Abstract
The endoplasmic reticulum (ER) is the primary site for folding and quality control for proteins destined to the cell surface and intracellular organelles. A variety of cellular insults alter ER homeostasis to disrupt protein folding, cause the accumulation of misfolded proteins, and activate an autophagic response. However, the molecular signaling pathways required for ER stress-induced autophagy are largely unknown. Recently, we discovered that a novel-type protein kinase C family member (PKCθ) is required for ER stress-induced autophagy. We show that ER stress, in a Ca2+-dependent manner, induces PKCθ phosphorylation within the activation loop and localization with LC3-II in punctate cytoplasmic structures. Pharmacological inhibition, siRNA-mediated knockdown, or transdominant-negative mutant expression of PKCθ block the ER stress-induced autophagic response. PKCθ activation is not required for autophagy induced by amino acid starvation, and PKCθ activation in response to ER stress does not require either the mTOR kinase or the unfolded protein response signaling pathways. Herein, we review and discuss the significance of these findings with respect to regulation of autophagy in response to ER stress.
Keywords: unfolded protein response, protein kinase Cθ, calcium, autophagy, endoplasmic reticulum, autophagosome
ER Stress-Induced Autophagy
The endoplasmic reticulum (ER) is the site where proteins enter the secretory pathway for trafficking to the cell surface and intracellular organelles. Protein folding in the ER is highly regulated and only properly folded proteins can bypass quality control surveillance and traffic in an anterograde direction to the Golgi compartment. Misfolded proteins are retained in the ER through interaction with protein chaperones and are eventually targeted for degradation by either retrotranslocation into the cytosol for degradation by the 26S proteasome or by autophagic digestion. A variety of conditions, such as pathogen infection, nutrient deprivation (such as the absence of glucose) as well as nutrient excess (such as the overabundance of lipids or glucose), inflammation, alterations in ER lumenal Ca2+ or redox status, genetic mutation, toxic chemicals, etc., disrupt protein folding reactions in the ER and cause the accumulation of unfolded proteins. These conditions are collectively referred to as ‘ER stress.’ Eukaryotic cells constantly monitor the fidelity of client protein folding in the ER and can dynamically adjust protein synthesis, degradation, and folding capacity to prevent accumulation of misfolded proteins. These processes are signaled through the unfolded protein response (UPR). In metazoan cells, the UPR is regulated through three ER-localized transmembrane proteins (PERK, IRE1, and ATF6) that sense the accumulation of unfolded proteins in the ER lumen.1,2 IRE1 is a bifunctional protein kinase and endoribonuclease that cleaves an unconventional 26 base intron in Xbp1 mRNA to generate a translational frame shift that produces a potent basic leucine zipper (bZIP)-containing transcription factor. PERK phosphorylates eukaryotic translation factor 2 on the alpha subunit (eIF2α) to attenuate mRNA translation initiation. eIF2α phosphorylation paradoxically increases translation of Atf4 mRNA which encodes a transcription factor required to activate the majority of the UPR-induced genes. ATF6 contains a bZIP-transcription factor domain in its cytosolic amino terminus. Upon activation of the UPR, ATF6 transits to the Golgi complex where it is cleaved by the site 1 and site 2 proteases S1P and S2P and traffics to the nucleus to activate transcription of genes encoding protein chaperones and machinery of ER-associated protein degradation (ERAD). Because alterations in UPR signaling are associated with numerous diseases ranging from diabetes mellitus to neurodegenerative disease, under-standing how the UPR is regulated and how the UPR maintains cellular homeostasis should provide insight into pathologies associated with many disease processes. Recently, several investigators have independently reported that autophagy is induced by ER stress and is critical for cell survival under conditions of ER stress.3–7
Autophagy is a highly conserved pathway in eukaryotic cells that evolved to degrade bulk cytoplasmic material. Autophagy, a process of ‘self-eating,’ has been classically studied in response to energy deprivation, such as that resulting from nutritional starvation of amino acids or fatty acids.8 Autophagy also provides an important function in the clearance of aggregated or misfolded proteins, such as those that accumulate in neurodegenerative diseases.9 It is thought that ER stress-induced autophagy evolved as a mechanism to degrade misfolded proteins in the ER that cannot be degraded by ER-associated proteasomal degradation. We recently reported that PKCθ mediates ER stress-induced autophagy.10 Treatment of cells with chemical agents that induce ER stress, such as tunicamycin that inhibits N-linked glycosylation or thapsigargin that depletes ER lumenal Ca2+ stores, increase PKCθ phosphorylation. Pharmacological inhibition, siRNA-mediated knockdown, or trans-dominant negative mutant expression of PKCθ activity blocks ER stress-induced autophagy. PKCθ was originally identified and characterized as a critical factor for induction of interleukin-2 expression in response to activation of NFκB, AP-1 and NFAT during T cell receptor/CD3-induced T cell activation.11 Although some reports indicated that PKCθ mediates insulin resistance through serine phosphorylation of IRS-1 in liver or skeletal muscle,12–14 its role in other tissues has remained an enigma. Our studies provide a new role for PKCθ in regulation of ER stress-induced autophagy.
