Abstract
Ovarian cancer is 1 of the most significant malignancies in the Western world, and the antiangiogenesis strategy has been postulated for prevention and treatment of ovarian cancers. Kaempferol is a natural flavonoid present in many fruits and vegetables. The antiangiogenesis potential of kaempferol and its underlying mechanisms were investigated in two ovarian cancer cell lines, OVCAR-3 and A2780/CP70. Kaempferol mildly inhibits cell viability but significantly reduces VEGF gene expression at mRNA and protein levels in both ovarian cancer cell lines. In chorioallantoic membranes of chicken embryos, kaempferol significantly inhibits OVCAR-3-induced angiogenesis and tumor growth. HIF-1α, a regulator of VEGF, is downregulated by kaempferol treatment in both ovarian cancer cell lines. Kaempferol also represses AKT phosphorylation dose dependently at 5 to 20 μM concentrations. ESRRA is a HIF-independent VEGF regulator, and it is also downregulated by kaempferol in a dose-dependent manner. Overall, this study demonstrated that kaempferol is low in cytotoxicity but inhibits angiogenesis and VEGF expression in human ovarian cancer cells through both HIF-dependent (Akt/HIF) and HIF-independent (ESRRA) pathways and deserves further studies for possible application in angio prevention and treatment of ovarian cancers.
INTRODUCTION
Ovarian cancer is one of the most important malignancies for women in the Western world, ranking as the fifth leading cause of cancer-related deaths (1). Due to a lack of effective biomarkers for screening, nearly 60–70% of ovarian cancers are diagnosed at advanced stages (2), with a poor prognosis of about 30% for a 5-yr survival rate (3). Treatment of ovarian cancers involves surgery and chemotherapy, but is often not effective because of problems with drug resistance (4) and later relapse in patients (5).
Angiogenesis is the process of developing new blood vessels and plays an important role in tumor growth (6). In a normal adult, angiogenesis is virtually quiescent, with only 0.01% of endothelial cells undergoing cell division (7). In contrast, tumor growth requires active angiogenesis, and antiangiogenesis becomes a rational anticancer strategy (7). Vascular endothelial growth factor (VEGF) is the most pivotal positive regulator of angiogenesis (6), and VEGF gene expression is found in many human tumors including ovarian cancers (8).
VEGF gene expression is regulated by oxygen tension, growth factors, hormones, and oncogenes (9). Hypoxia induces VEGF expression through hypoxia-inducible factor 1 (HIF-1), which is composed of HIF-1α and HIF-1β subunits, with the former one being inducible (10). In normoxia, the PI3 kinase/AKT pathway is implicated in the regulation of HIF-mediated VEGF responses (11). Growth factors and inflammatory cytokines, including epidermal growth factor, transforming growth factor (TGF), interleukin-1 (IL-1), IL-6, also stimulate expression of the VEGF gene (9). Estrogens activate VEGF expression through estrogen receptors (ERs) and the estrogen response element (ERE) (12). Proto-oncogene c-Myc enforces cellular proliferation and growth in tumors (13) and cooperates with HIF-1 in inducing VEGF expression (14). Myc has been recently reported to be regulated by PI3K/AKT pathways (15). Whereas regulation of VEGF through PI3Kinase/AKT and HIF is considered the classical pathway, a pathway involving peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PPARGC1A) and estrogen-related receptor alpha (ESRRA) was recently discovered to be independent of HIF (16). This pathway goes through ESRRA, an orphan nuclear receptor that has a high degree of sequence similarity and intense cross-talk to ERs (17).
Flavonoids are natural polyphenols present in a wide variety of fruits and vegetables (19) and have been shown to inhibit cancer development (20,21). It has been reported that dietary flavonoids reduce the risk to cardiovascular disease (22), prostate cancer (23), colorectal cancer (24), and renal cancer (19) in humans. Flavonoids have also been found to inhibit cell growth and proliferation (25) and induce cell toxicity (26,27) in cancer cells. Kaempferol [3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one] is among the most common dietary flavonoids and recently has been shown to possess antioxidant and antitumor properties. Kaempferol was found to inhibit proliferation of human breast and lung cancer cells (28,29), inhibit ER-α expression in breast cancer cells (28), and induce apoptosis in glioblastoma cells (30) and in lung cancer cells by activation of MEK-MAPK (29). Kaempferol also exhibited an anti-inflammatory effect through inhibition of IL-4 (31), COX-2 and CRP expression and downregulation of NFκ B pathway in liver cells (32). In human studies, a significant 40% decrease in ovarian cancer incidence was found for the highest quintile of kaempferol intake as compared to the lowest quintile (33). However, the effect and mechanism by which kaempferol inhibits ovarian cancer cell proliferation and tumor formation is not yet clear. In this study, kaempferol was investigated for its effects on angiogenesis and VEGF expression in ovarian cancer cells and the underlying mechanisms for this effect, including the conventional AKT-HIF and novel PPARGC1A-ESRRA pathways.
MATERIALS AND METHODS
Cell Culture
Two human ovarian cancer cell lines, OVCAR-3 (mutant p53) and A2780/CP70 (wild-type p53) (34,35), were maintained in RPMI 1640 medium supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin (VWR, West Chester, PA), and 10% US-qualified fetal bovine serum (Invitrogen, Grand Island, NY) in a humidified incubator with 5% CO2 at 37°C.
