Abstract
Small-molecule inhibition of extracellular proteins that activate membrane receptors has proved to be extremely challenging. Diversity-oriented synthesis and small-molecule microarrays enabled the discovery of robotnikinin, a small molecule that binds the extracellular Sonic Hedgehog (Shh) protein and blocks Shh-signaling in cell lines, human primary keratinocytes and a synthetic model of human skin. Shh pathway activity is rescued by small-molecule agonists of Smoothened, which functions immediately downstream of the Shh receptor Patched.
Sonic Hedgehog (Shh), the most widely characterized of the Hedgehog homologues, is essential for proper embryonic development.1,2,3 The Shh pathway involves the auto-cleavage of full length Shh into an active 20 kD N-terminal fragment (ShhN), which binds to its 12-pass transmembrane receptor, Patched (Ptc1), reversing its inhibitory effect on Smoothened (Smo). One effect of this de-repression is the activation of Gli transcription factors, which regulate the transcription of target genes that include Gli1 and Ptc1.1
Several synthetic and natural small-molecule modulators of Smo, an apparent member of the family of G protein-coupled receptors (GPCRs), have been discovered using cell-based phenotypic screens.4 Shh signaling antagonists that bind to Smo include cyclopamine (3), SANT1 (4), and Cur-61414 (5).5,6 Shh signaling agonists that bind to Smo include the synthetic small molecules purmorphamine (6) and Hh-Ag1.2 (SAG; 7).7,8 Furthermore, small molecules which inhibit Shh signaling downstream of Smo, GANT61 (8) and GANT58 (9), have also been reported (Supplementary Fig. 1 online).9 The discovery of small-molecule modulators of Shh signaling provides an avenue to regulate the activity of a pathway implicated in medulloblastoma, basal cell carcinoma (BCC), pancreatic cancer, prostate cancer, and developmental disorders.2,10,11 Clinical trials in BCC and pancreatic cancer involving the Smo antagonists GDC-0449 (10, Phase I: NCT00607724) and IPI-926 (11, Phase I: NCT00761696) are currently underway.12,13
None of the reported synthetic Shh pathway inhibitors is known to target the Shh protein itself. To our knowledge, all reported examples of discoveries of small-molecule Shh signaling modulators resulted from the use of cell-based phenotypic assays. Target-based discovery of modulators of Shh signaling was expected to provide a complementary approach.
Small-molecule microarray (SMM)-based screens have enabled the discovery of small molecules that bind target proteins of interest and modulate the cellular functions of their targets.14,15,16 In this system, small molecules have been linked covalently to a glass surface and screened for binding to either a purified protein or an epitope-tagged protein in a complex cell lystate.17,18 Here, we report a screen of bacterially expressed ShhN using SMMs containing a collection of approximately 10,000 diversity-oriented synthesis (DOS) compounds and natural products arrayed on a single microscope slide.19
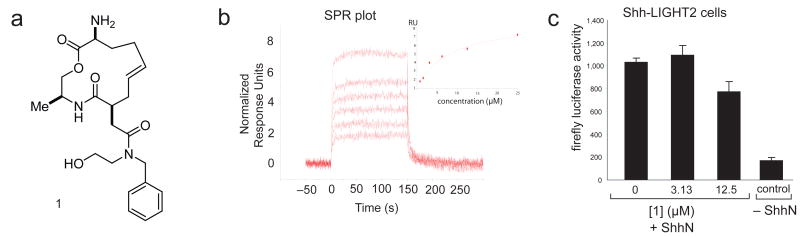
In the ShhN SMM screen, some structurally related macrocycles emerged as intriguing assay positives. A representative macrocycle, 1 (Fig. 1a) was retested for binding to ShhN via surface plasmon resonance (SPR) (Fig. 1b). The compound exhibited binding to ShhN in a concentration-dependant fashion with a KD of 9 μM, determined by fitting steady-state data. To our knowledge, this is the first reported discovery of a small molecule capable of binding to the ShhN protein.
Figure 1. Characterization of SMM hit, 1.
(a) Structure of 1. (b) SPR plot of 1 binding to purified ShhN. The plot shows normalized response units (RUs) on the y-axis and time (s) on the x-axis. The concentrations plotted are 0.78 μM, 1.56 μM, 3.13 μM, 6.25 μM, 12.5 μM, and 25 μM, in order of increasing normalized RUs. (c) Luminescence plots for a Gli-dependent firefly luciferase reporter gene assay of 1 at the indicated concentrations. ShhN represents a positive control for medium containing ShhN palmitoylated at the N-terminus. The assays were performed at 0.25 % (v/v) DMSO. Each value represents the average of five experiments, with the error bar denoting the standard deviation.
