Abstract
Lymphocytes involved in intestinal immune response are found in organized immune inductive sites of the gut-associated lymphoid tissues (GALT) such as Peyer’s patches (PP), mesenteric lymph nodes (MLN) and diffuse effector sites of gut epithelium and lamina propria (LP). β7 integrins are responsible for efficient trafficking and retention of lymphocytes in these sites. Naïve and effector lymphocytes use α4β7 integrin to extravasate from blood to gut mucosal tissues of GALT, MLN and LP via interactions with Mucosal Addressin Cell Adhesion Molecule-1 (MAdCAM-1). The αEβ7 integrin facilitates retention of effector and memory lymphocytes in the gut epithelial layer via interactions with E-cadherin. Mucosal dendritic cells (DCs) regulate the expression of the gut homing receptors α4β7 integrin and the chemokine receptor CCR9 on activated effector and regulatory lymphocytes in a retinoic acid-dependent manner. CD103 (αE integrin) identifies a subset of mucosal DCs in MLN and small intestine LP that have an enhanced ability to induce gut-tropic receptors on responding lymphocytes. The interactions between β7 integrin and their ligands are also implicated in the pathogenesis and progression of inflammatory bowel diseases (IBDs), intestinal parasitic infections and graft-versus-host diseases. During intestinal inflammation, β7 integrin-dependent and -independent pathways contribute to lymphocytes recruitment to the intestinal tissues and disease pathogenesis. Recent works have explored the potential of therapeutic targeting of α4 and β7 integrins in IBDs. Here, we review the current understanding of the role of β7 integrins in intestinal lymphocyte trafficking and retention in health and disease.
Keywords: Integrins, homing, retention, lymphocytes, intestinal tissues, intestinal inflammation
Introduction
The gastrointestinal tract is exposed to continuous antigenic challenges including food antigens, bacterial antigens of the normal bacterial flora and pathogens. The intestinal immune system therefore must be able to defend against pathogens while maintaining tolerance to the normal bacterial flora and food antigens. Many immune cells interact to achieve this task including lymphocytes, dendritic cells (DC), macrophages and epithelial cells [1-3]. Lymphocytes involved in intestinal immune response are found in organized immune inductive sites of the gut-associated lymphoid tissues (GALT) such as Peyer’s patches (PPs), in draining gut mesenteric lymph nodes (MLNs) as well as in diffuse effector sites of gut epithelium and lamina propria (LP) [4]. The trafficking and retention of lymphocytes in these distinct tissue sites require orchestrated adhesion to vascular endothelium and migration of lymphocytes through the blood vessel walls. These processes are thought to follow a sequence which includes lymphocyte rolling, chemokine-mediated activation and subsequent firm adhesion, followed by lymphocyte transendothelial migration into intestinal tissues. The main cell surface molecules involved in this adhesion cascade are selectins, chemokines and their receptors, immunoglobulin superfamily molecules and integrins [5-8].
The efficient homing and retention of lymphocytes to the gut is dependent on the β7-containing integrins, α4β7 and αEβ7 integrin as well as the chemokine CCL25 and its receptor CCR9 [9-13]. α4β7 integrin binds Mucosal Addressin Cell Adhesion Molecule-1 (MAdCAM-1), which is constitutively expressed on high endothelial venules (HEVs) of PPs and MLNs as well as on postcapillary venules of gut LP. Both naïve and effector lymphocytes use α4β7 integrin to extravasate from blood to gut mucosal tissues of GALT, MLN and LP via interactions with MAdCAM-1 [11, 14]. The αEβ7 integrin facilitates retention of effector and memory lymphocytes in the gut epithelial layer via interactions with E-cadherin [15, 16]. In addition to their roles in mediating lymphocyte trafficking and localization in the gut, the interactions between β7 integrins and their respective ligands have been implicated in mediating the formation of secondary lymphoid structures [17], in protective immunity against mucosal pathogens [18-20], in the pathogenesis and progression of gut inflammation including inflammatory bowel disease (IBD) [21-23], and intestinal graft-versus-host disease (GVHD) [24]. These conclusions are based on in vitro and in vivo studies that primarily involved function-blocking mAbs to β7 integrins and their ligands [22], β7- and αE-integrins deficient mice [11, 15] as well as various models of IBD, intestinal infections and GVHD [19, 24, 25]. Studies performed with antibodies to α4β7 or MAdCAM-1 or with β7 deficient mice indicated that the homeostatic lymphocyte recruitment to intestinal tissues is highly regulated and dependent on β7 integrins [11, 14, 26]. During intestinal inflammation, both β7 integrin-dependent and -independent pathways contribute to lymphocyte recruitment to the intestinal tissues and disease pathogenesis in mouse models of ileitis, colitis and intestinal infections [21, 22, 27-29].
The past few years have witnessed great progress in our understanding of how gut-associated DC regulate the expression of α4β7 integrin and CCR9 on activated effector and regulatory lymphocytes in a retinoic acid (RA) dependent manner [2, 30-32] and the critical importance of CD103+ mucosal DCs [33, 34]. CD103 (αE integrin) serves as a marker of mucosal DC subsets associated with essential immune activities, including antigen presentation [35], induction of Foxp3+ regulatory T cells (Treg) [36, 37], generation of gut-tropic CD8+ effector T cells [33] and retinoic acid receptor (RAR) signaling [38, 39]. Furthermore, recent works have explored the potential of targeting α4 and β7 integrins in IBDs [25, 40, 41] with one specific strategy (i.e. natalizumab) already approved by the FDA for the treatment of Crohn’s disease and multiple sclerosis (MS) [42, 43].
This review focuses on the role of β7 integrins in intestinal lymphocyte trafficking and retention in health and disease. To this end we will review the activation and expression of β7 integrins and their endothelial ligands. We will also discuss the therapeutic targeting of these molecules for the inhibition of lymphocyte trafficking during inflammatory diseases of the gastrointestinal tract, with a special emphasis on IBD.
Integrins
Integrins are transmembrane cell adhesion receptors composed of noncovalently associated α and β subunits that bind to cell-surface ligands, soluble ligands and extracellular matrix proteins [44]. These adhesive interactions are essential for lymphocyte recirculation, migration into inflammatory sites, and recognition of foreign antigens, survival and proliferation [45-47]. Vertebrates express 18 α and 8 β subunits that combine to generate at least 24 different integrin heterodimers. They are subdivided into subfamilies based on their distinct β subunits. Each subfamily has distinct structural, tissue-restricted expression and functional characteristics. The β2 (CD18) and β7 integrins are leukocyte-specific, and are mainly involved in cell to cell adhesion [45, 48]. So far at least 14 members of the integrin heterodimers belonging to the β1, β2, β7 and αv are known to be expressed on immune cells [46, 49-51].
Both α and β integrin subunits are type I transmembrane glycoproteins with distinct and large extracellular domains, a single pass transmembrane domain and, with the exception of β4 integrin, a short cytoplasmic tail [45, 49]. Extracellular domains from each subunit contribute to the ligand binding site of the heterodimers. Of the 18 α subunits, 9 contain a domain of about 200 amino acids known as inserted (I) domain, or von Willebrand factor A domain, which comprise the ligand-binding sites of these integrins. Integrins require divalent cations (Ca2+, Mg2+, and Mn2+) that bind to a metal ion-dependent adhesion site (MIDAS) required for ligand binding. The β I-like domain is similar to the α I domain with MIDAS located at the center and two adjacent metal ion binding sites known as the ligand-induced metal ion-binding site (LIMBS) and adjacent to metal ion dependent adhesion site (ADMIDAS). As revealed from mutational studies, LIMBS and ADMIDAS regulate the MIDAS, which act as coordinators of ligand binding in a positive and a negative manner, respectively. As such, LIMBS and ADMIDAS are regulators of adhesion and de-adhesion [45, 49]. Site-directed mutations have been introduced into the ADMIDAS that constitutively keep the integrin α4β7 integrin in the adhesive state [52]. The cytoplasmic domains of α and β subunits of integrin are determinants of the ability of integrins to bind ligands. These cytoplasmic domains have been suggested to associate with each other at the membrane-proximal regions, thereby constraining the integrin in the inactive state [53, 54]. Thus, the integrin heterodimers exist in multiple conformational states with low, intermediate and high affinity conformers [55]. These dynamic properties play an important role during leukocyte migration in the immune system.
Regulation of β7 integrin expression
Integrin α4β7
α4β7 integrin is constitutively expressed on naïve T and B cells at a relatively low level [56]. It is also expressed on NK cells, stimulated monocytes, macrophages and eosinophils. Of note, higher levels of α4β7 expression on eosinophils are observed in hypereosinophilic patients, where its expression is maintained on gut-derived eosinophils but downregulated on lung-derived eosinophils [57]. The majority of naïve CD8+ recent thymic emigrants (RTEs) in mice also express α4β7 integrin [58]. The expression of this integrin is increased on IgA-secreting plasma cells and memory gut-homing CD4+ subsets (α4β7 high memory T cells) [59]. By contrast, the majority of memory T cells that circulate to non-mucosal tissues lack expression of β7 integrins [56, 60]. Instead, these α4β7 negative memory cells express the related α4β1 integrin, which binds to vascular cell adhesion molecule-1 (VCAM-1). The upregulation of α4β7 on the memory or activated gut-homing T cells and circulating B cells is induced by RA produced by intestinal DCs [32, 61]. It is not known whether RA regulates expression of α4 or β7 subunits individually. Mora et al observed increased α4 integrin mRNA after CD8+ T cell priming with PP DCs [62]. On the other hand, using RA responsive element reporter mice, Svensson et al found that CD103+ (αE integrin) MLN DC induced expression of α4β7 and CCR9 on CD8+ T cells in a RAR signaling dependent manner [38].
Integrin αEβ7
αEβ7 is expressed by only 2% of circulating blood lymphocytes, but more than 90% of intraepithelial lymphocytes (IEL) and a minority of lamina propria lymphocytes (LPL) [63]. CD103 (αE) is also expressed on some CD4+ T cells, CD8+, DCs and mast cells. Naïve recent thymic emigrants can enter the intestinal epitheliium in α4β7 and CCR9 dependent manner and then proliferate and acquire CD62LloCD103high phenotype, like the resident IEL [58]. Of note, CD103 expression defines a unique population of mouse and human CD4+ natural T reg cells as well as a subset of DC uniquely imprinting gut homing receptors on effector and regulatory T lymphocytes [33, 34, 36, 64]. It has been proposed that α4β7 + T cells differentiate within gut epithelial tissues into αEβ7 + T cells. This may be regulated by TGF-β, locally secreted by intestinal epithelial cells [65, 66]. TGF-β regulates the expression of αEβ7 by increasing the expression of αE and β7 mRNA transcripts and the cell surface expression of αEβ7, while decreasing α4 mRNA [66]. This TGF-β-dependent switch from α4β7 + LPL to αEβ7 + IEL cells involves activation and inactivation of Smad signaling molecules, which are involved in TGF-β signaling. In support to this idea, Smad7 transgenic mice, which antagonize TGF-β /Smad signaling, failed to induce αEβ7 on T cells, thereby resulting in a reduced number of IEL cells [67]. By using a deletion analysis with β7 integrin promotor reporter constructs, Lim et al identified two potentially interactive TGF-β1 response elements, regulating the expression of the β7 gene [68]. The adhesion of αEβ7 T cells to epithelial E-cadherin is promoted by CCL25, suggesting that CCR9 on T cells regulates the expression and function of the co-expressed CD103 upon the arrival of these cells in small intestinal epithelium where the CCR9 ligand CCL25 is constitutively expressed. Thus αEβ7 is induced on T cells after they entered into small intestinal epithelium [69].
