Abstract
Myocarditis, an inflammatory disease of heart muscle, is an important cause of dilated cardiomyopathy worldwide. Viral infection is also an important cause of myocarditis, and the spectrum of viruses known to cause myocarditis has changed in the past 2 decades. Several new diagnostic methods, such as cardiac magnetic resonance imaging, are useful for diagnosing myocarditis. Endomyocardial biopsy may be used for patients with acute dilated cardiomyopathy associated with hemodynamic compromise, those with life-threatening arrhythmia, and those whose condition does not respond to conventional supportive therapy. Important prognostic variables include the degree of left and right ventricular dysfunction, heart block, and specific histopathological forms of myocarditis. We review diagnostic and therapeutic strategies for the treatment of viral myocarditis. English-language publications in PubMed and references from relevant articles published between January 1, 1985, and August 5, 2008, were analyzed. Main keywords searched were myocarditis, dilated cardiomyopathy, endomyocardial biopsy, cardiac magnetic resonance imaging, and immunotherapy.
ACCF/AHA/ESC = American College of Cardiology Foundation/American Heart Association/European Society of Cardiology; CK-MB = creatine kinase—MB isoenzyme; DCM = dilated cardiomyopathy; ECMO = extracorporeal membrane oxygenation; EF = ejection fraction; LV = left ventricular; LVEF = left ventricular ejection fraction; MRI = magnetic resonance imaging; PCR = polymerase chain reaction
Myocarditis is an important and often unrecognized cause of dilated cardiomyopathy (DCM). It is defined as inflammation of the heart muscle that may be identified by clinical or histopathologic criteria. Recent developments in the diagnosis and treatment of patients with suspected myocarditis include improved histologic criteria and use of cardiac magnetic resonance imaging (MRI). The aim of this review is to provide a contemporary evidence-based approach to evaluation and treatment of patients with suspected myocarditis. Main keywords searched are as follows: myocarditis, dilated cardiomyopathy, endomyocardial biopsy, cardiac magnetic resonance imaging, and immunotherapy. Articles were screened on the premise of importance, quality, and relevance.
REPORT OF A CASE
A 19-year-old and otherwise healthy woman presented to her primary care physician with a report of increasing dyspnea on exertion of 2 or 3 days' duration. She had had an upper respiratory tract infection 3 weeks previously. Chest radiography showed mild cardiac enlargement, and subsequent transthoracic echocardiography revealed a small circumferential pericardial effusion. The patient was treated with ibuprofen for presumed postviral pericarditis.
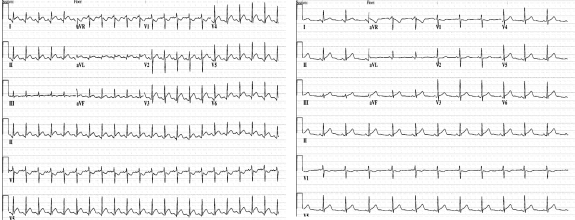
Two days later, the patient was found unconscious in her shower. Electrocardiography revealed diffuse ST-segment elevation throughout the precordial leads, with 1.0-mm PR-segment depression in leads I and II (Figure 1). She underwent immediate coronary catheterization, which showed normal coronary arteries, and was subsequently transferred to a tertiary referral center for further evaluation. On arrival, she was intubated and sedated, with a heart rate of 125 beats/min and a blood pressure of 94/60 mm Hg.
FIGURE 1.
Electrocardiograms. Left, At hospital admission, sinus tachycardia at 130 beats/min, with diffuse ST-segment elevation and 1.0 mm of PR-segment depression in leads I and II. Right, Two days after admission, normal sinus rhythm at 85 beats/min and resolution of ST-segment abnormalities.
