Abstract
At the most severe end of the spectrum of acute coronary syndromes is ST-segment elevation myocardial infarction (STEMI), which usually occurs when a fibrin-rich thrombus completely occludes an epicardial coronary artery. The diagnosis of STEMI is based on clinical characteristics and persistent ST-segment elevation as demonstrated by 12-lead electrocardiography. Patients with STEMI should undergo rapid assessment for reperfusion therapy, and a reperfusion strategy should be implemented promptly after the patient's contact with the health care system. Two methods are currently available for establishing timely coronary reperfusion: primary percutaneous coronary intervention and fibrinolytic therapy. Percutaneous coronary intervention is the preferred method but is not always available. Antiplatelet agents and anticoagulants are critical adjuncts to reperfusion. This article summarizes the current evidence-based guidelines for the diagnosis and management of STEMI. This summary is followed by a brief discussion of the role of noninvasive stress testing in the assessment of patients with acute coronary syndrome and their selection for coronary revascularization.
ACC = American College of Cardiology; ACE = angiotensin-converting enzyme; ACS = acute coronary syndrome; AHA = American Heart Association; APTT = activated partial thromboplastin time; ASSENT = Assessment of the Safety of a New Thrombolytic; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CCTA = coronary computed tomographic angiography; CHF = congestive heart failure; CLARITY = Clopidogrel as Adjunctive Reperfusion Therapy; ECG = electrocardiography; GP = glycoprotein; GUSTO = Global Utilization of Strategies to Open Occluded Arteries; HF = heart failure; ICH = intracerebral hemorrhage; IV = intravenous; LBBB = left bundle branch block; LMWH = low-molecular-weight heparin; LV = left ventricular; LVEF = LV ejection fraction; MI = myocardial infarction; NSTEMI = non—ST-segment elevation MI; NTG = nitroglycerin; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation MI; TIMI = Thrombolysis in Myocardial Infarction; UA = unstable angina; UFH = unfractionated heparin
Unlike unstable angina/non—ST-segment elevation myocardial infarction (UA/NSTEMI), ST-segment elevation myocardial infarction (STEMI) is characterized by total occlusion of the infarct-related artery. Evidence from several randomized clinical trials during the past 2 decades has established the importance of the open artery theory, which states that prompt and complete restoration of flow in the occluded artery decreases infarct size, preserves left ventricular (LV) function, and improves survival rates.1 Two types of strategies are currently available for the judicious establishment of coronary reperfusion: pharmacological (fibrinolysis) and mechanical (primary percutaneous coronary intervention [PCI]).2,3 Regardless of the mode of reperfusion, the overarching concept is to minimize total ischemic time, which is defined as the time from the onset of symptoms of STEMI to the initiation of reperfusion therapy. The 2004 STEMI guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA)4 and the 2007 focused update of these guidelines5 recommend that all patients with STEMI undergo rapid assessment for reperfusion therapy and that a reperfusion strategy be implemented promptly after the patient's contact with the medical system. The goal is to initiate fibrinolytic therapy within 30 minutes (door-to-needle time or first medical contact—to-needle time) and to achieve intracoronary balloon inflation within 90 minutes (door-to-balloon time or first medical contact—to-balloon time) of the patient's arrival at the hospital or first contact with the medical system.
REPERFUSION THERAPY
Fibrinolysis
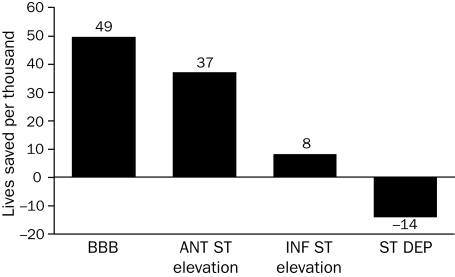
An overview of the results of 9 trials by the Fibrinolytic Therapy Trialists' Collaborative Group comparing the outcomes of patients undergoing fibrinolytic therapy and those of controls demonstrated statistically significant absolute reductions in 35-day mortality rates of approximately 30 per 1000 for patients who arrived at the hospital within 6 hours of the onset of symptoms and of approximately 20 per 1000 for patients who arrived 7 to 12 hours after the onset of symptoms.6 Benefit was observed among patients with ST-segment elevation or left bundle branch block (LBBB) at the time of presentation, irrespective of age, sex, blood pressure, heart rate, or a history of myocardial infarction (MI) or diabetes. The greatest benefit was observed among patients with LBBB or anterior STEMI (Figure 1).
FIGURE 1.
Effect of fibrinolytic therapy on mortality risk according to findings on admission electrocardiography. ANT ST = anterior ST-segment; BBB = bundle branch block; INF ST = inferior ST-segment; ST DEP = ST-segment depression.
From Lancet,6 with permission from Elsevier.
Fibrinolytic therapy is currently indicated, in the absence of contraindications (Table 1), for patients with STEMI who have experienced symptom onset within the previous 12 hours and in whom electrocardiography (ECG) demonstrates ST-segment elevation of more than 0.1 mV in at least 2 contiguous precordial leads or at least 2 adjacent limb leads, or new or presumably new LBBB.4
TABLE 1.
Contraindications and Cautions for Fibrinolysis as Treatment for Patients With ST-Segment Elevation Myocardial Infarction
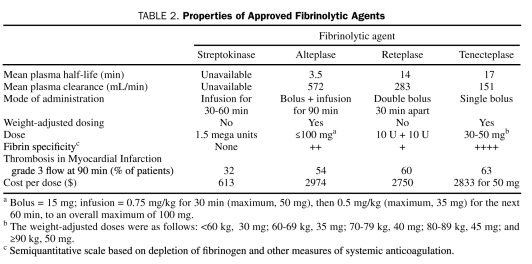
The fibrinolytic agents currently approved for treating patients with STEMI include streptokinase, alteplase, reteplase, and tenecteplase (Table 2). The TIMI (Thrombolysis in Myocardial Infarction), phase 1 trial randomly assigned 290 patients with evolving acute MI to alteplase (the first tissue plasminogen activator to be produced through recombinant DNA technology) or to streptokinase. Alteplase was far superior in achieving coronary reperfusion; twice as many occluded infarct-related arteries opened after 90 minutes with alteplase than with streptokinase.7 The GUSTO (Global Utilization of Strategies to Open Occluded Arteries)-I trial, which involved more than 40,000 patients, compared the clinical efficacy of accelerated alteplase with that of streptokinase.8 The 30-day mortality rates were significantly lower with accelerated alteplase (6.3%) than with streptokinase (7.3%; relative risk reduction, 14%; P=.001). Newer fibrin-specific lytic agents have longer half-lives and can be administered as bolus agents; although these agents have demonstrated no further improvements in survival, they offer the convenience of easier administration and can be delivered more rapidly in the emergency department. The GUSTO-III trial found no significant difference in 30-day mortality rates among patients treated with reteplase (7.47%) and those treated with accelerated alteplase (7.24%; P=.54).9 Tenecteplase therapy was assessed in the ASSENT (Assessment of the Safety of a New Thrombolytic)-2 trial, which randomly assigned 16,949 patients to weight-based single-bolus tenecteplase or to accelerated alteplase infusion.10 The 30-day mortality rates were virtually identical; this outcome met the predefined criteria for equivalence. As a single-bolus agent, tenecteplase has become the most widely used fibrin-specific agent.
TABLE 2.
Properties of Approved Fibrinolytic Agents
Primary PCI
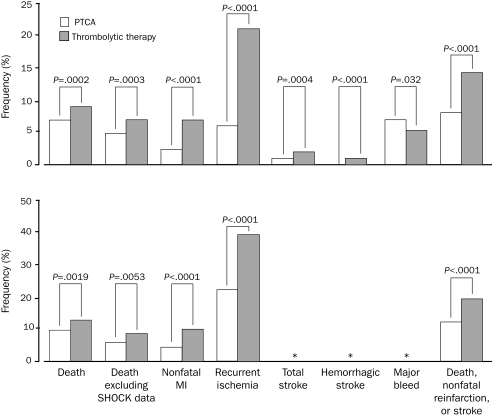
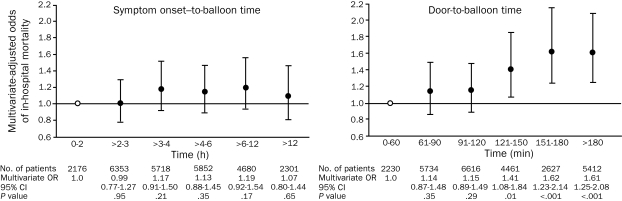
A meta-analysis of 23 randomized clinical trials that compared primary PCI with fibrinolytic therapy demonstrated that PCI was better than fibrinolysis in reducing the incidence of short-term and long-term adverse outcomes, including death (Figure 2).3 Although the clinical superiority of primary PCI is clear, the main challenge lies in the ability to implement such a strategy promptly (maintaining a first medical contact—to-balloon time of <90 minutes). A multivariate adjusted analysis found that, for patients undergoing primary PCI, increases in the door-to-balloon time (especially by >2 hours) were associated with higher mortality rates (Figure 3).11
FIGURE 2.
Short-term (top) and long-term (bottom) clinical outcomes of patients treated with either primary percutaneous transluminal coronary angioplasty (PTCA) or thrombolytic therapy. MI = myocardial infarction; SHOCK = Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock? An asterisk indicates that data are unavailable.
