Introduction
Both periodic leg movements in sleep (PLMS) and restless legs syndrome (RLS) are common conditions in elderly populations. In this paper, we review relevant knowledge regarding their prevalence and associated conditions, discuss technical considerations related to the polysomnographic characterization of PLMS in relation to age, evaluate possible manifestations of these conditions in dementia and offer some brief perspectives on treatment considerations. Although PLMS does not approach 100% sensitivity and 100% specificity as a marker of RLS (specificity lags because many patients without RLS still demonstrate PLMS), both conditions show a high prevalence in the older adult. PLMS and RLS are defined from different data sources (polysomnographic criteria for PLMS, clinical criteria for RLS), though their considerable overlap has led many researchers1 to argue that PLMS represents the single best objective marker of a condition (i.e., RLS) that may be complex and variegated, and may have somewhat unique characterization in geriatrics2.
PLMS: Prevalence and Associated Factors
PLMS are stereotypic, repetitive, non-epileptiform movements of the lower limbs typically occurring during NREM sleep but occasionally occurring in REM sleep and in some situations discernible in the waking state as well. PLMS typically consist of dorsiflexion of the anterior tibialis muscle, although movements may involve the hip or be confined to the great toe (extensor hallucis longus). PLMS represent a physiologic finding made with polysomnography or ankle-mounted actigraphy. The best single-night estimate of PLMS prevalence is about 45% in an unselected geriatric population derived from the San Diego area3. In a population of similar demographics, over 85% of elderly subjects showed a mean PLMS Index (PLMS per hour of sleep) in excess of 5.04 across 5 nights of recordings. Somewhat lower estimates of PLMS prevalence (29.0 %) have also been reported5. Longitudinal data on whether PLMS increase with aging are scarce but disagree, with some studies showing increases6 and other studies show no change7 over time.
A substantial number of studies have examined symptomatic correlates of PLMS in middle-aged and aged populations and have shown decidedly mixed results. Complaints of poor and/or altered sleep architecture were not apparent in conjunction with PLMS in the early studies of Bixler and Kales5, 8 and Mosko and colleagues9. Ancoli-Israel et al3 noted that the myoclonus index (based solely on recorded movements without reference to EEG) was related to a history of kicking at night and some history of respiratory symptoms, but many symptoms that might be expected to be correlated with disrupted sleep (e.g., lower total sleep time, prolonged sleep onset latency) were not. The strongest single correlate of PLMS in that study was the report of the estimated number of awakenings on the night of recording. Another study in the elderly noted relationships between PLMS and sleep latency problems but not nocturnal awakenings10. Youngstedt et al11 examined PLMS polysomnographically without reference to arousal and noted relationships with lower total sleep times and wake after sleep onset. By contrast, across a broader age range of subjects, Mendelson12 evaluated leg movements accompanied by EEG arousals and was unable to find relationships between PLMS and symptoms. Montplaisir et al13 noted no differences in PLMS among controls, individuals with insomnia and individuals with hypersomnia, and Karadeniz et al14 found no modifications in macrosleep or microsleep architecture associated with the presence of PLMS in 40-64 year old patients with insomnia. Hornyak et al15 reported no association between PLMS and sleep quality in non-RLS insomniac patients. More recently, Carrier et al16 reported that the presence of PLMS was unrelated to polysomnographic measurements of sleep quality in a group of 70 normal subjects between the ages of 40 and 60, although in male subjects a significant (but very small size) effect was noted for lower sleep quality in association with PLMS Index (number of movements per sleep hour) greater than 10.
The absence of associations between subjectively and/or objectively disturbed sleep and PLMS in many of these studies stands in contrast to early published case series of patients from Sleep Disorders Clinics where the diagnosis of the condition appeared to be clearly related to disturbed sleep17, 18. However, these were highly selected, clinic-based populations and also included a substantial portion of patients with frank RLS, which is less ambiguously related to poor sleep and in fact, poor sleep is a feature associated with diagnosis2 (see section below). Thus, the meaning of such studies for the clinical correlates of PLMS in the general population remains doubtful. Of note is a recent epidemiologic study suggesting that the clinical conditions of RLS and reported leg jerking may be relatively tightly coupled19, but because PLMS in that study were defined atypically (i.e., via self-report and not electrophysiologically), it is difficult to draw inferences from these data. Some have claimed that when PLMS are defined symptomatically by the concurrent presence of sleep complaints (i.e., periodic leg movement disorder, PLMD), specific polysomnographic features (e.g., higher number of PLMS with arousal) may distinguish this group of patients from those with RLS20.
Technical Considerations in the Recording of PLMS as a Function of Age
A possible reason that many of the aforementioned studies may have failed to find unequivocal relationships between PLMS and symptoms is the large night-to-night variability that exists in the nightly measurement of PLMS, particularly in the aged4, 21-23. Extensive variability in the measurement of PLMS would be expected to introduce error into the detection of any relationships between such metrics and symptoms. Potentially the variability could also underlay the inconsistencies across those few longitudinal studies to date. Factors influencing the variability in PLMS between nights remain ill-defined. Some have speculated that, in the patient with RLS, extreme variability in sleep length and/or quality may impact upon the variability1, such that if an individual sleeps very little on a given night their PLMS Index will be low by necessity. Some data support that the variability of the PLMS index is higher in RLS than in other sleep disorders patients24, but higher mean levels make this conclusion equivocal on a purely statistical basis. Other studies of inter-night variability of PLMS in RLS suggest considerably less variability25. Considerable night-to-night variability also occurs in PLMS in elderly individuals who do not have apparent RLS, as has been noted in several studies4, 21-23.