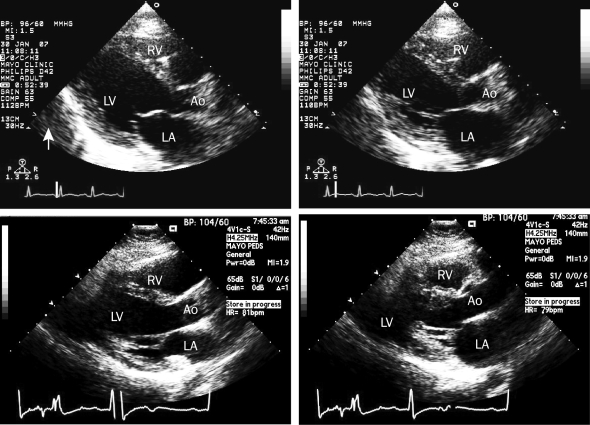
Cardiopulmonary examination showed an S3 gallop with no audible murmur or rub. The patient's jugular venous pressure was elevated at 8 cm H2O, and scattered crackles were found on lung examination. Echocardiography revealed a left ventricular ejection fraction (LVEF) of 15% to 20% with generalized hypokinesis and small pericardial effusion (Figure 2). Laboratory findings included a white blood cell count of 27.2 × 109/L (reference ranges shown parenthetically) (3.5-10.5 × 109/L), a creatinine kinase—MB isoenzyme (CK-MB) fraction level of 22 ng/mL (<6.2 ng/mL; to convert to μg/L, multiply by 1.0), and a troponin T level of 0.9 ng/mL (<0.01 ng/mL; to convert to μg/L, multiply by 1.0). Emergent right heart catheterization with endomyocardial biopsy was performed. The biopsy specimen showed active lymphocytic myocarditis (Figures 3 and 4). Inotropic support was initiated with dobutamine; gentle afterload reduction was initiated with nitroglycerin.
FIGURE 2.
Echocardiograms. At hospital admission, parasternal long-axis view showing ventricular diastole (upper left) and systole (upper right) with an estimated ejection fraction of 20% and a small pericardial effusion (arrow). Two months after admission, parasternal long-axis view showing ventricular diastole (bottom left) and systole (bottom right) with an estimated ejection fraction of 55%. Ao = aorta; LA = left atrium; LV = left ventricle; RV = right ventricle.
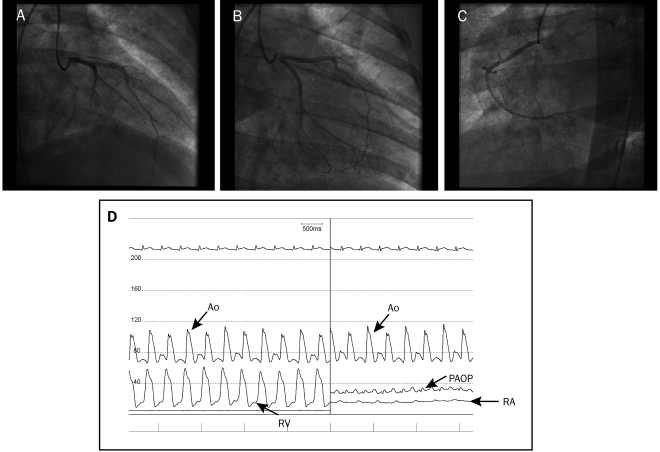
FIGURE 3.
Coronary angiograms of left and right coronary arteries. A and B, Normal left main coronary artery, left anterior descending artery, and left circumflex artery and their respective branches. C, Normal right coronary artery and its respective branches. D, Hemodynamic tracings of aortic (Ao) pressure and right ventricular (RV) pressure, showing right-sided systolic pressures to be one-half of systemic pressures; Ao pressure, pulmonary artery occlusive pressure (PAOP), and right atrial (RA) pressure, showing severely elevated left-sided pressures (mean, 30 mm Hg) and moderately elevated right-sided filling pressures (mean right atrial pressure, 15 mm Hg). Pulmonary arteriolar resistance ([mean pulmonary artery pressure — pulmonary artery occlusive pressure]/cardiac output) was normal at 1.47 Wood unit, suggesting that the elevated right-sided systolic pressures were secondary to left ventricular dysfunction and not intrinsic pulmonary disease. Cardiac output calculated with the Fick formula (cardiac output = stroke volume × heart rate) was normal at 5.2 L/min due to a heart rate of 135 beats/min; however, stroke volume was severely decreased at 39 mL.
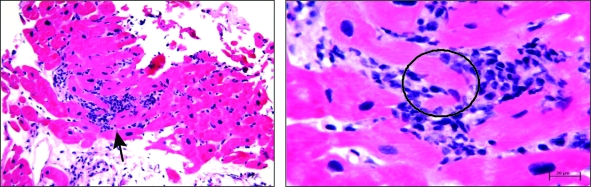
FIGURE 4.
Endomyocardial biopsy specimens. Left, Low-power view showing diffuse lymphocytic infiltration of myocardium (arrow). Right, High-power view showing lymphocytic infiltration with myocyte destruction and surrounding myocardial edema (circle).
The patient was weaned from hemodynamic support and extubated; low doses of β-blocker and angiotensin-converting enzyme inhibitor were initiated. At 6-week follow-up, her LVEF had improved to 66%. Subsequently, endomyocardial tissue was analyzed with polymerase chain reaction (PCR) and found to be positive for Epstein-Barr virus.