How is PKCθ Activated in Response to ER Stress?
There is accumulating evidence that Ca2+ release from the ER is a primary signal to initiate an autophagic response.10,15 In addition, recent studies have shown that accumulation of unfolded proteins in the ER lumen causes Ca2+ leak into the cytosol, possibly through the inositol trisphosphate (IP3) receptor.16 Our findings indicate that ER stress-induced Ca2+-flux from the ER causes PKCθ activation to signal autophagy. In mammals, the PKC family comprises 11 isoforms that are classified into three subfamilies based on their structural features. The classical PKCs (cPKC) possess both C1 and C2 domains that can bind to diacylglycerol (DAG) and Ca2+, respectively, whereas novel PKCs (nPKC) lack a C2 domain, and atypical PKCs (aPKC) lack both C1 and C2 domains.17 PKCθ is a member of the nPKC family that should not be directly regulated by Ca2+. However, chelation of intracellular calcium blocks ER stress-induced PKCθ phosphorylation and autophagy. This indicates that a Ca2+-dependent signaling pathway is activated upstream of PKCθ during ER stress-induced autophagy. It is possible that phospholipase C may mediate the Ca2+-dependent activation of PKCθ in response to ER stress. Phospholipase C [PLC] converts phosphatidylinositol bisphosphate (PIP2) to DAG and inositol trisphosphate (IP3) in response to receptor tyrosine kinase or G-protein-coupled receptor activation. PLC-delta possesses a Ca2+-binding domain that is indispensable for its activation and intracellular localization.18 Liang et al., report that PKC-dependent induction of c-Myc expression occurs immediately in response to thapsigargin treatment, but with much slower kinetics in response to other ER stressors.19 In their analysis, the PLC inhibitor U73122 blocks c-Myc induction by thapsigargin, suggesting that PLC-PKC signaling is activated by Ca2+-release from the ER. Alternatively, inhibition of PLC may reduce cellular levels of IP3 to reduce Ca2+-release from the ER.
In budding yeast, mTOR kinase regulates the phosphorylation of Atg13. In a highly conserved pathway, nutritional starvation inactivates mTOR kinase. Inactivation of mTOR reduces the phosphorylation of Atg13, thereby promoting its interaction with additional cofactors, to induce autophagy. 20,21 However, we do not observe a decrease in mTOR kinase activity during ER stress. Our findings suggest that PKCθ activation mediates an mTOR-independent, Ca2+-dependent, pathway of autophagy. Hoyer-Hansen et al., report that an increase in cytosolic Ca2+ induces autophagy through Ca2+/calmodulin-dependent kinase kinase-β-mediated activation of AMP-activated protein kinase (AMPK). AMPK represses mTOR kinase.15 However, in our studies, AMPK phosphorylation and mTOR kinase activity are not changed during ER stress. Furthermore, PKCθ activation is not required for induction of autophagy in response to amino acid starvation. Where Hoyer-Hansen et al., use a low concentration of thapsigargin for 24 hours, we treat cells with a higher concentration for a few hours and do not observe mTOR inactivation. However, upon treatment with thapsigargin for more than 16 hrs, mTOR kinase activity is reduced. Therefore, it is possible that PKCθ is required for autophagy in response to acute ER stress that does not elicit mTOR kinase inactivation, whereas prolonged ER stress leads to mTOR kinase inactivation, possibly as a consequence of secondary effects of ER stress that may activate alternative stress signaling cascades.
