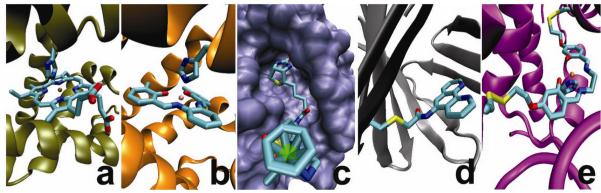
Figure 4. Strategies for non-native cofactor incorporation into a protein scaffold.
Native cofactor substitution of heme b in myoglobin for a, Fe-porphycene and b, 3,3-Cr salophen, exploiting the structural and dative-bonding similarities between the native cofactor and the surrogate. Affinity tagging of the catalyst with a protein-selective linker such as the biotin-streptavidin couple, c, allows for versatile attachment of non-native cofactors. Covalent strategies include a single attachment strategy as in d, showing the adipocyte lipid binding protein (ALBP) linked through Cys117 to a phenanthrolene complex; and dual covalent attachment as in e, showing dual anchoring of a Mn-salen complex into the myoglobin scaffold. Description of streptavidin complex obtained from ref91. Crystal structure of Fe porphycene, Cr(3,3′-Me2-salophen)-Mb complex, biotin-streptavidin complex, and phenanthroline-ALBP were obtained from the Protein Databank (PDB codes: 2D6C, 1J3F, 2QCB, and 1A1894, respectively). Computer model of Mb(L72C/Y103C) generated from an overlay with native heme b structure.