Several other potential age-associated aspects related to characterization of the movements have been examined. Within bouts of PLMS, both duration of movements26 and intermovement interval (IMI)9 were unrelated to age. Nicholas et al26, however, noted the IMI for waking periodic leg movements decreased with age. An analysis of time of night effects noted similarly that older subjects, who had an earlier time-of-night predominance in their PLMS, also had shorter IMI's27. Finally, given the known disruption of sleep continuity with aging, it is hardly surprising that several studies have reported that, consistent with greater fragmentation of sleep with aging, the number of PLMS with arousals or awakening increase with age12, 17, 28. Heightened reactivity to movements cannot be assumed to crossover into the autonomic domain, however, since Gosselin et al29 reported that older subjects had reduced magnitude of heart rate variability accompanying PLMS relative to younger subjects.
RLS: Prevalence and Associated Factors
RLS is a clinical syndrome, now consensually agreed upon to be defined by four cardinal features: a) the urge to move legs often accompanied by unpleasant leg sensations; b) the urge to move or unpleasant sensations begin or worsen during periods of inactivity; c) the urge to move or unpleasant sensations are relieved by movement; d) the urge to move or unpleasant sensations are worse in the evening or at night2. Supportive features include: positive family history, treatment responsiveness, a finding of PLMS (measured electrophysiologically).
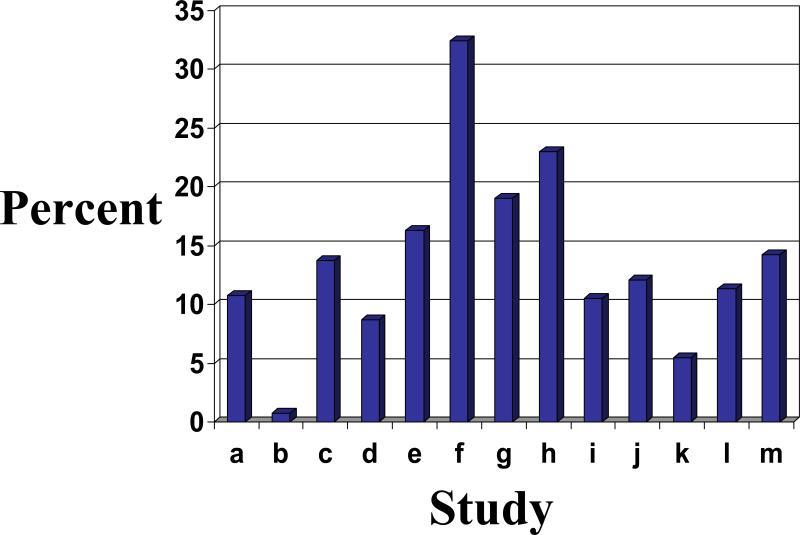
Many30-35 but not all19, 36-41 epidemiologic studies have suggested that the prevalence of RLS increases with age. Interpretation of age effects in population-based work is complicated by the fact that the upper age limit varies among studies and various studies employ different definitions of RLS. The wide range of prevalence figures is depicted in Figure 1. Because of the wide range of peak prevalence (from 50-59 to as high as 80 and above) reported in these studies, it remains unclear whether RLS shows true age dependence (i.e., the likelihood of its prevalence increases nearly linearly with chronologic age), or whether the age effect is better characterized as age-related (i.e., encompassing a distinct chronologic window of vulnerability)42. Additionally, because these studies encompass diverse populations from the United States32, 33, 38, 40, the United Kingdom33, 34, 40, French and non-speaking French Canadian provinces31, Germany33, 37, 39, 40, Scandinavian countries19, 30, France35, 40, Italy33, 36, 41 and Spain33, 40, age differences in peak prevalence could represent the partial influence of varying genetic predispositions for RLS, consistent with several gene loci that have been implicated with the condition43, 44.
Figure 1. Prevalence of RLS Across Various Studies.
Prevalence figures reflect oldest age groups with peak prevalence with data averaged for men and women when available. Studies (and references) are as follows: a36, b 34, c 37, d33, e39, f38, g32, h31,i30, j41, k40, l35, m19.
Many conditions have been associated with RLS in these epidemiologic studies. Perhaps because many of the RLS definitions used encompass elements of disturbed sleep, only a limited number of prevalence studies have presented data on relationships between RLS and independently ascertained insomnia questions. Not surprisingly, RLS was associated with complaints of poor sleep in those studies19, 30, 34, 40. More commonly, medical/psychiatric conditions have been studied in association with RLS including: hypertension and cardiovascular disease30, 33, 39, diabetes and/or possible neuropathy32, 33, 39, depressive symptomatology30, 34, 37 or poor mental health32, 33, musculoskeletal disease33, hypothyroidism39, renal disease34, habitual lack of exercise32, smoking33, 39. Not every study reports data on all the aforementioned conditions, and particular medication classes have also been associated with RLS (and/or PLMS) (see section below on Treatment Considerations). At least some of these conditions (e.g., cardiovascular disease depressive symptoms, diabetes, sedentary lifestyle) would be expected to occur in higher frequency in elderly populations, however there is only limited evidence that age prevalence per se could be due primarily to any one of these factors. Such a contention would require that observed age prevalence no longer occurred subsequent to multivariate adjustment for any particular associated factor. To date, no study has shown this.