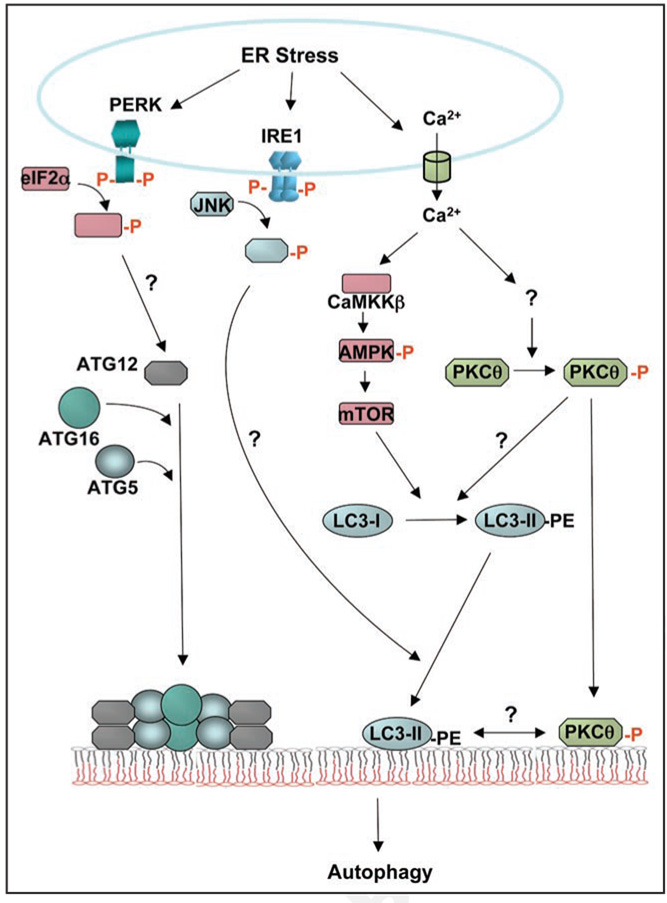
Although increasing evidence supports the notion that ER stress signaling intersects with the autophagic response, the molecular signaling pathways are not known. Ogata et al. report that ER stress-induced autophagy is regulated through IRE1 interaction with TRAF2 to mediate JNK activation.6 In contrast, Kouroku et al., demonstrate that PERK/eIF2α-mediated induction of ATG12 is required for ER stress-induced autophagy (Fig. 1).5,6 However, although cells with either IRE1α deletion, or deficiency in eIF2α phosphorylation display reduced ER stress-induced autophagy, PKCθ phosphorylation is not altered in response to ER stress. ATF6α deletion has no effect on ER stress-induced autophagy or PKCθ phosphorylation. Thus, UPR signaling is not required for PKCθ-activation. These findings suggest that PKCθ may modulate a different step in ER stress-induced autophagy.
Figure 1.
ER stress-induced signaling pathways that mediate autophagy. During induction of autophagy, the ubiquitin-like modification complexes of ATG12 and ATG8 (LC3) play critical roles in the dynamic changes in membranes that coalesce to form autophagosomes. These two ubiquitin-like proteins are targeted to the membrane through interaction with ATG5 and ATG16, or lipidation with phosphatidyle-thanolamine (PE), respectively. The ATG5—ATG12-ATG16 complex promotes conversion of LC3-I (cytosolic soluble form) to LC3-II (membranous lipidated form). In response to ER stress, PERK-mediated phosphorylation of eIF2α induces transcription of ATG12.5 In addition, ER stress-induced activation of the IRE1-JNK pathway is required for recruitment of LC3-II to the autophagosome.6 Our results show that PKCθ activation is also critical for ER stress-induced autophagy. ER stress induces Ca2+ release from the ER that is required to increase PKCθ phosphorylation. The phosphorylated form of PKCθ is targeted to LC3-II-containing punctate membranes in the cytosol. 10 As PKCθ is a member of the novel PKC family, it is unlikely that Ca2+ directly activates PKCθ. It is also proposed that Ca2+ release from the ER stimulates a CaMKK/AMPK-dependent pathway that inhibits mTORC1 to induce autophagy. 15
What is the Role of PKCθ Activation During ER Stress-Induced Autophagy?