CAUSE AND PATHOPHYSIOLOGY
The pathogenesis of myocarditis has been studied previously in animal models. Viruses enter cardiac myocytes and macrophages through specific receptors, inciting a cytotoxic effect.1,2 The exact incidence of myocarditis is difficult to ascertain. However, one study suggests that myocarditis is the cause of sudden cardiac death in 8.6% of cases and is identified in up to 9% of routine postmortem examinations.3 Most studies of myocarditis report a slight male predominance.4,5 Although the cause in individual cases of myocarditis often is unidentified, specific and treatable causes that should be investigated include infections, systemic autoimmune diseases, and hypersensitivity to certain medications6 (Table). In addition, human immunodeficiency virus, Chagas disease, and nutritional causes should be investigated in appropriate clinical settings.
TABLE.
Causes of Myocarditis
Seroepidemiological studies have linked enteroviruses with myocarditis through the co-occurrence of increased enterovirus titers and a clinical syndrome of acute heart failure.7 Rarely, viruses could be cultured from the heart tissue of patients with fatal acute myocarditis. Since the development of molecular techniques to examine endomyocardial tissue, many other viruses and viral coinfections have been recognized.8,9
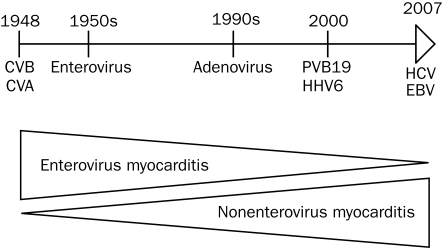
Because the ability to diagnose viral infection has improved with the advent of these molecular biologic techniques, case reports and series have associated DCM with about 20 viruses. As the prevalence of enteroviruses decreased, the prevalence of adenovirus increased after 1995.10 More recently, parvovirus B19 has been the most commonly detected viral genome.9,11 Although the pathogenic role of enteroviruses in myocarditis and chronic DCM is well established, whether parvovirus B19 is incidental or pathogenic in acute myocarditis is still unclear.12 Hepatitis C virus has been associated with myocarditis in Japan, and influenza virus, cytomegalovirus, and Epstein-Barr virus have been identified in some patients with acute and chronic myocarditis (Figure 5).
FIGURE 5.
Evolution of viral causes of myocarditis over time. CVA = coxsackievirus A; CVB = coxsackievirus B; EBV = Epstein-Barr virus; HCV = hepatitis C virus; HHV6 = human herpesvirus 6; PV-B19 = parvovirus B19.
The clinical spectrum of viral cardiomyopathy can be classified as fulminant, acute, or chronic. Viremia is followed by cardiomyocyte infection. In the first phase, acute infection of cardiac myocytes results in myocyte death and activation of the innate immune response, including interferon gamma, natural killer cells, and nitric oxide.13,14 Antigen-presenting cells phagocytize released viral particles and cardiac proteins and migrate out of the heart to regional lymph nodes. Most patients recover, but a subset has progression to a second phase, consisting of an adaptive immune response. In this response, antibodies to viral proteins, and to some cardiac proteins (including cardiac myosin and β1 or muscarinic receptors), are produced, and effector T cells proliferate. In the third phase, the immune response is down-regulated, and fibrosis replaces a cellular infiltrate in the myocardium. Under neurohumoral stimulation and hemodynamic stress, the ventricles dilate, leading to chronic cardiomyopathy. Additionally, in the third phase, viral genome may persist in the heart or inflammatory mechanisms may persist and contribute to ventricular dysfunction.6,15
CLINICAL PRESENTATION AND DIAGNOSIS
The clinical presentation of viral myocarditis varies from nonspecific electrocardiographic abnormalities and mild viral illness to acute hemodynamic compromise or sudden cardiac death. However, most patients are asymptomatic. In the European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases, 72% of patients had dyspnea, 32% had chest pain, and 18% had arrhythmias.16 The condition of some patients with acute focal myocarditis mimics a diagnosis of myocardial infarction, with acute onset of chest pain, tachyarrhythmia, or sudden death.17
Physical examination findings are variable but may provide insight into other underlying causes. The findings can include tachycardia, laterally displaced point of maximal impulse, soft S1 sounds, S3 or S4 gallop, lymphadenopathy (sarcoidosis), rash (hypersensitivity), polyarthritis, subcutaneous nodules, or erythema marginatum (acute rheumatic fever). Levels of cardiac biomarkers, including CK-MB, troponin I, and troponin T, are elevated in a minority of cases (indicating myocardial damage). However, one study found that only 35% of patients with suspected myocarditis had elevated troponin levels, providing a sensitivity of 53%.18 The serum concentration of troponin I is increased more frequently than that of CK-MB fractions in patients with acute myocarditis.15 Electrocardiography may show nonspecific ST-T wave changes, ST elevation mimicking acute myocardial infarction, or various degrees of blockade of the atrioventricular node. The presence of Q waves or bundle branch block is associated with increased rates of heart transplant or death.4,19
Echocardiography is an important component of the diagnostic work-up to establish left ventricular (LV) function and to rule out other causes of heart failure, such as valvular, congenital, or amyloid heart disease. Classic findings include global hypokinesis with or without pericardial effusion. Echocardiographic features suggestive of myocarditis are often nonspecific but can be helpful in identifying a fulminant course. Felker et al20 developed echocardiographic criteria to help differentiate between fulminant and acute myocarditis. Patients with fulminant myocarditis had near-normal LV diastolic dimensions; increased septal thickness at presentation was thought to be secondary to acute myocardial edema.20 In addition, right ventricular systolic function was found to be an independent predictor of death or myocardial transplant in patients with acute myocarditis.21 Coronary angiography usually reveals normal coronary arteries, although myocarditis may affect patients with coronary artery disease.