From Lancet,3 with permission from Elsevier.
FIGURE 3.
Relationship between time intervals and mortality in patients with ST-segment elevation myocardial infarction who are undergoing primary percutaneous coronary intervention. Error bars indicate 95% confidence intervals (CIs). Open circle indicates reference value. OR = odds ratio.
From JAMA,11 with permission. Copyright 2000, American Medical Association. All rights reserved.
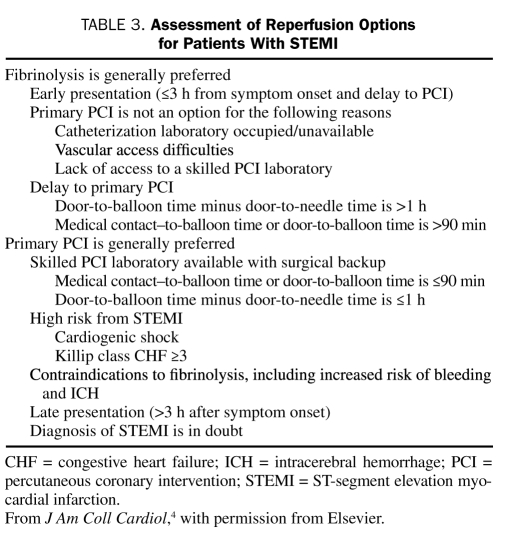
The ACC/AHA guidelines state that the selection of either fibrinolysis or primary PCI as the appropriate reperfusion strategy should depend on the clinical scenario (Table 3).4,5 Primary PCI, with or without stenting, is generally preferable if it is rapidly available because it yields better outcomes than fibrinolysis.
TABLE 3.
Assessment of Reperfusion Options for Patients With STEMI
Time Since Onset of Symptoms. The effectiveness of both fibrinolytic therapy and primary PCI diminishes with the passage of time12,13; however, the ability of PCI to produce a patent infarct-related artery is much less time-dependent.14 Thus, PCI is generally preferred for patients who arrive at the hospital late after the onset of symptoms (>3 hours). In contrast, clinical trials have shown that early initiation of fibrinolytic therapy (within the first 2-3 hours after the onset of symptoms) may lead to outcomes that are similar to or better than those achieved with PCI.15,16
Risk Related to STEMI. STEMI carries a continuum of risk, and multiple risk-prediction tools are available for estimating both short-term (4-6 weeks) and long-term (1-6 years) prognosis.17-19 In general, the higher the estimated risk of mortality with fibrinolysis, the greater the survival benefit of PCI.20 The SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock?) trial demonstrated that 1-year survival rates for patients with cardiogenic shock were better with early coronary revascularization (53%) than with no or late revascularization (66%).21 Observational data from the National Registry of Myocardial Infarction suggest that PCI is superior to fibrinolysis for patients with congestive heart failure (CHF) of Killip class II or higher.22
Availability of Skilled PCI Laboratory. The 2005 ACC/AHA/SCAI (Society for Coronary Angiography and Interventions) guideline update for PCI defines the conditions that must be met if physicians are to capitalize on the advantages of PCI. One of these conditions is the availability of a skilled PCI team, defined as one that performs at least 75 PCI procedures per year, at least 11 of which are primary PCI procedures for STEMI. Another condition is the availability of an appropriate laboratory, one in which at least 200 PCI procedures are performed annually, at least 36 of which are for STEMI.23 Additional requirements include the availability of cardiac surgery (rapid transfer is acceptable in some cases) and round-the-clock coverage with a skilled staff. If these criteria are not met, the time required to transport the patient to a skilled facility is a factor in determining the appropriate strategy. According to the ACC/AHA guidelines, fibrinolytic therapy is preferable to PCI if the estimated difference between the door-to-balloon time and the door-to-needle time is greater than 1 hour.4,5
Contraindications to Fibrinolysis. Primary PCI is the preferred strategy for patients with absolute or relative contraindications to fibrinolysis (Table 1). For patients who are at high risk of bleeding complications, especially intracerebral hemorrhage (ICH), PCI should be strongly considered. If PCI is unavailable, the benefit of fibrinolysis should be balanced against the risk of bleeding.
Prehospital Fibrinolytic Therapy
A meta-analysis of 6 randomized trials comparing prehospital and in-hospital fibrinolytic therapy for acute MI showed that prehospital fibrinolysis significantly decreased all-cause hospital mortality rates (odds ratio, 0.83; 95% confidence interval, 0.70-0.98).24 The estimated time to fibrinolysis was 104 minutes for the prehospital group and 162 minutes for the in-hospital fibrinolysis group (P=.007).
The CAPTIM (Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial Infarction) trial directly compared the outcomes of 840 patients treated with prehospital fibrinolysis or primary PCI within 6 hours of the onset of STEMI. The results showed no significant difference in the incidence of the primary end point (a composite of death, nonfatal reinfarction, and nonfatal disabling stroke) at 30 days between the group treated with prehospital fibrinolysis (8.2%) and those treated with primary PCI (6.2%).25
The ACC/AHA guidelines state that it is reasonable to initiate prehospital fibrinolytic therapy if a physician is present in the ambulance or if the emergency medical services system provides full-time paramedics who have the equipment necessary for performing and transmitting the results of 12-lead ECG (class IIa recommendation).4
ANTI-ISCHEMIC THERAPY
Nitroglycerin
Nitroglycerin (NTG) is intended primarily to relieve ischemic pain for patients with STEMI; it may also act as a vasodilator for patients with associated LV failure. The GISSI-3 (Third Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico) trial and the ISIS-4 (Fourth International Study of Infarct Survival) trial found that NTG had no beneficial effect on mortality rates among patients with suspected MI.26,27 The standard dose of NTG is 0.4 mg, taken sublingually every 5 minutes for a total of 3 doses. If this does not relieve chest discomfort, intravenous (IV) NTG can be administered.
Analgesia
The updated guidelines contain a class I recommendation for the use of morphine in managing the pain associated with STEMI5; however, for the pain associated with UA/NSTEMI, the recommendation for morphine has been downgraded to class IIa.
The administration of nonsteroidal anti-inflammatory drugs (both nonselective and cyclooxygenase-2 selective agents, except for aspirin) should be discontinued when a patient presents with STEMI, and these drugs should be avoided during the period of hospitalization because they are associated with cardiovascular risks.5
β-Blockers
β-Blockers should be administered to patients with STEMI regardless of the planned reperfusion strategy. β-Blockers decrease the rates of recurrent ischemia and reinfarction among patients receiving concomitant fibrinolytic therapy.28,29 In a recent study of patients treated with either fibrinolytic therapy or PCI, β-blockers substantially reduced the rates of all-cause mortality, cardiovascular mortality, and recurrent nonfatal MI.30
The 2007 update to the ACC/AHA STEMI guidelines recommends that oral β-blocker therapy be initiated within the first 24 hours after the onset of symptoms for all patients without contraindications such as heart failure (HF), evidence of a low-output state, increased risk of cardiogenic shock, or other relative contraindications to β-blockade, including a PR interval of more than 0.24 seconds, second- or third-degree heart block, active asthma, or reactive airway disease (class I).5 It is reasonable to administer IV β-blockers to patients who are hypertensive at the time of presentation (class IIa). Greater caution has been suggested with the early use of IV β-blockers, because the COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) trial31 found that their use was associated with an increased risk of cardiogenic shock among patients with any of the following features: age 70 years or older, systolic blood pressure lower than 120 mm Hg, sinus tachycardia of 110 beats/min or higher, heart rate lower than 60 beats/min, or increased time since the onset of symptoms of STEMI.
Calcium Channel Blockers
Verapamil substantially reduced the rates of mortality and major events among patients who had MI and were not in HF.32 Diltiazem was associated with a statistically significant reduction in cardiac events at 2 years among patients without pulmonary congestion.33 The ACC/AHA guidelines state that it is reasonable to give verapamil or diltiazem to patients with STEMI for whom β-blockers are ineffective or contraindicated for relief of ongoing ischemia or control of a rapid ventricular response (with atrial fibrillation or atrial flutter) in the absence of CHF, LV dysfunction, or atrioventricular block (class IIa recommendation).4 The dihydropyridine calcium antagonist nifedipine (immediate-release form) is contraindicated for patients with STEMI. The newer dihydropyridines (eg, amlodipine) have not been tested for patients with STEMI.
