Abstract
Purpose
To describe tachyphylaxis to intravitreal bevacizumab (IVB) in patients with exudative age-related macular degeneration (AMD).
Methods
We retrospectively reviewed the records of 59 consecutive patients treated with IVB at the National Eye Institute over a 14 month period, and identified cases demonstrating loss of treatment efficacy as revealed by spectral domain optical coherence tomography. We defined tachyphylaxis as a loss of therapeutic response to IVB 28±7 days after administration in an eye which had previously demonstrated a therapeutic response in the same time interval.
Results
Five patients (6 eyes) were identified as developing tachyphylaxis following repeated treatment with IVB. High-dose IVB (2.50mg) did not restore therapeutic response in these patients. Bilateral tachyphylaxis to IVB was seen following an episode of unilateral post-injection anterior uveitis. After the first treatment of IVB, the median time taken to develop tachyphylaxis was 100 weeks (range: 31-128 weeks), and the median number of IVB treatments to the development of tachyphylaxis was 8 treatments (range: 5-10).
Conclusion
Tachyphylaxis can occur following long-term intravitreal use of bevacizumab in patients with AMD. The precise mechanism of tachyphylaxis is unclear, but both local and/or systemic factors may be involved.
Keywords: Age-related macular degeneration, anti-angiogenic treatment, bevacizumab, intravitreal injection, neovascularization, optical coherence tomography, tachyphylaxis, vascular endothelial growth factor
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of blindness in the developed world among people over 50 years of age1 . Exudative AMD, characterized by the development of choroidal neovascularization (CNV), represents the most common form of AMD resulting in severe visual loss2 . Biologic therapies have emerged as effective agents in the treatment of exudative AMD. Ranibizumab (Lucentis; Genentech, Inc., San Francisco, CA) is a humanized monoclonal antibody fragment targeting vascular endothelial growth factor (VEGF) that is currently FDA approved for the treatment of exudative AMD3 . Bevacizumab (Avastin; Genentech, Inc., San Francisco, CA), a humanized monoclonal antibody targeting VEGF, has gained wide acceptance as an “off-label” agent for the treatment of exudative AMD4 .
As experience using biologic agents to treat exudative AMD increases, a better understanding of the long-term effects of these agents is beginning to evolve. One of these effects may be tachyphylaxis, which is defined as a diminished therapeutic response to a drug following repeated administration over time. Tachyphylaxis has been documented in the setting of other biologic agents including β-interferon, infliximab, and natalizumab5-7 . Quantitative analysis of optical coherence tomography (OCT) volumes measured in patients with exudative AMD treated with intravitreal ranibizumab and intravitreal bevacizumab has demonstrated an attenuation of the ability of repeated injections to reduce retinal volume, suggesting tachyphylaxis8, 9 . The purpose of this study was to further describe and characterize the tachyphylactic response following intravitreal bevacizumab.
In this report we describe longitudinal findings in a retrospective case series of patients with exudative AMD treated with intravitreal bevacizumab (IVB) who developed tachyphylaxis following long-term treatment. Patient response to treatment was followed with spectral-domain high-resolution OCT. These patients were identified from a population of patients treated with IVB in a referral retina practice setting. Our findings illustrate the clinical scenarios in which tachyphylaxis to IVB can arise and highlight this phenomenon as a potential challenge in the long-term treatment of exudative AMD using biologic-based anti-angiogenics.