We also find that phosphorylated PKCθ localizes with EGFPLC3 in punctate cytoplasmic structures. In addition, phosphorylated PKCθ is enriched in microsome fractions with endogenous LC3-II. In our unpublished findings, we observe that the nPKC inhibitor Rottlerin causes excessive expansion of ER membranes in thapsigargin-treated immortalized hepatocyte cells. It is possible that in the absence of autophagy, ER-derived membranes accumulate in response to ER stress, as suggested by Bernales et al.3 These findings suggest that PKCθ activation and membrane localization may play an important role in the dynamic membrane changes that occur during ER stress-induced autophagy.
Acknowledgements
R.J.K. is an investigator of the Howard Hughes Medical Institute and supported by NIH grants DK042394, HL052173 and HL057346.
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Schroder M, Kaufman RJ. Divergent roles of IRE1alpha and PERK in the unfolded protein response. Curr Mol Med. 2006;6:5–36. doi: 10.2174/156652406775574569. [DOI] [PubMed] [Google Scholar]
- 3.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II) Hum Mol Genet. 2007;16:618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- 5.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:30–39. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 6.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 9.Ventruti A, Cuervo AM. Autophagy and neurodegeneration. Curr Neurol Neurosci Rep. 2007;7:443–451. doi: 10.1007/s11910-007-0068-5. [DOI] [PubMed] [Google Scholar]
- 10.Sakaki K, Wu J, Kaufman RJ. Protein kinase C-theta is required for autophagy in response to stress in the endoplasmic reticulum. J Biol Chem. 2008 doi: 10.1074/jbc.M710209200. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman A, Villalba M. Protein kinase C-theta (PKC theta): a key enzyme in T cell life and death. J Biochem. 2002;132:841–846. doi: 10.1093/oxfordjournals.jbchem.a003295. [DOI] [PubMed] [Google Scholar]
- 12.Gao Z, Wang Z, Zhang X, Butler AA, Zuberi A, Gawronska-Kozak B, et al. Inactivation of PKCtheta leads to increased susceptibility to obesity and dietary insulin resistance in mice. Am J Physiol Endocrinol Metab. 2007;292:E84–E91. doi: 10.1152/ajpendo.00178.2006. [DOI] [PubMed] [Google Scholar]
- 13.Haasch D, Berg C, Clampit JE, Pederson T, Frost L, Kroeger P, et al. PKCtheta is a key player in the development of insulin resistance. Biochem Biophys Res Commun. 2006;343:361–368. doi: 10.1016/j.bbrc.2006.02.177. [DOI] [PubMed] [Google Scholar]
- 14.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 16.Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 17.Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 18.Yagisawa H. Nucleocytoplasmic shuttling of phospholipase C-delta1: a link to Ca2+ J Cell Biochem. 2006;97:233–243. doi: 10.1002/jcb.20677. [DOI] [PubMed] [Google Scholar]
- 19.Liang SH, Zhang W, McGrath BC, Zhang P, Cavener DR. PERK (eIF2alpha kinase) is required to activate the stress-activated MAPKs and induce the expression of immediateearly genes upon disruption of ER calcium homoeostasis. Biochem J. 2006;393:201–209. doi: 10.1042/BJ20050374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 Suppl 2:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheong H, Yorimitsu T, Reggiori F, Legakis JE, Wang CW, Klionsky DJ. Atg17 regulates the magnitude of the autophagic response. Mol Biol Cell. 2005;16:3438–3453. doi: 10.1091/mbc.E04-10-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]