Inhibitors of the Renin-Angiotensin-Aldosterone System
Several large randomized trials of angiotensin-converting enzyme (ACE) inhibitors administered to patients with STEMI, as well as a meta-analysis, have found statistically significant decreases in mortality rates.26,27,34 The greatest reduction in the mortality rate occurred within the first 5 days after an MI; this finding underscores the importance of early treatment. The ACC/AHA guidelines recommend the administration of an oral ACE inhibitor within the first 24 hours after an MI to patients with anterior STEMI, pulmonary congestion, or an LV ejection fraction (LVEF) of less than 40%, in the absence of hypotension or other known contraindications, such as clinically relevant renal failure, a history of bilateral stenosis of the renal arteries, or known allergy to ACE inhibitors (class I). This treatment should also be considered for patients without these features (class II).4 An angiotensin receptor blocker should be administered to patients with STEMI who cannot tolerate ACE inhibitors and who have either clinical or radiologic signs of HF or an LVEF of less than 40% (class I).4
Magnesium
Although early studies suggested that magnesium might reduce the mortality rates associated with STEMI,35,36 later studies showed that it had no benefit.27,37 Intravenous magnesium is currently indicated only for patients with documented magnesium deficits and for those with torsades de pointes—type ventricular tachycardia.5
ANTITHROMBOTIC THERAPY
The goals of antithrombotic therapy for patients with STEMI are to establish and maintain patency of the infarct-related artery, limit the consequences of myocardial ischemia, enhance myocardial healing, and reduce the likelihood of recurrent events. These goals can be realized with a combination of antiplatelet and anticoagulant agents, which serve as ancillary therapy to reperfusion. Aspirin is the standard antiplatelet agent. Although the latest data on clopidogrel show benefit, glycoprotein (GP) IIb/IIIa inhibitors have shown no discernible benefit to date. The 2007 update to the ACC/AHA guidelines contains a class I recommendation stating that all patients with STEMI who are undergoing reperfusion therapy (including those who receive streptokinase) should receive an anticoagulant for a minimum of 48 hours and preferably for the duration of the index hospitalization, up to 8 days.5 Patients with STEMI have the choice of 3 anticoagulants: unfractionated heparin (UFH), enoxaparin, and fondaparinux. For patients undergoing PCI, bivalirudin is also an option. Regimens other than UFH are recommended if anticoagulant therapy is given for more than 48 hours because prolonged treatment with UFH is associated with the risk of heparin-induced thrombocytopenia. It is reasonable to administer anticoagulants to patients who are not undergoing reperfusion (class IIa recommendation).5
Aspirin
Aspirin should be given as early as possible to all patients with suspected STEMI, at a dose between 162 and 325 mg (to be chewed), and its administration should be continued indefinitely at a daily dose of 75 to 162 mg.4 The ISIS-2 (Second International Study of Infarct Survival) trial conclusively showed the efficacy of aspirin in reducing mortality rates among patients with evolving acute MI.38
Clopidogrel
Two recent studies have provided data about the efficacy of clopidogrel in enhancing pharmacological reperfusion for patients with STEMI.
The CLARITY (Clopidogrel as Adjunctive Reperfusion Therapy)-TIMI 28 trial randomly assigned 3491 patients aged 75 years or younger, who were treated with a standard fibrinolytic regimen and aspirin, to receive either clopidogrel (a 300-mg loading dose followed by 75 mg once daily) or placebo.39 Clopidogrel therapy was associated with a statistically significant reduction of 36% in the odds of the composite end point (an occluded infarct-related artery as demonstrated by angiography; death or recurrent MI before angiography) (clopidogrel, 15%; placebo, 21.7%; P<.001). By 30 days after the initiation of therapy, clopidogrel therapy was associated with a statistically significant reduction of 20% in the odds of the composite end point of death from cardiovascular causes, recurrent MI, or recurrent ischemia leading to the need for urgent revascularization (clopidogrel, 11.6%; placebo, 14.1%; P=.03). The rates of major bleeding and ICH were similar in the 2 groups.
In the COMMIT/CSS-2 (Second Chinese Cardiac Study) trial, 45,852 patients with suspected acute MI (93% had STEMI) were randomly allocated to 75 mg/d of clopidogrel or matching placebo.40 Treatment was to continue until discharge or for as long as 4 weeks in the hospital (mean, 15 days for survivors). Patients allocated to clopidogrel exhibited a statistically significant proportional reduction of 9% in the incidence of death, reinfarction, or stroke (clopidogrel, 9.2%; placebo, 10.1%; P=.002) and a statistically significant proportional reduction of 7% in the incidence of death due to any cause (clopidogrel, 7.5%; placebo, 8.1%; P=.03). No statistically significant increase in the incidence of major bleeding was noted with clopidogrel.
The 2007 update to the ACC/AHA guidelines states that 75 mg/d of oral clopidogrel should be added to aspirin for patients with STEMI, regardless of whether they undergo reperfusion with fibrinolytic therapy or do not receive reperfusion therapy (class I recommendation).5 It is reasonable to administer an oral loading dose of 300 mg of clopidogrel to patients aged 75 years or younger. Treatment with clopidogrel should continue for at least 14 days (class I recommendation); long-term maintenance therapy (eg, for 1 year) is reasonable (class IIa recommendation).5
Several recent studies have examined whether treatment with clopidogrel before PCI for patients with recent STEMI is superior to clopidogrel treatment initiated at the time of PCI in preventing major adverse cardiovascular events. The PCI-CLARITY study was a prospectively planned analysis of the 1863 patients who underwent PCI after mandated angiography in the CLARITY-TIMI 28 trial.41 Patients were randomly assigned to receive either clopidogrel or placebo initiated with fibrinolysis and given until coronary angiography, which was performed 2 to 8 days later. Patients undergoing coronary artery stenting were given open-label clopidogrel after diagnostic angiography. Overall, pretreatment with clopidogrel resulted in a statistically significant reduction of 41% in the incidence of cardiovascular death, MI, or stroke from the time of randomization through 30 days thereafter (7.5% vs 12.0%; odds ratio, 0.59; P=.001). No significant difference was found between groups in the rates of TIMI major or minor bleeding. Pretreatment with clopidogrel before PCI has been given a class I recommendation (level of evidence, A) by the ACC/AHA/SCAI (Society for Angiography and Interventions) PCI Guidelines.42,43 This recommendation is based on the results of the PCI-CLARITY trial, the PCI-CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial, the CREDO (Clopidogrel for the Reduction of Events During Observation) trial, and a meta-analysis, which showed that clopidogrel pretreatment was associated with substantial benefits in reducing cardiovascular end points.41
Glycoprotein IIb/IIIa Inhibitors
Glycoprotein IIb/IIIa inhibitors have been combined with fibrinolytic agents in an effort to improve the likelihood of achieving TIMI 3 flow. Although initial angiographic studies showed higher TIMI 3 flow rates at 60 to 90 minutes after the administration of these agents,44,45 later clinical studies showed an increase in the incidence of bleeding complications and no advantage in terms of mortality.
The GUSTO-V trial randomly assigned 16,588 patients within 6 hours of evolving STEMI to standard-dose reteplase or half-dose reteplase and full-dose abciximab.46 The rate of the primary end point of 30-day mortality was similar between the 2 groups. Nonfatal in-hospital reinfarction rates were lower in the combination therapy group, but these lower rates did not translate into a survival benefit at 1 year.47 Combination therapy was associated with a substantial increase in major bleeding complications and an increase in ICH rates among patients older than 75 years.
The ASSENT-3 trial randomly assigned 6095 patients with STEMI to 1 of 3 tenecteplase-based regimens: full-dose tenecteplase with UFH, full-dose tenecteplase with enoxaparin, or half-dose tenecteplase plus abciximab plus weight-adjusted, reduced-dose UFH.48 Like the GUSTO-V trial, this trial showed that the combination of abciximab and half-dose tenecteplase was not associated with lower mortality rates than was full-dose tenecteplase; however, this combination was associated with substantially lower rates of in-hospital infarction and refractory ischemia. Notably, the rate of major bleeding (other than ICH, the rates of which were similar in the 2 groups) was substantially higher in the abciximab group.
Thus, in the absence of a benefit in mortality rates, combination pharmacological reperfusion with GP IIb/IIIa inhibitors and a half-dose fibrinolytic agent is generally not recommended.4 The use of GP IIb/IIIa inhibitors for patients undergoing primary PCI is discussed in “Facilitated PCI.”
Unfractionated Heparin
Unfractionated heparin has been the mainstay of STEMI treatment for more than 40 years. One study found that the administration of smaller dose, weight-adjusted heparin to patients with STEMI who were treated with fibrinolysis resulted in similar rates of 30-day mortality, recurrent infarction, and ICH but in lower rates of major bleeding and refractory ischemia than did the administration of heparin at doses stratified by weight.49 Another study found that an activated partial thromboplastin time (APTT) within a range of 50 to 70 seconds was associated with the lowest 30-day rates of mortality, stroke, and bleeding and with fewer instances of refractory ischemia than was an APTT higher than 70 seconds.50 The 2007 update of the ACC/AHA guidelines recommends an initial UFH bolus of 60 U/kg (maximum, 4000 U), followed by an initial infusion of 12 U/kg/h (maximum, 1000 U/h) for 48 hours after fibrinolysis, with a target APTT of 1.5 to 2 times the upper limit of normal (approximately 50-70 seconds).5
Low-Molecular-Weight Heparin
A number of recent trials have compared low-molecular-weight heparin (LMWH) with UFH or placebo for patients with STEMI. The CREATE (Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment Evaluation) trial randomly assigned 15,570 patients with STEMI to the subcutaneous administration of either reviparin (not currently approved in the United States) or placebo twice daily for 7 days. The incidence of the primary composite outcome of death, MI, or stroke was significantly lower with reviparin at both 7 days (reviparin, 9.6%; placebo, 11%) and 30 days (reviparin, 11.8%; placebo, 13.6%).51 There was a small absolute increase in the risk of life-threatening bleeding, but the benefits of therapy outweighed the risks. In the CLARITY-TIMI 28 trial, which compared LMWH and UFH, treatment with LMWH was associated with a significantly lower rate of a closed infarct-related artery or death or MI before angiography (LMWH, 13.5%; UFH, 22.5%) and with a significantly lower rate of cardiovascular death or recurrent MI through 30 days (LMWH, 6.9%; UFH, 11.5%).52 The rates of ICH and of major bleeding through 30 days were similar in the 2 groups. The ASSENT-3 trial found that enoxaparin plus full-dose tenecteplase achieved a significantly better outcome than UFH plus full-dose tenecteplase; the rate of mortality, in-hospital reinfarction, or in-hospital refractory ischemia was 11.4% for the enoxaparin group and 15.4% for the UFH group (P=.0002).48 A meta-analysis of 14 trials involving 25,280 patients with STEMI found that, compared with placebo, LMWH (given for 4 to 8 days) decreased the rate of reinfarction by approximately 25% and the rate of death by approximately 10%.53 The administration of UFH during hospitalization did not prevent reinfarction or death. The EXTRACT (Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment)-TIMI 25 trial randomly assigned 20,506 patients with STEMI who were scheduled to undergo fibrinolysis to receive either enoxaparin throughout the index hospitalization or weight-based UFH for at least 48 hours.54 The incidence of the primary end point of death or MI through 30 days was 12.0% for the UFH group and 9.9% for the enoxaparin group (relative risk reduction, 17%; P<.001). The incidence of major bleeding was higher with the enoxaparin strategy (2.1% vs 1.4%), but the net clinical benefit clearly favored enoxaparin over UFH.
