells
SFKs have been implicated in tumor cell invasion and motility in different cancers. To establish a role for Fyn in promoting tumor cell motility in EGFR-driven glioblastomas, genetic inhibition of Fyn was studied in a panel of EGFR-expressing glioblastoma cell lines, including two low passage patient cultures. SFK activating phosphorylation of these lines was highly concordant with their expression of EGFR (Fig. 2A). RNAi mediated knockdown of Fyn significantly decreased tumor cell migration through matrigel as compared to untransfected cells or cells transfected with scrambled siRNA sequences (Fig. 2B). Fyn knockdown for each individual cell line was significantly correlated with its reduction in invasion (r=0.96, p=0.0403; Supplementary Fig. S3). Consistent with this observation, transfection of Fyn into U87MG cells, which express low levels of EGFR and Fyn and relatively low SFK phosphorylation (Fig. 2A), significantly promoted tumor cell migration (Fig. 2B).
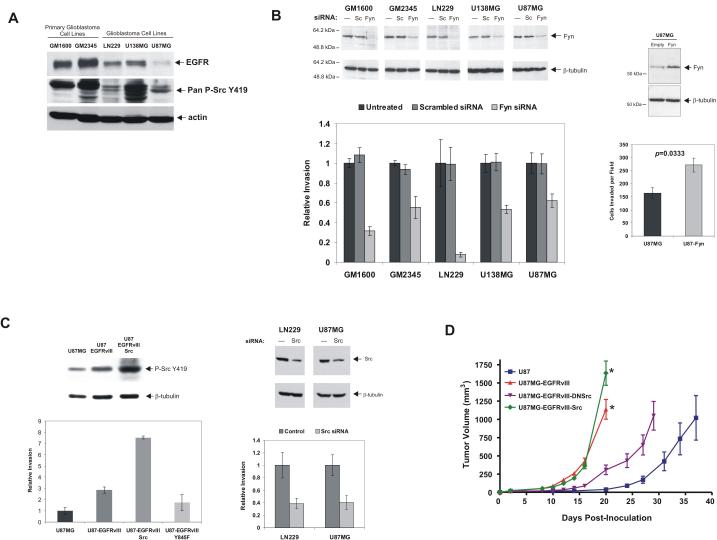
Figure 2.
Fyn and Src promote motility of EGFR-expressing glioblastoma cells. A, Primary glioblastoma cultures and established glioblastoma cell lines expressing EGFR exhibit high levels of activating SFK phosphorylation. U87MG cells express low levels of EGFR and have relatively low phospho-SFK activation. B, Glioblastoma cell lines transfected with Fyn-specific siRNAs (Fyn) demonstrated 50-90% reduction in tumor cell invasion through Matrigel compared to control scrambled siRNA (Sc) (mean P=0.017). U87MG cells transfected with Fyn cDNA were more invasive in vitro. C, EGFRvIII expression promotes SFK phosphorylation and U87 cell invasion through Matrigel. Constitutively active Src significantly enhances invasion and total SFK phosphorylation; an EGFRvIII Y845F mutant suppresses invasion. Right, siRNA mediated silencing of Src in LN229 and U87MG cells inhibits invasion. D, Subcutaneous U87 xenograft growth is significantly increased by EGFRvIII expression. Src overexpression further enhances xenograft growth whereas dominant negative Src curbs the proliferative effect of EGFRvIII. n=5 mice with 2 tumors per mouse for all time points. *p=0.03.
Src is also a mediator of aberrant EGFR signaling promoting glioblastoma motility and survival
Genetic disruption of Src enhances the efficacy of the targeted anti-EGFR antibody mAb 806 in glioblastoma xenografts (26), therefore we examined whether Src is also a phosphorylation target and mediator of aberrant EGFR signaling. Like Fyn, Src co-precipitated with EGFRvIII and wild type EGFR in U87MG cells (Fig. 1D).