Cell Viability Assay
Kaempferol’s effects on OVCAR-3 and A2780/CP70 cell viability were colorimetrically determined with a “CellTiter 96 Aqueous One Solution Cell Proliferation Assay” kit from Promega (Madison, WI). Cells (8 × 103/well) were seeded into 96-well plates and incubated for 16 h before being treated with 0 to 80 μM kaempferol (Sigma, St. Louis, MO) in triplicates for another 24 h with DMSO as solvent control. Cells were then washed twice with phosphate-buffered saline (PBS), introduced with 100 μl medium containing 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), incubated at 37°C for 2 h, and measured for OD values at 490 nm. A linear standard curve was generated by seeding different amount of cells (0 to 1 × 104) and treating with medium containing DMSO only. Cell viability was expressed as percentage of control from 3 independent experiments.
qRT-PCR
The effects of kaempferol on several genes’ mRNA level were determined by quantitative reverse-transcription PCR (qRT-PCR). Cells (5 × 105) were seeded in 60-mm dishes and incubated for 16 h before treatment with kaempferol. For time course of VEGF mRNA expression, both cell lines were treated with 40 μM kaempferol for 0 to 24 h, and cells were harvested in TRIzol reagent (Invitrogen, Grand Island, NY) and stored in −80°C until analysis. For VEGF mRNA expression in response to kaempferol doses, both cell lines were treated with 0 to 40 μM kaempferol for 24 h before RNA was extracted, reconstituted in DEPC-treated water, and checked for integrity by agarose-gel electrophoresis. RNA samples were quantitated at OD 260/280, and 1 μg RNA was introduced to reverse transcription with AMV reverse transcriptase from Promega (Madison, WI). cDNA equivalent to 80 ng RNA was amplified by real-time PCR for various genes in triplicate with RT2 SYBR Green qPCR Master Mix (SuperArray, Frederick, MD) and a Chromo4™ real-time detector coupled to a DNA Engine® thermal cycler (Bia-Rad, Hercules, CA). Primers for GAPDH, HIF-1α, HIF-1β, and ESRRA were chosen from the Primer-Bank Web site (http://pga.mgh.harvard.edu/primerbank/), and primers for VEGF and PPARGC1A were designed from the Primer3 Web site (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Primer sequences are listed in Table 1. The PCR program was set as follows: 95°C 10 min (95°C 20s, 58°C 45 s, 72°C 20 s, 77°C 1 s, read plate) × 50; 72°C 5 min; 58°C 1 min; melting curve (65°C to 95°C by 0.5°C increments). A standard curve for each gene was generated from serial dilutions of PCR products to monitor amplification efficiency and to relatively quantify mRNA abundance. RNA samples without reverse transcription served as a non-reverse-transcription (−RT) control, and water served as a nontemplate control (NTC). Arbitrary units of each gene were derived from a corresponding standard curve, and mRNA abundance was normalized to GAPDH levels and expressed as percentages of control for statistical analysis.
TABLE 1.
Primer sequences of genes analyzed by qRT-PCRa
| Gene | GenBank Accession | Primers | Amplicon Size |
|---|---|---|---|
| VEGF | NM 001025368 | AAG GAG GAG GGC AGA ATC AT | 226 |
| ATC TGC ATG GTG ATG TTG GA | |||
| GAPDH | NM 002046 | CAT GAG AAG TAT GAC AAC AGC CT | 113 |
| AGT CCT TCC ACG ATA CCA AAG T | |||
| HIF-1α | NM 001530 | ATC CAT GTG ACC ATG AGG AAA TG | 126 |
| CTC GGC TAG TTA GGG TAC ACT T | |||
| HIF-1β | NM 001668 | CTG CCA ACC CCG AAA TGA CAT | 117 |
| GCC GCT TAA TAG CCC TCT GG | |||
| ESRRA | NM 004451 | GTC CAA AGG GTT CCT CGG AG | 221 |
| GGA TGC CAC ACC ATA GTG GTA | |||
| PPARGC1A | NM 013261 | TGG GTA GCC CAT CAA AAT GT | 176 |
| TGG TAC TTA CCA CGG CAT GA |
Abbreviations are as follows: qRT-PCR, quantitative reverse-transcription polymerase chain reaction; VEGF, vascular endothelial growth factor; GAPDH, glyderaldehyde-3-phosphate dehydrogenase; HIF, hypoxia-inducible factor; ESRRA, estrogen-related receptor alpha; PPARGC1A, peroxisome proliferator-activated receptor gamma coactivator 1 alpha.
ELISA
Secreted VEGF protein levels were analyzed by sandwich ELISA with a Quantikine Human VEGF Immunoassay Kit from R&D Systems (Minneapolis, MN) targeting VEGF165 in cell culture supernates. Cells (8 × 103/well) were seeded into 96-well plates and incubated for 16 h before treatment with kaempferol for 24 h. Culture supernates were collected for VEGF assay, and cell numbers were quantitated with MTS-based assay as mentioned above. VEGF levels, as determined following the manufacturer’s instructions, were normalized to cell numbers for each treatment. A total of 4 independent experiments, each in duplicates, was assayed, and the mean VEGF protein level from each duplicate was used for statistical analysis. For phosphorylated ERK1/ERK2 and AKT, DuoSet IC kits (R&D Systems, Minneapolis, MN) were used to develop sandwich ELISA, measuring phospho-ERK1 (T202/Y204)/phospho-ERK2 (T185/Y187), and phospho-AKT (Pan) (S473) in cell lysates, respectively. Cells (5 × 105) were seeded into 60-mm dishes and incubated for 16 h before treatment with kaempferol for 24 h. Cell lysates were analyzed for p-ERK and p-AKT levels as per instructions, and total protein levels in lysates were determined with BCA Protein Assay Kit (Pierce, Rockford, IL) to normalize p-ERK and p-AKT abundance. A total of 4 independent experiments with triplicates each were performed, and averages from each triplicate were used for statistical analysis.