Recent advances in the diagnosis of myocarditis have centered on the development of newer technologies to more precisely identify cardiac inflammation. An article by Skouri et al22 reviewed noninvasive imaging studies for detecting myocardial inflammation. The importance of noninvasive cardiac imaging stems from the low sensitivity of the Dallas criteria in diagnosing myocarditis histologically. To diagnose myocarditis with 80% sensitivity, an estimated 17 endomyocardial biopsies are necessary, leading many experts to believe there is a real need for practical noninvasive imaging studies to aid in diagnosing and managing acute DCM.23
Cardiac MRI may be useful for diagnosing myocarditis associated with edema, hyperemia, or fibrosis sensitive sequences.24 Friedrich et al25 concluded that cardiovascular MRI has promise in diagnosing myocarditis and showed an evolution of contrast enhancement from focal to disseminated disease during a 2-week period. Newer techniques, including segmented inversion recovery gradient-echocardiography pulse sequences, have improved contrast enhancement of the myocardium and allowed visualization of small myocardial injuries, increasing the sensitivity of detecting active myocarditis.
In a study of 32 patients with suspected myocarditis, Mahrholdt et al26 found that contrast enhancement was present in 88% of patients, and biopsy samples from the area of enhancement showed active acute or chronic myocarditis in 90% of patients. The overall study findings concluded that focal myocardial gadolinium enhancement, coupled with regional wall motion abnormalities on echocardiography, yielded a positive predictive value of 71% and a negative predictive value of 100%. In a study by Yelgec et al27 that involved 20 patients with suspected myocarditis, 5 patients underwent endomyocardial biopsy, the specimens of which showed normal findings, and subsequent contrast-enhanced cardiac MRI revealed evidence of active myocarditis. This illustrates the potential of cardiac MRI to identify regions of myocarditis and to increase the sensitivity of endomyocardial biopsy.
Histologic examination of heart tissue is required to confirm the diagnosis of myocarditis. However, the utility of endomyocardial biopsy is limited because of sampling error from patchy inflammatory infiltrates and variability in observer interpretation.28 In a large case series, the sensitivity of endomyocardial biopsy was only 35% compared to a clinical criterion standard that included recovery of myocardial function.29 Immunostains for cell specific markers such as T lymphocytes (CD3) or macrophages (CD68) or human leukocyte antigens have a sensitivity of up to 50%, which is much better than routine histologic techniques.30,31 A recent case series suggests that the presence of inflammation as defined by immunoperoxidase stains may predict the subsequent risk of death or heart transplant.12 The presence of viral genomes in heart tissue from patients with acute myocarditis may predict adverse events. The absence of viral genomes in patients with chronic myocarditis may identify a subset of patients who will respond to a short course of immunosuppression.32,33
The current recommendations from an American College of Cardiology Foundation/American Heart Association/European Society of Cardiology (ACCF/AHA/ESC) scientific statement support a limited role for endomyocardial biopsy in the evaluation of patients with cardiomyopathy. The class I indications are limited to patients with new-onset heart failure (<2 weeks) associated with a normal or dilated left ventricle with hemodynamic compromise and to patients with new-onset heart failure of 2 weeks to 3 months' duration with a dilated left ventricle, ventricular arrhythmia, or high degree atrioventricular blockade or to patients whose condition fails to respond to treatment in 1 to 2 weeks.34
TREATMENT
The treatment of viral myocarditis varies by clinical presentation. Acute heart failure should be managed according to the current guidelines of the ACCF/AHA/ESC and the Heart Failure Society of America.35-38 Experimental models of murine myocarditis generally support the guideline-based treatment recommendations that apply to forms of noninflammatory DCM and that have been studied in clinical trials. Hemodynamically stable patients with DCM and symptomatic heart failure may benefit from angiotensin-converting enzyme inhibition or angiotensin receptor blockade. In euvolemic patients with DCM, β-adrenergic blockade may improve LV function, heart failure symptoms, and decrease inflammation. Patients who have persistent heart failure symptoms despite optimal management with angiotensin and adrenergic pathway inhibition may benefit from aldosterone antagonists, such as eplerenone or spironolactone. Diuretics should be used to optimize intravascular volume. The use of anticoagulation is similar to that in patients with nonischemic DCM, and anticoagulation is usually indicated in the setting of concomitant atrial fibrillation or arterial or venous thromboembolism. In patients with severe myocarditis and symptomatic hypotension, parenteral inotropes, including phosphodiesterase inhibitors (eg, milrinone) or adrenergic agonists (eg, dobutamine or dopamine) may be required.