Stable expression of constitutively active Src (Y528F) in U87-EGFRvIII cells greatly enhanced tumor cell migration, whereas expression of an EGFR Y845F mutant, a critical site for cooperative Src/EGFR interaction (26), resulted in significantly diminished migration through matrigel (Fig. 2C). Consistent with these findings, siRNA silencing of Src also effectively reduced in vitro invasion of EGFR-expressing LN229 glioblastoma cells (Fig. 2C). These results indicated that Src was an effector of EGFR-mediated tumor cell migration.
To examine the effect of Src signaling on EGFR-mediated glioblastoma pathogenesis in vivo, we assessed the effects of constitutively active Src (Y528F) or dominant negative (DN)Src (K296R/Y528F) on the growth of subcutaneous U87 glioblastoma xenografts. EGFRvIII conferred significantly faster tumor growth relative to parental counterparts, which was greatly abrogated by DNSrc (Fig. 2D). The constitutively activated Src allele did not initially enhance growth of EGFRvIII expressing tumors, but eventually conferred a modest growth enhancement once the tumors had become very large (Fig. 2D, p=0.03). These data demonstrate that Src is an effector of EGFRvIII-promoted glioblastoma pathogenesis in vivo. Moreover, because the DNSrc is known to affect all Src family members, it suggested therapeutic potential for Src inhibitors targeting whole SFK family.
Dasatinib blocks SFK activity and inhibits glioblastoma invasion
Having shown that Fyn and Src are effectors of EGFR-mediated pathogenesis, and not excluding the possibility that other SFK members may also play a role in this process, we examined the efficacy of pharmacologic inhibition with the dual Src/Abl inhibitor dasatinib, which inhibits the entire family of Src kinases. Dasatinib has been approved for use in patients with imatinib-resistant leukemias (31, 32) and is being evaluated for use in numerous solid cancers (14, 33-35). 50 nM dasatinib inhibited SFK phosphorylation in a panel of glioblastoma cell lines, a dose that blocks SFK activation in other types of cancer cell lines and is clinically achievable (Fig. 3A) (14, 35, 36). Significant inhibition of migration through matrigel was also observed after treatment with 50 nM dasatinib (Fig. 3B). U87MG cells express very little EGFR and have very low levels of SFK activation as noted above (Fig. 2A), and were relatively insensitive to dasatinib (Fig. 3C). EGFRvIII increased SFK phosphorylation in U87MG cells and sensitized them to dasatinib in matrigel migration assays (Fig. 3C).
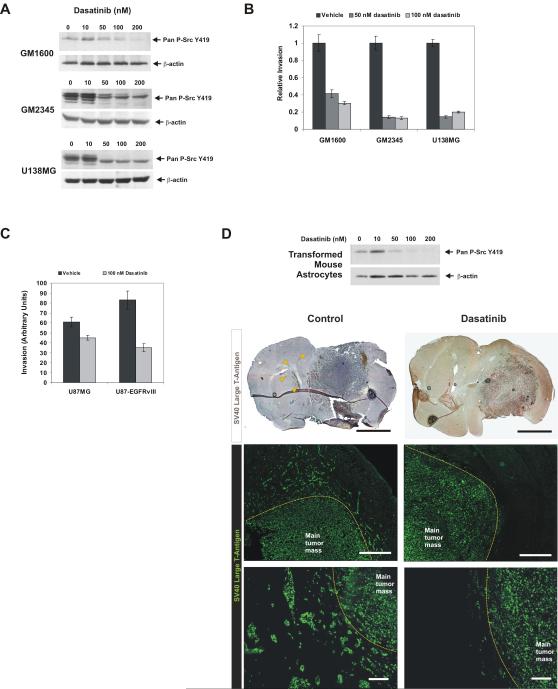
Figure 3.
Dasatinib inhibits SFK activity and tumor cell invasion. A, B, Dasatinib inhibits pan-SFK Y419 phosphorylation and glioblastoma cell invasion in vitro. C, EGFRvIII expression increased U87 cell invasion but sensitized them to dasatinib. D, Dasatinib blocks pan-SFK Y419 phosphorylation of SV40 large T-antigen and H-Ras transformed mouse astrocytes. Mice intracranially implanted with transformed mouse astrocytes were treated with dasatinib (n=10) or vehicle (n=10). Brain sections from treated mice were stained for SV40 large T-antigen to detect tumor cells and visualized by DAB or fluorescence. Arrowheads indicate invasive tumor cells far from the main tumor mass in control mice. Yellow dotted lines indicate borders of the main tumor mass. Scale bars: Top, 2 mm. Center, 500 μm. Bottom, 100 μm.