MAdCAM-1
In rodents MAdCAM-1 is constitutively expressed by mucosal endothelial cells of PPs, MLNs, and LP of the small and large intestine, the lactating mammary gland as well as sinus-lining cells in the spleen [70]. Human MAdCAM-1 is expressed at similar sites and additionally expressed in non-gastrointestinal organs like thymic medulla, tonsils, nasal associated lymphoid tissues, pancreas and brain [71]. MAdCAM-1 is expressed transiently in developing PLNs, suggesting that the gut-associated mucosal tissue-restricted expression of MAdCAM-1 is developmentally regulated and that tissue specificity is only acquired after birth [72, 73]. Fetal expression of MAdCAM-1 is found in the microvessels of many lymphoid and non-lymphoid extraintestinal tissues, including PLNs, thymus, spleen, pancreas, skin, muscle, and kidney [73]. This finding suggests that MAdCAM-1 is a primordial homing receptor that may carry out a broader, non-gut specific immune cell trafficking throughout the body [74]. MAdCAM-1 expression is upregulated on inflamed venules in chronic inflammatory diseases such as in IBD, diabetes, primary sclerosing cholangitis, liver cirrhosis [75], chronic relapsing experimental autoimmune encephalomyelitis as well as in the genital tract of mice following infection with chlamaydia trachomatis [76]. During active IBD, MAdCAM-1 is aberrantly expressed in extraintestinal endothelial tissues including in joints, eyes, skin and liver [77, 78]. Leung et al also identified expression of MAdCAM-1 on the surface of fibroblasts and melanoma cell lines, indicating that MAdCAM-1 also plays a wider regulatory role in non-endothelial cells, leukocyte transmigration across connective tissues, or the homotypic interaction of some malignant melanoma cells [79].
The transcriptional regulation of MAdCAM-1 expression is not well understood. TNF-α, IL-1β and lymphotoxin, but not IFNγ induce expression of MAdCAM-1 [80-83]. Administration of TNF-α enhances expression of MAdCAM-1 in the intestine, colon and MLN [84]. MAdCAM-1 expression on the endothelial surface is also dependent on phosphatidylinsositol 3-kinase (PI-3K) and AKT [85]. Induction of MAdCAM-1 expression by TNF-α stimulation requires tyrosine kinases, p38, p42/22 mitogen activated protein kinases, NF-kB and poly-ADP ribose polymerase (PARP) [81]. The homeobox gene NKx2-3 has been suggested as a potential target gene that regulates the expression of MAdCAM-1. NKx2-3 mutant mice lack MAdCAM-1 and manifest disordered intestinal and secondary lymphoid tissue architecture where T and B cells normally segregate [86, 87]. Moreover, these mice display decreased number and size of PP, and reduced numbers of CD4+ and IgA+ plasma cells in intestinal villi [86, 87]. MAdCAM-1-deficient mice also showed a reduced size of PPs, deceased number of IgA-secreting plasma cells in the small intestinal LP and defective intestinal IgA response upon oral immunization [88].
Role of β7 integrins in secondary lymphoid tissue development
The fact that β7-deficient mice are viable, healthy and display no alteration in their B cell and T cell development suggests that β7 integrins are not required for lymphocyte development [11]. Furthermore, a recent study using a hematopoietic cell-restricted deficiency for β7 and β1 integrins has excluded an essential role for these integrins during hematopoiesis [89]. However, β7 integrins play an important role in secondary lymphoid tissue development where they promote immune homeostasis. Thus, β7-deficient mice have an impaired development of GALT [11], which is likely secondary to the homing defect and not to defective lymphocyte development. The α4β7/MAdCAM-1 pathway has been implicated in localizing fetal lymphoid tissue inducer cells (LTi) to the intestine to deliver the early signals for PP organogenesis [17]. As suggested from the NKX2.3 mutant study, MAdCAM-1 seems to be essential for the maintenance of mucosal lymphoid tissue architectures like the PP and the marginal zone of the spleen [87]. However, a recent study in MAdCAM-1 knockout mice showed that β7-integrin /MAdCAM-1 pathway is dispensable for embryonic PP development [88]. Thus the previously reported effect on PP development in NKX2.3 mutant mice may be related to seeding of the PPi to anlagen or to other effects of NKX2.3. α4β7 and α4β1 integrins are also expressed in isolated lymphoid follicles (ILFs), which are presumably derived from cryptopatches (CP), localized at the base of the villi in LP. Antibody blockade, β7 integrin-deficient mice and bone marrow reconstitution experiments have demonstrated a role of α4β7/MAdCAM-1 interaction in ILF development but not in CP formation [90].
Role of α4β7 integrin/MAdCAM-1 interaction in homeostatic lymphocyte trafficking to the intestinal tissues
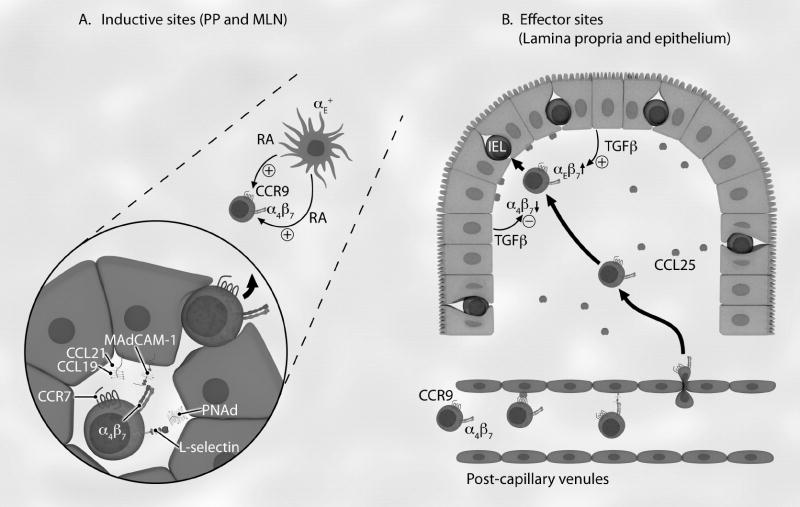
Naïve lymphocytes continuously recirculate between the blood and the secondary lymphoid organs in search for their cognate antigens. Since naïve lymphocytes are poorly responsive to inflammatory signals, they are mostly excluded from non-lymphoid tissues. This restricted lymphocyte trafficking pattern is determined by the expression of adhesion molecules and chemokine receptors on lymphocytes and their specific ligands on endothelial cells. Lymphocyte exit from the blood to secondary lymphoid organs occurs across HEVs following a sequential adhesion cascade (Fig. (1)) [7, 91, 92].
Fig. (1). Role of β7 integrins in intestinal lymphocyte homing and retention.
Lymphocytes involved in the intestinal immune response are organized in two compartments: inductive (A) and effector (B) sites. (A) Naïve lymphocytes (round cells) enter inductive sites through HEVs (polygonal) in PPs and MLNs, where they roll through L-selectin interactions with PNAd and become activated by surface-bound CCL19 and CCL21 that bind to CCR7. Lymphocyte α4β7 integrin interacts with MAdCAM-1 to mediate rolling and, after activation, firm adhesion. Once transmigrated into the parenchyma, lymphocytes encounter dendritic cells presenting antigen in the T cell zone of PP and MLN. RA produced by CD103 (αE)+ dendritic cells induce or enhance expression of gut-trophic receptors α4β7 integrin and CCR9 chemokine receptor. Once activated in PP, the mucosal lymphoblasts then enter the gut-draining MLN. (B) When effector cells return back to the intestinal LP, CCR9+α4β7 + activated lymphocytes preferentially leave the circulation via MAdCAM-1 and CCL25 (small circles) expressing LP-associated post-capillary venuels. Some of these α4β7 +CCR9+ LPLs are then destined to reside in the intestinal epithelium, attracted by CCL25. Switching from α4β7 + LPL to αEβ7+ IEL occurs by the effect of TGF-β, locally secreted by intestinal epithelium (cuboidal). As a result, most IELs express only αEβ7 integrin. The αEβ7+ IEL cells reside between epithelial cells via interaction with E-cadherin and this interaction is likely promoted by CCR9/CCL25 signaling. Thick arrows indicate migration; thin arrows indicate secretion of soluble mediators.
Under homeostatic conditions, naïve lymphocytes first roll using L-selectin (CD62L) and α4β7 integrins binding to peripheral lymph node addressins (PNAd) and MAdCAM-1 respectively, which are expressed on the HEVs of PP and MLN. Rolling naïve lymphocytes activate their integrins by the engagement of the chemokine receptor CCR7 with chemokines CCL19 and CCL21 presented on HEVs [7, 91-94]. Signaling through heterotrimeric G-protein coupled signaling pathways leads to changes in integrin conformation and affinity [95]. The activated α4β7 integrin then allows firm adhesion of the naïve lymphocytes to MAdCAM-1 on the HEVs. Once adhered, lymphocytes transmigrate across HEVs into the parenchyma of lymphoid tissues (Fig. (1)) [7, 91, 92].
MAdCAM-1 in MLN is decorated with a mucin domain, which may support L-selectin dependent rolling in young mice [96]. MAdCAM-1 contains two immunoglobulin-like domains, which mediate firm adhesion via interaction with α4β7 [97, 98]. Homing of effector and memory lymphocytes with high α4β7 expression to the LP does not require L-selectin and is entirely dependent on interaction between α4β7 and MAdCAM-1 [26]. This suggests that α4β7/MAdCAM-1 interaction can mediate both rolling and firm adhesion [96-98]. CD44 present in close proximity to α4β7 has been proposed to mediate rolling of activated T cell subsets [99]. CCL25 and MAdCAM-1, constitutively expressed on LP post-capillary venules, can recruit α4β7 +CCR9+ T cells from the circulation, which is followed by the migration of T cells into the LP [12, 26, 31, 100]. Thus, α4β7-MAdCAM-1 and CCR9-CCL25 interactions are critical in effector T-cell localization to intestinal mucosa [26, 100].