Despite maximal oral and parenteral medical therapy, patients with acute myocarditis may require mechanical circulatory support. Data from case series suggest that ventricular assist devices may provide a bridge to transplant or to recovery in patients with acute myocarditis.39,40 Extracorporeal membrane oxygenation (ECMO) has also been used as a short-term bridge to transplant or recovery, but usually in patients with sustained ventricular arrhythmias, in whom support with ventricular assist devices would be less effective. In a case series, Chen et al41 reported that 80% of patients who received ECMO therapy were bridged to recovery.
Because patients generally present days to weeks after the initial viral infection, antiviral therapy has limited applicability in patients with acute viral myocarditis. Also, the sensitivity of endomyocardial biopsy for the diagnosis of viral genomes in the myocardium has not been reported. Nonetheless, antiviral agents have been evaluated for the treatment of acute myocarditis in animal models and in a few small case series. Ribavarin and interferon alpha improved survival in mice with acute myocarditis when administered at the time of virus inoculation.42,43 Antiviral therapy cannot be recommended for the treatment of acute myocarditis at this time; however, the role of antiviral therapy for more chronic myocarditis associated with persistent viral genomes is a matter of active clinical investigation.
A large body of experimental evidence suggests that acute and some chronic myocardial injury in myocarditis is due to an immune response involving T lymphocytes and autoreactive antibodies. However, data from the few randomized clinical trials suggest that on average patients with acute myocarditis do not benefit from immunosuppression. For example, the US Myocarditis Treatment Trial, in which 111 patients with histologically confirmed myocarditis were randomly assigned to placebo or to prednisone and either azathioprine or cyclosporine, showed no benefit in either transplant-free survival or change in LVEF.44 However, the duration of symptoms appears to be a major determinant of response to immunotherapy. A recent meta-analysis of immunosuppression and immunomodulation trials suggested that a symptom duration of less than 6 months was associated with a lack of active treatment benefit, and yet trials of DCM in which patients had symptoms for more than 6 months were generally positive. The difference in response was due largely to spontaneous improvement in the placebo arm participants who had symptoms for less than 6 months.45 In a trial of immunosuppression in patients with myocarditis and symptoms for more than 6 months, LVEF and New York Heart Association functional class improved after treatment with azathioprine and prednisone.46 In a recent trial of patients who had chronic myocarditis, no viral genomes, and symptomatic heart failure, treatment with azathioprine and prednisone also improved cardiac function and New York Heart Association functional class.34
After recovery from acute myocarditis, patients should be cautioned to refrain from aerobic activity for several months. Timing of resumption of aerobic exercise should be guided by the severity of acute injury and the degree of ongoing LV systolic dysfunction.47 For patients with ongoing systolic dysfunction, counseling on lifestyle modifications is vital, including a low-sodium diet, fluid restriction, and avoidance of nonsteroidal anti-inflammatory medications.