To determine whether dasatinib could effectively inhibit glioblastoma invasion in vivo, we used an intracranial transformed murine astrocyte model that produces invasive astrocytic tumors and reflects hallmarks of human glioblastomas (23, 24). These tumor cells are transformed with SV40 large T-antigen and are specifically detectable by immunohistochemistry. Treament of with dasatinib in vitro confirmed that SFK phosphorylation could be inhibited (Fig. 3D). Following orthotopic injection, tumor cells diffusely invaded out of the central tumor mass both as infiltrating single cells and as small clusters of cells, some disseminating large distances into the opposite hemisphere (Fig. 3D). Dasatinib treatment dramatically inhibited tumor cell invasion, resulting in more focally localized central tumor masses with little dispersal of tumor cells (Fig. 3D). These results clearly demonstrate that dasatinib can block glioblastoma cell invasion into the surrounding brain parenchyma in vivo.
Dasatinib promotes tumor regression and apoptosis and prolongs survival in an EGFRvIII-expressing intracranial glioblastoma model
To study the effects of dasatinib in human glioblastomas endogenously expressing EGFRvIII, we utilized another orthotopic model in which human glioblastomas are excised from patients and serially propagated as subcutaneous tumors in mice for subsequent intracranial implantation (Fig. 4A). One drawback of most intracranial human glioblastoma xenograft models is that EGFRvIII expression and EGFR gene amplification cannot be maintained long-term in culture, therefore they are unable to model endogenous oncogenic EGFR signaling. This system, featuring luciferase expression for non-invasive bioluminescence monitoring during therapy (37), has been shown to maintain key molecular alterations and facilitates pre-clinical testing of agents on human glioblastomas expressing endogenous EGFRvIII (38). The GBM39 line was chosen as it expresses mutant EGFRvIII (25).
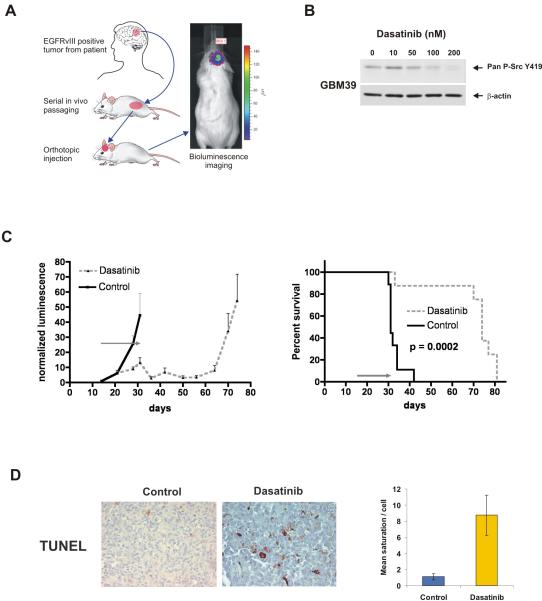
Figure 4.
Dasatinib promotes tumor regression and survival in an orthotopic human glioblastoma xenograft expressing endogenous EGFRvIII. A, The EGFRvIII positive, bioluminescence enabled human glioblastoma line GBM39 was derived from a patient tumor and serially passaged in mouse flanks. Tumors were processed for intracranial injection into host animals and monitored by bioluminescence imaging. B, Pan-SFK Y419 phosphorylation of GBM39 cells was inhibited by dasatinib. C, Normalized bioluminescence of vehicle-treated control (n=9) or dasatinib-treated (n=10) mice intracranially injected with GBM39 cells showing decreased bioluminescence in the treatment group, which is sustained following completion of therapy at 31 days. Kaplan-Meier survival analysis shows significant survival extension in dasatinib treated animals (p=0.0002). The gray arrow indicates the 17-day treatment period. D, TUNEL positivity was significantly higher in dasatinib-treated tumors at the end of the treatment period (31 days post-tumor injection). Right, quantification of TUNEL staining by quantitative image analysis.