Altered iron metabolism represents a condition associated with RLS deserving special note in the context of aged populations. Following the report of O'Keeffe45 that elderly RLS patients were likely to demonstrate low serum ferritin levels, several important lines of evidence have pursued this line of work. Although frank anemia and lower serum iron may not always be apparent in association with RLS, neuroimaging and cerebrospinal fluid studies have suggested that total brain iron concentrations are reduced in RLS, which would be consistent with an alteration in blood/brain transport46, 47. Because anemia is a common problem in elderly populations, it is possible that such derangements in some aspect of iron metabolism underlie the high prevalence of RLS in the aged. Few studies have tested this hypothesis in elderly populations. O'Keeffe48 published a small additional case series suggesting that serum ferritin levels <50 ng/ml were significantly more likely in elderly patients with recent onset RLS, but other population-based studies show a more complex picture. Although RLS were not accompanied by low levels of serum ferritin or by higher levels of soluble transferrin receptor in one population-based study, it appeared that mid-range levels (c.f. highest ranges) of serum iron and transferrin saturation may have exerted protective effects49. Neither anemia (defined by hemoglobin levels less than 2 SDs below gender- expected values) nor ferritin values were significant factors in RLS in another study of a German population across a broader age range (20-79)39. By contrast, in a northern Italian elderly population (South Tyrol), lower serum iron and higher soluble transferrin receptor levels (often seen in early stage anemia) were related to RLS41. Finally, recent data have suggested that cerebrospinal fluid (CSF) ferritin in older, late onset RLS patients were unrelated to onset of symptoms and that elderly patients had higher levels of CSF ferritin than younger patients50. These data cast doubt on whether iron deficiency may be a relevant risk factor for RLS presenting in the aged population, unless the condition also had early onset.
RLS in Dementia
The recent NIH Diagnosis and Workshop Conference regarding definitions of RLS2 acknowledged that different definitions of the syndrome might be relevant in geriatrics and children. Although dementia patients may be too verbally compromised to express their condition in language, several essential features such as rubbing or kneading legs, excessive motor activity in lower limbs (including fidgeting and pacing), and worsening of leg discomfort during rest or activity and its diminishment with activity are suggestive of the condition, as is the temporally specific occurrence of leg discomfort or motor activity in the late afternoon and/or early evening. Although not explicitly described in NIH Conference Report, wandering (c.f., pacing), a widely acknowledged clinical management issue in dementia patients51, may represent an heretofore unrecognized RLS phenotype of particular note and importance in geriatric care. For example, typical treatments for wandering may involve medications that exert dopaminergic blockage, which might otherwise be expected to worsen, rather than improve, the wandering behaviors (see section below on Treatment Considerations).
Wandering has long been recognized as one of the most difficult and intractable components of dementia in general52 and Alzheimer's Disease (AD), specifically53. Patients may place themselves at grave risk wandering outside their homes, and their recovery often involves police and other emergency service personnel54. Several studies have noted that nocturnal wandering was the most distressing of all sleep-related behaviors of AD patients53, 55. Wandering represents a particular conundrum for clinical researchers attempting to understand the mechanisms underlying these peculiar behaviors. Some descriptive research has focused on tracking the specific vectors of the ambulation56, whereas others have focused on wandering as escape-like behavior57. Perhaps the most thorough neurobiologic perspective on wandering to date has been the work of Duffy and colleagues who have suggested that wandering may be a function of a modified processing system in which aberrant visual attention systems undermine spatial orientation58, 59 or, as so aptly stated by Duffy: “Alzheimer's patients do not get lost because they have forgotten where they are going, rather, they get lost because they cannot keep track of where they have been.” p. xi ref 52. Also of mechanistic interest are the positron emission tomography (PET) studies of Meguro and colleagues60, 61, who showed that wandering AD patients (relative to those who do not wander) demonstrated lower dopamine uptake in caudate and putamen; those decreases also correlated with decreased cerebral glucose utilization in frontal and temporal, but not parietal, regions. Comparable findings have been reported more recently by Rolland et al62. These results in wandering AD patients bear resemblance to some neuroimaging findings in RLS patients, who showed reduced dopamine terminal storage63, 64. However, these parallels assume a major role for nigrostriatal dysfunction in RLS, which may be open to question (see section below on Parkinsonism).
Within the context of the NIH Workshop statement2, there is now increasing recognition that at least some cases of wandering could represent unrecognized RLS, and the tendency for wandering to occur in the early evening hours65 is consistent with RLS in dementia. Obviously a patient with a long-standing personal history suggestive of RLS or other (perhaps younger) family members with known RLS would also serve as partial corroboration for RLS in the dementia patient. Of note in this regard is that wanderers were more likely to have lifelong patterns of walking or strolling when stressed, at least as recalled retrospectively by family members56. Factors associated with RLS in the general population (anemia, diabetes, musculoskeletal disease or neuropathy), when present in a dementia patient who wanders, also might be suggestive of RLS. Curiously, as a group, AD patients were reported by their caregivers to be no more likely to have RLS symptoms or leg twitches than elderly controls66, implying that only a subset of patients show such symptomatology. RLS patients have been reported recently to have deficits in cognitive tasks implicated in pre-frontal cortex localization, which have been interpreted as the effects of sleep loss67. These data can be viewed as broadly compatible with the presence of RLS in dementia.