Western Blot
Cells (5 × 105) were seeded in 60-mm dishes and incubated for 16 h before treatment with kaempferol for 24 h. After double wash with cold PBS, cells were harvested with RIPA buffer freshly supplemented with 3 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 1 mM sodium vanadate (activated), and 1 mM PMSF (Sigma, St. Louis, MO). Total protein levels were assayed with a BCA Protein Assay Kit (Pierce, Rockford, IL), and lysates (50 μg total protein) were separated by 10% SDS-PAGE and blotted into nitrocellulose membrane with a Mini-Protean 3 System (Bio-Rad Laboratories, Hercules, CA). For immunodetection, antibodies against HIF-1α, HIF-1β (BD Biosciences, San Jose, CA), and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) were applied and signals visualized with a SuperSignal West Pico Complete Mouse IgG Detection Kit and x-ray film (Pierce Biotechnology, Rockford, IL). Protein bands were quantitated with Quantity One software (Bio-Rad Laboratories, Hercules, CA), normalized to corresponding GAPDH bands, and expressed as percentages of control. A total of 3 independent experiments were carried out for statistical analysis.
Chicken Embryo Chorioallantoic Membrane (CAM) Assay
OVCAR-3 cells (3 × 106) were suspended in 100 μl medium (serum-free, 4°C), mixed with 50 μl liquid BD Matrigel Basement Membrane Matrix High Concentration (BD Biosciences, San Jose, CA), supplemented with or without 20-μM kaempferol at 4°C, and implanted in the CAM of 9-day-old chicken embryos. After 5-day incubation at 37.5°C, tumor implants were dissected out, photographed, weighed, and counted under a dissecting microscope for branching blood vessels. A total of 5 chicken embryos were included in each treatment group.
Statistical Analysis
Average values of replicates were collected from independent experiments and analyzed by analysis of variance (ANOVA) and post hoc test (2-sided Dunnett’s t) with SPSS 15.0 (SPSS Inc., Chicago, IL) to test both overall differences and specific differences between each treatment and control. A P value of less than 0.05 was considered significant.
RESULTS
Kaempferol Mildly Inhibits Cell Viability in Ovarian Cancer Cell Lines
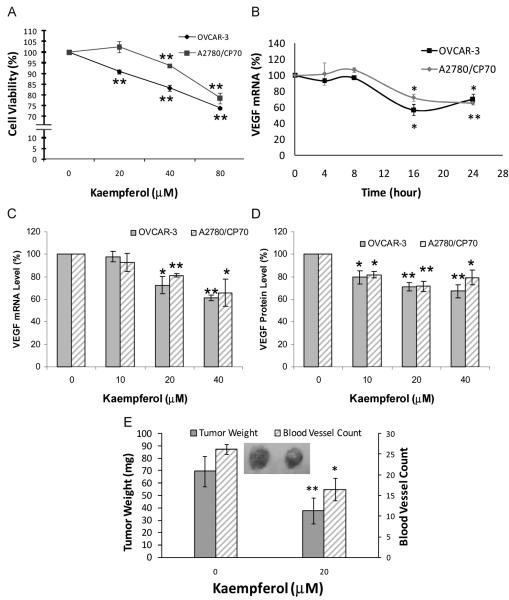
OVCAR-3 and A2780/CP70 cells were treated with kaempferol for 24 h and assayed for cell viability. As shown in Fig. 1A, OVCAR-3 cell viability was inhibited to 91% by 20-μM kaempferol treatment (P < 0.01), and to 74% at 80-μM kaempferol concentration (P < 0.001). For A2780/CP70 cells, the viability was slightly promoted to 102% at 20-μM kaempferol concentration (P > 0.38), and then inhibited down to 94% and 79% by 40-μM and 80-μM kaempferol treatments, respectively (P < 0.001). An overall inhibitory effect on cell viability was observed for both cell lines, and A2780/CP70 cells appeared more resistant than OVCAR-3 cells to the inhibiting effect of kaempferol (P < 0.05).
FIG. 1.
Kaempferol’s effect on cell viability, VEGF expression, tumorigenesis, and angiogenesis in ovarian cancer cell lines. A: Cells (8 × 103/well) were seeded in 96-well plates, incubated for 16 h, and treated with kaempferol for 24 h. Cell viability was colorimetrically determined by a MTS-based method and expressed as percentages of control. Data represents mean ± SE from 3 independent experiments. B: Cells (5 × 105) were seeded in 60-mm dishes, incubated 16 h, and treated with 40-μM kaempferol for 0 to 24 h. Cells were harvested in TRIzol Reagent and stored in −80 °C until analysis. RNA was extracted; reverse transcribed with AMV reverse transcriptase; and quantitated by SYBR Green-based, real-time PCR. VEGF mRNA levels were normalized by GAPDH abundance and expressed as percentages of control. Data represents mean ± SE from 2 independent experiments. C: Cells (5 × 105) were seeded in 60-mm dishes, incubated 16 h, and treated with 0 to 40 μM kaempferol for 24 h. RNA was extracted with TRIzol Reagent, reverse-transcribed with AMV reverse transcriptase, and quantitated by SYBR Green-based, real-time PCR. VEGF mRNA levels were normalized by GAPDH abundance and expressed as percentages of control. Data represents mean ± SE from 3 independent experiments. D: Cells (8 × 103/well) were seeded in 96-well plates, incubated for 16 h, and treated with kaempferol for 24 h. Culture supernates were collected and analyzed for VEGF165 by ELISA, and cell numbers were determined by MTS-based assay. VEGF levels were normalized by cell numbers and expressed as percentages of control. Data represents mean ± SE from 4 independent experiments. E: OVCAR-3 cells (3 × 106) were suspended in 100 μ l serum-free medium at 4°C, mixed with 50 μl cold BD Matrigel, supplemented with or without 20-μM kaempferol at 4°C, implanted in the chorioallantoic membrane of 9-day-old chicken embryos, and incubated for 5 days at 37.5°C before tumors were weighed and counted for blood vessels. Data represents mean ± SE from 5 chicken embryos. *P < 0.05 as compared to control. **P < 0.01 as compared to control.