METHODS
We retrospectively reviewed the charts of 59 consecutive patients who presented to the National Eye Institute with exudative AMD and were treated with IVB from July, 2007 to August, 2008. Patients were treated on a pro re nata (prn) basis at the discretion of the treating ophthalmologist based on an OCT-guided treatment protocol similar to that previously described in a clinical trial using ranibizumab10 . Patients were seen approximately every four weeks. A comprehensive ophthalmic examination including best-corrected visual acuity (BCVA) and spectral domain OCT imaging using the Cirrus™ HD-OCT system (Carl Zeiss Meditec, Dublin, CA) was performed at every visit. HD-OCT images were captured using the high-resolution 5-line raster protocol positioned through the foveal region, as well as the 512×128 macular cube protocol covering a 6×6mm area centered approximately at the fovea. Careful inspection of the entire macular area covered was performed by the treating ophthalmologist. If intraretinal/subretinal fluid was seen on Cirrus™ HD-OCT, the patient was treated with IVB (1.25mg). If the macula appeared free of intraretinal and subretinal fluid, treatment was deferred and the patient was asked to return in 4 weeks for repeat evaluation. Eyes were not retreated until intraretinal and/or subretinal fluid was observed on repeat examination. Measurements of central subfield thickness were derived from the 512×128 macular cube scans and calculated using software intrinsic to the Cirrus™ OCT system. This retrospective study was performed with informed patient consent and conducted under a protocol approved by the local institutional review board (IRB) and in accordance with the ethical standards stated in the 1964 Declaration of Helsinki.
From the population of patients followed and treated with IVB using the treatment paradigm described above, individual cases demonstrating evidence of tachyphylaxis were identified and characterized. As tachyphylaxis is defined as a loss of drug efficacy with repeated administration, only eyes that had a definite previous therapeutic response to IVB (either 1.25mg or 2.5mg), defined as complete or near-complete resolution of intraretinal or subretinal fluid 28±7 days following treatment as evaluated on HD-OCT imaging, were considered as candidates. Among these, treated eyes that then subsequently failed to respond similarly to later IVB treatments (either at a 1.25mg or 2.5mg dose), as demonstrated by the persistence of or increased intraretinal/subretinal fluid 28±7 days following treatment, were identified as demonstrating evidence of tachyphylaxis.
RESULTS
We identified a total of six eyes (five patients) patients who met the criteria for tachyphylaxis. All patients were Caucasian, with a median age of 77 years (range: 69-81 years). Four of the five patients were female. The median time taken to develop tachyphylaxis was 100 weeks (range: 31-128 weeks) after the first treatment with IVB in either the eye developing tachyphylaxis or the fellow eye, and 60 weeks (range: 33-121 weeks) after the first treatment with IVB in the eye developing tachyphylaxis. The median number of IVB treatments to the development of tachyphylaxis was 8 treatments (range: 5-10) considering IVB treatment in the eye developing tachyphylaxis or the fellow eye, and 6 treatments (range: 3-9) considering IVB treatment in the eye developing tachyphylaxis. A detailed description of each case is provided below.
Case 1
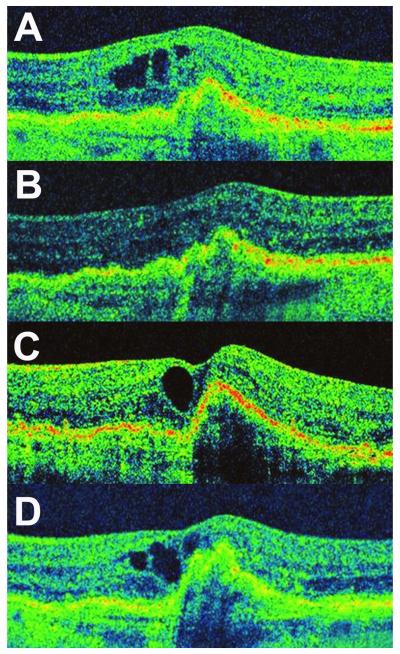
An 81 year-old female presented with complaints of visual distortion in the left eye. BCVA was 20/25 OD, and 20/63 OS. Dilated fundus examination revealed large drusen OU, and exudative AMD OS. OCT demonstrated the presence of a drusenoid pigment epithelial detachment (PED) in the fovea with overlying intraretinal cystic fluid (Figure 1A). The patient’s left eye was treated with IVB (1.25mg), which resulted in resolution of the intraretinal fluid. The patient was followed approximately every four weeks and was treated with additional treatments of IVB in the left eye when the intraretinal fluid was observed to recur on HD-OCT. Complete resolution of intraretinal fluid in the macula was achieved after each IVB treatment up to and including the fifth IVB treatment (1.25mg) (Figure 1B). However, following the sixth treatment with IVB (1.25mg) the intraretinal fluid failed to resolve (Figure 1C). At this point, the IVB treatment was administered at a higher dose (2.50mg), but this did not result in any diminution of the intraretinal fluid (Figure 1 D). Final BCVA in the treated eye was 20/100. The time from the first IVB treatment to documentation of tachyphylaxis was 100 weeks.