A discussion of the phenotypic presentation of RLS in neurodegenerative disease (such as AD) invariably raises the issue of RLS in Parkinsonism, which can often but not invariably be accompanied by dementia (e.g., Lewy Body Dementia). Although RLS and Parkinsonism are both characterized broadly as dopamine deficient conditions, one could expect the prevalence of the former to be considerably higher in the latter. Some data suggest that PLMS prevalence is higher in Parkinson's Disease (PD) than in control populations68, however, evidence to date linking PD and RLS has been negative34, 36, 49, 69, mixed70, 71 or, in one study, positive72. Low serum ferritin has been shown to a relevant mediating variable for older onset RLS in PD70, however, as described above, there is some evidence of decreased salience of reduced iron stores as relevant for later onset RLS50. The relative independence of RLS and PD may reflect the relative contribution of putative postsynaptic and /or diencephalospinal dysfunction in RLS73. Abnormalities of flexor reflex and the sensory abnormalities accompanying RLS would be compatible with dysfunction of dopaminergic efferents and afferents within the dorsal horn that are rarely, if ever, seen in PD73. Comparisons of single photon emission computed tomography (SPECT) of the dopamine transporter in age-matched RLS and PD patients showed better preserved binding in the RLS patients74. Finally, a small neuropathologic case series of RLS patients did not demonstrate Lewy Bodies or alpha-synuclein deposits (exceedingly common in PD), again reiterating the relative independence of the two conditions75.
Treatment Considerations
Empirical evidence for treatment options for RLS and symptomatic PLMS (i.e., Periodic Limb Movement Disorder, PLMD) have been summarized in a Practice Parameters publication from the American Academy of Sleep Medicine (AASM) 76 and an accompanying review of empirical evidence as of 200277. These publications clearly indicate the efficacy of levo-dopa and the dopamine agonists, pergolide, pramipexole and ropinirole, as effective for RLS and PLMD. Other dopamine agonists, as well as amantadine and selegiline, were viewed as possibly effective. With the exception of ropinirole (which has an FDA-approved indication for use in RLS), usage of all of these other medications constitute used off-label use. Since the publication of the AASM guidelines, a number of phase III, multi-site, randomized clinical trials of some of these medications (e.g., ropinirole) have been published that confirm the original report78, 79. Some data also suggest utility of dopamine agonists having longer half-lives, such as cabergoline80, and, under development in transdermal formulation, rotigotine81 and lisuride82 . In practice, oral pramipexole and ropinirole probably see the most widespread current usage. The two can be distinguished by primarily hepatic (ropinirole) versus renal (pramipexole) excretion and by half-life (6 hours for ropinirole; 8-12 hours for pramipexole). Iron supplementation may also be useful in selected cases, but as suggested above, this may be less relevant for the aged population.
In the wandering dementia patient with a history of RLS or a medical condition associated with RLS, an empirical trial of a low dose dopaminergic agonist (0.25-0.5 mg ropinirole; 0.125-0.25 mg pramipexole) may represent an avenue of treatment, however, because of possible dopamine-induced psychosis, judicious usage and careful dose escalation is advised. An initial approach should examine potential exacerbating medications already in use before adding new ones. In non-demented populations, some evidence suggests that selective serotonin-reuptake inhibitors33, 83, as well as anti-depressants such as venlafaxine83, and mirtazapine84, 85 can be associated with PLMD and/or RLS, though one recent study presented data to the contrary86.
Case reports with atypical anti-psychotics (olanzapine, quetiapine, risperidone) having at least partial blockade at specific dopaminergic receptors (e.g., olanzapine at D1-D4, quetiapine at D1/D2, risperidone at D2) have been reported to both aggravate RLS or PLMS87-89, but also treat nocturnal wandering in one case series in AD patients90. Neuroleptic use was significant34 or borderline significant37 in predicting RLS in population-based studies. At least in theory, for the dementia patient with longstanding RLS whose evening wandering and agitation represents a continuation or exacerbation of their pre-morbid condition, such medication would be expected only to aggravate, rather than improve, the behavior. In a secondary analysis of double-blind, placebo-controlled trial of risperidone in dementia patients, baseline wandering predicted higher rates of falls at 2.0 mg/day but was protective at 1.0 mg, though it was unclear whether any of the wandering patients may have represented unrecognized RLS91. In normal adults, quetiapine (25 mg and 100 mg) was shown to improve polysomnographically defined sleep architecture in a double-blind, placebo-controlled trial, though the number of PLMS were increased under the higher dose92. Taken together, these results suggest that usage of atypical anti-psychotics to treat wandering (an off-label indication) should be entertained cautiously with careful ascertainment of pre-morbid predisposition for RLS and/or PLMS.
Summary
PLMS and RLS are exceedingly common in ambulatory, non-institutionalized, non-cognitively impaired elderly populations and may occur in demented patients as well, where they may be manifested by signs of late afternoon and evening wandering. Risk factors operating for these conditions in geriatrics include many of the same factors acknowledged to be of importance in middle-aged patients (e.g., diabetes, neuropathy/radiculopathy, renal insufficiency, cardiovascular disease) but occurring with relatively high prevalence in the elderly and thus particularly salient in this age group. Altered iron metabolism may also be a risk if the RLS is longstanding. It must never be assumed that PLMS, in the absence of frank RLS symptomatology represents a cause of poor sleep or daytime sleepiness in old age. Ample evidence demonstrates that many geriatric patients present with PLMS that have no symptomatic correlate. In such cases, intervention would be premature and unnecessary. There are no natural history data that suggest that the presence of asymptomatic PLMS is a harbinger for any later pathology, and overlap between RLS and Parkinsonism remains in doubt. Nonetheless, symptomatic RLS is a major problem for many geriatric patients and deserves full recognition as a highly treatable condition. Particularly in the dementia population with nocturnal wandering (as a potential sign of RLS) implementation of new treatment should be carefully entertained, and cessation of potentially aggravating medications always should be considered initially.