Dasatinib treatment of GBM39 cells grown in culture confirmed its ability to block SFK phosphorylation (Fig. 4B). Treatment of mice with established intracranial GBM39 tumors with a 17 day course of dasatinib promoted substantial tumor regression as determined by bioluminescence monitoring, and significantly extended survival (Fig. 4C, p=0.0002).
Immunohistochemical analysis of treated tumors relative to controls at the end of the treatment period (31 days post-injection, 17 treatment days) demonstrated significant inhibition of phospho-SFK and phospho-FAK (Supplementary Fig. S4) at the time of maximal tumor shrinkage, with resurgent SFK phosphorylation at the time of recurrence (71 days), suggesting that the effects were mediated by inhibition of SFK signaling. The treated tumors exhibited increased apoptosis relative to controls as measured by TUNEL (Fig. 4D) and cleaved caspase-3 (data not shown) staining. Taken together, these results demonstrate that dasatinib can provide significant survival benefit, promote apoptosis, and reduce tumor burden of glioblastomas expressing endogenous EGFRvIII.
Combined EGFR and SFK inhibition enhances tumor regression and prolongs survival of mice bearing EGFRvIII/SFK driven glioblastomas
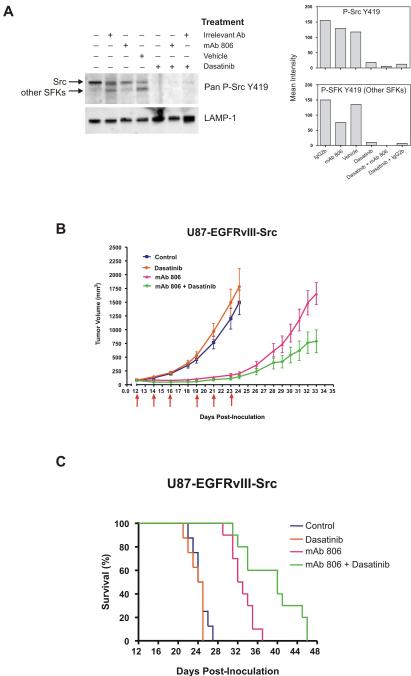
Genetic disruption of Src significantly enhances the efficacy of the anti-EGFR antibody mAb 806 on EGFRvIII-expressing U87 xenografts (26). This raised the possibility that pharmacologic disruption of SFK signaling could enhance the sensitivity of EGFRvIII-expressing tumors to mAb 806. U87 glioblastoma cells expressing EGFRvIII and constitutively active Src (U87-EGFRvIII-Src) were treated in vitro with mAb 806, dasatinib, or a combination of both (Fig 5A and Supplementary Fig. S5). As expected, mAb 806 did not inhibit the activity of the constitutively active Src but decreased the phosphorylation of other SFK members. Treatment with dasatinib alone or in combination with mAb 806 completely inhibited both Src and SFK phosphorylation.
Figure 5.
Combined EGFR and SFK inhibition enhances tumor regression and prolongs survival. A, U87-EGFRvIII-Src cells co-expressing EGFRvIII and activated Src were treated in vitro with mAb 806 (100 μg/ml), dasatinib (100 nM), or a combination of both, then probed for phospho-Src by western blot. The lysosomal marker LAMP-1 is shown as a loading control. Right, quantification of band intensities after normalization to LAMP-1. B, mAb 806 substantially reduced growth of U87 subcutaneous xenografts expressing EGFRvIII and constitutively active Src, while dasatinib alone had no effect. Combined dasatinib and mAb 806 treatment demonstrated significant additive anti-tumor benefit. C, Survival of mice implanted with subcutaneous U87-EGFRvIII-Src xenografts reflected the tumor growth curves of the various treatments. Mice treated with a combination of mAb 806 and dasatinib demonstrated significant survival benefit compared to monotherapy.