Figure 1.
Tachyphylaxis to Intravitreal Bevacizumab in Case 1 (Left Eye).
(A) HD-OCT scan obtained on presentation demonstrating the presence of a drusenoid pigment epithelial detachment with intraretinal cystic fluid spaces; IVB treatment was initiated at this point. (B) HD-OCT scan demonstrated absence of intra- or sub-retinal fluid after each injection, and the macula remained fluid-free sixty-one days following the fifth consecutive IVB treatment (C) Twenty-eight days following the sixth IVB treatment, HD-OCT imaging demonstrated the failure of intraretinal fluid to resolve. In response to this, a seventh IVB treatment of a higher dose (2.5mg) was administered but failed to result in resolution of intraretinal fluid when evaluated 35 days later (D).
Case 2
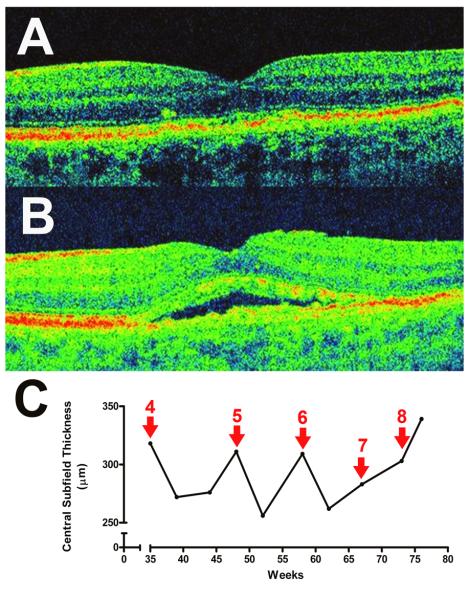
A 71 year-old male presented with vision loss in the left eye. BCVA was 20/400 OD and 20/50 OS. The right eye had previously been diagnosed with exudative AMD and had been treated with two treatments of IVB (1.25mg). Dilated fundus examination demonstrated a disciform scar in the right macula, and evidence of exudative AMD in the left eye with subretinal fluid observed on HD-OCT imaging. IVB treatment was initiated in the left eye. Over the next 6 months, the left eye received a series of IVB (1.25mg) treatments, each resulting in a complete resolution of subretinal fluid approximately 4 weeks following treatment as observed on HD-OCT (Figure 2A). However, 4 weeks following the eighth treatment, the subretinal fluid failed to resolve, and this persisted even after an additional treatment with IVB (1.25mg) was administered (Figure 2B, 2C). Final BCVA in the treated eye was 20/40. Clinical definition for tachyphylaxis was met 77 weeks after IVB treatment was initiated in the left eye.
Figure 2.
Tachyphylaxis to Intravitreal Bevacizumab (IVB) in Case 2 (Left Eye). A series of repeated IVB treatments was initiated after the detection of exudative age-related macular degeneration.(A) HD-OCT scan 30 days following a fifth injection of IVB (1.25mg), demonstrating a fluid-free macula (B) Post-treatment resolution of retinal fluid post-treatment was maintained until the eighth IVB treatment. Twenty-three days following the eighth treatment with intravitreal bevacizumab (1.25mg), OCT imaging documented the persistence of subretinal fluid underneath the fovea. (C) Plot of central subfield thickness over time (weeks) during treatment displays the anatomical response to early IVB treatment and the development of tachyphylaxis later in the course. Red arrows represent treatment with intravitreal bevacizumab (1.25mg), while adjacent numbers in red indicate the treatment number since the first treatment in that eye (at time = 0 weeks).