PROGNOSIS AND OUTCOME
The prognosis for patients with acute myocarditis varies and depends on clinical presentation, ejection fraction (EF), and pulmonary artery pressure.48,49 Several case reports and studies suggest that patients with fulminant myocarditis and hemodynamic compromise at presentation have better outcomes than those with acute nonfulminant myocarditis.50-53 In a prospective study, Mc Carthy et al54 used clinical features to classify patients with biopsy-proven myocarditis and found that 93% of those with fulminant myocarditis were alive at 11-year follow-up compared with 45% of those with acute nonfulminant myocarditis. The results of these studies have shown the necessity of early recognition of risk factors for fulminant myocarditis and subsequent aggressive early hemodynamic support. Lee et al55 conducted a retrospective study to identify which clinical risk factors predict a course of fulminant myocarditis. They found that, in general, the group of patients with fulminant myocarditis and acute myocarditis had higher pulse rates, lower blood pressure levels, higher C-reactive protein levels, higher cardiac biomarker levels, wider QRS complexes, and decreased LVEFs on admission compared with the nonfulminant group. Despite a higher in-hospital mortality rate in the fulminant group in the study by Lee et al,55 results of other studies have shown excellent long-term prognosis for patients who are treated with aggressive hemodynamic support.
Several mechanisms have been identified by which fulminant myocarditis results in persistent LV dysfunction less frequently than acute nonfulminant myocarditis. Kühl et al9 postulated that persistence of viral genome leads to chronic inflammation, thus diminishing the recovery of LV function. They found that, in patients with clearance of viral genome, EF improved from 50% to 58%, and in patients with persistence of viral genome, EF decreased from 54% to 51%, both findings are statistically significant. These results underscore the importance of immunohistochemical findings on endomyocardial biopsy that are suggestive of chronic inflammation as a negative prognostic indicator. In contrast with patients who have acute myocarditis, patients who have evidence of chronic inflammation may respond to immunomodulatory therapy.
CONCLUSION
Suspected viral myocarditis is an important cause of cardiomyopathy that presents diagnostic and therapeutic challenges. The initial evaluation should include electrocardiography, echocardiography, and often contrast-enhanced cardiac MRI. Patients with presentations suggestive of ischemia should usually undergo coronary angiography. Patients with ventricular tachycardia, hemodynamic instability, or high-grade atrioventricular block should usually undergo endomyocardial biopsy. Polymerase chain reaction techniques may facilitate precise viral genomic diagnosis, which may guide future therapies. Patients with a fulminant presentation should be aggressively supported therapeutically because outcomes are particularly favorable if they survive the initial injury.
All patients should receive standard heart failure care as outlined in the ACC/AHA/ESC, and Heart Failure Society of America guidelines. Ongoing trials of antiviral treatment such as the use of interferon beta may lead to the use of specific antiviral treatment in the future. Conversely, patients with chronic DCM and no evidence of viral genome in the heart tissue may benefit from immunosuppressive therapy if preliminary results are confirmed in randomized, controlled trials.
A recent viral infection should always be considered as a cause of acute DCM, being mindful that the spectrum of viruses that cause myocarditis continues to change. Cardiac MRI is useful for diagnosing acute myocarditis. Finally, endomyocardial biopsy is indicated in patients with hemodynamic compromise, heart block, or ventricular tachycardia or those whose condition fails to respond to standard care.
Supplementary Material
REFERENCES
- 1.Klingel K, Stephan S, Sauter M, et al. Pathogenesis of murine enterovirus myocarditis: virus dissemination and immune cell targets. J Virol 1996;70(12):8888-8895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klingel K, Sauter M, Bock CT, Szalay G, Schnorr JJ, Kandolf R. Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol. 2004May;193(2-3):101-117 Epub 2003 Aug 14 [DOI] [PubMed] [Google Scholar]