Mice subcutaneously implanted with U87-EGFRvIII-Src cells were treated with mAb 806 or dasatinib either alone or in combination. mAb 806 significantly inhibited the growth of U87-EGFRvIII-Src xenografts (Fig. 5B, mean tumor volume at day 24 of 1500 ± 225 mm3 versus 200 ± 35 mm3 for control and mAb 806, respectively, p < 0.0001). Dasatinib alone was not effective, likely because it was given at a dose that did not impact tumor growth. However, addition of dasatinib significantly enhanced the efficacy of mAb 806 (mean tumor volume at day 33 of 1655 ± 200 mm3 for mAb 806 alone versus 790 ± 210 mm3 for combination group, p < 0.008) (Fig. 5B), Data from survival analysis (Fig. 5C) reflected the differences seen in the growth curves (Fig. 5B), in which mice treated with both mAb 806 and dasatinib demonstrated significantly longer survival relative to the benefit observed with mAb 806 treatment alone (p < 0.0001). These data demonstrate that dasatinib significantly enhances the efficacy of anti-EGFR therapy in EGFR and Src-activated glioblastomas.
SFKs are overexpressed and phosphorylated in association with p-EGFR in glioblastoma clinical samples
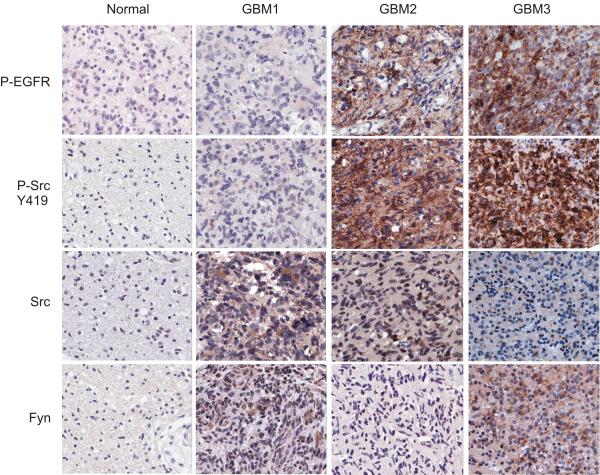
The preclinical data presented above demonstrated that the SFKs are effectors of oncogenic EGFR signaling in glioblastoma. To determine whether the signaling relationships uncovered in the cell lines and xenograft models shown above could also be detected in a large cohort of patients, we performed immunohistochemical analysis of two glioblastoma tissue microarrays containing 252 tumor cores and 91 matched normal cores from 140 patients. Consistent with the reported frequency of activating EGFR amplification or mutation in 45% of glioblastomas (1), EGFR phosphorylation was detected in 44% of tumor samples in our tissue microarrays (P<0.0001), suggesting that this was a representative dataset. Fyn and Src expression were both significantly elevated in the tumor cores relative to the matched normal brain samples (Fig. 6). More importantly, SFK phosphorylation was significantly correlated with pEGFR in tumor samples (R=0.56, P<1 × 10-13). Phospho-FAK Y397, a key effector of SFKs, was also highly and significantly correlated with SFK phosphorylation in patients (R=0.71; P<2.9 × 10-13, data not shown). These data indicate that EGFR and SFK are frequently coactivated and delineate a signaling pathway from EGFR to SFKs in glioblastoma patients.
Figure 6.
SFK activating phosphorylation is frequently observed and correlates with EGFR phosphorylation in glioblastoma patients. Glioblastoma tissue microarrays representing tumor and matched normal tissue cores from 140 patients were immunohistochemically stained for P-EGFR, P-SFK, Src, and Fyn. Representative staining results from 4 spots are shown: a normal tissue core and 3 independent tumor cores. Staining of normal tissues was largely negative. Some tumors exhibited Src and Fyn expression but not P-EGFR or P-SFK (GBM1). Tumors exhibiting P-EGFR also displayed P-SFK, regardless of whether they expressed Src or Fyn (GBM2 and GBM3).