Fondaparinux
The clinical efficacy and safety of fondaparinux was evaluated in the OASIS (Organization for the Assessment of Strategies for Ischemic Syndromes)-6 trial, which randomly assigned 12,092 patients with STEMI to receive either treatment with fondaparinux (2.5 mg/d, started early and given for up to 8 days) or usual care (placebo for those for whom UFH was not indicated, or UFH for up to 48 hours followed by placebo for up to 8 days).55 The composite end point of death or reinfarction at 30 days was significantly lower for patients treated with fondaparinux (9.7%) than for patients in the control group (11.2%; hazard ratio, 0.86; P=.008), with an absolute risk reduction of 1.5%. Overall, bleeding was not increased, with a tendency toward fewer bleeding complications with fondaparinux. Patients undergoing primary PCI, however, exhibited a trend toward a higher rate of death or MI with fondaparinux (2.5-5.0 mg administered intravenously) than with UFH. The number of patients with catheter thrombosis was 0 in the UFH group and 22 in the fondaparinux group (P<.001). The 2007 update of the ACC/AHA guidelines states that fondaparinux should not be used as the sole anticoagulant during PCI but should be coupled with an additional agent that has anti-IIa activity (such as UFH or bivalirudin) so that the risk of catheter complications can be ameliorated.5
Bivalirudin
The HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial randomly assigned 3602 patients with STEMI who were undergoing primary PCI to treatment with UFH plus a GP IIb/IIIa inhibitor or to treatment with bivalirudin alone.56 The incidence of the primary end point of net adverse clinical events, defined as death, MI, ischemic target vessel revascularization, stroke, or major bleeding at 30 days, was 24% lower in the group treated with bivalirudin alone (9.2%) than in the group receiving UFH plus a GP IIb/IIIa inhibitor (12.1%; P=.005), primarily because of a 40% reduction in the rate of major bleeding (bivalirudin group, 4.9%; UFH group, 8.3%; P<.001). The incidence of major adverse cardiac events was similar in the 2 groups (bivalirudin group, 5.4%; UFH group, 5.5%; P>.99). After a 1-year follow-up period, the rate of cardiac-related mortality was 43% lower and the rate of all-cause mortality was 31% lower (absolute reductions, 1.7% and 1.4%) in the group receiving bivalirudin monotherapy than in the group receiving UFH plus GP IIb/IIIa inhibitors. Although the risk of acute stent thrombosis within 24 hours was higher in the bivalirudin group, no statistically significant difference was seen between the groups at 30 days and at 1 year. Thus, for patients with STEMI who are undergoing primary PCI, administering bivalirudin alone appears to reduce major bleeding complications, decrease cardiac mortality rates, and improve overall survival rates.
FACILITATED PCI
Facilitated PCI refers to a strategy of planned immediate PCI after the administration of an initial pharmacological regimen aimed at improving the patency of coronary arteries before the procedure. Such regimens have included GP IIb/IIIa inhibitors, full-dose or reduced-dose fibrinolytic therapy, and the combination of a GP IIb/IIIa inhibitor and a reduced-dose fibrinolytic agent. Facilitated PCI is an attempt to capitalize on the timeliness of pharmacological reperfusion and the superior outcome of PCI. Despite the potential advantages of this strategy, clinical trials of facilitated PCI have not shown any benefit in improving outcomes.
The ASSENT-4 PCI trial randomly assigned 1667 patients with STEMI of less than 6 hours' duration to standard PCI or PCI preceded by the administration of full-dose tenecteplase.57 The trial was terminated prematurely because the in-hospital mortality rate was significantly higher in the facilitated PCI group (6%) than in the standard PCI group (3%; P=.01). The primary end point, a composite of death, shock, and CHF within 90 days, was significantly higher with facilitated PCI (18.6%) than with primary PCI (13.4%; P=.0045). Patients assigned to facilitated PCI also experienced more strokes and ischemic complications than those assigned to primary PCI.
A quantitative review of 17 trials showed that, compared with primary PCI, facilitated PCI was associated with substantially higher rates of short-term mortality, nonfatal reinfarction, urgent target-vessel revascularization, total and hemorrhagic stroke, and major bleeding.58 The increased rates of adverse events with the facilitated approach were seen mainly in regimens based on fibrinolytic therapy, whereas no significant differences were observed in efficacy or safety between primary PCI and PCI facilitated with a GP IIb/IIIa inhibitor.
The FINESSE (Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events) trial randomly assigned patients (in a 1:1:1 fashion) to early administration of combination therapy with reduced-dose reteplase and abciximab (n=828), early administration of abciximab alone followed by PCI (n=818), or abciximab alone administered just before PCI (n=806). The primary end point was the composite of all-cause mortality or complications after MI within 90 days.59 On arrival at the catheterization laboratory, the percentage of patients with an open artery before PCI was higher in the reteplase plus abciximab group (61%) than in the abciximab group (26%) or the primary PCI group (25%; P<.001). The percentage of patients experiencing the primary end point was 9.8% in the combination-facilitated PCI group, 10.5% in the abciximab-facilitated PCI group, and 10.7% in the primary PCI group (P=.55). TIMI major or minor bleeding occurred more often in the combination-facilitated PCI group than in either the abciximab-facilitated PCI group or the primary PCI group (P<.001).
The 2007 update of the STEMI guidelines gives a class IIb recommendation (level of evidence, C) to the selective use of a facilitated strategy with regimens other than full-dose fibrinolytic therapy for subgroups of high-risk patients when PCI is not available within 90 minutes, provided the risk of bleeding is low.5
ASSESSMENT OF REPERFUSION
Early assessment of reperfusion is essential for determining the success of therapy. Although angiographic assessment of epicardial flow has been the criterion standard for determining the success of reperfusion, such an assessment is currently considered inadequate because studies have shown that microvascular perfusion may be impaired even when TIMI grade 3 flow and less than 50% coronary narrowing have been achieved.60,61 Furthermore, techniques that are more readily available and noninvasive are needed for assessing the early success of pharmacological reperfusion.
One such simple and readily available technique is evaluation of ECG ST-segment resolution. A resolution of more than 50% of ST-segment elevation at 60 to 90 minutes after the initiation of therapy is a good indicator of improved myocardial perfusion and is associated with enhanced recovery of LV function, reduced infarct size, and improved prognosis.60-65
According to the ACC/AHA guidelines, it is reasonable to monitor the pattern of ST-segment elevation, cardiac rhythm, and clinical symptoms during the 60 to 180 minutes after the initiation of fibrinolytic therapy (class IIa recommendation).4 Noninvasive findings suggestive of reperfusion include relief of symptoms, maintenance or restoration of hemodynamic or electrical stability or both, and a reduction of at least 50% in the initial ST-segment elevation injury pattern as demonstrated by follow-up ECG 60 to 90 minutes after the initiation of therapy. In contrast, persistence of unrelenting ischemic chest pain, absence of resolution of the qualifying ST-segment elevation, and hemodynamic or electrical instability are generally indicators of failed pharmacological reperfusion and the need to consider rescue PCI.