Kaempferol Inhibits VEGF Expression in Ovarian Cancer Cell Lines
Both cell lines were treated with kaempferol and assayed for VEGF mRNA and protein levels by qRT-PCR and ELISA, respectively. As shown in Fig. 1B, 40-μM kaempferol treatment did not influence VEGF mRNA expression within 8 h. However, VEGF mRNA levels were downregulated to 57% in OVCAR-3 cells (P < 0.05) and 72% in A2780/CP70 cells (P < 0.05) at Hour 16 and remained at close levels at Hour 24. For 24-h kaempferol treatment (Fig. 1C), VEGF mRNA expression was downregulated by 20-μM kaempferol treatment to 73% (P < 0.05) and 81% (P < 0.001) in OVCAR-3 and A2780/CP70 cells, respectively. Significant further downregulation was also observed for 40-μM kaempferol treatment in both cell lines. Both cell lines showed concentration dependent inhibition on VEGF mRNA levels by kaempferol treatment, and no significant difference between OVCAR-3 and A2780/CP70 cells was observed.
VEGF protein levels in cell culture supernates were also downregulated by kaempferol treatments (Fig. 1D). The levels of VEGF protein were 80% (P < 0.05) and 82% (P < 0.05) of controls in OVCAR-3 and A2780/CP70 cells, respectively, at 10-μM kaempferol concentration. Further inhibition was observed for higher kaempferol treatments in a concentration-dependent and significant manner, and both cell lines appeared similar in their response to kaempferol’s inhibition on VEGF protein levels.
Kaempferol Inhibits Tumorigenesis and Angiogenesis in Chicken Embryo CAMs
Kaempferol was tested for its effects on in vivo angiogenesis. Chicken embryo CAMs were implanted with OVCAR-3 cells suspended in cold liquid matrigel, which gels quickly at raised temperature. This cancer cell-containing gel becomes a tumor in chicken embryo and continues to grow and induce angiogenesis within the vasculature-rich CAM due to the lack of an immune system in chicken embryos. Growth and angiogenesis of tumor implants are inhibited by inclusion of 20-μM kaempferol. As shown in Fig. 1E, the implanted cancer cells grow to a tumor of 70 mg, with 26 blood vessels counted. Inclusion of 20-μM kaempferol in this implant, however, reduced tumor growth down to 38 mg (P < 0.01) and inhibited blood vessel development to 16 (P < 0.05). A typical photograph (Fig. 1E) was shown to contrast the 2 tumors with or without kaempferol in terms of both tumor size and angiogenesis.
Kaempferol Inhibits HIF-1α Protein Expression in Ovarian Cancer Cell Lines
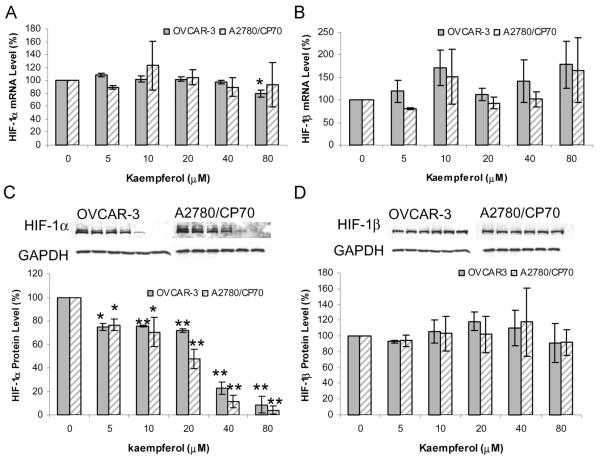
The effect of kaempferol on HIF-1α and HIF-1β gene expression was investigated at both the mRNA and protein levels by qRT-PCR and Western blot, respectively. As shown in Fig. 2A, kaempferol treatment did not have an obvious effect on HIF-1α mRNA levels except for an 80-μM treatment on OVCAR-3 cells, which downregulated HIF-1α mRNA level to 80% (P = 0.010). For HIF-1β mRNA, no appreciable effects were found in both cell lines (P > 0.40), leaving a random distribution and an inconsistent pattern (Fig. 2B).
FIG. 2.