Case 3
A 69 year-old female presented with a complaint of visual distortion in the right eye. The left eye had a previous history of exudative AMD that was treated with photodynamic therapy. The right eye had more recently developed exudative AMD and had been treated with 7 previous intravitreal injections of ranibizumab (0.50mg) prior to presentation to our clinic. Visual acuity on presentation was 20/50 OD, and hand-motion OS. Clinical examination demonstrated exudative AMD in the right eye with subretinal fluid under the fovea, and a disciform scar in the left eye. For financial reasons, the patient elected to be treated with IVB. Treatment with IVB at a 1.25mg dose was initiated in the right eye but failed to resolve the subretinal fluid. A higher dose of IVB at the 2.50mg dose was instituted 4 weeks later and this was successful in completely resolving the subretinal fluid (Figure 3A). This higher dose (2.50mg) of IVB was administered 3 additional times, each resulting in complete fluid resolution. Following the fifth treatment with IVB (2.50mg), the subretinal fluid again failed to resolve (Figure 3B). Final BCVA in the right eye was 20/32. Tachyphylaxis in this patient developed 31 weeks after the first treatment with IVB (2.50mg). The total number of anti-angiogenic injections (bevacizumab and ranibizumab) received in this case prior to development of tachyphylaxis was 15, the largest number of treatments received by a single eye in our series.
Figure 3.
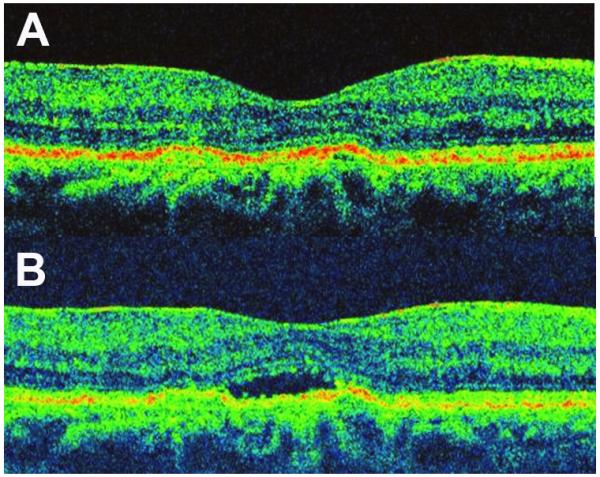
Tachyphylaxis to Intravitreal Bevacizumab (IVB) in Case 3 (Right Eye).
(A) OCT imaging 37 days following the initial injection of intravitreal bevacizumab at the higher dose of 2.50mg, demonstrated the complete resolution of subretinal fluid. Response to IVB at the 2.5mg dose was maintained over the next 3 treatments but 28 days following the fifth treatment, the subretinal fluid failed to resolve as seen on OCT (B)
Case 4
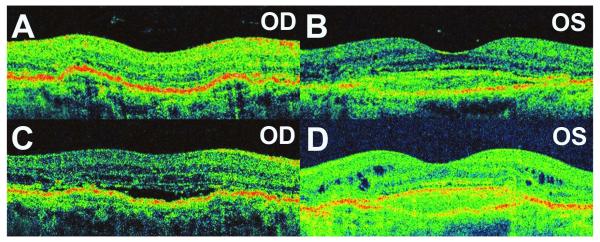
A 79 year-old female presented with a report of new-onset, visual distortion in the right eye. The left eye had a previous history of exudative AMD that had been treated with photodynamic therapy. BCVA was 20/50 OD, and 20/800 OS. Fundus examination demonstrated exudative AMD OD and a disciform scar OS. HD-OCT confirmed the presence of central serous PEDs with adjacent subretinal fluid. Treatments with IVB (1.25mg) resulted in near complete resolution of the subretinal fluid (Figure 4A). However, this response was not durable and after the ninth treatment with IVB (1.25mg), the subretinal fluid failed to resolve significantly (Figure 4B). A higher dose (2.50mg) of IVB was then administered, but this similarly did not resolve the subretinal fluid (Figure 4C). Final BCVA was 20/50 OD. The loss of clinical response in this patient developed 121 weeks following the first treatment with IVB in this eye.
Figure 4.
Tachyphylaxis to Intravitreal Bevacizumab in Case 4 (Right Eye).