IMMEDIATE OR EMERGENCY INVASIVE STRATEGY AND RESCUE PCI
Rescue PCI is defined as PCI within 12 hours after failed fibrinolysis for patients with continuing or recurrent myocardial ischemia. One early study, the RESCUE (Randomized Evaluation of Salvage Angioplasty with Combined Utilization of Endpoints) trial, demonstrated a lower mortality rate and decreased frequency of a composite end point of death or CHF at 30 days when PCI was performed within 8 hours after the onset of symptoms for patients with anterior STEMI for whom fibrinolytic therapy had failed.66
More recently, the MERLIN (Middlesbrough Early Revascularization to Limit Infarction) trial, the REACT (Rescue Angioplasty Versus Conservative Treatment or Repeat Thrombolysis) trial, and 3 meta-analyses have reaffirmed the benefits of rescue PCI.67-71 The REACT trial involved 427 patients with acute MI for whom thrombolysis had failed (as determined by <50% resolution of ST-segment elevation on ECG at 90 minutes). Within 6 hours of pain onset, these patients were randomly assigned to repeated thrombolysis, conservative treatment, or rescue PCI.68 The primary end point was a composite of death, reinfarction, stroke, or severe HF within 6 months. The rate of event-free survival was 84.6% for patients treated with rescue PCI, 70.1% for those receiving conservative therapy, and 68.7% for those undergoing repeated thrombolysis (overall, P=.004). The 2007 update of the STEMI guidelines gives a class I recommendation to a strategy of coronary angiography with intent to perform PCI (or emergency coronary artery bypass grafting [CABG]) for patients with STEMI (aged <75 years) who have received fibrinolytic therapy and have cardiogenic shock, severe CHF (Killip class III), or hemodynamically compromising ventricular arrhythmias.5 It is reasonable to perform rescue PCI for patients with hemodynamic or electrical instability, persistent ischemic symptoms, or a moderate or large area of myocardium at risk (class IIa recommendation).5
PCI AFTER SUCCESSFUL FIBRINOLYSIS OR FOR PATIENTS NOT UNDERGOING PRIMARY REPERFUSION
The “late open artery” hypothesis suggested that late patency of an infarct artery is associated with improved LV function, increased electrical stability, and the provision of collateral vessels to other coronary beds for protection against future events. The Occluded Artery Trial randomly assigned 2166 patients with a totally occluded infarct artery 3 to 28 days after MI (approximately 20% of whom received fibrinolytic therapy) to optimal medical therapy and PCI with stenting or to optimal medical therapy alone.72,73 The 4-year cumulative end point (composite of death, reinfarction, or class IV HF) was 17.2% in the PCI group and 15.6% in the medical therapy group (hazard ratio, 1.16; 95% confidence interval, 0.92-1.45; P=.20). Reinfarction rates tended to be higher in the PCI group, and this difference may have attenuated any benefit in LV remodeling.
The 2007 update of the STEMI guidelines contains a class IIb recommendation for PCI of a hemodynamically significant stenosis in a patent infarct artery more than 24 hours after STEMI.4 Percutaneous coronary intervention of a totally occluded infarct artery more than 24 hours after STEMI is not recommended for patients with asymptomatic 1- or 2-vessel disease if they are hemodynamically and electrically stable and they exhibit no evidence of severe ischemia.
NONINVASIVE TESTING
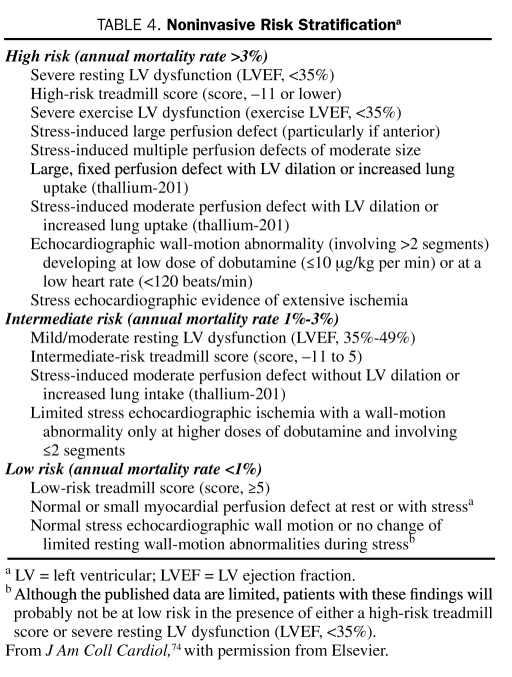
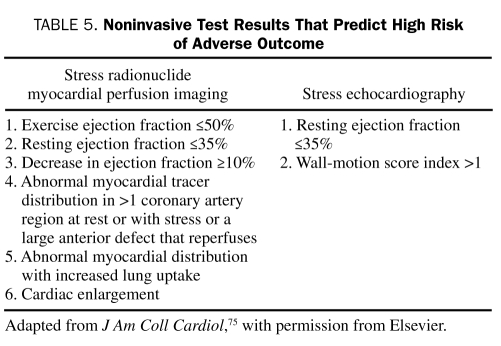
The goals of noninvasive testing are to determine whether ischemia is present in patients with a low or intermediate likelihood of coronary artery disease (CAD) and to estimate prognosis. High-risk patients, including those with refractory angina, hemodynamic compromise, or severe LV dysfunction, should be immediately considered for early coronary angiography and revascularization because noninvasive risk stratification would probably not differentiate a subgroup of patients with risk low enough to allow avoidance of coronary angiography. The results of noninvasive tests and the corresponding approximate mortality rates are shown in Table 4. Table 5 lists results that predict a high risk of future cardiac events. These results are derived from studies involving patients with all types of CAD. The markers of high risk, as shown, are either evidence of ischemia or LV dysfunction (either at rest or stress-induced).74,76,77 Recent recurrence of ischemic rest pain, especially if associated with ECG changes or other signs of instability, is a contraindication to stress testing.
TABLE 4.
Noninvasive Risk Stratificationa
TABLE 5.
Noninvasive Test Results That Predict High Risk of Adverse Outcome
TIMING OF NONINVASIVE TEST
Noninvasive stress testing is recommended for low- or intermediate-risk patients with UA/NSTEMI who have been free of ischemia at rest (or with low-level activity) and free of HF for a minimum of 12 to 24 hours.78 One study found that, although the diagnostic and prognostic values of the results of symptom-limited exercise tests performed before discharge and after 1 month in men with UA/NSTEMI were similar, the earlier test identified patients who experienced adverse events during the first month, and this was the time during which approximately half of all events occurred during the first year.79 These findings illustrate the importance of early noninvasive testing for risk stratification.
The optimal time for performing stress testing after STEMI has not yet been determined. The 2004 ACC/AHA STEMI guidelines recommend that noninvasive stress testing be performed either in the hospital or early after discharge for stable patients who have not undergone coronary angiography.4
SELECTION OF NONINVASIVE TEST
The ACC/AHA guidelines state that the choice of stress test should be based on the results of resting ECG, the ability to perform exercise, local expertise, and the technologies available.78 Exercise treadmill testing is recommended as the first-line test of choice for patients who can exercise and whose ECG results are free of baseline ST-segment abnormalities, such as resting ST-segment depression (≥0.10 mV), LV hypertrophy, bundle branch block, pre-excitation, or digoxin effect. If ST-segment abnormalities exist, an imaging modality such as nuclear imaging (single-photon emission computed tomography with either thallium-201 or technetium Tc 99m compounds as the radioactive tracer) or echocardiographic imaging should be added, and the exercise tolerance component of the test should be preserved because this test provides valuable and important prognostic information.80 Pharmacological stress testing (using vasodilators such as adenosine or adrenergic stimulators such as dobutamine for patients with asthma) with imaging is recommended when physical limitations (eg, arthritis, amputation, severe peripheral vascular disease, severe chronic obstructive pulmonary disease, or general debility) preclude adequate exercise stress. Although stress myocardial perfusion imaging or stress echocardiographic imaging is slightly more sensitive than ECG stress testing alone and has greater prognostic value,81,82 it is generally cost-effective only for higher-risk patients.83 Neither single-photon emission computed tomography nor echocardiography has proved superior for the purpose of risk stratification for patients with acute coronary syndrome (ACS). Resting echocardiography can, however, evaluate local and global LV function and can detect complications of MI, such as mural thrombi, aneurysms, valvular dysfunction, ventricular septal rupture, and pericardial effusion. For women, the optimal testing strategy is less well defined than that for men, but evidence suggests that imaging studies are superior to exercise ECG evaluation.84
Cardiac magnetic resonance imaging and coronary computed tomographic angiography (CCTA) are newer imaging modalities that hold promise as alternative or supplementary imaging modalities for assessing patients who present with chest pain syndromes.85-87 Cardiac magnetic resonance imaging can assess cardiac function, perfusion, and viability in the same setting. Coronary computed tomographic angiography provides valuable anatomic information and has a high negative predictive value; that is, if no evidence of either calcified or noncalcified (soft/fibrous) plaque is found, then it is highly unlikely that the patient's symptoms are the result of ACS.85,86,88 The disadvantages of CCTA are exposure to a high dose of radiation (8-24 mSv) and the absence of a functional or physiologic assessment. The 2007 ACC/AHA UA/NSTEMI guidelines recommend CCTA as a reasonable alternative to stress testing for patients with negative results from 12-lead ECG and cardiac biomarker tests and a low or intermediate probability of CAD (class IIa recommendation).