The generation of β7-deficient mice by targeted gene deletion has contributed to our understanding of the critical role of this homeostatic pathway [11]. These mice have PPs that are dramatically reduced in size and cellularity due to severely reduced homing of naïve lymphocytes. The numbers of LPL and IEL are reduced [11]. Using in vivo migration assays, lymphocytes from these mice were found to show severely decreased migration into PPs, while migration into MLN was much less affected due to the overlapping function of L-selectin [11, 101]. Intravital microscopy studies of exteriorized PPs from β7-deficient mice showed that lymphocytes roll normally along the endothelium, demonstrating that L-selectin is sufficient to mediate rolling [11, 102], yet they failed to firmly adhere to or transmigrate through the endothelium [11, 102]. The generation of mice lacking both β7 integrin and L-selectin confirmed the role of these two homing receptors in regulating the lymphocyte migration across HEVs in lymphoid tissues. Lymphocytes from these double deficient mice were unable to migrate into PPs, MLN or PLN [103]. These mice also have fewer resident cells in PPs compared to β7 integrin-deficient mice, showing an additional role of L-selectin in lymphocyte migration to PPs [101]. The recent analysis of MAdCAM-1-deficient mice phenocopied β7-deficient mice, confirming that α4β7-MAdCAM-1 is the major pathway in lymphocyte trafficking to the intestinal tissues [88].
Role of mucosal DCs in imprinting gut-homing receptors
Upon encountering dendritic cells expressing relevant antigenic peptide-MHC complexes, naïve lymphocyte become activated, proliferate and differentiate to effector or memory cells in lymphoid tissues. The differentiation into effector or memory cells is accompanied by the up-regulation of receptors that interact with inflammation-induced endothelial adhesion molecules and inflammatory chemokines. Effector lymphocytes then migrate to extralymphoid tissues and sites of inflammation, where they execute their function [92, 104].
Following lymphocyte activation in PPs and MLNs, DCs present in GALT sites induce the selective and rapid up-regulation of gut-homing receptors, α4β7 and CCR9 on activated lymphocytes. As a result of such induction of small intestinal-homing receptors, effector cells then home to the LP through their interaction with MAdCAM-1 on LP associated venules and CCL25 produced by small intestinal epithelial cells [30, 31, 93, 105, 106]. The molecular mechanism responsible for up-regulation of these gut homing receptors was discovered by the seminal study of Iwata and colleagues, who identified that mucosal DCs, but not spleen or skin DCs, express relatively high levels of retinal dehydrogenase (RALDH), an enzyme required for the generation of the vitamin A metabolite retinoic acid (RA) [32]. They showed that while vitamin A deficiency in mice results in decreased expression of α4β7 integrin on effector and memory T cells and reduced LPL numbers, it has no effect on L-selectin, E-or P-selectin ligand expression on skin-homing effector cells [32]. RA is sufficient to induce expression of α4β7 and CCR9 on activated T cells in the absence of DC in vitro. Pharmacological inhibition of RALDH inhibits GALT DCs imprinting of gut-tropism [32]. This ability to induce the up-regulation of α4β7 and CCR9 on gut-tropic effector lymphocytes is not a property of all GALT-associated DCs. It is rather seen only in DCs expressing CD103 [33]. RA has been shown to up-regulate gut homing receptors on CD8+, CD4+, B cells and naturally occurring CD4+CD25+Foxp3+ T reg [30, 31, 61, 93, 107]. Moreover, RA in combination with TGF-β has been implicated in the peripheral generation of Foxp3+ T reg from naïve T cells [37] and to synergize with IL6 and IL-5 in mediating class switching to IgA in B cells [61]. Peripheral induction of Treg cells is accompanied by up-regulation of α4β7 and CCR9, allowing their efficient homing to small intestinal tissues where they are required to maintain immune homeostasis [108]. RA also enhances the frequency and function of Foxp3+ T reg cells and inhibits IL-6 and TGF-β–driven induction of proinflammatory Th17 cells in vitro [109]. While LP macrophages (CD11c-CD11b+) secrete IL-10 and RA and induce differentiation of Foxp3+ Treg, LP CD11chiCD11b+ DCs secrete TGF-β and induce Th17 responses [110]. These findings illustrate the complex scenario with regard to the mucosal environment in directing the generation of immune responses and the role of APCs to regulate the balance between immune tolerance and responsiveness in the gut.
The route of antigen entry, presence or absence of adjuvant and antigen dose have been also identified as important contributing factors in regulating the efficiency of CCR9 and α4β7 expression on T cells primed in MLN [31, 33, 38]. Furthermore, peripheral tissues and MLN stromal cells exhibit instructive roles in the imprinting of these gut-homing receptors on activated T cells [111-113].
Role of α4β7-integrin for lymphocyte trafficking to intestinal tissues during inflammatory bowel disease
The pathological manifestations of inflammatory diseases including IBD result from the dysregulated recruitment of leukocytes into sites of inflammation where they inflict robust cytokine-mediated tissue injury [13, 92, 93, 114]. The inflammatory bowel diseases are chronic inflammatory disorders of gastrointestinal tissues and include Crohn’s disease (CD) and ulcerative colitis (UC). The development of IBD requires aberrant immune responses against bacterial antigens (normal flora or pathogenic bacteria) from the lumen in genetically susceptible individuals. Key features of CD include discontinuous ulceration and transmural inflammation, which can affect any site of the gastrointestinal tract but most commonly involve the terminal ileum and colon. The inflammation is associated with aggregation of macrophages that form granulomata as well as CD4+ T cells which produce TNF-α, IFNγ and IL-12. UC primarily affects the colon, leading to continuous and superficial mucosal ulceration that extends proximally from the rectum. UC is associated with increased leukocyte infiltration in the LP, superficial crypt abscess and expression of IL-13 and IL-5 [115, 116]. Recently, IL-17- producing Th17 cells have been associated with the pathogenesis of IBD. While TGF-β and IL-6 drive the differentiation of Th17 cells from naïve T cells, IL-23 plays a critical role in the maintenance and expansion of Th17 cells [117, 118]. Although the direct role of IL-17 in IBD pathogenesis remains less convincing [1, 119], IL-23 has been implicated as a major player in the induction of intestinal inflammation in both innate and adaptive models of colitis [120, 121]. Interestingly, a polymorphism in the human IL-23R gene has been associated with the incidence of IBD [122]. A blocking antibody against IL-23 p19 that targets IL-23 (but not IL-12) ameliorated Th17-mediated intestinal inflammation [123], suggesting a role of Th17 cells in IBD. Currently, little is known about the molecular mechanisms of Th17 cell trafficking into sites of inflamed intestinal tissues and the kinetics of α4β7 integrin expression under conditions favoring Th17 cells differentiation. IL-17 expressing T cell that lack β7 integrin show about 50% reduced homing to MLNs and LP (Smith E. and Ley K., 2007 unpublished observation).
Animal models of IBD and diseased human tissues underscored the role of gut-homing effector and regulatory T lymphocytes in IBD pathogenesis (Table 1) [25, 124, 125]. In the earliest study using cotton-top tamarins, which spontaneously develop a chronic colitis similar to human UC, infusion of function blocking anti α4- or α4β7-integrin antibodies was found to ameliorate disease symptoms [126, 127]. Similarly, antibodies specific for integrin β7 and MAdCAM-1 reduced recruitment of lymphocytes to colitic colon and abrogated symptoms of colitis in a mouse model of IBD where naïve CD4+CD45RBhigh T cells were transferred into immunodeficient SCID mice [22]. In a model of colitis induced by intramural injection of peptidoglycan-polysaccharide to rat colon, anti-MAdCAM-1 Ab significantly attenuated lymphocyte recruitment and colonic damage [128]. MAdCAM-1 also plays a role in lymphocyte recruitment into inflamed small intestine in SAMP1/Yit mice, a spontaneous mouse model of human CD. Anti-MAdCAM-1 Ab inhibited adhesion of T cell to microvessels of terminal ileum and attenuated established ileitis [129]. In our studies using this ileitis model, anti-MAdCAM-1 and α4β7 integrin alone failed to attenuate ileitis [27]. The reasons for this discrepancy is presently unknown, but may lie in the kinetic onset of inflammation, age of mice used, the dose or the treatment regime of anti-MAdCAM-1 mAb used. During inflammation, other adhesion molecules are induced, suggesting redundancy among adhesion molecules. As a result, lymphocytes display non-gut specific trafficking into inflamed intestinal tissues. Consistent with this idea, ileitis in SAMP1/Yit mice is inhibited by blocking at least two integrins using an anti-α4 integrin antibody, which interferes with both α4β7 and α4β1 integrins [27]. Moreover, a combination of MAdCAM-1 and L-selectin blockade attenuated the ileitis, suggesting that L-selectin plays a relevant role in ileitis. In the SAMP1/Yit mouse model, over 55% of α4β7 + CD4 effector cells in the MLN co-expressed L-selectin. Interestingly, CD4+CD62L+α4β7 + cells produced more TNF-α than CD62Lnegative cells following stimulation with anti-CD3, indicating functional association between expression of α4β7 and L-selectin [27]. TNFΔARE mice show dysregulated and increased TNF-α expression due to lack of the ARE regulatory element [130]. In the TNFΔARE model, mice genetically lacking β7 integrin showed very mild intestinal pathology [28]. However when TNFΔARE mice on C57BL6 background were crossed with integrin β7 deficient mice, the progeny did not show attenuated ileitis (Rivera-Nieves, J, unpublished results).
Table 1.