(A) Thirty-one days following the fourth injection of intravitreal bevacizumab (1.25mg), the subretinal fluid appeared resolved on HD-OCT. (B) Thirty days following the ninth treatment with intravitreal bevacizumab (1.25mg), the subretinal fluid failed to resolve. (C) Graphical representation of response to intravitreal bevacizumab, displaying change in central subfield thickness over time. Red arrows represent treatment with intravitreal bevacizumab (1.25mg), while numbers in red indicate the treatment number since the first treatment in that eye (at time = 0 weeks). Blue arrow represents treatment with intravitreal bevacizumab (2.50mg).
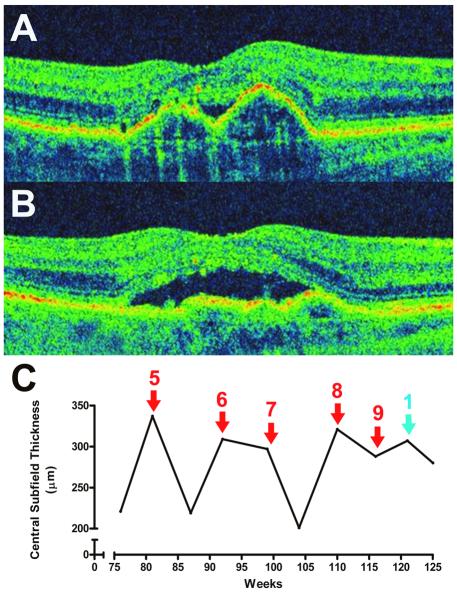
Case 5
A 77 year-old woman presented with bilateral exudative AMD in both eyes, with no history of previous anti-angiogenic treatment. BCVA was 20/25 OD, and 20/63 OS. The left eye was treated with IVB (1.25mg), and the right eye was given the same treatment one week later. Both eyes responded to this treatment initially with complete resolution of intraretinal and subretinal fluid (Figure 5A, 5B), and the patient continued to receive treatment in both eyes at the present dose. However, after 4 injections in the left eye and 2 injections in the right eye, the patient developed marked iritis in the left eye with 3+ cells and flare. No posterior segment inflammation was noted. A vitreous tap was performed in the left eye and intravitreal antibiotic treatment was instituted for empiric treatment of infectious endophthalmitis. When cultures of vitreous fluid failed to demonstrate any evidence of infection, the diagnosis of sterile inflammatory reaction to IVB was made. Signs and symptoms of inflammation resolved 10 days later with topical corticosteroids. The patient continued to receive IVB after this episode with topical corticosteroid given for a few days before and subsequent to IVB treatment. No further episodes of anterior uveitis occurred.
Figure 5.
Tachyphylaxis to Intravitreal Bevacizumab (IVB) in Case 5 (Both Eyes).
(A) OCT imaging 28 days following treatment with the initial treatment with IVB (1.25mg) in the right eye, demonstrating complete resolution of subretinal fluid . (B) OCT imaging 50 days following IVB treatment (1.25mg) in the left eye, demonstrating near complete resolution of intraretinal and subretinal fluid. (C) Thirty days following the third injection on intravitreal bevacizumab (1.25mg) in the right eye, the subretinal fluid persisted. (D) Twenty-eight days following 2.50mg IVB, the intraretinal fluid persisted in the left eye.
One month after the anterior uveitis had resolved in the left eye, the right eye was treated again with IVB (1.25mg). Following this injection, the subretinal fluid failed to resolve completely in the right eye (Figure 5C). The patient received subsequent IVB treatment (1.25mg) in the left eye, and this too failed to result in complete resolution of intraretinal fluid. Following this, the IVB dose was increased to 2.50mg but this did not result in complete fluid resolution (Figure 5D). Final BCVA was 20/40 OD, and 20/125 OS. The time to development of tachyphylaxis in the right eye was 33 weeks after the first treatment with IVB in the right eye, while the time to development of tachyphylaxis in the left eye was 42 weeks after the first treatment with IVB in the left eye.