Kaempferol’s effect on HIF gene expression. A and B: Cells (5 × 105) were seeded in 60-mm dishes, incubated 16 h, and treated with kaempferol for 24 h. RNA was extracted with TRIzol Reagent, reverse-transcribed with AMV reverse transcriptase, and quantitated by SYBR Green-based, real-time PCR. HIF-1α and HIF-1β mRNA levels were normalized by GAPDH abundance and expressed as percentages of control. Data represents mean ± SE from 3 independent experiments. C and D: Cells (5 × 105) were seeded in 60-mm dishes, incubated for 16 h, and treated with kaempferol for 24 h. Cells were harvested with RIPA buffer, and cell lysates were separated by SDS-PAGE and blotted into nitrocellulose membrane for immunodetection. Chemiluminescent signals were captured by x-ray film and quantitated by imaging software. HIF-1α and HIF-1β protein levels were normalized by GAPDH and expressed as percentages of control. Typical blots were shown for both cell lines, and data represents mean ± SE from 3 independent experiments. *P < 0.05 as compared to control. **P < 0.01 as compared to control.
HIF-1α protein levels showed intense and consistent down-regulation by kaempferol treatment (Fig. 2C). A 5-μM kaempferol treatment led to inhibition of HIF-1α protein to 75% in OVCAR-3 cells (P < 0.01), and a 10-μM treatment reduced HIF-1α protein to 70% in A2780/CP70 cells (P < 0.05). Higher concentrations of kaempferol resulted in greater inhibition, with the levels of HIF-1α protein at 80-μM kaempferol down to 9% and 4% in OVCAR-3 and A2780/CP70 cancer cells, respectively (P < 0.001). For the concentration-dependent inhibition of HIF-1α protein levels, no significant difference between OVCAR-3 and A2780/CP70 cells was observed. HIF-1β proteins were not affected by kaempferol treatment, with levels distributed from 91% to 118% (P > 0.50) randomly for both cell lines (Fig. 2D).
Kaempferol Inhibits p-AKT But Not p-ERK Levels in Ovarian Cancer Cell Lines
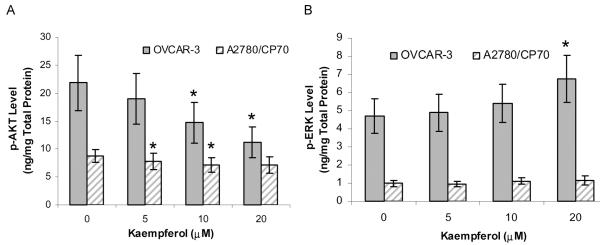
The levels of the signal transduction molecules, phosphorylated AKT and ERK, were determined with ELISA. As shown in Fig. 3A, p-AKT levels were downregulated from 21.8 ng/mg total protein (TP) at control to 11.2 ng/mg TP at 20-μM kaempferol treatment in OVCAR-3 cells, and from 8.8 ng/mg TP at control to 7.1 ng/mg TP at 20-μM kaempferol treatment in A2780/CP70 cells, showing a concentration dependent and significant inhibition in both cell lines (P < 0.05). Phosphorylation of ERK is promoted by kaempferol treatment (Fig. 3B). The p-ERK levels were increased from 4.7 ng/mg TP at control to 6.7 ng/mg TP at 20 μM in OVCAR-3 cells (P < 0.05), showing a concentration-dependent promotion effect by kaempferol treatment; however, kaempferol did not affect p-ERK level in A2780/CP70 cells (P = 0.18). Both signaling molecules were found at much higher levels in OVCAR-3 cells than in A2780/CP70 cells, and kaempferol’s effect on these signaling molecules was also more pronounced in OVCAR-3 cells (Fig. 3).
FIG. 3.
Kaempferol’s effect on p-AKT and p-ERK levels. A and B: Cells (5 × 105) were seeded in 60-mm dishes, incubated for 16 h, and treated with kaempferol for 24 h. Cell lysates were analyzed for p-AKT and p-ERK with ELISA, and for total protein with BCA assay. Levels of p-AKT and p-ERK were normalized by total protein. Data represents mean ± SE from 4 independent experiments. *P < 0.05 as compared to control.
Kaempferol Inhibits ESRRA mRNA Expression in Ovarian Cancer Cell Lines
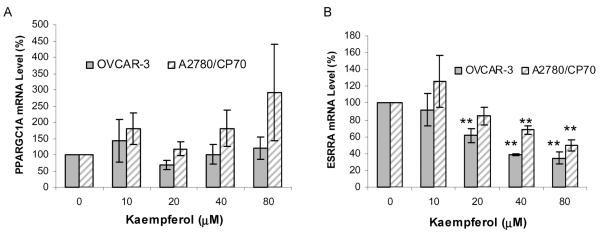
Kaempferol’s effect on PPARGC1A and ESRRA was examined by qRT-PCR in both cell lines. As shown in Fig. 4A, no significant change was found on PPARGC1A mRNA levels, resulting in a random fluctuation of mRNA levels with increasing doses of kaempferol. For ESRRA, the mRNA levels were inhibited to 62% in OVCAR-3 cells by a 20-μM treatment (P < 0.01) and to 68% in A2780/CP70 cells at 40-μM kaempferol (P < 0.01; Fig. 4B). Further inhibition effects were observed for higher kaempferol treatments in a concentration-dependent manner. A2780/CP70 cells also show higher resistance than OVCAR-3 cells to kaempferol treatment (P < 0.01), with a significant inhibition of ESRRA mRNA levels observed only at 40- and 80-μM treatments.
FIG. 4.