Effects of Anti-Adhesion Molecule Blockade in Animal Models of IBD (Adapted and modified from [25])
| IBD Model | Adhesion molecules blocked by mAb | Effect | Reference |
|---|---|---|---|
| CTT | α4 integrin | attenuation of acute but not chronic inflammatory infiltrates | [126] |
| α4β7 integrin | attenuation of colitis | [127] | |
| Gαi2 -/- | α4 integrin | exacerbation of colitis in long term treatment | [186] |
| CD4+CD45RBhigh | α4β7 integrin | reduction in severity of colitis | [22] |
| MAdCAM-1 | |||
| DSS | MAdCAM-1 | reduction in colonic injury | [203] |
| DSS | MAdCAM-1 + CCL20 | attenuation of T and B lymphocytes accumulation | [204] |
| DSS | MAdCAM-1 | no effect on the colitis | [205] |
| VCAM-1 | attenuation of colitis | ||
| SAMP1/Yit | MAdCAM-1 | amelioration of ileitis; attenuation of established ileitis | [129] |
| SAMP1/Yit | ICAM-1 + VCAM-1 | reduction of ileitis | [206] |
| SAMP1/YitFc | α4β7 integrin | no effect on the severity of acute and chronic ileitis | [27] |
| MAdCAM-1 | |||
| α4 integrin | attenuation of ileitis | ||
| MAdCAM-1 + L-selectin | |||
| TNP-OVA/ IL2-/- | αEβ7 | Prevention of colitis; Amelioration of established colitis | [165] |
CTT, Cotton-top tamarins; Gαi2, heterotrimeric G protein subunit Gαi2; DSS, Dextran sulfate sodium; SAMP/Yit, senescence-accelerated mouse; TNP: 2,4,6-trinitrophenol
Differential mechanisms in lymphocyte recruitment during intestinal inflammation have also been demonstrated in colitis models. In colitis models primarily involving CD4+ T cell effector function, such as IL-2-/- mice and adoptive transfer of CD4+CD45Rbhigh cells into SCID mice, β7 integrins are not required for T lymphocyte localization to intestine and for colitis pathogenesis [21]. The onset of colitis in recipients of T cells from β7-deficient mice was delayed over 9 weeks but eventually developed at 25 weeks, suggesting β7 integrins may be required during the early course of induction of colitis [21]. Using a similar transfer model in RAG-/- mice, Park et al found reduced colitis over 11 weeks in mice reconstituted with CD4+CD45RBhigh T cells from β7-integrin deficient mice compared to wild type mice [131]. Although the latter study did not examine the inflammation score beyond 11 weeks, they suggested a protective role of β7 integrins deficiency in reducing colitis progression.
Taken together, the role of β7 integrin in colitis models remains inconclusive. Plausible explanations include stage-specific roles of this integrin in the pathogenesis of colitis, implying differential involvement of adhesion molecules in the early versus late gut inflammatory responses. In this regard the kinetics of the cytokines produced may vary depending on Th1/Th2/Th17 polarization condition of the inflammation. Activation of murine CD4+ T cells under Th1 promoting conditions favors α4β7 expression compared to activation under Th2 promoting conditions [132]. There is also a positive correlation between α4β7 expression on human CD4+ T cells and their ability to produce IFN-γ [132]. Alternatively, other pathways that involve different adhesion molecules or non-lymphocyte cell populations may be involved. In line with the latter hypothesis, abundant presence of neutrophils and eosinophils in UC has been reported. Presence of these granulocytes also correlates with disease severity and gastrointestinal dysfunction [133]. In a dextran sodium sulphate-induced colitis model, recruitment of eosinophils has been shown to be dependent on β2-integrin/ICAM-1 interactions but not on α4β7 integrin [134]. Similarly, lymphocytes may also use αLβ2 (LFA-1) to compensate for a lack of the conventional gut homing α4β7/MAdCAM-1 interaction for their recruitment to large intestine. Moreover, adoptive transfer of CD4+CD62L+ T cells has been shown to induce chronic colitis, suggesting the involvement of L-selectin in the pathogenesis of UC [135]. Alternatively L-selectin serves as a marker of naïve T cells which are known to induce colitis. L-selectin ligands have been detected in chronic inflammatory sites [136, 137].
The role of β7 integrins for Treg trafficking is not well understood. Previous work on characterization of different subsets of regulatory T cells identified α4β7 + Treg that induce IL-10highTGF-βlow secondary suppressor T cells [138]. In a CD4+CD45RBhigh T cell adoptive transfer model of colitis, those integrin β7-deficient Treg cells with severely impaired gut homing still possess in vivo suppressive effect [139]. It is likely that this suppressive effect is exerted at the induction sites of GALT where it is able to inhibit the initial priming and differentiation of colitogenic T cells through cell-cell contact inhibition. As discussed in a previous section, lymphocytes use L-selectin and β7 integrin to home to GALT. About 50% of Foxp3+ Treg cells express L-selectin which can bind to MAdCAM-1 [140]. Thus, MLN is a likely site where β7-deficient Treg cells accumulate, interact with APCs and inhibit the initial priming and differentiation of pathogenic Th cells, thereby preventing the development of colitis. This concept is in agreement with recent studies in adoptive transfer models of colitis demonstrating that CCR7-/- naïve Treg [141] and CCR4-/- Treg [142] cells exhibited delayed accumulation in PLN and MLN, which in turn correlated with their impaired ability to inhibit development of colitis. Alternatively, Treg cells likely use β7 integrin-independent pathways to migrate to the effector sites of LP, where they restrain the responses of effector T cells through secretion of anti-inflammatory cytokines IL-10 and TGF-β. It is still unknown whether Treg cells use similar homing receptors like effector T cells to migrate to the same intestinal tissues and whether Treg cell migration to the intestinal effector sites like LP is really important to induce their suppressive effect.
β7 integrins and intestinal parasitic infections
The efficient recruitment of appropriately polarized CD4+ T lymphocyte to the intestine is crucial for resolution of intestinal parasite infection. Using a Trichuris muris-driven large intestinal inflammation, it has been demonstrated that while mice treated with ant-α4 antibodies did not expel parasites as efficiently as rat IgG control treated mice, those treated with anti-β7 or anti-MAdCAM-1 did [29]. Blockade of α4β7, α4β1, αEβ7 and MAdCAM-1 failed to prevent recruitment of CD4+ T cells to the large intestine during T.muris infection [29]. Similarly, β7-deficient mice are protected when infected with T.muris compared to wild type mice [19]. In contrast, during infection with Trichinella spiralis, a small-intestine nematode, β7 deficient mice displayed a delayed recruitment of leukocytes to the small intestine and delayed worm expulsion [19]. These findings show a difference between large and small intestine in terms of the dependency for α4β7/MAdCAM-1 interaction, suggesting compartmentalized usage of adhesion molecules along the length of the intestine.
β7 integrins and intestinal GVHD
β7 integrins are also involved in the development of graft-versus-host disease (GVHD). GVHD is a serious and often fatal complication of allogenic hematopoietic cell transplantation. Acute GVHD (aGVHD) is an immune syndrome mediated by donor-derived CTL that recognize and attack the recipient’s tissues such as gastrointestinal tract, liver and skin as foreign [143]. Donor-derived CTLs use L-selectin and α4β7 to migrate to GALT such as MLN and PP [144]. Absence of β7 on donor CTL [145, 146] or interference with MAdCAM-1 [24] ameliorates GVHD-associated intestinal injury without affecting the beneficial graft versus tumor (GVT) effects. Murai et al demonstrated that PPs are a key location for the donor T cell activation and subsequent trigger of GVHD [147]. Mice lacking PP and mice in which donor T cell migration to PP is blocked by anti-MAdCAM-1 blocking antibodies show protection from GVHD [147]. However, recent studies did not reproduce the same beneficial effects using anti-MAdCAM-1 and anti-CD62L blocking antibodies [148, 149]. The initiation of aGVHD was prevented only in splenectomized B6-LTα-/- recipients or using anti-MAdCAM-1 and anti-CD62L blocking antibodies in splenectomized Balb/c recipients [149]. This finding emphasizes a need for blocking T cell access to all SLOs including spleen to prevent aGVHD and demonstrates a redundancy of priming sites at different anatomical locations within SLOs. aGVHD can proceed irrespective of the original priming site [149].
αEβ7 integrin and its ligand E-cadherin
The other β7 integrin, αEβ7, has been implicated in retention of lymphocytes within epithelial tissues via binding to E-cadherin [10]. Thus, αE-deficient mice display a reduction in the number of lymphocytes residing in the intestinal epithelium and LP [15]. E-cadherin is the only known ligand for αEβ7 integrin. However, the reduction of LPL in αE-deficient mice [15] and the blockade of lymphocyte binding to gut microvascular endothelial cells by anti-αE integrin antibody [150] suggest the presence of other as yet unidentified ligand(s) in the LP-associated venules, which do not express E-cadherin. It thus remains important to identify what retains LPL expressing αEβ7 integrin in the LP. Moreover, expression of αE does not endow T cells with migratory behavior [151]. Adoptively transferred CD103-deficient CTL cells localize to the epithelium or LP like their wild type counterparts after antiviral immune response [152]. Thus, αEβ7 integrin does not act as a homing receptor for effector CTL cells and as such its role in the IEL and LPL multi-step adhesion cascade remains unclear.
Although CD103-deficient mice have a reduced number of IELs, they still have considerable numbers of IELs [15], demonstrating the existence of alternative mechanisms to retain IELs within small intestine epithelial tissues. IELs express multiple β1 integrins that can interact with components of epithelial basement membranes and mucosal mesenchymal cells [153, 154], but β1 integrins are not required to retain IELs within intestinal epithelia [154]. Similarly, mice chimeric for β1 associated α chains, α4, α5 and αV, have normal numbers of IELs [155]. However, very late antigen-1 (VLA-1, α1β1 integrin) deficient mice have reduced number of small intestine IELs [156]. The apparent discrepancy of this study with the former two reports is not clear, but may be related to deficiency of VLA-1 expression in many non-hematopoietic cells including small intestinal epithelial cells [157].
As mentioned earlier, αE integrin has been shown to be expressed on various immune cells including CD8+ T cells, CD4+CD25+ Treg and DC. The expression of CD103 in particular on mucosal DCs is associated with diverse immune functions including induction of gut homing receptor expression [33], RAR signaling [38, 39] and Foxp3+ Treg differentiation [36, 37]. These functions of CD103+ DC are not a general property of intestinal DCs but are peculiar to CD103+ DCs residing in the MLN and small intestine LP [39]. However, CD103 is not required to imprint gut tropic receptors, as MLN DCs from CD103-deficient and WT mice are equally efficient at inducing gut tropic receptors on activated T cells [39]. It thus seems that expression of CD103 is just a marker of a DC subset.
The recognition of epithelial cells is likely to be important in understanding the role of cross talk between intestinal epithelium and IEL cells [3, 158]. For instance, CTL and γδ IEL cells in close proximity to the mucosal intestinal epithelial tissues may have broad functions including cytolytic, cytokine/chemokine secretion as well as modulating adaptive immune responses [159]. Furthermore, the binding of CD103+ DC to its ligand E-cadherin on epithelial cells may ‘condition’ their function by the influence of factors from the intestinal microenvironment [2, 160-162]. Such conditioned DCs fail to release inflammatory cytokines and to support differentiation of Th1 cells. Interestingly, in human it has been identified that one of such conditioning factor from epithelial cells include the cytokine thymic stromal lymphopoietin (TSLP) [163]. Thus, with the other factors released from epithelial cells such as RA and TGF-β, TSLP may induce the development of tolerogenic CD103+ DCs [164]. The conditioned CD103+ DCs then constitutively migrate to MLN, where they may induce development of Treg that reduce intestinal inflammation through secretion of immunosuppresvive cytokines [2, 160, 161]. A subset of CD103+ DCs in MLN promotes the conversion of naïve T reg into Foxp3+ T reg cells in TGF-β and RA dependent manner [36]. CD103+ DCs in MLN also regulate the development of CD4+CD45RBhigh T cell mediated colitis [34].