Acknowledgments
This work was supported by the following grants: AG-020269; AG-025688; NS-35345; NS-050595; AT-00611 and a grant from the Alzheimer's Association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen RP. The resurrection of periodic limb movements (PLM): leg activity monitoring and the restless legs syndrome (RLS). Sleep Med. 2005 Sep;6(5):385–387. doi: 10.1016/j.sleep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003 Mar;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991 Dec;14(6):496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 4.Youngstedt SD, Kripke DF, Klauber MR, Sepulveda RS, Mason WJ. Periodic leg movements during sleep and sleep disturbances in elders. J Gerontol A Biol Sci Med Sci. 1998 Sep;53(5):M391–394. doi: 10.1093/gerona/53a.5.m391. [DOI] [PubMed] [Google Scholar]
- 5.Bixler EO, Kales A, Vela-Bueno A, Jacoby JA, Scarone S, Soldatos CR. Nocturnal myoclonus and nocturnal myoclonic activity in the normal population. Res Commun Chem Pathol Pharmacol. 1982 Apr;36(1):129–140. [PubMed] [Google Scholar]
- 6.Coleman R, Bliwise D, Sajben N, et al. Epidemiology of periodic leg movements during sleep. In: Guilleminault C, Lugaresi E, editors. Sleep/Wake Disorders: Natural History, Epidemiology, and Long-Term Evolution. Raven Press; New York: 1983. pp. 217–229. [Google Scholar]
- 7.Gehrman P, Stepnowsky C, Cohen-Zion M, Marler M, Kripke DF, Ancoli-Israel S. Long-term follow-up of periodic limb movements in sleep in older adults. Sleep. 2002 May 1;25(3):340–343. doi: 10.1093/sleep/25.3.340. [DOI] [PubMed] [Google Scholar]
- 8.Kales A, Bixler EO, Soldatos CR, Vela-Bueno A, Caldwell AB, Cadieux RJ. Biopsychobehavioral correlates of insomnia, part 1: Role of sleep apnea and nocturnal myoclonus. Psychosomatics. 1982 Jun;23(6):589–600. doi: 10.1016/S0033-3182(82)73359-6. [DOI] [PubMed] [Google Scholar]
- 9.Mosko SS, Dickel MJ, Paul T, et al. Sleep apnea and sleep-related periodic leg movements in community resident seniors. J Am Geriatr Soc. 1988 Jun;36(6):502–508. doi: 10.1111/j.1532-5415.1988.tb04019.x. [DOI] [PubMed] [Google Scholar]
- 10.Bliwise D, Petta D, Seidel W, Dement W. Periodic leg movements during sleep in the elderly. Arch Gerontol Geriatr. 1985 Oct;4(3):273–281. doi: 10.1016/0167-4943(85)90009-3. [DOI] [PubMed] [Google Scholar]
- 11.Youngstedt SD, Kripke DF, Elliott JA, Klauber MR. Circadian abnormalities in older adults. J Pineal Res. 2001 Oct;31(3):264–272. doi: 10.1034/j.1600-079x.2001.310311.x. [DOI] [PubMed] [Google Scholar]
- 12.Mendelson WB. Are periodic leg movements associated with clinical sleep disturbance? Sleep. 1996 Apr;19(3):219–223. doi: 10.1093/sleep/19.3.219. [DOI] [PubMed] [Google Scholar]
- 13.Montplaisir J, Michaud M, Denesle R, Gosselin A. Periodic leg movements are not more prevalent in insomnia or hypersomnia but are specifically associated with sleep disorders involving a dopaminergic impairment. Sleep Med. 2000 Apr 1;1(2):163–167. doi: 10.1016/s1389-9457(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 14.Karadeniz D, Ondze B, Besset A, Billiard M. Are periodic leg movements during sleep (PLMS) responsible for sleep disruption in insomnia patients? Eur J Neurol. 2000 May;7(3):331–336. doi: 10.1046/j.1468-1331.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 15.Hornyak M, Trenkwalder C. Restless legs syndrome and periodic limb movement disorder in the elderly. J Psychosom Res. 2004 May;56(5):543–548. doi: 10.1016/S0022-3999(04)00020-0. [DOI] [PubMed] [Google Scholar]
- 16.Carrier J, Frenette S, Montplaisir J, Paquet J, Drapeau C, Morettini J. Effects of periodic leg movements during sleep in middle-aged subjects without sleep complaints. Mov Disord. 2005 Sep;20(9):1127–1132. doi: 10.1002/mds.20506. [DOI] [PubMed] [Google Scholar]
- 17.Coleman RM, Miles LE, Guilleminault CC, Zarcone VP, Jr., van den Hoed J, Dement WC. Sleep-wake disorders in the elderly: polysomnographic analysis. J Am Geriatr Soc. 1981 Jul;29(7):289–296. doi: 10.1111/j.1532-5415.1981.tb01267.x. [DOI] [PubMed] [Google Scholar]
- 18.Roehrs T, Zorick F, Sicklesteel J, Wittig R, Roth T. Age-related sleep-wake disorders at a sleep disorder center. J Am Geriatr Soc. 1983 Jun;31(6):364–370. doi: 10.1111/j.1532-5415.1983.tb05748.x. [DOI] [PubMed] [Google Scholar]
- 19.Bjorvatn B, Leissner L, Ulfberg J, et al. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med. 2005 Jul;6(4):307–312. doi: 10.1016/j.sleep.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Eisensehr I, Ehrenberg BL, Noachtar S. Different sleep characteristics in restless legs syndrome and periodic limb movement disorder. Sleep Med. 2003 Mar;4(2):147–152. doi: 10.1016/s1389-9457(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 21.Bliwise DL, Carskadon MA, Dement WC. Nightly variation of periodic leg movements in sleep in middle aged and elderly individuals. Arch Gerontol Geriatr. 1988 Dec;7(4):273–279. doi: 10.1016/0167-4943(88)90010-6. [DOI] [PubMed] [Google Scholar]