DISCUSSION
We have retrospectively reviewed the records of 59 consecutive patients diagnosed with exudative AMD who were treated with IVB on a prn basis over a 14-month period at the National Eye Institute. Among this cohort, we have identified and characterized 6 eyes from 5 patients that developed tachyphylaxis to IVB treatment. In these eyes, the amount of therapeutic response was adequate and successful in resolving all intraretinal and/or subretinal fluid on OCT early in the course of treatment, but the therapeutic response then diminished as a function of time and the increasing number of treatments. A recent report9 has also described tachyphylaxis following intravitreal bevacizumab. In this report, we have described additional features of this tachyphylactic response such as the persistence of tachyphylaxis following administration of high-dose bevacizumab and development of tachyphylaxis in both eyes of a patient following unilateral post-injection anterior uveitis. Based on our observations, we speculate on the possible mechanisms by which this phenomenon of tachyphylaxis may have arisen in this clinical context.
In eyes developing tachyphylaxis while being treated with the 1.25mg dose of IVB, a therapeutic response could not be achieved with the subsequent administration of a higher 2.5mg dose. In one patient (case 3), initial IVB at the 1.25mg dose did not result in a therapeutic response but a higher dose of 2.5mg successfully achieved a fluid-free macula. However, tachyphylaxis also subsequently developed with repeated administrations at this dose. Although a progressive dose escalation beyond 2.5mg was not performed, these observations suggest that increasing the dose of IVB in patients who develop tachyphylaxis may not be readily effective in restoring a complete therapeutic response in all cases.
In one patient in our series (case 3), we observed the development of tachyphylaxis following multiple treatments with both intravitreal ranibizumab and bevacizumab, suggesting the possibility that both biologics, in sharing a common therapeutic molecular target, may have both contributed to the emergence of tachyphylaxis. One mechanism for tachyphylaxis may involve the response of tissue to chronic blockage of signaling mediated by vascular endothelial growth factor (VEGF). Macrophages have been implicated in the pathogenesis of CNV as sources of VEGF, as well as inducing VEGF secretion by retinal pigment epithelium (RPE) cells11-13 . The macrophages located within the choroidal neovascular tissue may respond to VEGF blockade by upregulating the production of VEGF. This hypothesis is supported by recent findings that macrophages located in surgically excised human CNV membranes in eyes that previously received IVB are increased in density and proliferative activity14 , akin to those found in CNV following PDT treatment15, 16 . These findings suggest that, in response to chronic VEGF blockade following long-term use of IVB, a compensatory response by proliferating macrophages may overcome further therapeutic attempts to block VEGF signaling and contribute to the development of tachyphylaxis.
We also observed tachyphylaxis in both eyes of one patient following an episode of sterile uveitis in one eye treated with IVB. This case suggests that a systemic immune response may be involved in the mechanism of tachyphylaxis. Acute uveitis following intravitreal injection of bevacizumab and ranibizumab has been previously described 3, 17 . In one series, the majority of cases of acute uveitis following treatment occurred after multiple injections, indicating that a necessary sensitization or priming may be required in order that an immune response can be mounted against the therapeutic agent 17 . One component of this systemic immune response may take the form of neutralizing antibodies. Although bevacizumab is a monoclonal antibody that has been “humanized” to reduce its antigenicity in patients, it may not be however completely non-immunogenic. Neutralizing antibodies formed against other therapeutic humanized monoclonal antibodies such as natalizumab have been previously reported in treated patients5, 6 . Detectable levels of bevacizumab have also been found in serum following intravitreal injection18 , and may thus be recognized as an antigen by the systemic immune system. Interestingly, results from a large randomized clinical trial have previously demonstrated that antibodies against ranibizumab develop following intravitreal administration, and that the titers of these antibodies increase with increasing duration of treatment3 . Although the analysis performed in this study did not find an association between the presence of anti-ranibizumab antibodies and a decrease in clinical response, a more recent quantitative OCT subanalysis of patients with exudative AMD treated with long-term intravitreal ranibizumab indicated that tachyphylaxis may indeed occur, as evidenced by a decreasing ability of repeated ranibizumab treatments to reduce retinal thickness 8 .
In addition to proposed local and/or systemic inflammatory mechanisms described above, several other possibilities may explain the tachyphylactic response observed.