αEβ7 integrin and GI inflammation
The role of αEβ7 integrin in IBD is not clear. By using 2, 4, 6-trinitrophenol (TNP)-OVA immunized IL2-/- mice, Ludviksson et al demonstrated a critical role of colonic localization of αEβ7-expressing CD4+ LPL in the induction and maintenance of colitis [165]. They showed that treatment with anti-αEβ7 mAb prevented colitis and ameliorated established colitis [165]. However, colitogenic CD4+CD45RBhigh T cells from wild-type or CD103-deficient mice equally transferred the disease into SCID mice, indicating CD103 is not required for the accumulation of CD4+ T cells in the colon and the development of colitis [34]. A similar study also indicated that β7 integrins are not required in the development of colitis in IL-2-/- mice and in an adoptive transfer model [21].
Recent studies have shown that CD4+CD103+ T cells regulate colitis induced by CD4+CD45Rbhigh cells through their production of IL-10 [34, 64, 166]. However, in the SAMP1/YitFc mouse model of ileitis the CD4+CD103high subset failed to attenuate disease [167]. In the TNFΔARE model of ileitis, in which TNF-α production is dysregulated, CD8+CD103high T cells attenuate ileitis induced by adoptive transfer of CD4+ T cells isolated from TNFΔARE mice [168]. However, the regulatory effect in this model is different from that afforded by CD4+CD103high T cells as it appears to be mediated by TGF-β and not by IL-10 [34, 64, 169], mimicking the functional phenotype of Th3 cells [170] and CD8+ Treg cells induced by HIV proteins and peptides [171]. Indeed, neutralizing antibodies against TGF-β reversed the attenuating effect of co-transferring CD45RBlow and the protective effect of feeding the hapten in TNBS-induced colitis [166]. In humans, a regulatory role for CD8+CD103high T cells has also been reported after stimulation with allo-antigens [172].
In TNFΔARE mice, inflammatory mediators appear to enhance the regulatory function of the CD8+CD103high subset. Thus, in comparison to WT controls, fewer cells isolated from TNFΔARE mice were required to inhibit CD4+ proliferation [168]. Notably, IFN-γ present at high levels during the entire disease time course directly stimulates CD8+ regulatory T cells to produce TGF-β [173]. TGF-β in turn increases the expression of CD103 [174] and positively regulates its own production (Fig. (1)) [175]. Thus, the crosstalk between T cell subsets appears to modulate the balance between effector and regulatory functions.
The expression of CD103 on donor CD8+ CTL is required for the retention of these cells within the intestinal epithelium and for the development of GVHD. Of interest, the degree of expression of this integrin correlates with the extent of epithelial cell destruction and thus the clinical manifestation of GVHD [65]. Thus, CD8+ CTL deficient in CD103 migrate to intestinal epithelium normally, but fail to be retained in the epithelium and their number is decreased compared to wild type CD8+ CTL. This in turn resulted in a defective ability of CD103-deficient CD8+ CTL to transfer intestinal GVHD [65]. As discussed in a previous section, this integrin is upregulated on CD8 IEL precursors, derived from α4β7 + CD8+ cells in the gut lymph nodes, which migrate to the intestinal epithelial compartment and differentiate under the influence of TGF-β, which is produced locally in the intestine. Removal of MLN demonstrated a significant reduction in the number of CD103+ CD8+ CTL in the lamina propria and gut epithelium, suggesting a role of gut LNs in the pathology of GVHD [176]. Apart from its role in the retention of T cells in intestinal epithelium expressing E-cadherin, the expression of CD103 on CTL with T cell receptor for cognate alloantigen on gut epithelium could provide a costimulatory signal for IEL proliferation and target cell lysis activity that promotes epithelial destruction and cellular cytotoxicity [177].
Additional roles of β7-integrins in immune responses
The α4β7 integrin and MAdCAM-1 adhesion pathway may have additional immunological functions other than acting as a lymphocyte homing receptor. MAdCAM-1 has been reported to be expressed on follicular DCs (FDCs) in PPs and MLN [178], possibly providing a co-stimulatory signal that mediates antigen-specific T cell proliferation [179]. Similarly, recombinant MAdCAM-1-Ig can enhance T lymphocyte activation of murine MLN lymphocytes expressing α4β7 integrin [180]. Costimulation of T cells can also be triggered via anti-α4β7 integrin antibodies [181]. Here it is important to note that, FDCs expressing MAdCAM-1 do not present antigenic peptides. Nevertheless, the abundantly expressed MAdCAM-1 on the processes of FDCs in germinal centers may mediate B cell adhesion and activation [182-184]. Alternatively, MAdCAM-1 mediated costimulation likely originates from the MAdCAM-1 expressing mesenchymal stromal cells in the secondary lymphoid tissues. However, there are no in vivo studies that show a direct interaction of MAdCAM-1 positive FDCs with α4β7 integrin in mediating costimulation of lymphocytes.
Anti-integrin antibody-mediated costimulation could also modulate T cell responses during Ag presentation, such as polarizing or skewing the Th1/Th2 biased immune response. During the formation of the immunological synapse, engagement of α4β1 integrin with specific mAbs induces recruitment of this integrin to the peripheral supramolecular activation cluster of the immunological synapse, leading to the differentiation of Th1 cells [185]. In support of this idea, a recent study using a Trichuris muris-mediated large intestinal inflammation model showed that treatment of C57BL6 mice with anti-α4 mAbs acts as a costimulatory signal via α4β7 or α4β1 interaction, resulting in a dominant Th1 polarization in the MLN and parasite persistence and a chronic T. muris infection [29]. Similar results have been also observed in an in vitro polarization system using MLN cells from naïve C57BL6 mice. The addition of anti-α4 antibody to Th2 polarized T cells significantly reduced IL-13 production by MLN cells compared to untreated cells [29]. It is likely that such costimulation is triggered via α4β7 or α4β1 with a resulting dominant Th1 polarization in the MLN, which prevents parasite expulsion [29]. It is also tempting to speculate that the exacerbated colitis seen in Gαi2 -/- mice following long term treatment with anti-α4 antibodies may be partly explained by a Th1-biased immune response [186]. Consistent with this idea, increased production of the proinflammatory Th1 cytokines IFNγ and TNF-α has been found in anti-α4-treated mice [186]. Moreover, blockade of lymphocyte α4β1 integrin interaction with VCAM-1 increases T cell apoptosis [187]. This is an interesting finding in light of prevailing evidence that augmented intestinal LP T-cell activation and thus resistance to apoptosis is central to the pathogenesis of IBD [188]. Hence, targeting MAdCAM-1 or α4/α4β7 integrin by antibody blockade is directed against receptors with different biological roles that do not only involve lymphocyte trafficking but are also involved in the generation of the immune response and in the differentiation of Th1/Th2 lymphocytes and survival. As such, this raises the possibility that the therapeutic efficacy of humanized mAbs against α4 and α4β7 integrins in IBDs might not be only mediated by blocking lymphocyte trafficking.
β7 integrin as therapeutic target in GI inflammation
Because of their critical role in leukocyte trafficking from blood to the intestinal tissues, α4β7 integrin and MAdCAM-1 have emerged as anti-adhesion therapeutic targets in IBD [25, 40, 41, 189]. Blocking dysregulated recruitment of inflammatory cells into the gut and thus the development of intestinal inflammation has prompted interest of pharmaceutical companies to develop anti-adhesion therapies for IBD. Natalizumab, a humanized IgG4 therapeutic monoclonal antibody is directed against both α4β1- and α4β7- integrins, thereby blocking α4β1/VCAM-1 and α4β7/MAdCAM-1 interactions of lymphocytes with endothelium [40, 190]. This antibody has shown efficacy for treatment of MS [191] and CD [43]. The success in treatment of these immune-mediated inflammatory diseases has been attributed to blocking trafficking adhesion molecules used by lymphocytes. Natalizumab was temporarily suspended from the market, because three out of several thousand patients who had received the drug developed progressive multifocal leukoencephalopathy (PML) [192]. PML is a demyelinating disease of the central nervous system caused by the reactivation of the JC virus, a human polyoma virus, in the setting of immunodeficiency. All these PML patients had received additional immunosuppresants. Thus, natalizumab might cause global inhibition of α4 integrin-mediated host defense functions, such as entry of JC-virus specific CTL to brain to control this opportunistic latent virus infection. This safety issue raised an interest to develop a more intestinal tissue-specific therapy with a mAb against α4β7 (MLN02) in UC [23]. After review, natalizumab has been reintroduced as a therapy for MS and CD patients that do not receive other immunosuppressant treatments [193].
Novel therapeutic approaches could exploit the recent development in our understanding of the structure-function of α4β7 integrin [131, 194], in identifying the cytoplasmic factors involved in integrin activation [195, 196] and in vivo delivery of small interfering RNA (siRNA) targeted to leukocyte β7 integrin [197]. Recent studies by Shimaoka and colleagues identified that the ADMIDAS site in the ectodomain of the β subunit of α4β7 regulates α4β7 integrin adhesiveness [131]. They showed that mutation of ADMIDAS constitutively keeps the integrin in the adhesive state, which dramatically impaired gut accumulation of activated T lymphocytes in an immunodeficient transfer colitis model and thus resulted in attenuated colitis [131]. Thus the balance in adhesion and deadhesion of α4β7 integrin may regulate lymphocyte trafficking to the gut and colitis progression [131]. Similarly, knock-in mice (α4-R/AGFFKR mice) in which perturbing a putative cytoplasmic membrane-proximal salt bridge in α4 integrin resulted in constitutively activated integrin, suggesting that the putative membrane-proximal cytoplasmic regulates the proper adhesive dynamics of the integrin. As such, lymphocytes from α4-R/AGFFKR mice exhibit increased firm adhesion to PP venules in vivo and suppressed migration to both normal and DSS-induced inflamed gut [194]. The α4 integrin cytoplasmic domain binds to paxillin [195]. Mutation of the paxillin binding site in the α4 integrin resulted in blockade of α4-paxillin interaction, which in turn selectively impaired recruitment of mononuclear cells to inflammatory sites [195, 198]. The YDRREY sequence within the β7 cytoplasmic domain interacts with focal adhesion kinase and Src family kinases, thus controlling clustering and adhesion of the intergin α4β7 [199]. Using a DSS colitis model, systemic delivery of leukocyte-directed cyclin D1 siRNA coupled to gut specific β7 integrin attenuated colitis through suppression of aberrant mononuclear cell proliferation and reduction of expression of Th1 proinflammatory cytokines such as TNF-α and IL-12 [197].