- 22.Mosko SS, Dickel MJ, Ashurst J. Night-to-night variability in sleep apnea and sleep-related periodic leg movements in the elderly. Sleep. 1988 Aug;11(4):340–348. [PubMed] [Google Scholar]
- 23.Edinger JD, McCall WV, Marsh GR, Radtke RA, Erwin CW, Lininger A. Periodic limb movement variability in older DIMS patients across consecutive nights of home monitoring. Sleep. 1992 Apr;15(2):156–161. [PubMed] [Google Scholar]
- 24.Hornyak M, Kopasz M, Feige B, Riemann D, Voderholzer U. Variability of periodic leg movements in various sleep disorders: implications for clinical and pathophysiologic studies. Sleep. 2005 Mar 1;28(3):331–335. [PubMed] [Google Scholar]
- 25.Sforza E, Haba-Rubio J. Night-to-night variability in periodic leg movements in patients with restless legs syndrome. Sleep Med. 2005 May;6(3):259–267. doi: 10.1016/j.sleep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Nicolas A, Michaud M, Lavigne G, Montplaisir J. The influence of sex, age and sleep/wake state on characteristics of periodic leg movements in restless legs syndrome patients. Clin Neurophysiol. 1999 Jul;110(7):1168–1174. doi: 10.1016/s1388-2457(99)00033-4. [DOI] [PubMed] [Google Scholar]
- 27.Culpepper WJ, Badia P, Shaffer JI. Time-of-night patterns in PLMS activity. Sleep. 1992 Aug;15(4):306–311. [PubMed] [Google Scholar]
- 28.Chabli A, Michaud M, Montplaisir J. Periodic arm movements in patients with the restless legs syndrome. Eur Neurol. 2000;44(3):133–138. doi: 10.1159/000008221. [DOI] [PubMed] [Google Scholar]
- 29.Gosselin N, Lanfranchi P, Michaud M, et al. Age and gender effects on heart rate activation associated with periodic leg movements in patients with restless legs syndrome. Clin Neurophysiol. 2003 Nov;114(11):2188–2195. doi: 10.1016/s1388-2457(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 30.Ulfberg J, Nystrom B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001 Nov;16(6):1159–1163. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 31.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994 Dec;17(8):739–743. [PubMed] [Google Scholar]
- 32.Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000 Jul 24;160(14):2137–2141. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 33.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002 Jul;53(1):547–554. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 34.Van De Vijver DA, Walley T, Petri H. Epidemiology of restless legs syndrome as diagnosed in UK primary care. Sleep Med. 2004 Sep;5(5):435–440. doi: 10.1016/j.sleep.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Tison F, Crochard A, Leger D, Bouee S, Lainey E, El Hasnaoui A. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology. 2005 Jul 26;65(2):239–246. doi: 10.1212/01.wnl.0000168910.48309.4a. [DOI] [PubMed] [Google Scholar]
- 36.Wenning GK, Kiechl S, Seppi K, et al. Prevalence of movement disorders in men and women aged 50-89 years (Bruneck Study cohort): a population-based study. Lancet Neurol. 2005 Dec;4(12):815–820. doi: 10.1016/S1474-4422(05)70226-X. [DOI] [PubMed] [Google Scholar]
- 37.Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology. 2000 Mar 14;54(5):1064–1068. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 38.Nichols DA, Allen RP, Grauke JH, et al. Restless legs syndrome symptoms in primary care: a prevalence study. Arch Intern Med. 2003 Oct 27;163(19):2323–2329. doi: 10.1001/archinte.163.19.2323. [DOI] [PubMed] [Google Scholar]
- 39.Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004 Jan 26;164(2):196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- 40.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005 Jun 13;165(11):1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 41.Hogl B, Kiechl S, Willeit J, et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005 Jun 14;64(11):1920–1924. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 42.Brody JA, Schneider EL. Diseases and disorders of aging: an hypothesis. J Chronic Dis. 1986;39(11):871–876. doi: 10.1016/0021-9681(86)90035-4. [DOI] [PubMed] [Google Scholar]
- 43.Ferini-Strambi L, Bonati MT, Oldani A, Aridon P, Zucconi M, Casari G. Genetics in restless legs syndrome. Sleep Med. 2004;5:301–304. doi: 10.1016/j.sleep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Bonati MT, Ferini-Strambi L, Aridon P, Oldani A, Zucconi M, Casari G. Autosomal dominant restless legs syndrome maps on chromosome 14q. Brain. 2003;126:1485–1492. doi: 10.1093/brain/awg137. [DOI] [PubMed] [Google Scholar]
- 45.O'Keeffe ST, Gavin K, Lavan JN. Iron status and restless legs syndrome in the elderly. Age Ageing. 1994 May;23(3):200–203. doi: 10.1093/ageing/23.3.200. [DOI] [PubMed] [Google Scholar]
- 46.Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000 Apr 25;54(8):1698–1700. doi: 10.1212/wnl.54.8.1698. [DOI] [PubMed] [Google Scholar]
- 47.Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001 Jan 23;56(2):263–265. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- 48.O'Keeffe ST. Secondary causes of restless legs syndrome in older people. Age Ageing. 2005 Jul;34(4):349–352. doi: 10.1093/ageing/afi066. [DOI] [PubMed] [Google Scholar]