We examined the activity of 1 in Shh-LIGHT2 cells (ATCC, Manassas VA),20 which is an NIH3T3 cell line with a Gli-dependent firefly luciferase reporter. These cells have been widely used to demonstrate the efficacy of Shh pathway inhibitors (cyclopamine) and activators (purmorphamine and SAG).4,6,21 Shh pathway activity was inferred by measuring firefly luciferase levels after a 30 h incubation with compound in the presence of N-palmitoylated ShhN. The compound exhibited moderate Shh pathway inhibition (Fig. 1c) and did not demonstrate cytotoxicity at any of the experimental concentrations based on a cell titer viability assay run in parallel (see Supplementary methods online). This raised the possibility that the ShhN-binding was related to the moderate Shh pathway inhibition.
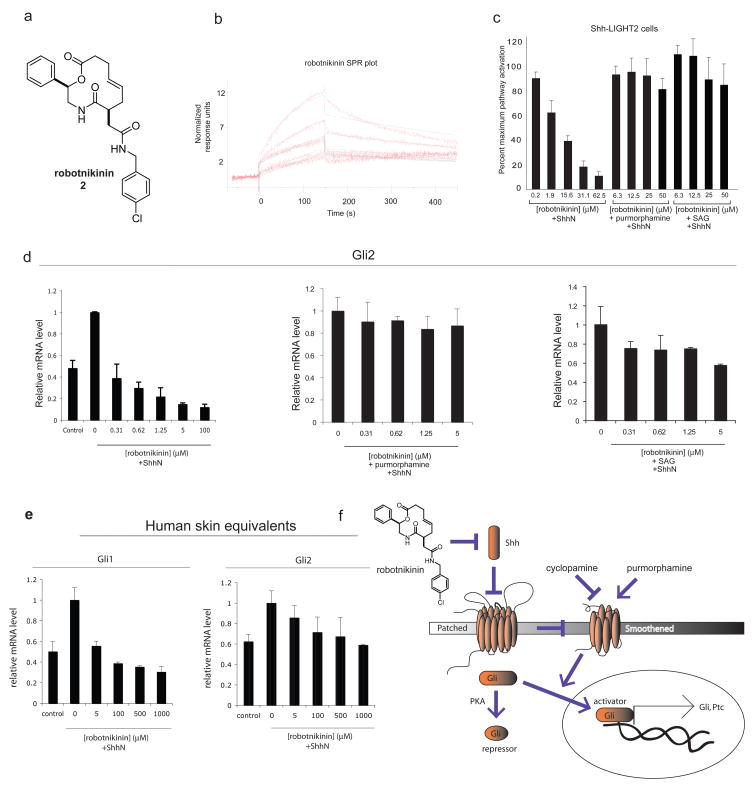
As part of an ongoing SAR effort, we identified a 12-membered macrocycle, which we have named robotnikinin (2), that showed increased ShhN-binding and displayed a significantly longer dissociation time than 1. Based on SPR experiments, robotnikinin (Fig. 2a) demonstrated ShhN-binding capacity at concentrations between 1.56 μM and 25 μM, with a KD of 3.1 μM derived from kinetic data (Fig. 2b). When this compound was tested in an NIH3T3 line transformed with a Gli-luciferase construct, or Shh-LIGHT2 cells11, it showed concentration-dependent inhibition of ShhN-induced pathway activation (Fig. 2c). Additionally, at concentrations above 30 μM the macrocycle exhibited comparable inhibition relative to treatment with 6.25 μM cyclopamine in Shh-LIGHT2 cells. No significant cytotoxicity was observed as judged by cell titer measurements using a cell viability assay.
Figure 2. Robotnikinin.