Finally, RA may also have therapeutic potential by inducing differentiation of naïve B and T cells into small intestinal-homing, IgA-producing ASCs [61] and Foxp3+ T reg [37], respectively. Thus, RA supplementation may have therapeutic potential to harness the mucosal immune response, either to immune responsiveness or unresponsiveness/oral tolerance [2, 200, 201]. One promising application might be to generate regulatory T cells by activating ex vivo naïve T cells in the presence of TGF-β and RA with a characteristic gut homing phenotype imprinting by RA [36].
Conclusions
Our understanding of the mechanisms of intestinal lymphocyte trafficking and retention in health and disease has progressed in the past few years. DCs in the mucosal environment ‘imprint’ gut-homing capacity by increasing the selective expression of α4β7 integrin and CCR9 chemokine receptor on activated effector and regulatory T lymphocytes in a RA dependent manner. Although, the expression of CD103 integrin on DCs has been associated with many immune functions, it is currently unclear whether CD103 is just a marker of a DC subset or whether its binding to E-cadherin actually changes immune regulation. The physiological lymphocyte recruitment to intestinal tissues is highly regulated and depends on the classical gut specific homing receptor α4β7 and its ligand MAdCAM-1. Inflammatory conditions in the intestine seem to invoke a redundant upregulated expression of inflammatory adhesion molecule receptors and ligands such as α4β1 and VCAM-1, LFA-1 and ICAM-1 and L-selectin. This results in dysregulated leukocyte recruitment to chronic inflamed intestinal tissues and thus perpetuation of the inflammatory disease. Accordingly, future anti-adhesion molecule therapies in IBD need to optimize the strategy to block such redundant non-tissue-specific pathways. Blockade of α4 integrins has been already approved for treatment of MS and CD. Other specific approaches targeting β7 integrins including modulating the activation of α4β7 integrin, identifying cytoplasmic factors activating α4 integrin and in vivo delivery of siRNA targted to leukocyte β7 integrin are in active research. Beyond its role in blocking lymphocyte trafficking, the anti-adhesion molecules therapeutic strategy may have effects in lymphocyte costimulation, polarization and survival. Such therapeutic targeting should also take into account the multiple roles of adhesion molecules in inflammation and host defense, as blocking them could be a ‘double-edged sword’ [202].
Acknowledgments
Our research is supported by NIH DK 57880.
Abbreviations
- CD
Crohn’s disease
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- GALT
Gut-associated lymphoid tissue
- GI
Gastrointestinal
- GVHD
Graft-versus-host disease
- IEL
Intraepithelial lymphocyte
- IBD
Inflammatory bowel disease
- LP
Lamina propria
- LPL
Lamina propria lymphocyte
- MAdCAM-1
Mucosal Addressin Cell Adhesion Molecule-1
- MLN
Mesenteric lymph node
- PP
Peyer’s patch
- RA
Retinoic acid
- RTE
Recent thymic emigrant
- UC
Ulcerative colitis
Footnotes
Conflict of interest The authors declare no competing financial interests.
References
- 1.Kelsall BL. J Pathol. 2008;214:242–59. doi: 10.1002/path.2286. [DOI] [PubMed] [Google Scholar]
- 2.Coombes JL, Powrie F. Nat Rev Immunol. 2008;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artis D. Nat Rev Immunol. 2008;8:411–20. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, kiyono H, Pabst R, Russell MW. Mucosal Immunol. 2008;1:31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- 5.Butcher EC. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 6.Springer TA. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 7.von Andrian UH, Mackay CR. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 8.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 9.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 10.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Nature. 1994;372:190–3. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 11.Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, Rajewsky K, Muller W. Nature. 1996;382:366–70. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 12.Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. J Exp Med. 2000;192:761–8. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salmi M, Jalkanen S. Immunol Rev. 2005;206:100–13. doi: 10.1111/j.0105-2896.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 14.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 15.Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM. J Immunol. 1999;162:6641–9. [PubMed] [Google Scholar]
- 16.Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. Curr Opin Cell Biol. 2000;12:563–8. doi: 10.1016/s0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 17.Mebius RE, Schadee-Eestermans IL, Weissman IL. Cell Adhes Commun. 1998;6:97–103. doi: 10.3109/15419069809004464. [DOI] [PubMed] [Google Scholar]
- 18.Rott LS, Rose JR, Bass D, Williams MB, Greenberg HB, Butcher EC. J Clin Invest. 1997;100:1204–8. doi: 10.1172/JCI119633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artis D, Humphreys NE, Potten CS, Wagner N, Muller W, McDermott JR, Grencis RK, Else KJ. Eur J Immunol. 2000;30:1656–64. doi: 10.1002/1521-4141(200006)30:6<1656::AID-IMMU1656>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 20.Kuklin NA, Rott L, Feng N, Conner ME, Wagner N, Muller W, Greenberg HB. J Immunol. 2001;166:1894–902. doi: 10.4049/jimmunol.166.3.1894. [DOI] [PubMed] [Google Scholar]
- 21.Sydora BC, Wagner N, Lohler J, Yakoub G, Kronenberg M, Muller W, Aranda R. Clin Exp Immunol. 2002;129:35–42. doi: 10.1046/j.1365-2249.2002.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. J Immunol. 1997;158:2099–106. [PubMed] [Google Scholar]
- 23.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, Dube R, Cohen A, Steinhart AH, Landau S, Aguzzi RA, Fox IH, Vandervoort MK. N Engl J Med. 2005;352:2499–507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 24.Ueha S, Murai M, Yoneyama H, Kitabatake M, Imai T, Shimaoka T, Yonehara S, Ishikawa S, Matsushima K. J Leukoc Biol. 2007;81:176–85. doi: 10.1189/jlb.0306231. [DOI] [PubMed] [Google Scholar]
- 25.Rivera-Nieves J, Gorfu G, Ley K. Inflamm Bowel Dis. 2008;14:1715–1735. doi: 10.1002/ibd.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. J Immunol. 1994;152:3282–93. [PubMed] [Google Scholar]
- 27.Rivera-Nieves J, Olson T, Bamias G, Bruce A, Solga M, Knight RF, Hoang S, Cominelli F, Ley K. J Immunol. 2005;174:2343–52. doi: 10.4049/jimmunol.174.4.2343. [DOI] [PubMed] [Google Scholar]
- 28.Apostolaki M, Manoloukos M, Roulis M, Wurbel MA, Muller W, Papadakis KA, Kontoyiannis DL, Malissen B, Kollias G. Gastroenterology. 2008;134:2025–35. doi: 10.1053/j.gastro.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 29.Bell LV, Else KJ. Parasite Immunol. 2008;30:163–70. doi: 10.1111/j.1365-3024.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- 30.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 31.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. J Exp Med. 2003;198:963–9. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. J Exp Med. 2005;202:1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. J Immunol. 2007;178:6861–6. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 36.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svensson M, Johansson-Lindbom B, Zapata F, Jaensson E, Austenaa LM, Blomhoff R, Agace WW. Mucosal Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Andrian UH, Engelhardt B. N Engl J Med. 2003;348:68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- 41.Yonekawa K, Harlan JM. J Leukoc Biol. 2005;77:129–40. doi: 10.1189/jlb.0804460. [DOI] [PubMed] [Google Scholar]
- 42.Lebwohl M, Tyring SK, Hamilton TK, Toth D, Glazer S, Tawfik NH, Walicke P, Dummer W, Wang X, Garovoy MR, Pariser D. N Engl J Med. 2003;349:2004–13. doi: 10.1056/NEJMoa030002. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnalek P, Zadorova Z, Palmer T, Donoghue S. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 44.Hynes RO. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 45.Hynes RO. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 46.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Annu Rev Immunol. 2004;22:157–80. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- 47.Kinashi T. Nat Rev Immunol. 2005;5:546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 48.Humphries JD, Byron A, Humphries MJ. J Cell Sci. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo BH, Carman CV, Springer TA. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Proc Natl Acad Sci U S A. 2007;104:15823–8. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Nature. 2007;449:361–5. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Salas A, Springer TA. Nat Struct Biol. 2003;10:995–1001. doi: 10.1038/nsb1011. [DOI] [PubMed] [Google Scholar]
- 53.Lu C, Takagi J, Springer TA. J Biol Chem. 2001;276:14642–8. doi: 10.1074/jbc.M100600200. [DOI] [PubMed] [Google Scholar]
- 54.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Science. 2003;302:103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 55.Takagi J, Petre BM, Walz T, Springer TA. Cell. 2002;110:599–11. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 56.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. J Immunol. 1994;153:517–28. [PubMed] [Google Scholar]
- 57.Brandt EB, Zimmermann N, Muntel EE, Yamada Y, Pope SM, Mishra A, Hogan SP, Rothenberg ME. Clin Exp Allergy. 2006;36:543–53. doi: 10.1111/j.1365-2222.2006.02456.x. [DOI] [PubMed] [Google Scholar]
- 58.Staton TL, Habtezion A, Winslow MM, Sato T, Love PE, Butcher EC. Nat Immunol. 2006;7:482–8. doi: 10.1038/ni1319. [DOI] [PubMed] [Google Scholar]
- 59.Schweighoffer T, Tanaka Y, Tidswell M, Erle DJ, Horgan KJ, Luce GE, Lazarovits AI, Buck D, Shaw S. J Immunol. 1993;151:717–29. [PubMed] [Google Scholar]
- 60.Rott LS, Briskin MJ, Andrew DP, Berg EL, Butcher EC. J Immunol. 1996;156:3727–36. [PubMed] [Google Scholar]
- 61.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 62.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. J Exp Med. 2005;201:303–16. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cepek KL, Parker CM, Madara JL, Brenner MB. J Immunol. 1993;150:3459–70. [PubMed] [Google Scholar]