Patients with definite ACS who are not scheduled for coronary angiography and left ventriculography should have their LV function assessed by echocardiography or another imaging modality because LV function is a powerful determinant of prognosis and greatly affects therapeutic options.89,90
SELECTION FOR CORONARY ANGIOGRAPHY
The results of stress testing should be discussed with the patient (or family) and used to help determine the advisability of coronary angiography. Patients for whom noninvasive stress testing yields high-risk findings should be referred for coronary angiography and revascularization. The VANQWISH (Veterans Affairs Non-Q-Wave Infarction Strategies In-Hospital) trial used the results of symptom-limited thallium exercise treadmill testing at 3 to 5 days after MI to direct the need for angiography among 442 patients with non—Q-wave MI who were randomly assigned to an early conservative treatment strategy.91 Among patients who met the VANQWISH stress test criteria for coronary angiography, 51% were found to have surgical CAD and showed favorable outcomes after revascularization.92 Similarly, a benefit of revascularization was found for patients with ischemia demonstrated by stress testing after thrombolytic therapy for STEMI.93 Angiography provides detailed structural information that helps in determining prognosis and directing further revascularization plans (either PCI or CABG); when combined with LV angiography, it allows an assessment of global and regional LV function.
CORONARY REVASCULARIZATION
Coronary revascularization (PCI or CABG) is performed to improve prognosis, relieve symptoms, prevent ischemic complications, and improve functional capacity. Important factors that must be considered when proceeding from diagnostic angiography to revascularization are coronary anatomy, ventricular function, anticipated life expectancy, comorbid conditions, functional capacity, severity of symptoms, and quantity of viable myocardium at risk. Because of differences in the underlying pathophysiology, the indications for coronary angiography and revascularization differ for patients with UA/NSTEMI and those with STEMI.
PERCUTANEOUS CORONARY INTERVENTION
The term PCI refers to a family of percutaneous techniques that includes percutaneous transluminal coronary angioplasty, intracoronary stenting, and atheroablative technologies such as atherectomy. Currently, 80% to 85% of PCIs involve balloon dilation and coronary stenting, whereas other devices are used for specific lesions and patient subsets. Metal stents provide the advantage of maintaining the patency of stenosed or occluded arteries and preventing restenosis. However, they constitute a powerful thrombogenic and atherogenic nidus in the vessel wall and can lead to subacute stent thrombosis and chronic complications of in-stent restenosis caused by neointimal hyperplasia and the proliferation of smooth muscle cells. To counteract this problem, investigators developed drug-eluting stents that are coated with immune modulators (most commonly sirolimus or paclitaxel). These stents arrest the cell cycle and limit local smooth muscle proliferation, thereby dramatically reducing in-stent restenosis and target vessel revascularization.94 The same mechanism delays the protective process of stent endothelialization and leads to increased rates of subacute stent thrombosis. This makes it necessary to administer thienopyridine therapy for at least 1 year and preferably longer. The 2 most commonly used drug-eluting stents are the sirolimus-coated stent and the paclitaxel-coated stent; studies have shown that these stents are equally effective.95,96
CABG VS PCI
Coronary artery bypass grafting is recommended by both the 2007 UA/NSTEMI guidelines and the 2004 STEMI guidelines as the preferred revascularization strategy for patients with significant left main disease (>50% stenosis) or with 3-vessel or 2-vessel disease with significant proximal stenosis of the left anterior descending artery and abnormal LV function (LVEF <50 %) (class I recommendation).4,78 The 2007 UA/NSTEMI guidelines also state that it is reasonable to perform CABG with the internal mammary artery for patients with multivessel disease and treated diabetes mellitus (class IIa recommendation).78 In the absence of these features, either PCI (with suitable coronary anatomy) or CABG is recommended for patients with single or multivessel coronary disease who have a large or moderate area of viable myocardium and who exhibit high-risk criteria on noninvasive testing.4,78
Most of the large trials on which the current recommendations for PCI or CABG are based, however, used balloon angioplasty rather than stenting. Several recent trials have compared the outcomes achieved with PCI using stents with those achieved by CABG for patients with multivessel coronary disease. The SoS (Stent or Surgery) trial was a randomized, controlled trial comparing the outcomes achieved by these 2 procedures for 988 patients with multivessel disease. Both the initial results at a median follow-up of 2 years97 and the 6-year results98 showed a survival advantage for patients randomly assigned to CABG. In contrast, the results of a meta-analysis of 4 randomized trials that compared the outcomes achieved by CABG (n=1533) with those achieved by PCI with multiple stenting using bare-metal stents (n=1518) for patients with multisystem disease showed no statistically significant difference between the 2 groups in the primary composite end point of death, MI, and stroke or in mortality rates at 1 year after the initial procedures.99 The need for repeat revascularization procedures, however, remained high after PCI.
The most recent trial, SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery), randomly assigned 1800 patients with previously untreated 3-vessel or left main CAD (or both) to either PCI with drug-eluting stents or CABG.100 The rate of the primary end point (death from any cause, stroke, MI, or repeat revascularization) was significantly higher for the PCI group (17.8%) than for the CABG group (12.4%; P=.002), primarily because of a higher rate of repeat revascularization (PCI, 13.5%; CABG, 5.9%; P<.001). As a result, the criterion for noninferiority was not met. At 12 months, the rates of death and MI were similar between the 2 groups. Continuous improvements in the design and composition of drug-eluting stents101 and advances in PCI technology and adjunctive therapy may render PCI equivalent to CABG for patients such as these with complex anatomy and advanced disease.
CONCLUSION
The past decade has seen enormous advances in antithrombotic therapies and catheter-based coronary interventions, and these advances have dramatically improved the outlook for patients with ACS. Patients with STEMI require immediate reperfusion therapy with either primary PCI or fibrinolysis. Primary PCI is generally preferred if intracoronary balloon inflation can be achieved within 90 minutes after the first medical contact. Cardiac stress testing has become an increasingly sophisticated and important tool for the noninvasive evaluation of patients with ACS. The debate about whether to use CABG or PCI continues, and several large randomized trials are ongoing with the goal of establishing the superiority of either revascularization strategy over the other for patients with multivessel disease.
Supplementary Material
On completion of this article, you should be able to: (1) identify the optimal reperfusion strategy for ST-segment elevation myocardial infarction in a given clinical scenario and recognize the importance of adjunctive pharmacological therapies, (2) list the various stress test methods for detection of significant coronary artery stenoses, and (3) discuss the relative risks and benefits of percutaneous coronary intervention and coronary artery bypass grafting.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Braunwald E. The open-artery theory is alive and well—again [editorial]. N Engl J Med. 1993;329(22):1650-1652 [DOI] [PubMed] [Google Scholar]
- 2.Boersma E, Mercado N, Poldermans D, Gardien M, Vos J, Simoons ML. Acute myocardial infarction. Lancet 2003;361(9360):847-858 [DOI] [PubMed] [Google Scholar]
- 3.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361(9351):13-20 [DOI] [PubMed] [Google Scholar]
- 4.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). J Am Coll Cardiol. 2004;44(3):E1-E211 [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, Hand M, Armstrong PW, et al. 2004 Writing Committee 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2008;117(6):e162] Circulation 2008January15;117(2):296-329 Epub 2007 Dec 10 [DOI] [PubMed] [Google Scholar]
- 6.Fibrinolytic Therapy Trialists' (FTT) Collaborative Group Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients [published correction appears in Lancet. 1994;343(8899):742] Lancet 1994;343(8893):311-322 [PubMed] [Google Scholar]
- 7.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase: clinical findings through hospital discharge. Circulation 1987;76(1):142-154 [DOI] [PubMed] [Google Scholar]
- 8.GUSTO Investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329(10):673-682 [DOI] [PubMed] [Google Scholar]
- 9.Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO III) Investigators A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med. 1997;337(16):1118-1123 [DOI] [PubMed] [Google Scholar]
- 10.Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet 1999;354(9180):716-722 [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA 2000;283(22):2941-2947 [DOI] [PubMed] [Google Scholar]
- 12.Zeymer U, Tebbe U, Essen R, Haarmann W, Neuhaus KL, ALKK-Study Group Influence of time to treatment on early infarct-related artery patency after different thrombolytic regimens. Am Heart J. 1999;137(1):34-38 [DOI] [PubMed] [Google Scholar]