Tachyphylaxis may arise from mechanisms in which the basic physiology of the affected retina has undergone alterations, such as a change in CNV lesion type, associated loss of RPE function, or the emergence of chronic inflammatory changes. Permanent structural damage to the vascular walls of the choroidal neovascular (CNV) complex may also develop in patients with chronic disease. This structural damage could conceivably result in permanent abnormal vascular permeability and persistent exudation that is no longer amenable to anti-VEGF treatment. As the phenomenon of tachyphylaxis is only recently but increasingly recognized, a number of plausible explanations may be raised, and future investigations may indeed be required to clarify them.
In summary, we have described tachyphylaxis to IVB in a cohort of AMD patients receiving long-term treatment with this agent. The mechanisms underlying the development of this phenomenon are unclear; however, local and/or systemic factors may be involved in its pathogenesis. With continued long-term use of anti-VEGF biologic agents, tachyphylaxis may become an increasing therapeutic problem in patients with AMD. A more thorough understanding of the mechanisms responsible for the development of tachyphylaxis may help establish treatment strategies to avoid or circumvent this phenomenon.
Summary Statement (50 words): Tachyphylaxis, described as a decrease in therapeutic drug response following repeated administration, was documented in 5 patients receiving multiple intravitreal bevacizumab injections for exudative age-related macular degeneration. The clinical features of the tachyphylactic response, the time-line of its emergence, and hypotheses concerning its pathogenesis are described in this report.
Acknowledgments
This work has been supported by the National Eye Institute Intramural Research Program. None of the authors has proprietary interests related to the material in this manuscript.
REFERENCES
- 1.Bressler NM, Bressler SB, Congdon NG, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–4. doi: 10.1001/archopht.121.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 4.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. e5. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Fox EJ, Vartanian TK, Zamvil SS. The immunogenicity of disease-modifying therapies for multiple sclerosis: clinical implications for neurologists. Neurologist. 2007;13:355–62. doi: 10.1097/NRL.0b013e318148c08e. [DOI] [PubMed] [Google Scholar]
- 6.Calabresi PA, Giovannoni G, Confavreux C, et al. The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology. 2007;69:1391–403. doi: 10.1212/01.wnl.0000277457.17420.b5. [DOI] [PubMed] [Google Scholar]
- 7.Svenson M, Geborek P, Saxne T, Bendtzen K. Monitoring patients treated with anti-TNF-alpha biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology (Oxford) 2007;46:1828–34. doi: 10.1093/rheumatology/kem261. [DOI] [PubMed] [Google Scholar]
- 8.Keane PA, Liakopoulos S, Ongchin SC, et al. Quantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:3115–20. doi: 10.1167/iovs.08-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology. 2008;115:2199–205. doi: 10.1016/j.ophtha.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–83. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–26. [PubMed] [Google Scholar]
- 12.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–85. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–92. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 14.Tatar O, Yoeruek E, Szurman P, et al. Effect of bevacizumab on inflammation and proliferation in human choroidal neovascularization. Arch Ophthalmol. 2008;126:782–90. doi: 10.1001/archopht.126.6.782. [DOI] [PubMed] [Google Scholar]
- 15.Tatar O, Adam A, Shinoda K, et al. Influence of verteporfin photodynamic therapy on inflammation in human choroidal neovascular membranes secondary to age-related macular degeneration. Retina. 2007;27:713–23. doi: 10.1097/IAE.0b013e318042d3b0. [DOI] [PubMed] [Google Scholar]
- 16.Tatar O, Adam A, Shinoda K, et al. Expression of VEGF and PEDF in choroidal neovascular membranes following verteporfin photodynamic therapy. Am J Ophthalmol. 2006;142:95–104. doi: 10.1016/j.ajo.2006.01.085. [DOI] [PubMed] [Google Scholar]
- 17.Wickremasinghe SS, Michalova K, Gilhotra J, et al. Acute Intraocular Inflammation after Intravitreous Injections of Bevacizumab for Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmology. 2008 doi: 10.1016/j.ophtha.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114:855–9. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]