- 64.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Proc Natl Acad Sci U S A. 2002;99:13031–6. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. J Exp Med. 2005;201:1647–57. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kilshaw PJ, Murant SJ. Eur J Immunol. 1991;21:2591–7. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki R, Nakao A, Kanamaru Y, Okumura K, Ogawa H, Ra C. Int Immunol. 2002;14:339–45. doi: 10.1093/intimm/14.4.339. [DOI] [PubMed] [Google Scholar]
- 68.Lim SP, Leung E, Krissansen GW. Immunogenetics. 1998;48:184–95. doi: 10.1007/s002510050422. [DOI] [PubMed] [Google Scholar]
- 69.Ericsson A, Svensson M, Arya A, Agace WW. Eur J Immunol. 2004;34:2720–9. doi: 10.1002/eji.200425125. [DOI] [PubMed] [Google Scholar]
- 70.Kraal G, Schornagel K, Streeter PR, Holzmann B, Butcher EC. Am J Pathol. 1995;147:763–71. [PMC free article] [PubMed] [Google Scholar]
- 71.Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 72.Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. Proc Natl Acad Sci U S A. 1996;93:11019–24. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salmi M, Alanen K, Grenman S, Briskin M, Butcher EC, Jalkanen S. Gastroenterology. 2001;121:853–64. doi: 10.1053/gast.2001.27968. [DOI] [PubMed] [Google Scholar]
- 74.Ley K, Burns C. Gastroenterology. 2001;121:1008–10. doi: 10.1053/gast.2001.28635. [DOI] [PubMed] [Google Scholar]
- 75.Salmi M, Andrew DP, Butcher EC, Jalkanen S. J Exp Med. 1995;181:137–49. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelly KA, Rank RG. Infect Immun. 1997;65:5198–208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adams DH, Eksteen B. Nat Rev Immunol. 2006;6:244–51. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 78.Adams DH, Eksteen B, Curbishley SM. Gut. 2008;57:838–48. doi: 10.1136/gut.2007.122168. [DOI] [PubMed] [Google Scholar]
- 79.Leung E, Kanwar RK, Kanwar JR, Krissansen GW. Immunol Cell Biol. 2003;81:320–7. doi: 10.1046/j.1440-1711.2003.t01-1-01175.x. [DOI] [PubMed] [Google Scholar]
- 80.Cuff CA, Schwartz J, Bergman CM, Russell KS, Bender JR, Ruddle NH. J Immunol. 1998;161:6853–60. [PubMed] [Google Scholar]
- 81.Oshima T, Pavlick KP, Laroux FS, Verma SK, Jordan P, Grisham MB, Williams L, Alexander JS. Am J Physiol Cell Physiol. 2001;281:C1096–105. doi: 10.1152/ajpcell.2001.281.4.C1096. [DOI] [PubMed] [Google Scholar]
- 82.Ando T, Langley RR, Wang Y, Jordan PA, Minagar A, Alexander JS, Jennings MH. BMC Physiol. 2007;7:10. doi: 10.1186/1472-6793-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. J Exp Med. 1996;183:1461–72. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. J Leukoc Biol. 1999;65:349–55. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 85.Ogawa H, Binion DG, Heidemann J, Theriot M, Fisher PJ, Johnson NA, Otterson MF, Rafiee P. Am J Physiol Cell Physiol. 2005;288:C272–81. doi: 10.1152/ajpcell.00406.2003. [DOI] [PubMed] [Google Scholar]
- 86.Wang CC, Biben C, Robb L, Nassir F, Barnett L, Davidson NO, Koentgen F, Tarlinton D, Harvey RP. Dev Biol. 2000;224:152–67. doi: 10.1006/dbio.2000.9749. [DOI] [PubMed] [Google Scholar]
- 87.Pabst O, Forster R, Lipp M, Engel H, Arnold HH. Embo J. 2000;19:2015–23. doi: 10.1093/emboj/19.9.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schippers A, Leuker C, Pabst O, Kochut A, Prochnow B, Gruber AD, Leung E, Krissansen GW, Wagner N, Muller W. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 89.Bungartz G, Stiller S, Bauer M, Muller W, Schippers A, Wagner N, Fassler R, Brakebusch C. Blood. 2006;108:1857–64. doi: 10.1182/blood-2005-10-007658. [DOI] [PubMed] [Google Scholar]
- 90.Wang C, McDonough JS, McDonald KG, Huang C, Newberry RD. J Immunol. 2008;181:4052–61. doi: 10.4049/jimmunol.181.6.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.von Andrian UH, Mempel TR. Nat Rev Immunol. 2003;3:867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 92.Luster AD, Alon R, von Andrian UH. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 93.Agace WW. Nat Rev Immunol. 2006;6:682–92. doi: 10.1038/nri1869. [DOI] [PubMed] [Google Scholar]
- 94.Johansson-Lindbom B, Agace WW. Immunol Rev. 2007;215:226–42. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 95.Carman CV, Springer TA. Curr Opin Cell Biol. 2003;15:547–56. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 96.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. Nature. 1993;366:695–8. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 97.Briskin MJ, McEvoy LM, Butcher EC. Nature. 1993;363:461–4. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- 98.Shyjan AM, Bertagnolli M, Kenney CJ, Briskin MJ. J Immunol. 1996;156:2851–7. [PubMed] [Google Scholar]
- 99.Siegelman MH, Stanescu D, Estess P. J Clin Invest. 2000;105:683–91. doi: 10.1172/JCI8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Svensson M, Marsal J, Ericsson A, Carramolino L, Broden T, Marquez G, Agace WW. J Clin Invest. 2002;110:1113–21. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steeber DA, Tang ML, Zhang XQ, Muller W, Wagner N, Tedder TF. J Immunol. 1998;161:6638–47. [PubMed] [Google Scholar]
- 102.Kunkel EJ, Ramos CL, Steeber DA, Muller W, Wagner N, Tedder TF, Ley K. J Immunol. 1998;161:2449–56. [PubMed] [Google Scholar]
- 103.Wagner N, Lohler J, Tedder TF, Rajewsky K, Muller W, Steeber DA. Eur J Immunol. 1998;28:3832–9. doi: 10.1002/(SICI)1521-4141(199811)28:11<3832::AID-IMMU3832>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 104.Ley K, Kansas GS. Nat Rev Immunol. 2004;4:325–35. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 105.Stagg AJ, Kamm MA, Knight SC. Eur J Immunol. 2002;32:1445–54. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 106.McLachlan JB, Jenkins MK. Proc Am Thorac Soc. 2007;4:439–42. doi: 10.1513/pats.200606-137MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siewert C, Menning A, Dudda J, Siegmund K, Lauer U, Floess S, Campbell DJ, Hamann A, Huehn J. Eur J Immunol. 2007;37:978–89. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- 108.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 110.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 111.Edele F, Molenaar R, Gutle D, Dudda JC, Jakob T, Homey B, Mebius R, Hornef M, Martin SF. J Immunol. 2008;181:3745–9. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 112.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. J Exp Med. 2008;205:2483–90. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ahrendt M, Hammerschmidt SI, Pabst O, Pabst R, Bode U. J Immunol. 2008;181:1898–907. doi: 10.4049/jimmunol.181.3.1898. [DOI] [PubMed] [Google Scholar]
- 114.Eksteen B, Liaskou E, Adams DH. Inflamm Bowel Dis. 2008;14:1298–312. doi: 10.1002/ibd.20453. [DOI] [PubMed] [Google Scholar]
- 115.Xavier RJ, Podolsky DK. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 116.Strober W, Fuss I, Mannon P. J Clin Invest. 2007;117:514–21. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weaver CT, Hatton RD, Mangan PR, Harrington LE. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 118.Kastelein RA, Hunter CA, Cua DJ. Annu Rev Immunol. 2007;25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 119.Steinman L. J Exp Med. 2008;205:1517–22. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. J Exp Med. 2006;203:2473–83. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. J Exp Med. 2006;203:2485–94. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. Science. 2006;314:1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 124.Strober W, Fuss IJ, Blumberg RS. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 125.Strober W, Fuss IJ. Adv Exp Med Biol. 2006;579:55–97. doi: 10.1007/0-387-33778-4_5. [DOI] [PubMed] [Google Scholar]
- 126.Podolsky DK, Lobb R, King N, Benjamin CD, Pepinsky B, Sehgal P, deBeaumont M. J Clin Invest. 1993;92:372–80. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hesterberg PE, Winsor-Hines D, Briskin MJ, Soler-Ferran D, Merrill C, Mackay CR, Newman W, Ringler DJ. Gastroenterology. 1996;111:1373–80. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- 128.Hokari R, Kato S, Matsuzaki K, Iwai A, Kawaguchi A, Nagao S, Miyahara T, Itoh K, Sekizuka E, Nagata H, Ishii H, Iizuka T, Miyasaka M, Miura S. Clin Exp Immunol. 2001;126:259–65. doi: 10.1046/j.1365-2249.2001.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matsuzaki K, Tsuzuki Y, Matsunaga H, Inoue T, Miyazaki J, Hokari R, Okada Y, Kawaguchi A, Nagao S, Itoh K, Matsumoto S, Miura S. Clin Exp Immunol. 2005;140:22–31. doi: 10.1111/j.1365-2249.2005.02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 131.Park EJ, Mora JR, Carman CV, Chen J, Sasaki Y, Cheng G, von Andrian UH, Shimaoka M. J Clin Invest. 2007;117:2526–38. doi: 10.1172/JCI31570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abramson O, Qiu S, Erle DJ. Immunology. 2001;103:155–63. doi: 10.1046/j.0019-2805.2001.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Forbes E, Murase T, Yang M, Matthaei KI, Lee JJ, Lee NA, Foster PS, Hogan SP. J Immunol. 2004;172:5664–75. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 134.Forbes E, Hulett M, Ahrens R, Wagner N, Smart V, Matthaei KI, Brandt EB, Dent LA, Rothenberg ME, Tang M, Foster PS, Hogan SP. J Leukoc Biol. 2006;80:330–41. doi: 10.1189/jlb.1105643. [DOI] [PubMed] [Google Scholar]
- 135.Mudter J, Wirtz S, Galle PR, Neurath MF. Pathobiology. 2002;70:170–6. doi: 10.1159/000068150. [DOI] [PubMed] [Google Scholar]
- 136.Rosen SD. Am J Pathol. 1999;155:1013–20. doi: 10.1016/S0002-9440(10)65201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rosen SD. Annu Rev Immunol. 2004;22:129–56. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 138.Stassen M, Fondel S, Bopp T, Richter C, Muller C, Kubach J, Becker C, Knop J, Enk AH, Schmitt S, Schmitt E, Jonuleit H. Eur J Immunol. 2004;34:1303–11. doi: 10.1002/eji.200324656. [DOI] [PubMed] [Google Scholar]
- 139.Denning TL, Kim G, Kronenberg M. J Immunol. 2005;174:7487–91. doi: 10.4049/jimmunol.174.12.7487. [DOI] [PubMed] [Google Scholar]
- 140.Huehn J, Siegmund K, Hamann A. Curr Top Microbiol Immunol. 2005;293:89–114. doi: 10.1007/3-540-27702-1_5. [DOI] [PubMed] [Google Scholar]