Kaempferol’s effect on PPARGC1A and ESRRA mRNA level. A and B: Cells (5 × 105) were seeded in 60-mm dishes, incubated 16 h, and treated with kaempferol for 24 h. RNA was extracted with TRIzol Reagent, reverse-transcribed with AMV reverse transcriptase, and quantitated by SYBR Green-based, real-time PCR. PPARGC1A and ESRRA mRNA levels were normalized by GAPDH abundance and expressed as percentages of control. Data represents mean ± SE from 3 independent experiments. **P < 0.01 as compared to control.
DISCUSSION
The risk of ovarian cancer increases with age and with the use of oral contraceptive pills (18). Few studies have been done to relate lifestyles to ovarian cancer risks, although ovarian cancer risk has been consistently associated with a high intake of saturated fat and a low intake of vegetables (36). Flavonoids, as an abundant ingredient in fruits and vegetables, are believed to play an important role in anticarcinogenesis through their antioxidant, antiestrogenic, antiproliferative, antiangiogenic, and anti-inflammatory properties (33). Kaempferol is a flavonoid widely and abundantly distributed in diet, and an impressive 40% decrease in ovarian cancer incidence with kaempferol intake reported by a recent study suggests kaempferol as a potential chemoprevention agent but calls for more intense studies on its effect and mechanism of action in ovarian cancers (33).
Obviously, kaempferol’s effects in reducing ovarian cancer risks can not be explained solely for its direct cytotoxicity on ovarian cancer cells, as our experiments on ovarian cancer cell lines only revealed a very mild inhibitory effect on cell viability. Besides, very few chemicals can distinguish well between tumor cells and normal tissues, and a strong cytotoxicity is normally associated with severe side effects because of the killing of healthy cells. As a widely distributed dietary flavonoid, kaempferol does not cause any severe side effects in either healthy people or ovarian cancer patients, nor does it kill ovarian cancer cells directly and strongly. However, kaempferol could possibly inhibit ovarian cancer cells through an indirect mechanism, antiangiogenesis.
Ovarian cancer cells are known to secrete VEGF to recruit vascular endothelial cells for angiogenesis (37,38). Angiogenesis is critical in tumor growth, invasion, and metastasis (39,40). Therefore, VEGF is one of the most significant and direct targets in an antiangiogenesis strategy. Our experiments discovered time- and dose-dependent inhibition on VEGF expression in ovarian cancer cells by kaempferol, with a greater effect at protein levels than mRNA levels. A 10-μM kaempferol treatment significantly inhibited VEGF protein secretion down to 80%; but for VEGF mRNA, a concentration of 20 μM was needed to reach similar VEGF inhibition. This difference possibly reflects an amplification effect in translating mRNA to proteins in ovarian cancer cells where the signal for VEGF expression was amplified along the central dogma in genetics. By comparing kaempferol’s inhibitory effects on cell viability and VEGF expression, it further proves that kaempferol mainly performs its function through antiangiogenesis rather than killing cells directly: a 10-μM kaempferol treatment inhibits VEGF protein down to 80%, a slightly higher 20-μM kaempferol treatment inhibits VEGF mRNA down to 80%, but a eight-fold higher concentration (80 μM) of kaempferol is needed to inhibit cell viability down to 80% in A2780/CP70 ovarian cancer cells. In our chicken embryo CAM assay, kaempferol also reduced tumor size significantly. This should be explained more as a result of antiangiogenesis rather than direct inhibition of tumor growth because a 20 μM kaempferol only inhibits OVCAR-3 cell viability down to 91% in our cell viability assay, whereas the tumor weight was reduced down to 54% (from 70 to 38 mg).
Although our results indicate that kaempferol works on ovarian cancer cells through inhibiting VEGF expression rather than killing cancer cells directly, inhibition of VEGF is more than just antiangiogenesis. It, in turn, has an indirect effect in suppressing growth of cancer cells. Originally thought to recruit vascular endothelial cells through a paracrine mode, VEGF was later found to also act in an autocrine manner to promote growth of tumor cells themselves (41). Ovarian tumors are known to express VEGF receptors (42), and VEGF secreted by ovarian cancer cells can act in both autocrine and paracrine fashions to promote growth of tumor cells. Moreover, VEGF can act as a survival factor through enhanced expression of B-cl-2 and survivin, protecting cancer cells against apoptosis (6). By targeting VEGF in ovarian cancer cells, kaempferol inhibits angiogenesis in tumors and represses tumor growth and survival indirectly.
Our experiments further explored mechanisms through which kaempferol inhibits VEGF expression and angiogenesis in ovarian cancer cells. It is known that hypoxia stimulates VEGF expression through inducing HIF-1 transcriptional factors; and under normoxia, the PI3 kinase and AKT signaling pathway is implicated in HIF-1-mediated responses (43). Like apigenin, the other dietary flavonoid studied for this pathway (10), kaempferol inhibits HIF-1α protein levels in a concentration dependent manner; but HIF-1α mRNA levels are only inhibited at high kaempferol concentration (80 μM) in ovarian cancer cells. This again indicates an amplification effect in the translation process. HIF-β is a constitutively expressed subunit and as expected is not affected by kaempferol treatment at mRNA or protein levels. Phosphorylated AKT in this pathway was downregulated by kaempferol treatment up to 20 μM in a concentration-dependent manner, and it proves AKT-HIF-VEGF as a working mechanism at physiologically relevant kaempferol concentrations, which is typically below 20 μM in vivo. Normal population kaempferol intake is assumed to be the 0.8 to 11.0 mg/day in the Nurses’ Health Study (33). We also examined another signaling molecule, p-ERK, and our results showed that ERK pathway is not involved in kaempferol’s effect on VEGF inhibition.