- 3.Fabre A, Sheppard MN. Sudden adult death syndrome and other nonischaemic causes of sudden cardiac death. Heart 2006March;92(3):316-320 Epub 2005 May 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnani JW, Danik HJ, Dec GW, Jr, DiSalvo TG. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151(2):463-470 [DOI] [PubMed] [Google Scholar]
- 5.Caforio AL, Calabrese F, Angelini A, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007June;28(11):1326-1333 Epub 2007 May 9 [DOI] [PubMed] [Google Scholar]
- 6.Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation 2006;113(6):876-890 [DOI] [PubMed] [Google Scholar]
- 7.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343(19):1388-1398 [DOI] [PubMed] [Google Scholar]
- 8.Dec GW, Jr, Palacios IF, Fallon JT, et al. Active myocarditis in the spectrum of acute dilated cardiomyopathies: clinical features, histologic correlates, and clinical outcome. N Engl J Med. 1985;312(14):885-890 [DOI] [PubMed] [Google Scholar]
- 9.Kühl U, Pauschinger M, Seeberg B, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 2005September27;112(13):1965-1970 Epub 2005 Sep 19 [DOI] [PubMed] [Google Scholar]
- 10.Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction: evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42(3):466-472 [DOI] [PubMed] [Google Scholar]
- 11.Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006October10;114(15):1581-1590 Epub 2006 Oct 2 [DOI] [PubMed] [Google Scholar]
- 12.Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation 2008August5;118(6):639-648 Epub 2008 Jul 21 [DOI] [PubMed] [Google Scholar]
- 13.Matsumori A, Yamada T, Suzuki H, Matoba Y, Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br Heart J. 1994;72(6):561-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaragoza C, Ocampo C, Saura M, et al. The role of inducible nitric oxide synthase in the host response to coxsackievirus myocarditis. Proc Natl Acad Sci U S A 1998;95(5):2469-2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowlton KU, Badorff C. The immune system in viral myocarditis: maintaining the balance [editorial]. Circ Res. 1999;85(6):559-561 [DOI] [PubMed] [Google Scholar]
- 16.Hufnagel G, Pankuweit S, Richter A, Schönian U, Maisch B. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID): first epidemiological results. Herz 2000;25(3):279-285 [DOI] [PubMed] [Google Scholar]
- 17.Dec GW, Jr, Waldman H, Southern J, Fallon JT, Hutter AM, Jr, Palacios I. Viral myocarditis mimicking acute myocardial infarction. J Am Coll Cardiol. 1992;20(1):85-89 [DOI] [PubMed] [Google Scholar]
- 18.Lauer B, Niederau C, Kühl U, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997;30(5):1354-1359 [DOI] [PubMed] [Google Scholar]
- 19.Morgera T, Di Lenarda A, Dreas L, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. 1992;124(2):455-467 [DOI] [PubMed] [Google Scholar]
- 20.Felker GM, Boehmer JP, Hruban RH, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36(1):227-232 [DOI] [PubMed] [Google Scholar]
- 21.Mendes LA, Picard MH, Dec GW, Hartz VL, Palacios IF, Davidoff R. Ventricular remodeling in active myocarditis: Myocarditis Treatment Trial. Am Heart J. 1999;138(2, pt 1):303-308 [DOI] [PubMed] [Google Scholar]
- 22.Skouri HN, Dec GW, Friedrich MG, Cooper LT. Noninvasive imaging in myocarditis. J Am Coll Cardiol. 2006;48(10):2085-2093 Epub 2006 Nov 1 [DOI] [PubMed] [Google Scholar]
- 23.Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64(10):1235-1245 [DOI] [PubMed] [Google Scholar]
- 24.Friedrich MG, Sechtem U, Schulz-Menger J, et al. International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53(17):1475-1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 1998;97(18):1802-1809 [DOI] [PubMed] [Google Scholar]
- 26.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 2004March;109(10):1250-1258 Epub 2004 Mar 1 [DOI] [PubMed] [Google Scholar]
- 27.Yelgec NS, Dymarkowski S, Ganame J, Bogaert J. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur Radiol. 2007September;17(9):2211-2217 Epub 2007 Mar 15 [DOI] [PubMed] [Google Scholar]
- 28.Baughman KL. Diagnosis of myocarditis: Death of Dallas criteria. Circulation 2006;113(4):593-595 [DOI] [PubMed] [Google Scholar]
- 29.Narula J, Khaw BA, Dec GW, et al. Diagnostic accuracy of antimyosin scintigraphy in suspected myocarditis. J Nucl Cardiol. 1996;3(5):371-381 [DOI] [PubMed] [Google Scholar]
- 30.Maisch B, Portig I, Ristic A, Hufnagel G, Pankuweit S. Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus: a status report. Herz 2000;25(3):200-209 [DOI] [PubMed] [Google Scholar]
- 31.Herskowitz A, Ahmed-Ansari A, Neumann DA, et al. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. J Am Coll Cardiol. 1990;15(3):624-632 [DOI] [PubMed] [Google Scholar]