- 49.Berger K, von Eckardstein A, Trenkwalder C, Rothdach A, Junker R, Weiland SK. Iron metabolism and the risk of restless legs syndrome in an elderly general population--the MEMO-Study. J Neurol. 2002 Sep;249(9):1195–1199. doi: 10.1007/s00415-002-0805-2. [DOI] [PubMed] [Google Scholar]
- 50.Earley CJ, Connor JR, Beard JL, Clardy SL, Allen RP. Ferritin levels in the cerebrospinal fluid and restless legs syndrome: effects of different clinical phenotypes. Sleep. 2005 Sep 1;28(9):1069–1075. doi: 10.1093/sleep/28.9.1069. [DOI] [PubMed] [Google Scholar]
- 51.Algase DL. Wandering in dementia. Annu Rev Nurs Res. 1999;17:185–217. [PubMed] [Google Scholar]
- 52.Silverstein NM, Flaherty G, Tobin TS. Dementia and Wandering Behavior: Concern for the Lost Elder. Springer Publishing Company; New York: 2002. [Google Scholar]
- 53.Logsdon RG, Teri L, McCurry SM, Gibbons LE, Kukull WA, Larson EB. Wandering: a significant problem among community-residing individuals with Alzheimer's disease. J Gerontol B Psychol Sci Soc Sci. 1998 Sep;53(5):P294–299. doi: 10.1093/geronb/53b.5.p294. [DOI] [PubMed] [Google Scholar]
- 54.Rowe MA, Glover JC. Antecedents, descriptions and consequences of wandering in cognitively-impaired adults and the Safe Return (SR) program. Am J Alzheimers Dis Other Demen. 2001 Nov-Dec;16(6):344–352. doi: 10.1177/153331750101600610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tractenberg RE, Singer CM, Cummings JL, Thal LJ. The Sleep Disorders Inventory: an instrument for studies of sleep disturbance in persons with Alzheimer's disease. J Sleep Res. 2003 Dec;12(4):331–337. doi: 10.1046/j.0962-1105.2003.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder LH, Rupprecht P, Pyrek J, Brekhus S, Moss T. Wandering. The Gerontologist. 1978;18:272–280. doi: 10.1093/geront/18.3.272. [DOI] [PubMed] [Google Scholar]
- 57.Nasman B, Bucht G, Eriksson S, Sandman P. Behavioural symptoms in the institutionalized elderly: relationship to dementia. Inter J Geriatr Psych. 1993;8(843849) [Google Scholar]
- 58.Kavcic V, Duffy CJ. Attentional dynamics and visual perception: mechanisms of spatial disorientation in Alzheimer's disease. Brain. 2003 May;126(Pt 5):1173–1181. doi: 10.1093/brain/awg105. [DOI] [PubMed] [Google Scholar]
- 59.Tetewsky SJ, Duffy CJ. Visual loss and getting lost in Alzheimer's disease. Neurology. 1999 Mar 23;52(5):958–965. doi: 10.1212/wnl.52.5.958. [DOI] [PubMed] [Google Scholar]
- 60.Meguro K, Yamaguchi S, Itoh M, Fujiwara T, Yamadori A. Striatal dopamine metabolism correlated with frontotemporal glucose utilization in Alzheimer's disease: a double-tracer PET study. Neurology. 1997 Oct;49(4):941–945. doi: 10.1212/wnl.49.4.941. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka Y, Meguro K, Yamaguchi S, et al. Decreased striatal D2 receptor density associated with severe behavioral abnormality in Alzheimer's disease. Ann Nucl Med. 2003 Oct;17(7):567–573. doi: 10.1007/BF03006670. [DOI] [PubMed] [Google Scholar]
- 62.Rolland Y, Payoux P, Lauwers-Cances V, Voisin T, Esquerre JP, Vellas B. A SPECT study of wandering behavior in Alzheimer's disease. Int J Geriatr Psychiatry. 2005 Sep;20(9):816–820. doi: 10.1002/gps.1362. [DOI] [PubMed] [Google Scholar]
- 63.Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology. 1999 Mar 23;52(5):932–937. doi: 10.1212/wnl.52.5.932. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Borreguero D, Odin P, Serrano C. Restless legs syndrome and PD: a review of the evidence for a possible association. Neurology. 2003 Sep 23;61(6 Suppl 3):S49–55. doi: 10.1212/wnl.61.6_suppl_3.s49. [DOI] [PubMed] [Google Scholar]
- 65.Martino-Saltzman D, Blasch BB, Morris RD, McNeal LW. Travel behavior of nursing home residents perceived as wanderers and nonwanderers. Gerontologist. 1991 Oct;31(5):666–672. doi: 10.1093/geront/31.5.666. [DOI] [PubMed] [Google Scholar]
- 66.Tractenberg RE, Singer CM, Kaye JA. Characterizing sleep problems in persons with Alzheimer's disease and normal elderly. J Sleep Res. 2006;15:97–103. doi: 10.1111/j.1365-2869.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS). Sleep Med. 2006 Jan;7(1):25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Wetter TC, Collado-Seidel V, Pollmacher T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson's disease and multiple system atrophy. Sleep. 2000 May 1;23(3):361–367. [PubMed] [Google Scholar]
- 69.Tan EK, Lum SY, Wong MC. Restless legs syndrome in Parkinson's disease. J Neurol Sci. 2002 Apr 15;196(12):33–36. doi: 10.1016/s0022-510x(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 70.Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol. 2002 Mar;59(3):421–424. doi: 10.1001/archneur.59.3.421. [DOI] [PubMed] [Google Scholar]
- 71.Nomura T, Inoue Y, Miyake M, Yasui K, Nakashima K. Prevalence and clinical characteristics of restless legs syndrome in Japanese patients with Parkinson's disease. Mov Disord. 2006;21:380–4. doi: 10.1002/mds.20734. [DOI] [PubMed] [Google Scholar]