Independent of HIF transcription factors, the PPARGC1A/ESRRA pathway is newly discovered that affects VEGF expression and angiogenesis (16). Kaempferol treatment has no effect on PPARGC1A mRNA levels, but it concentration dependently repressed ESRRA mRNA levels in ovarian cancer cells. This might be another mechanism through which kaempferol inhibits VEGF expression Estrogens bind to nuclear receptors ERα and ERβ, which are ligand-inducible transcription factors and stimulate transcription of many genes. Although no classical EREs are found in the 5′ regulatory regions, VEGF is an estrogen responsive gene with several AP-1 and Sp1 sites in this region to mediate estrogens’ effect (44) and a variant ERE 1.5 kb away from the transcription start (6). In fact, estrogens have been shown to induce VEGF mRNA levels in ER+ breast tumor cells (12), and kaempferol has been reported to inhibit ERα expression at both mRNA and protein levels in breast cancer cells (28). Although ESRRA does not respond to an estrogen stimulus, it is a downstream effector of PPARGC1A. ESRRA is also evolutionarily related to ERs and can efficiently bind to EREs that are commonly shared by many target genes (45). In fact, ESRRA expression has already been suggested as a negative prognostic factor for disease-free survival of ovarian cancer patients (17). Inhibition of ESRRA mRNA levels in ovarian cancer cells has suggested another pathway as kaempferol’s working mechanism in antiangiogenesis.
Overall, the dietary flavonoid, kaempferol, was found to be effective in inhibiting VEGF expression, angiogenesis, and cell viability through both the HIF-1α dependent and the HIF-1α independent pathways in ovarian cancer cells (Fig. 5). As a widely distributed natural product, kaempferol is low in toxicity, potent in antiangiogenesis, and deserves further study on possible applications in angio-prevention and therapy of ovarian cancers.
FIG. 5.
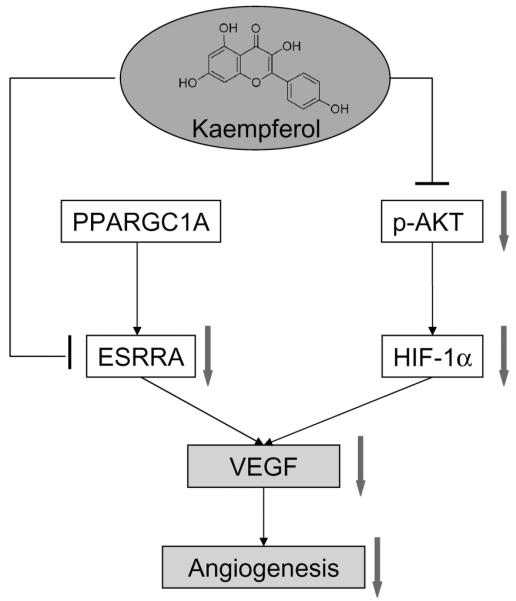
Proposed mechanism for kaempferol’s inhibition of angiogenesis and proliferation in ovarian cancer cells. Connector lines represent established pathways. Arrows indicate regulations by kaempferol treatment in experimental results. PPARGC1A, peroxisome proliferator-activated receptor gamma coactivator 1 alpha; ESRRA, estrogen-related receptor alpha; HIF-1α, hypoxia-inducible factor 1 alpha; VEGF, vascular endothelial growth factor.
ACKNOWLEDGMENTS
This research was supported by Grant P20 RR16477 from the National Center for Research Resources awarded to the West Virginia IDeA Network for Biomedical Research Excellence.
Contributor Information
Haitao Luo, Alderson-Broaddus College, Philippi, West Virginia, USA.
Gary O. Rankin, Joan C. Edwards School of Medicine, Marshall University, Huntington, West Virginia, USA
Lingzhi Liu, Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, West Virginia, USA.
Matthew K. Daddysman, Alderson-Broaddus College, Philippi, West Virginia, USA
Bing-Hua Jiang, Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, West Virginia, USA.
Yi Charlie Chen, Alderson-Broaddus College, Philippi, West Virginia, USA.