- 141.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. J Exp Med. 2007;204:735–45. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yuan Q, Bromley SK, Means TK, Jones KJ, Hayashi F, Bhan AK, Luster AD. J Exp Med. 2007;204:1327–34. doi: 10.1084/jem.20062076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Welniak LA, Blazar BR, Murphy WJ. Annu Rev Immunol. 2007;25:139–70. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 144.Dutt S, Ermann J, Tseng D, Liu YP, George TI, Fathman CG, Strober S. Blood. 2005;106:4009–15. doi: 10.1182/blood-2005-06-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Petrovic A, Alpdogan O, Willis LM, Eng JM, Greenberg AS, Kappel BJ, Liu C, Murphy GJ, Heller G, van den Brink MR. Blood. 2004;103:1542–7. doi: 10.1182/blood-2003-03-0957. [DOI] [PubMed] [Google Scholar]
- 146.Waldman E, Lu SX, Hubbard VM, Kochman AA, Eng JM, Terwey TH, Muriglan SJ, Kim TD, Heller G, Murphy GF, Liu C, Alpdogan O, van den Brink MR. Blood. 2006;107:1703–11. doi: 10.1182/blood-2005-08-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, Hamada H, Asakura H, Ishikawa H, Matsushima K. Nat Immunol. 2003;4:154–60. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 148.Welniak LA, Kuprash DV, Tumanov AV, Panoskaltsis-Mortari A, Blazar BR, Sun K, Nedospasov SA, Murphy WJ. Blood. 2006;107:410–2. doi: 10.1182/blood-2004-11-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beilhack A, Schulz S, Baker J, Beilhack GF, Nishimura R, Baker EM, Landan G, Herman EI, Butcher EC, Contag CH, Negrin RS. Blood. 2008;111:2919–28. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Strauch UG, Mueller RC, Li XY, Cernadas M, Higgins JM, Binion DG, Parker CM. J Immunol. 2001;166:3506–14. doi: 10.4049/jimmunol.166.5.3506. [DOI] [PubMed] [Google Scholar]
- 151.Austrup F, Rebstock S, Kilshaw PJ, Hamann A. Eur J Immunol. 1995;25:1487–91. doi: 10.1002/eji.1830250602. [DOI] [PubMed] [Google Scholar]
- 152.Lefrancois L, Parker CM, Olson S, Muller W, Wagner N, Schon MP, Puddington L. J Exp Med. 1999;189:1631–8. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ebert EC, Roberts AI. Cell Immunol. 1996;167:108–14. doi: 10.1006/cimm.1996.0013. [DOI] [PubMed] [Google Scholar]
- 154.Marsal J, Brakebusch C, Bungartz G, Fassler R, Agace WW. Eur J Immunol. 2005;35:1805–11. doi: 10.1002/eji.200425941. [DOI] [PubMed] [Google Scholar]
- 155.Arroyo AG, Taverna D, Whittaker CA, Strauch UG, Bader BL, Rayburn H, Crowley D, Parker CM, Hynes RO. J Immunol. 2000;165:4667–75. doi: 10.4049/jimmunol.165.8.4667. [DOI] [PubMed] [Google Scholar]
- 156.Meharra EJ, Schon M, Hassett D, Parker C, Havran W, Gardner H. Cell Immunol. 2000;201:1–5. doi: 10.1006/cimm.2000.1630. [DOI] [PubMed] [Google Scholar]
- 157.Lussier C, Basora N, Bouatrouss Y, Beaulieu JF. Microsc Res Tech. 2000;51:169–78. doi: 10.1002/1097-0029(20001015)51:2<169::AID-JEMT8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 158.Hayday A, Theodoridis E, Ramsburg E, Shires J. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 159.Kronenberg M, Havran WL. Immunol Rev. 2007;215:5–7. doi: 10.1111/j.1600-065X.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 160.Coombes JL, Maloy KJ. Semin Immunol. 2007;19:116–26. doi: 10.1016/j.smim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 161.Iliev ID, Matteoli G, Rescigno M. J Exp Med. 2007;204:2253–7. doi: 10.1084/jem.20062535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schlickum S, Sennefelder H, Friedrich M, Harms G, Lohse MJ, Kilshaw P, Schon MP. Blood. 2008;112:619–25. doi: 10.1182/blood-2008-01-134833. [DOI] [PubMed] [Google Scholar]
- 163.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Nat Immunol. 2005;6:507–14. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 164.Rescigno M, Lopatin U, Chieppa M. Curr Opin Immunol. 2008;20:669–75. doi: 10.1016/j.coi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 165.Ludviksson BR, Strober W, Nishikomori R, Hasan SK, Ehrhardt RO. J Immunol. 1999;162:4975–82. [PubMed] [Google Scholar]
- 166.Izcue A, Coombes JL, Powrie F. Immunol Rev. 2006;212:256–71. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 167.Olson TS, Bamias G, Naganuma M, Rivera-Nieves J, Burcin TL, Ross W, Morris MA, Pizarro TT, Ernst PB, Cominelli F, Ley K. J Clin Invest. 2004;114:389–98. doi: 10.1172/JCI20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. J Immunol. 2008;180:2573–80. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Leithauser F, Meinhardt-Krajina T, Fink K, Wotschke B, Moller P, Reimann J. Am J Pathol. 2006;168:1898–909. doi: 10.2353/ajpath.2006.050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Immunol Today. 1997;18:61–4. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 171.Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. J Immunol. 2002;168:2247–54. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- 172.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, ten Berge IJ. J Immunol. 2006;177:2775–83. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 173.Myers L, Croft M, Kwon BS, Mittler RS, Vella AT. J Immunol. 2005;174:7625–32. doi: 10.4049/jimmunol.174.12.7625. [DOI] [PubMed] [Google Scholar]
- 174.Hadley GA, Bartlett ST, Via CS, Rostapshova EA, Moainie S. J Immunol. 1997;159:3748–56. [PubMed] [Google Scholar]
- 175.Seder RA, Marth T, Sieve MC, Strober W, Letterio JJ, Roberts AB, Kelsall B. J Immunol. 1998;160:5719–28. [PubMed] [Google Scholar]
- 176.Zhou S, Ueta H, Xu XD, Shi C, Matsuno K. Int Immunol. 2008;20:385–94. doi: 10.1093/intimm/dxm153. [DOI] [PubMed] [Google Scholar]
- 177.Smyth LJ, Kirby JA, Cunningham AC. Clin Exp Immunol. 2007;149:162–70. doi: 10.1111/j.1365-2249.2007.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Szabo MC, Butcher EC, McEvoy LM. J Immunol. 1997;158:5584–8. [PubMed] [Google Scholar]
- 179.Lehnert K, Print CG, Yang Y, Krissansen GW. Eur J Immunol. 1998;28:3605–15. doi: 10.1002/(SICI)1521-4141(199811)28:11<3605::AID-IMMU3605>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 180.Viney JL, Jones S, Chiu HH, Lagrimas B, Renz ME, Presta LG, Jackson D, Hillan KJ, Lew S, Fong S. J Immunol. 1996;157:2488–97. [PubMed] [Google Scholar]
- 181.Teague TK, Lazarovits AI, McIntyre BW. Cell Adhes Commun. 1994;2:539–47. doi: 10.3109/15419069409014217. [DOI] [PubMed] [Google Scholar]
- 182.Carrasco YR, Batista FD. Curr Opin Immunol. 2006;18:286–91. doi: 10.1016/j.coi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 183.Carrasco YR, Batista FD. EMBO J. 2006;25:889–99. doi: 10.1038/sj.emboj.7600944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Allen CD, Cyster JG. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mittelbrunn M, Molina A, Escribese MM, Yanez-Mo M, Escudero E, Ursa A, Tejedor R, Mampaso F, Sanchez-Madrid F. Proc Natl Acad Sci U S A. 2004;101:11058–63. doi: 10.1073/pnas.0307927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bjursten M, Bland PW, Willen R, Hornquist EH. Eur J Immunol. 2005;35:2274–83. doi: 10.1002/eji.200526022. [DOI] [PubMed] [Google Scholar]
- 187.Leussink VI, Zettl UK, Jander S, Pepinsky RB, Lobb RR, Stoll G, Toyka KV, Gold R. Acta Neuropathol. 2002;103:131–6. doi: 10.1007/s004010100444. [DOI] [PubMed] [Google Scholar]
- 188.Neurath MF, Finotto S, Fuss I, Boirivant M, Galle PR, Strober W. Trends Immunol. 2001;22:21–6. doi: 10.1016/s1471-4906(00)01798-1. [DOI] [PubMed] [Google Scholar]
- 189.Goto A, Arimura Y, Shinomura Y, Imai K, Hinoda Y. Inflamm Bowel Dis. 2006;12:758–65. doi: 10.1097/00054725-200608000-00013. [DOI] [PubMed] [Google Scholar]
- 190.Steinman L. Nat Rev Drug Discov. 2005;4:510–8. doi: 10.1038/nrd1752. [DOI] [PubMed] [Google Scholar]
- 191.Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 192.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. N Engl J Med. 2005;353:375–81. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 193.Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E, Barkhof F, Radue EW, Jager HR, Clifford DB. N Engl J Med. 2006;354:924–33. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Imai Y, Park EJ, Peer D, Peixoto A, Cheng G, von Andrian UH, Carman CV, Shimaoka M. Blood. 2008;112:5007–15. doi: 10.1182/blood-2008-03-144543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Feral CC, Rose DM, Han J, Fox N, Silverman GJ, Kaushansky K, Ginsberg MH. J Clin Invest. 2006;116:715–23. doi: 10.1172/JCI26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hilden TJ, Nurmi SM, Fagerholm SC, Gahmberg CG. Ann Med. 2006;38:503–11. doi: 10.1080/07853890600969130. [DOI] [PubMed] [Google Scholar]
- 197.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Science. 2008;319:627–30. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cantor JM, Ginsberg MH, Rose DM. Immunol Rev. 2008;223:236–51. doi: 10.1111/j.1600-065X.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 199.Krissansen GW, Singh J, Kanwar RK, Chan YC, Leung E, Lehnert KB, Kanwar JR, Yang Y. Eur J Immunol. 2006;36:2203–14. doi: 10.1002/eji.200535324. [DOI] [PubMed] [Google Scholar]
- 200.von Boehmer H. J Exp Med. 2007;204:1737–9. doi: 10.1084/jem.20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mora JR, Iwata M, von Andrian UH. Nat Rev Immunol. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gonzalez-Amaro R, Mittelbrunn M, Sanchez-Madrid F. Immunology. 2005;116:289–96. doi: 10.1111/j.1365-2567.2005.02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shigematsu T, Specian RD, Wolf RE, Grisham MB, Granger DN. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1309–15. doi: 10.1152/ajpgi.2001.281.5.G1309. [DOI] [PubMed] [Google Scholar]
- 204.Teramoto K, Miura S, Tsuzuki Y, Hokari R, Watanabe C, Inamura T, Ogawa T, Hosoe N, Nagata H, Ishii H, Hibi T. Clin Exp Immunol. 2005;139:421–8. doi: 10.1111/j.1365-2249.2004.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Soriano A, Salas A, Sans M, Gironella M, Elena M, Anderson DC, Pique JM, Panes J. Lab Invest. 2000;80:1541–51. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 206.Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Gastroenterology. 2001;121:1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]