- 72.Krishnan PR, Bhatia M, Behari M. Restless legs syndrome in Parkinson's disease: a case-controlled study. Mov Disord. 2003 Feb;18(2):181–185. doi: 10.1002/mds.10307. [DOI] [PubMed] [Google Scholar]
- 73.Rye DB. Parkinson's disease and RLS: the dopaminergic bridge. Sleep Med. 2004 May;5(3):317–328. doi: 10.1016/j.sleep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 74.Linke R, Eisensehr I, Wetter TC, et al. Presynaptic dopaminergic function in patients with restless legs syndrome: are there common features with early Parkinson's disease? Mov Disord. 2004 Oct;19(10):1158–1162. doi: 10.1002/mds.20226. [DOI] [PubMed] [Google Scholar]
- 75.Pittock SJ, Parrett T, Adler CH, Parisi JE, Dickson DW, Ahlskog JE. Neuropathology of primary restless leg syndrome: absence of specific tau- and alpha-synuclein pathology. Mov Disord. 2004 Jun;19(6):695–699. doi: 10.1002/mds.20042. [DOI] [PubMed] [Google Scholar]
- 76.Littner MR, Kushida C, Anderson WM, et al. Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004 May 1;27(3):557–559. doi: 10.1093/sleep/27.3.557. [DOI] [PubMed] [Google Scholar]
- 77.Hening WA, Allen RP, Earley CJ, Picchietti DL, Silber MH. An update on the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004 May 1;27(3):560–583. doi: 10.1093/sleep/27.3.560. [DOI] [PubMed] [Google Scholar]
- 78.Walters AS, Ondo WG, Dreykluft T, Grunstein R, Lee D, Sethi K. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: a 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004 Dec;19(12):1414–1423. doi: 10.1002/mds.20257. [DOI] [PubMed] [Google Scholar]
- 79.Bogan RK, Fry JM, Schmidt MH, Carson SW, Ritchie SY. Ropinirole in the treatment of patients with restless legs syndrome: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2006 Jan;81(1):17–27. doi: 10.4065/81.1.17. [DOI] [PubMed] [Google Scholar]
- 80.Zucconi M, Oldani A, Castronovo C, Ferini-Strambi L. Cabergoline is an effective single-drug treatment for restless legs syndrome: clinical and actigraphic evaluation. Sleep. 2003 Nov 1;26(7):815–818. doi: 10.1093/sleep/26.7.815. [DOI] [PubMed] [Google Scholar]
- 81.Stiasny-Kolster K, Kohnen R, Schollmayer E, Moller JC, Oertel WH. Patch application of the dopamine agonist rotigotine to patients with moderate to advanced stages of restless legs syndrome: a double-blind, placebo-controlled pilot study. Mov Disord. 2004 Dec;19(12):1432–1438. doi: 10.1002/mds.20251. [DOI] [PubMed] [Google Scholar]
- 82.Benes H. Transdermal lisuride: short-term efficacy and tolerability study in patients with severe restless legs syndrome. Sleep Med. 2006 Jan;7(1):31–35. doi: 10.1016/j.sleep.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 83.Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005 Sep 15;58(6):510–514. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 84.Agargun MY, Kara H, Ozbek H, Tombul T, Ozer OA. Restless legs syndrome induced by mirtazapine. J Clin Psychiatry. 2002 Dec;63(12):1179. doi: 10.4088/jcp.v63n1214a. [DOI] [PubMed] [Google Scholar]
- 85.Bahk WM, Pae CU, Chae JH, Jun TY, Kim KS. Mirtazapine may have the propensity for developing a restless legs syndrome? A case report. Psychiatry Clin Neurosci. 2002 Apr;56(2):209–210. doi: 10.1046/j.1440-1819.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 86.Brown LK, Dedrick DL, Doggett JW, Guido PS. Antidepressant medication use and restless legs syndrome in patients presenting with insomnia. Sleep Med. 2005 Sep;6(5):443–450. doi: 10.1016/j.sleep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Pinninti NR, Mago R, Townsend J, Doghramji K. Periodic restless legs syndrome associated with quetiapine use: a case report. J Clin Psychopharmacol. 2005 Dec;25(6):617–618. doi: 10.1097/01.jcp.0000186870.75042.25. [DOI] [PubMed] [Google Scholar]
- 88.Kraus T, Schuld A, Pollmacher T. Periodic leg movements in sleep and restless legs syndrome probably caused by olanzapine. J Clin Psychopharmacol. 1999 Oct;19(5):478–479. doi: 10.1097/00004714-199910000-00017. [DOI] [PubMed] [Google Scholar]
- 89.Wetter TC, Brunner J, Bronisch T. Restless legs syndrome probably induced by risperidone treatment. Pharmacopsychiatry. 2002 May;35(3):109–111. doi: 10.1055/s-2002-31514. [DOI] [PubMed] [Google Scholar]
- 90.Meguro K, Meguro M, Tanaka Y, Akanuma K, Yamaguchi K, Itoh M. Risperidone is effective for wandering and disturbed sleep/wake patterns in Alzheimer's disease. J Geriatr Psychiatry Neurol. 2004 Jun;17(2):61–67. doi: 10.1177/0891988704264535. [DOI] [PubMed] [Google Scholar]
- 91.Katz IR, Rupnow M, Kozma C, Schneider L. Risperidone and falls in ambulatory nursing home residents with dementia and psychosis or agitation: secondary analysis of a double-blind, placebo-controlled trial. Am J Geriatr Psychiatry. 2004 Sep-Oct;12(5):499–508. doi: 10.1176/appi.ajgp.12.5.499. [DOI] [PubMed] [Google Scholar]
- 92.Cohrs S, Rodenbeck A, Guan Z, et al. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology (Berl) 2004 Jul;174(3):421–429. doi: 10.1007/s00213-003-1759-5. [DOI] [PubMed] [Google Scholar]