REFERENCES
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Fishman DA, Schwartz PE. Current approaches to diagnosis and treatment of ovarian germ cell malignancies. Curr Opin Obstet Gynecol. 1994;6:98–104. [PubMed] [Google Scholar]
- 3.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Perez RP, Godwin AK, Hamilton TC, Ozols RF. Ovarian cancer biology. Semin Oncol. 1991;18:186–204. [PubMed] [Google Scholar]
- 5.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9:2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- 7.Bertl E, Bartsch H, Gerhäuser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5:575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 8.Hartenbach EM, Olson TA, Goswitz JJ, Mohanraj D, Twiggs LB, et al. Vascular endothelial growth factor (VEGF) expression and survival in human epithelial ovarian carcinomas. Cancer Lett. 1997;121:169–175. doi: 10.1016/s0304-3835(97)00350-9. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 10.Fang J, Xia C, Cao Z, Zheng JZ, Reed E, et al. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 11.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, et al. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- 12.Sengupta K, Banerjee S, Saxena N, Banerjee SK. Estradiol-induced vascular endothelial growth factor-A expression in breast tumor cells is biphasic and regulated by estrogen receptor-alpha dependent pathway. Int J Oncol. 2003;22:609–614. [PubMed] [Google Scholar]
- 13.Ray S, Atkuri KR, Deb-Basu D, Adler AS, Chang HY, et al. MYC can induce DNA breaks in vivo and in vitro independent of reactive oxygen species. Cancer Res. 2006;66:6598–6605. doi: 10.1158/0008-5472.CAN-05-3115. [DOI] [PubMed] [Google Scholar]
- 14.Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27:7381–7893. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci USA. 2008;105:6584–6589. doi: 10.1073/pnas.0802785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 17.Gaillard S, Dwyer MA, McDonnell DP. Definition of the molecular basis for estrogen receptor-related receptor-alpha-cofactor interactions. Mol Endocrinol. 2007;21:62–76. doi: 10.1210/me.2006-0179. [DOI] [PubMed] [Google Scholar]
- 18.Banks E. The epidemiology of ovarian cancer. In: Bartlett JMS, editor. Ovarian Cancer, Methods and Protocols. Humana Press; Totowa, NJ: 2000. pp. 3–6. [Google Scholar]
- 19.Bosetti C, Rossi M, McLaughlin JK, Negri E, Talamini R, et al. Flavonoids and the risk of renal cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16:98–101. doi: 10.1158/1055-9965.EPI-06-0769. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez CA, Riboli E. Diet and cancer prevention: where we are, where we are going. Nutr Cancer. 2006;56:225–231. doi: 10.1207/s15327914nc5602_14. [DOI] [PubMed] [Google Scholar]
- 21.Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of intake of fruit, vegetables, and carotenoids and the risk of adult glioma. Am J Clin Nutr. 2007;85:877–886. doi: 10.1093/ajcn/85.3.877. [DOI] [PubMed] [Google Scholar]
- 22.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 23.Bosetti C, Bravi F, Talamini R, Parpinel M, Gnagnarella P, et al. Flavonoids and prostate cancer risk: a study in Italy. Nutr Cancer. 2006;56:123–127. doi: 10.1207/s15327914nc5602_1. [DOI] [PubMed] [Google Scholar]
- 24.Theodoratou E, Kyle J, Cetnarskyj R, Farrington SM, Tenesa A, et al. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:684–693. doi: 10.1158/1055-9965.EPI-06-0785. [DOI] [PubMed] [Google Scholar]
- 25.Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13:1611–1619. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 26.Plochmann K, Korte G, Koutsilieri E, Richling E, Riederer P, et al. Structure-activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch Biochem Biophys. 2007;460:1–9. doi: 10.1016/j.abb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Choi EJ, Kim T, Lee MS. Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells. Life Sci. 2007;80:1403–1408. doi: 10.1016/j.lfs.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Hung H. Inhibition of estrogen receptor alpha expression and function in MCF-7 cells by kaempferol. J Cell Physiol. 2004;198:197–208. doi: 10.1002/jcp.10398. [DOI] [PubMed] [Google Scholar]
- 29.Leung HW, Lin CJ, Hour MJ, Yang WH, Wang MY, et al. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem Toxicol. 2007;45:2005–2013. doi: 10.1016/j.fct.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Sharma V, Joseph C, Ghosh S, Agarwal A, Mishra MK, et al. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol Cancer Ther. 2007;6:2544–2453. doi: 10.1158/1535-7163.MCT-06-0788. [DOI] [PubMed] [Google Scholar]
- 31.Cortes JR, Perez-G M, Rivas MD, Zamorano J. Kaempferol inhibits IL-4-induced STAT6 activation by specifically targeting JAK3. J Immunol. 2007;179:3881–3887. doi: 10.4049/jimmunol.179.6.3881. [DOI] [PubMed] [Google Scholar]
- 32.García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, et al. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang liver cells. Eur J Pharmacol. 2007;557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, et al. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121:2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 34.Kolfschoten GM, Hulscher TM, Duyndam MC, Pinedo HM, Boven E. Variation in the kinetics of caspase-3 activation, Bcl-2 phosphorylation and apoptotic morphology in unselected human ovarian cancer cell lines as a response to docetaxel. Biochem Pharmacol. 2002;63:733–743. doi: 10.1016/s0006-2952(01)00895-4. [DOI] [PubMed] [Google Scholar]
- 35.Duyndam MC, van Berkel MP, Dorsman JC, Rockx DA, Pinedo HM, et al. Cisplatin and doxorubicin repress vascular endothelial growth factor expression and differentially down-regulate hypoxia-inducible factor I activity in human ovarian cancer cells. Biochem Pharmacol. 2007;74:191–201. doi: 10.1016/j.bcp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Risch HA, Jain M, Marrett LD, Howe GR. Dietary fat intake and risk of epithelial ovarian cancer. J Natl Cancer Inst. 1994;86:1409–1415. doi: 10.1093/jnci/86.18.1409. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005;94:209–231. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 38.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 39.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 40.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 41.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishida N, Yano H, Komai K, Nishida T, Kamura T, et al. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer. 2004;101:1364–1374. doi: 10.1002/cncr.20449. [DOI] [PubMed] [Google Scholar]
- 43.Jiang BH, Liu LZ. AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets. 2008;8:19–26. doi: 10.2174/156800908783497122. [DOI] [PubMed] [Google Scholar]
- 44.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, et al. Vascular Endothelial Growth Factor and Angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 45.Kallen J, Schlaeppi JM, Bitsch F, Filipuzzi I, Schilb A, et al. Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor alpha (ERRalpha): crystal structure of ERRalpha ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1alpha. J Biol Chem. 2004;279:49330–49337. doi: 10.1074/jbc.M407999200. [DOI] [PubMed] [Google Scholar]