Discussion
The inability of EGFR inhibitors to block PI3K signaling, either because of PTEN loss, compensatory activation of other receptor tyrosine kinases, or mutations rendering kinase inhibitor resistance, suggests that maintenance of persistent PI3K signaling may be one mechanism underlying clinical failure. The observation that genetic disruption of Src enhances the efficacy of the anti-EGFR mAb 806 (26) raised the possibility that Src is an effector of EGFRvIII signaling that may also limit response to EGFR targeted therapies. The present study sheds light on this question by demonstrating that EGFR and EGFRvIII physically associate with Fyn and Src and phosphorylate them on their active site. Genetic and pharmacologic inhibition of Src and Fyn block EGFR-dependent motility and tumor growth in vitro and in vivo. Most importantly, dasatinib enhances the efficacy of mAb 806 on tumors expressing EGFRvIII and persistent SFK activation in vivo. These results provide a compelling rationale for combining EGFR and SFK-targeted therapies in glioblastoma patients. Recent work in head and neck squamous cell cancer cell lines suggests that this may be generalized to multiple cancer types (39).
The transcripts of multiple SFKs were detected with varying expression patterns in all clinical glioblastoma samples examined (Fig. 1A). This suggests that multiple SFKs may be involved in glioblastoma pathogenesis in a non-redundant fashion. A v-src transgenic glioblastoma model (40) has suggested that SFKs are important in the development of malignant astrocytomas. High Lyn expression in glioblastoma has been described (41), and Yes was shown to modulate glioblastoma invasion (42). The focus here on Fyn and Src was motivated by their detection as potential EGFR effectors in two unbiased screens and does not preclude a role for other SFKs (Fig. 1A and B). Like Fyn and Src, Lyn siRNA also inhibited the motility of the panel of EGFR-expressing glioblastoma cells in vitro (data not shown), thus its importance cannot be excluded. Future studies will be needed for a broader understanding of the biology of each SFK member in glioblastoma pathogenesis and in mediating persistent EGFR signaling. Despite the complex expression of multiple SFKs, the pan-SFK inhibitor dasatinib was highly effective at blocking invasion, growth and survival of glioblastomas in vitro and in vivo (Fig. 3 and 4), suggesting that it is effective regardless of which SFKs are activated.
Du et al. recently described the development of a novel tyrosine kinase phosphorylation profiling method that identified Src as a frequently activated, dasatinib-sensitive target in glioblastoma (16). Similar to the results presented here, dasatinib treatment inhibited glioblastoma cell migration in vitro and significantly attenuated growth of orthotopic glioblastoma xenografts. That these similar results were arrived at independently through completely different approaches strengthens, through autonomous validation, the finding that SFKs are activated in glioblastomas and may be targeted with dasatinib. Moreover, the data described here provide mechanistic insight linking EGFR signaling to SFK activation and glioblastoma pathogenesis, further suggesting that combined SFK and EGFR inhibition may provide added therapeutic benefit.
SFKs are key intracellular components of many signaling pathways, including those that may facilitate escape from EGFR inhibition. Phosphorylation of PDGFR and c-Met by PDGF and HGF, respectively, leads to Y419 phosphorylation of SFKs (data not shown) suggesting that PDGFR and c-Met could also maintain SFK signaling in glioblastomas treated with EGFR inhibitors. Dasatinib significantly enhanced the efficacy of mAb 806 against EGFRvIII expressing tumors with persistent Src activation, raising the possibility that EGFR inhibition alone is not sufficient to fully limit SFK signaling and to promote glioblastoma regression. Future studies will be needed to assess whether c-Met, PDGFR, or other signaling pathways contribute to EGFR inhibitor clinical resistance by maintaining SFK as well as PI3K activation. Additionally, there are likely other mechanisms of SFK activation independent of EGFR as suggested by the decreased motility caused by Fyn or Src siRNA in parental U87MG cells with low EGFR expression (Fig. 2B and C).