- 13.De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004March16;109(10):1223-1225 Epub 2004 Mar 8 [DOI] [PubMed] [Google Scholar]
- 14.Brodie BR, Stuckey TD, Wall TC, et al. Importance of time to reperfusion for 30-day and late survival and recovery of left ventricular function after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 1998;32(5):1312-1319 [DOI] [PubMed] [Google Scholar]
- 15.Steg PG, Bonnefoy E, Chabaud S, et al. Comparison of Angioplasty and Prehospital Thrombolysis In acute Myocardial infarction (CAPTIM) Investigators Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation 2003;108(23):2851-2856 Epub 2003 Nov 17 [DOI] [PubMed] [Google Scholar]
- 16.Widimský P, Budesinský T, Vorác D, et al. ‘PRAGUE’ Study Group Investigators Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction: final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J. 2003;24(1):94-104 [DOI] [PubMed] [Google Scholar]
- 17.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000;102(17):2031-2037 [DOI] [PubMed] [Google Scholar]
- 18.Morrow DA, Antman EM, Giugliano RP, et al. A simple risk index for rapid initial triage of patients with ST-elevation myocardial infarction: an In-TIME II substudy. Lancet 2001;358(9293):1571-1575 [DOI] [PubMed] [Google Scholar]
- 19.Krumholz HM, Chen J, Wang Y, Radford MJ, Chen YT, Marciniak TA. Comparing AMI mortality among hospitals in patients 65 years of age and older: evaluating methods of risk adjustment. Circulation 1999;99(23):2986-2992 [DOI] [PubMed] [Google Scholar]
- 20.Kent DM, Schmid CH, Lau J, Selker HP. Is primary angioplasty for some as good as primary angioplasty for all? J Gen Intern Med. 2002;17:887-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochman JS, Sleeper LA, White HD, et al. SHOCK Investigators One-year survival following early revascularization for cardiogenic shock. JAMA 2001;285(2):190-192 [DOI] [PubMed] [Google Scholar]
- 22.Wu AH, Parsons L, Every NR, Bates ER. Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2). J Am Coll Cardiol. 2002;40:1389-1394 [DOI] [PubMed] [Google Scholar]
- 23.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation 2006;113(1):156-175 [DOI] [PubMed] [Google Scholar]
- 24.Morrison LJ, Verbeek PR, McDonald AC, Sawadsky BV, Cook DJ. Mortality and prehospital thrombolysis for acute myocardial infarction: a meta-analysis. JAMA 2000;283(20):2686-2692 [DOI] [PubMed] [Google Scholar]
- 25.Bonnefoy E, Lapostolle F, Leizorovicz A, et al. Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial Infarction (CAPTIM) Study Group Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: a randomised study. Lancet 2002;360(9336):825-829 [DOI] [PubMed] [Google Scholar]
- 26.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico GISSI-3: effect of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet 1994;343(8906):1115-1122 [PubMed] [Google Scholar]
- 27.ISIS-4 Collaborative Group ISIS-4: randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet 1995;345(8951):669-685 [PubMed] [Google Scholar]
- 28.Roberts R, Rogers WJ, Mueller HS, TIMI Investigators Immediate versus deferred β-blockade following thrombolytic therapy in patients with acute myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI) II-B study. Circulation 1991;83(2):422-437 [DOI] [PubMed] [Google Scholar]
- 29.Van de Werf F, Janssens L, Brzostek T, et al. Short-term effects of early intravenous treatment with a beta-adrenergic blocking agent or a specific bradycardiac agent in patients with acute myocardial infarction receiving thrombolytic therapy. J Am Coll Cardiol. 1993;22(2):407-416 [DOI] [PubMed] [Google Scholar]
- 30.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357(9266):1385-1390 [DOI] [PubMed] [Google Scholar]
- 31.COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366(9497):1622-1632 [DOI] [PubMed] [Google Scholar]
- 32.Effect of verapamil on mortality and major events after acute myocardial infarction (the Danish Verapamil Infarction Trial II—DAVIT II). Am J Cardiol. 1990;66(10):779-785 [DOI] [PubMed] [Google Scholar]
- 33.Multicenter Diltiazem Postinfarction Trial Research Group The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med. 1988;319(7):385-392 [DOI] [PubMed] [Google Scholar]
- 34.Latini R, Maggioni AP, Flather M, Sleight P, Tognoni G. ACE inhibitor use in patients with myocardial infarction: summary of evidence from clinical trials. Circulation 1995;92(10):3132-3137 [DOI] [PubMed] [Google Scholar]
- 35.Teo KK, Yusuf S, Collins R, Held PH, Peto R. Effects of intravenous magnesium in suspected acute myocardial infarction: overview of randomised trials. BMJ 1991;303(6816):1499-1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods KL, Fletcher S. Long-term outcome after intravenous magnesium sulphate in suspected acute myocardial infarction: the second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2). Lancet 1994;343(8901):816-819 [DOI] [PubMed] [Google Scholar]
- 37.Magnesium in Coronaries (MAGIC) Trial Investigators Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries (MAGIC) Trial: a randomised controlled trial. Lancet 2002;360(9341):1189-1196 [DOI] [PubMed] [Google Scholar]
- 38.ISIS-2 (Second International Study of Infarct Survival) Collaborative Group Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988;2(8607):349-360 [PubMed] [Google Scholar]
- 39.Sabatine MS, Cannon CP, Gibson CM, et al. CLARITY-TIMI 28 Investigators Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005March24;352(12):1179-1189 Epub 2005 Mar 9 [DOI] [PubMed] [Google Scholar]
- 40.Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366(9497):1607-1621 [DOI] [PubMed] [Google Scholar]
- 41.Sabatine MS, Cannon CP, Gibson CM, et al. Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005September14;294(10):1224-1232 Epub 2005 Sep 4 [DOI] [PubMed] [Google Scholar]
- 42.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention-summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47(1):216-235 [DOI] [PubMed] [Google Scholar]
- 43.King SB, III, Smith SC, Jr, Hirshfeld JW, Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2008;117(6):e161] Circulation 2008January15;117(2):261-295 Epub 2007 Dec 13 [DOI] [PubMed] [Google Scholar]
- 44.Antman EM, Giugliano RP, Gibson CM, et al. TIMI 14 Investigators Abciximab facilitates the rate and extent of thrombolysis: results of the thrombolysis in myocardial infarction (TIMI) 14 trial.a Circulation 1999;99(21):2720-2732 [DOI] [PubMed] [Google Scholar]
- 45.Strategies for Patency Enhancement in the Emergency Department (SPEED) Group Trial of abciximab with and without low-dose reteplase for acute myocardial infarction. Circulation 2000;101(24):2788-2794 [DOI] [PubMed] [Google Scholar]
- 46.Topol EJ, GUSTO V Investigators Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet 2001;357(9272):1905-1914 [DOI] [PubMed] [Google Scholar]
- 47.Lincoff AM, Califf RM, Van de Werf F, et al. GUSTO V Investigators Mortality at 1 year with combination platelet glycoprotein IIb/IIIa inhibition and reduced-dose fibrinolytic therapy vs conventional fibrinolytic therapy for acute myocardial infarction: GUSTO V randomized trial. JAMA 2002;288(17):2130-2135 [DOI] [PubMed] [Google Scholar]
- 48.Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction. Lancet 2001;358(9282):605-613 [DOI] [PubMed] [Google Scholar]
- 49.Curtis JP, Alexander JH, Huang Y, et al. ASSENT-2 and ASSENT-3 Investigators Efficacy and safety of two unfractionated heparin dosing strategies with tenecteplase in acute myocardial infarction (results from Assessment of the Safety and Efficacy of a New Thrombolytic Regimens 2 and 3). Am J Cardiol. 2004;94(3):279-283 [DOI] [PubMed] [Google Scholar]
- 50.Granger CB, Hirsch J, Califf RM, et al. Activated partial thromboplastin time and outcome after thrombolytic therapy for acute myocardial infarction: results from the GUSTO-I trial. Circulation 1996;93(5):870-878 [DOI] [PubMed] [Google Scholar]
- 51.Yusuf S, Mehta SR, Xie C, et al. CREATE Trial Group Investigators Effects of reviparin, a low-molecular-weight heparin, on mortality, reinfarction, and strokes in patients with acute myocardial infarction presenting with ST-segment elevation. JAMA 2005;293(4):427-435 [DOI] [PubMed] [Google Scholar]
- 52.Sabatine MS, Morrow DA, Montalescot G, et al. Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators Angiographic and clinical outcomes in patients receiving low-molecular-weight heparin versus unfractionated heparin in ST-elevation myocardial infarction treated with fibrinolytics in the CLARITY-TIMI 28 Trial. Circulation 2005December20;112(25):3846-3854 Epub 2005 Nov 15 [DOI] [PubMed] [Google Scholar]
- 53.Eikelboom JW, Quinlan DJ, Mehta SR, Turpie AG, Menown IB, Yusuf S. Unfractionated and low-molecular-weight heparin as adjuncts to thrombolysis in aspirin-treated patients with ST-elevation acute myocardial infarction: a meta-analysis of the randomized trials. Circulation 2005December20;112(25):3855-3867 Epub 2005 Dec 12 [DOI] [PubMed] [Google Scholar]
- 54.Antman EM, Morrow DA, McCabe CH, et al. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med. 2006April6;354(14):1477-1488 Epub 2006 Mar 14 [DOI] [PubMed] [Google Scholar]
- 55.OASIS-6 Trial Group Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA 2006April5;295(13):1519-1530 Epub 2006 Mar 14 [DOI] [PubMed] [Google Scholar]
- 56.Stone GW, Witzenbichler B, Guagliumi G, et al. HORIZONS-AMI Trial Investigators Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358(21):2218-2230 [DOI] [PubMed] [Google Scholar]
- 57.Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) Investigators Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 2006;367(9510):569-578 [DOI] [PubMed] [Google Scholar]