- 32.Cooper LT. The heat is off: immunosuppression for myocarditis revisited [editorial]. Eur Heart J. 2009;30(16):1936-1939 [DOI] [PubMed] [Google Scholar]
- 33.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009;30(16):1995-2002 [DOI] [PubMed] [Google Scholar]
- 34.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Eur Heart J. 2007December;28(24):3076-3093 Epub 2007 Oct 24 [DOI] [PubMed] [Google Scholar]
- 35.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) [published correction appears in J Am Coll Cardiol. 2006;47(7):1503-1505] J Am Coll Cardiol. 2005;46(6):e1-e82 [DOI] [PubMed] [Google Scholar]
- 36.Heart Failure Society of America Executive summary: HFSA 2006 comprehensive heart failure practice guideline. J Card Fail 2006;12(1):10-38 [DOI] [PubMed] [Google Scholar]
- 37.Swedberg K, Cleland J, Dargie H, et al. Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26(11):1115-1140 Epub 2005 May 18 [DOI] [PubMed] [Google Scholar]
- 38.Jessup M, Abraham WT, Casey DE, et al. American College of Cardiology American Heart Association Task Force on Practice Guidelines (2009 Writing Committee to update the 2005 Guidelines for the Evaluation and Management of Heart Failure). 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009April14;119:1977-2016 Epub 2009 Mar 2006 [DOI] [PubMed] [Google Scholar]
- 39.Martin J, Sarai K, Schindler M, van de Loo A, Yoshitake M, Beyersdorf F. MEDOS HIA-VAD biventricular assist device for bridge to recovery in fulminant myocarditis. Ann Thorac Surg. 1997;63(4):1145-1146 [DOI] [PubMed] [Google Scholar]
- 40.Bohn D, Macrae D, Chang AC. Acute viral myocarditis: mechanical circulatory support. Pediatr Crit Care Med. 2006;7(6)(suppl):S22-S24 [Google Scholar]
- 41.Chen YS, Wang MJ, Chou NK, et al. Rescue for acute myocarditis with shock by extracorporeal membrane oxygenation. Ann Thorac Surg. 1999;68(6):2220-2224 [DOI] [PubMed] [Google Scholar]
- 42.Matsumori A, Crumpacker CS, Abelmann WH. Prevention of viral myocarditis with recombinant human leukocyte interferon alpha A/D in a murine model. J Am Coll Cardiol. 1987;9(6):1320-1325 [DOI] [PubMed] [Google Scholar]
- 43.Okada I, Matsumori A, Matoba Y, Tominaga M, Yamada T, Kawai C. Combination treatment with ribavarin and interferon for coxsackie B3 replication. J Lab Clin Med. 1992;120(4):569-573 [PubMed] [Google Scholar]
- 44.Mason JW, O'Connell JB, Herskowitz A, et al. Myocarditis Treatment Trial Investigators A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med. 1995;333(5):269-275 [DOI] [PubMed] [Google Scholar]
- 45.Stanton C, Mookadam F, Cha S, et al. Greater symptom duration predicts response to immunomodulatory therapy in dilated cardiomyopathy. Int J Cardiol. 2008;128(1)38-41 [DOI] [PubMed] [Google Scholar]
- 46.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation 2001;104(1):39-45 [DOI] [PubMed] [Google Scholar]
- 47.Maron BJ, Ackerman MJ, Nishimura RA, Pyeritz RE, Towbin JA, Udelson JE. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol. 2005;45(8):1340-1345 [DOI] [PubMed] [Google Scholar]
- 48.Starling RC, Cooper LT, Dec GW, et al. Left ventricular diameter predicts recovery in acute cardiomyopathy: results of the IMAC 2 Trial [abstract 3002]. Circulation 2007;116:II671-II672 [Google Scholar]
- 49.Magnani JW, Danik HJ, Dec GW, Jr, DiSalvo TG. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151(2):463-470 [DOI] [PubMed] [Google Scholar]
- 50.Rockman HA, Adamson RM, Dembitsky WP, Bonar JW, Jaski BE. Acute fulminant myocarditis: long-term follow-up after circulatory support with left ventricular assist device. Am Heart J. 1991;121(3, pt 1):922-926 [DOI] [PubMed] [Google Scholar]
- 51.Chang AC, Hanley FL, Weindling SN, Wernovsky G, Wessel DL. Left heart support with a ventricular assist device in an infant with acute myocarditis. Crit Care Med. 1992;20(5):712-715 [DOI] [PubMed] [Google Scholar]
- 52.Amabile N, Fraisse A, Bouvenot J, Chetaille P, Ovaert C. Outcome of acute fulminant myocarditis in children. Heart 2006September;92(9):1269-1273 Epub 2006 Jan 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asaumi Y, Yasuda S, Morii I, et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. 2005October;26(20):2185-2192 Epub 2005 Jul 13 [DOI] [PubMed] [Google Scholar]
- 54.McCarthy RE, III, Boehmer JP, Hruban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342(10):690-695 [DOI] [PubMed] [Google Scholar]
- 55.Lee CH, Tsai WC, Hsu CH, Liu PY, Lin LJ, Chen JH. Predictive factors of a fulminant course in acute myocarditis [letter]. Int J Cardiol. 2006;109(1):142-145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.