Like other targeted cancer therapies, it will be important to determine which patients will likely benefit the most from combined EGFR and SFK inhibition. Somatic-activating mutations in EGFR are associated with increased sensitivity of non-small cell lung cancers and glioblastomas to EGFR inhibitors (28, 43, 44), however SFK activating mutations are rarely found in patient tumors. Combined with our finding that SFK activation is highly correlated with EGFR phosphorylation, this suggests that SFKs are downstream effectors frequently activated by mutated kinases such as EGFR or other aberrantly active pathways. Alternatively, the overexpression of SFKs in the absence of mutation may be all that is required. The data in this study indicate that patients with amplified or mutant EGFR coupled with high levels of SFK activation may stand to benefit the most from combined EGFR and SFK inhibition.
Utilizing a series of cell lines and mouse models, this study demonstrates a molecular circuitry linking EGFR/EGFRvIII with Fyn and Src to promote glioblastoma invasion and tumor progression. Clinical relevance of these findings is confirmed in a large cohort of tumor specimens, revealing that glioblastoma patients whose tumors exhibit activated EGFR signaling also frequently display activated Fyn and Src. These results demonstrate that Fyn and Src are clinically relevant targets and that their inhibition may augment the efficacy of anti-EGFR-targeted therapies.
Supplementary Material
Acknowledgments
Financial support: This work was supported by grants from the Brain Tumor Funders' Collaborative, the National Institute for Neurological Disorders and Stroke (NS050151) and the National Cancer Institute (CA119347 and CA108633), and an Accelerate Brain Cancer Cure Award to P.S.M. Additional NIH support was from grants NS049720 (C.D.J.) and CA097257 (C.D.J.). This work was also supported by the Harry Allgauer Foundation through The Doris R. Ullman Fund for Brain Tumor Research Technologies, a Henry E. Singleton Brain Tumor Fellowship to P.S.M., and a generous donation from the Ziering Family Foundation in memory of Sigi Ziering. K.V.L. was supported by a Leonard Heyman/American Brain Tumor Association Fellowship and a UCLA Tumor Cell Biology Training Grant funded by the National Cancer Institute (5T32CA09056). Microarray studies were supported by the UCLA DNA Microarray Facility. T.G.J. was supported by the NH&MRC of Australia (Project Grant: 433615).
References
- 1.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 4.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 5.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–72. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 6.Lal A, Glazer CA, Martinson HM, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–9. [PubMed] [Google Scholar]
- 7.Penar PL, Khoshyomn S, Bhushan A, Tritton TR. Inhibition of epidermal growth factor receptor-associated tyrosine kinase blocks glioblastoma invasion of the brain. Neurosurgery. 1997;40:141–51. doi: 10.1097/00006123-199701000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Martens T, Laabs Y, Gunther HS, et al. Inhibition of glioblastoma growth in a highly invasive nude mouse model can be achieved by targeting epidermal growth factor receptor but not vascular endothelial growth factor receptor-2. Clin Cancer Res. 2008;14:5447–58. doi: 10.1158/1078-0432.CCR-08-0147. [DOI] [PubMed] [Google Scholar]
- 9.Martin GS. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970;227:1021–3. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- 10.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12:1398–401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 11.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 12.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–14. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Ishizawar RC, Miyake T, Parsons SJ. c-Src modulates ErbB2 and ErbB3 heterocomplex formation and function. Oncogene. 2007;26:3503–10. doi: 10.1038/sj.onc.1210138. [DOI] [PubMed] [Google Scholar]
- 14.Song L, Morris M, Bagui T, Lee FY, Jove R, Haura EB. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res. 2006;66:5542–8. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Thomas SM, Xi S, et al. SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation, and invasion of head and neck cancer cells. Cancer Res. 2004;64:6166–73. doi: 10.1158/0008-5472.CAN-04-0504. [DOI] [PubMed] [Google Scholar]