- 58.Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials [published correction appears in Lancet. 2006;367(9523):1656] Lancet 2006;367(9510):579-588 [DOI] [PubMed] [Google Scholar]
- 59.Ellis SG, Tendera M, de Belder MA, et al. FINESSE Investigators Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358(21):2205-2217 [DOI] [PubMed] [Google Scholar]
- 60.de Lemos JA, Braunwald E. ST segment resolution as a tool for assessing the efficacy of reperfusion therapy. J Am Coll Cardiol. 2001;38(5):1283-1294 [DOI] [PubMed] [Google Scholar]
- 61.Gibson CM, Cannon CP, Murphy SA, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 2000;101(2):125-130 [DOI] [PubMed] [Google Scholar]
- 62.Ito H, Okamura A, Iwakura K, et al. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation 1996;93(11):1993-1999 [DOI] [PubMed] [Google Scholar]
- 63.Angeja BG, Gunda M, Murphy SA, et al. TIMI myocardial perfusion grade and ST segment resolution: association with infarct size as assessed by single photon emission computed tomography imaging. Circulation 2002;105(3):282-285 [DOI] [PubMed] [Google Scholar]
- 64.Schröder R, Dissmann R, Brüggemann T, et al. Extent of early ST segment elevation resolution: a simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol. 1994;24(2):384-391 [DOI] [PubMed] [Google Scholar]
- 65.de Lemos JA, Antman EM, Gibson CM, et al. Abciximab improves both epicardial flow and myocardial reperfusion in ST-elevation myocardial infarction: observations from the TIMI 14 trial. Circulation 2000;101(3):239-243 [DOI] [PubMed] [Google Scholar]
- 66.Ellis SG, da Silva ER, Heyndrickx G, et al. Randomized comparison of rescue angioplasty with conservative management of patients with early failure of thrombolysis for acute anterior myocardial infarction. Circulation 1994;90(5):2280-2284 [DOI] [PubMed] [Google Scholar]
- 67.Sutton AG, Campbell PG, Graham R, et al. A randomized trial of rescue angioplasty versus a conservative approach for failed fibrinolysis in ST-segment elevation myocardial infarction: the Middlesbrough Early Revascularization to Limit INfarction (MERLIN) trial. J Am Coll Cardiol. 2004;44(2):287-296 [DOI] [PubMed] [Google Scholar]
- 68.Gershlick AH, Stephens-Lloyd A, Hughes S, et al. REACT Trial Investigators Rescue angioplasty after failed thrombolytic therapy for acute myocardial infarction. N Engl J Med. 2005;353(26):2758-2768 [DOI] [PubMed] [Google Scholar]
- 69.Patel TN, Bavry AA, Kumbhani DJ, Ellis SG. A meta-analysis of randomized trials of rescue percutaneous coronary intervention after failed fibrinolysis. Am J Cardiol. 2006;97(12):1685-1690 Epub 2006 Apr 21 [DOI] [PubMed] [Google Scholar]
- 70.Collet JP, Montalescot G, Le May M, Borentain M, Gershlick A. Percutaneous coronary intervention after fibrinolysis: a multiple meta-analyses approach according to the type of strategy. J Am Coll Cardiol. 2006October3;48(7):1326-1335 Epub 2006 Sep 14 [DOI] [PubMed] [Google Scholar]
- 71.Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, et al. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol. 2007January30;49(4):422-430 Epub 2007 Jan 12 [DOI] [PubMed] [Google Scholar]
- 72.Occluded Artery Trial (OAT) Research Group Design and methodology of the Occluded Artery Trial (OAT). Am Heart J. 2005;150(4):627-642 [DOI] [PubMed] [Google Scholar]
- 73.Hochman JS, Lamas GA, Buller CE, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006December7;355(23):2395-2407 Epub 2006 Nov 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) [published correction appears in J Am Coll Cardiol. 2006;48(8):1731] J Am Coll Cardiol. 2002;40(8):1531-1540 [DOI] [PubMed] [Google Scholar]
- 75.O'Rourke RA, Chatterjee K, Dodge HT, et al. Guidelines for clinical use of cardiac radionuclide imaging, December 1986: a report of the American College of Cardiology/American Heart Association Task Force on Assessment of Cardiovascular Procedures (Subcommittee on Nuclear Imaging). J Am Coll Cardiol. 1986;8(6):1471-1483 [PubMed] [Google Scholar]
- 76.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003;108(9):1146-1162 [DOI] [PubMed] [Google Scholar]
- 77.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation 2003;108(11):1404-1418 [DOI] [PubMed] [Google Scholar]
- 78.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction [published correction appears in J Am Coll Cardiol. 2008;51(9):974] J Am Coll Cardiol. 2007;50(7):e1-e157 [DOI] [PubMed] [Google Scholar]
- 79.Larsson H, Areskog M, Areskog NH, et al. Should the exercise test (ET) be performed at discharge or one month later after an episode of unstable angina or non-Q-wave myocardial infarction? Int J Card Imaging 1991;7(1):7-14 [DOI] [PubMed] [Google Scholar]
- 80.Mark DB, Lauer MS. Exercise capacity: the prognostic variable that doesn't get enough respect. Circulation 2003;108(13):1534-1536 [DOI] [PubMed] [Google Scholar]
- 81.Santoro GM, Sciagrà R, Buonamici P, et al. Head-to-head comparison of exercise stress testing, pharmacologic stress echocardiography, and perfusion tomography as first-line examination for chest pain in patients without history of coronary artery disease. J Nucl Cardiol. 1998;5(1):19-27 [DOI] [PubMed] [Google Scholar]
- 82.Amanullah AM, Lindvall K. Prevalence and significance of transient--predominantly asymptomatic--myocardial ischemia on Holter monitoring in unstable angina pectoris, and correlation with exercise test and thallium-201 myocardial perfusion imaging. Am J Cardiol. 1993;72(2):144-148 [DOI] [PubMed] [Google Scholar]
- 83.Bateman TM, O'Keefe JH, Jr, Williams ME. Incremental value of myocardial perfusion scintigraphy in prognosis and outcomes of patients with coronary artery disease. Curr Opin Cardiol. 1996;11(6):613-620 [DOI] [PubMed] [Google Scholar]
- 84.Marwick TH, Anderson T, Williams MJ, et al. Exercise echocardiography is an accurate and cost-efficient technique for detection of coronary artery disease in women. J Am Coll Cardiol. 1995;26(2):335-341 [DOI] [PubMed] [Google Scholar]
- 85.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006October17;114(16):1761-1791 Epub 2006 Oct 2 [DOI] [PubMed] [Google Scholar]
- 86.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48(7):1475-1497 [DOI] [PubMed] [Google Scholar]
- 87.Klem I, Heitner JF, Shah DJ, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006April18;47(8):1630-1638 Epub 2006 Mar 27 [DOI] [PubMed] [Google Scholar]
- 88.Raff GL, Gallagher MJ, O'Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46(3):552-557 [DOI] [PubMed] [Google Scholar]
- 89.Multicenter Postinfarction Research Group Risk stratification and survival after myocardial infarction. N Engl J Med. 1983;309(6):331-336 [DOI] [PubMed] [Google Scholar]
- 90.Zaret BL, Wackers FJT, Terrin ML, et al. TIMI Study Group Value of radionuclide rest and exercise left ventricular ejection fraction is assessing survival of patients after thrombolytic therapy for acute myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II study. J Am Coll Cardiol. 1995;26(1):73-79 [DOI] [PubMed] [Google Scholar]
- 91.Boden WE, O'Rourke RA, Crawford MH, et al. Veterans Affairs Non-Q-Wave Infarction Strategies in Hospital (VANQWISH) Trial Investigators Outcomes in patients with acute non-Q-wave myocardial infarction randomly assigned to an invasive as compared with a conservative management strategy [published correction appears in N Engl J Med. 1998;339(15):1091] N Engl J Med. 1998;338(25):1785-1792 [DOI] [PubMed] [Google Scholar]
- 92.Goyal A, Samaha FF, Boden WE, Wade MJ, Kimmel SE. Stress test criteria used in the conservative arm of the FRISC-II trial underdetects surgical coronary artery disease when applied to patients in the VANQWISH trial. J Am Coll Cardiol. 2002;39(10):1601-1607 [DOI] [PubMed] [Google Scholar]
- 93.Madsen JK, Grande P, Saunamäki K, et al. DANAMI Study Group Danish multicenter randomized study of invasive versus conservative treatment in patients with inducible ischemia after thrombolysis in acute myocardial infarction (DANAMI). Circulation 1997;96(3):748-755 [DOI] [PubMed] [Google Scholar]
- 94.Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. 2006;354(5):483-495 [DOI] [PubMed] [Google Scholar]
- 95.Morice MC, Colombo A, Meier B, et al. REALITY Trial Investigators Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA 2006;295(8):895-904 [DOI] [PubMed] [Google Scholar]
- 96.Kaiser C, Brunner-La Rocca HP, Buser PT, et al. BASKET Investigators Incremental cost-effectiveness of drug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: randomised Basel Stent Kosten Effektivitäts Trial (BASKET) [published correction appears in Lancet. 2005;366(9503):2086] Lancet 2005;366(9489):921-929 [DOI] [PubMed] [Google Scholar]
- 97.SoS Investigators Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet 2002;360(9338):965-970 [DOI] [PubMed] [Google Scholar]
- 98.Booth J, Clayton T, Pepper J, et al. SoS Investigators Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008July22;118(4):381-388 Epub 2008 Jul 7 [DOI] [PubMed] [Google Scholar]
- 99.Mercado N, Wijns W, Serruys PW, et al. One-year outcomes of coronary artery bypass graft surgery versus percutaneous coronary intervention with multiple stenting for multisystem disease: a meta-analysis of individual patient data from randomized clinical trials. J Thorac Cardiovasc Surg. 2005;130(2):512-519 [DOI] [PubMed] [Google Scholar]
- 100.Serruys PW, Morice MC, Kappetein AP, et al. SYNTAX Investigators Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009March5;360(10):961-972 Epub 2009 Feb 18 [DOI] [PubMed] [Google Scholar]
- 101.Stone GW, Midei M, Newman W, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA 2008;299(16):1903-1913 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.