- 16.Du J, Bernasconi P, Clauser KR, et al. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nat Biotechnol. 2009;27:77–83. doi: 10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freije WA, Castro-Vargas FE, Fang Z, et al. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004;64:6503–10. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98:31–6. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skaggs BJ, Gorre ME, Ryvkin A, et al. Phosphorylation of the ATP-binding loop directs oncogenicity of drug-resistant BCR-ABL mutants. Proc Natl Acad Sci U S A. 2006;103:19466–71. doi: 10.1073/pnas.0609239103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Zhu S, Cloughesy TF, Liau LM, Mischel PS. p53 disruption profoundly alters the response of human glioblastoma cells to DNA topoisomerase I inhibition. Oncogene. 2004;23:1283–90. doi: 10.1038/sj.onc.1207244. [DOI] [PubMed] [Google Scholar]
- 21.Wang MY, Lu KV, Zhu S, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–9. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 22.Lu KV, Jong KA, Rajasekaran AK, Cloughesy TF, Mischel PS. Upregulation of tissue inhibitor of metalloproteinases (TIMP)-2 promotes matrix metalloproteinase (MMP)-2 activation and cell invasion in a human glioblastoma cell line. Lab Invest. 2004;84:8–20. doi: 10.1038/sj.labinvest.3700003. [DOI] [PubMed] [Google Scholar]
- 23.Blouw B, Song H, Tihan T, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4:133–46. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 24.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkaria JN, Yang L, Grogan PT, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–74. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 26.Johns TG, Perera RM, Vernes SC, et al. The efficacy of epidermal growth factor receptor-specific antibodies against glioma xenografts is influenced by receptor levels, activation status, and heterodimerization. Clin Cancer Res. 2007;13:1911–25. doi: 10.1158/1078-0432.CCR-06-1453. [DOI] [PubMed] [Google Scholar]
- 27.Johns TG, Stockert E, Ritter G, et al. Novel monoclonal antibody specific for the de2-7 epidermal growth factor receptor (EGFR) that also recognizes the EGFR expressed in cells containing amplification of the EGFR gene. Int J Cancer. 2002;98:398–408. doi: 10.1002/ijc.10189. [DOI] [PubMed] [Google Scholar]
- 28.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–24. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 29.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3'-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–6. [PubMed] [Google Scholar]
- 30.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 32.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 33.Finn RS, Dering J, Ginther C, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–26. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 34.Nam S, Kim D, Cheng JQ, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 35.Serrels A, Macpherson IR, Evans TR, et al. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5:3014–22. doi: 10.1158/1535-7163.MCT-06-0382. [DOI] [PubMed] [Google Scholar]
- 36.Luo FR, Yang Z, Camuso A, et al. Dasatinib (BMS-354825) pharmacokinetics and pharmacodynamic biomarkers in animal models predict optimal clinical exposure. Clin Cancer Res. 2006;12:7180–6. doi: 10.1158/1078-0432.CCR-06-1112. [DOI] [PubMed] [Google Scholar]
- 37.Dinca EB, Sarkaria JN, Schroeder MA, et al. Bioluminescence monitoring of intracranial glioblastoma xenograft: response to primary and salvage temozolomide therapy. J Neurosurg. 2007;107:610–6. doi: 10.3171/JNS-07/09/0610. [DOI] [PubMed] [Google Scholar]
- 38.Pandita A, Aldape KD, Zadeh G, Guha A, James CD. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer. 2004;39:29–36. doi: 10.1002/gcc.10300. [DOI] [PubMed] [Google Scholar]
- 39.Koppikar P, Choi SH, Egloff AM, et al. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:4284–91. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissenberger J, Steinbach JP, Malin G, Spada S, Rulicke T, Aguzzi A. Development and malignant progression of astrocytomas in GFAP-v-src transgenic mice. Oncogene. 1997;14:2005–13. doi: 10.1038/sj.onc.1201168. [DOI] [PubMed] [Google Scholar]
- 41.Stettner MR, Wang W, Nabors LB, et al. Lyn kinase activity is the predominant cellular SRC kinase activity in glioblastoma tumor cells. Cancer Res. 2005;65:5535–43. doi: 10.1158/0008-5472.CAN-04-3688. [DOI] [PubMed] [Google Scholar]
- 42.Kleber S, Sancho-Martinez I, Wiestler B, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13:235–48. doi: 10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 44.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–41. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.