(a) The structure of robotnikinin, which is the compound that resulted from follow-up chemistry efforts to optimize potency. (b) SPR curve of robotnikinin showing concentration-dependent binding to purified ShhN. Normalized RUs are plotted over a time course. The concentrations plotted are 1.56 μM, 3.13 μM, 6.25 μM, 12.5 μM, and 25 μM, in order of increasing RUs (c) Inhibition of Gli signaling by robotnikinin in Shh-LIGHT2 cells stimulated with medium containing ShhN palmitoylated at the N-terminus, relative to 6.25 μM cyclopamine (a small-molecule inhibitor of Smoothened). Shh-LIGHT2 cells stimulated with N-palmitoylated ShhN along with 3.6 μM purmorphamine or 100 nM SAG (small-molecule activator of Smoothened) showed negligible inhibition at the indicated concentrations of inhibitor. (d) Robotnikinin lowers levels of endogenous Gli2 mRNA (analyzed by qPCR) in primary human keratinocytes in a dose-dependent manner; this effect is blocked by the co-administration of Smo agonists. Note that there is some Gli expression in the absence of exogenous Shh due to the presence of a basal amount of Shh in the growth medium. (e) When analyzed by qPCR, synthetic human skin displayed Gli1 and Gli2 transcriptional repression in the presence of varying concentrations of robotnikinin. (f) Robotnikinin inhibits the induction of the Shh pathway. Our experiments support a mechanism involving inhibition of the actions of Shh, either directly or indirectly by interfering with a precursor complex.
To explore further the potential mechanism of Shh pathway inhibition involving direct perturbation of the ShhN protein complex, the same compounds were tested in a Ptc1−/− cell line derived from mouse embryos lacking Ptc1 function. The cell line had both Ptc1 alleles replaced with a β-galactosidase (β-gal) reporter.21 Because Ptc1 inhibits Hh pathway activation by repressing Smo function and is also a target gene, removing both Ptc1 alleles results in constitutive pathway activation and β-gal expression. Small-molecule pathway inhibitors that act downstream of Ptc1 remain active in this cell line. In the Ptc1−/− cell line, Shh pathway activity is proportional to the β-gal levels observed after 30 h of incubation with compound. No significant difference was observed when the Ptc1−/− cell line was treated with N-palmitoylated ShhN or low serum-containing culture medium, confirming that with the Ptc1 receptor absent, the Shh pathway is constitutively activated and ShhN does not increase pathway activation.21 Previous studies have demonstrated that cyclopamine, whose target (Smo) is downstream of Ptc16,22, is effective at ablating β-gal reporter activity in this cell line. In our study, treatment with 6.6 μM cyclopamine resulted in significant pathway inhibition. In contrast, no pathway inhibition was observed using robotnikinin at any of the concentrations tested after normalizing luminescence data for cell titer (Supplementary Fig. 2a online). These results support a model in which this small molecule inhibits the Shh pathway upstream of Ptc1 in Shh-LIGHT2 cells.
Our model predicts that treatment of Shh-LIGHT2 cells with the Smo agonists purmorphamine and SAG would override the inhibitory effect of robotnikinin since Smo functions downstream of Shh/Ptc1. When we tested the model by co-administering 3.6 μM purmorphamine in addition to various concentrations of robotnikinin, virtually all of the inhibitory effect was eliminated. This effect was recapitulated when 100 nM SAG was co-administered (Fig. 2c). Next, we sought to test our model in the context of a different cell line, without reliance on a reporter gene construct. C3H10T1/2 cells are an immortalized mouse mesenchymal stem cell line that differentiate to osteoblasts upon treatment with N-palmitoylated ShhN, with alkaline phosphatase (AP) as a reliable marker of the transformation.8,23 Robotnikinin showed dose-dependent inhibition of AP induction in the cell line in the presence of ShhN, but no detectable inhibition when 3.6 μM purmorphamine was co-administered (Supplementary Fig. 2b online). These data indicate that the inhibition and epistatic rescue of robotnikinin was not dependent on the Shh-LIGHT2 cell line. Consistent with its mode of action upstream of Smo and in contrast with other small-molecule modulators of Shh signaling, we also determined that at concentrations up to 25 μM, robotnikinin does not compete for Smo binding with BODIPY-cyclopamine in a Smo-overexpressing HEK293 cell line (Supplementary Fig. 2e online).
To obtain direct evidence of the ability of robitnikinin to block Shh signaling, we investigated its actions on two types of human primary cells on endogenous levels of transcripts resulting from Shh-induced genes. The first study used RT/PCR (qPCR) to measure quantitatively levels of mRNA for the Shh-induced transcription factors Gli1 and Gli2 in primary human keratinocytes. When primary human keratinocytes were stimulated with N-palmitoylated ShhN and incubated with robotnikinin, Gli2 and Gli1 transcription was markedly repressed by 30 h (Fig. 2d, Supplementary Fig. 2c online). Virtually all Gli2 transcription was abolished in the presence of 100 μM robotnikinin at the 30 h time point. When Smo agonists purmorphamine and SAG respectively, were co-administered with robotnikinin, an active Shh pathway phenotype was observed with a loss of response to robotnikinin, as evidenced by Gli2 and Gli1 mRNA levels (Fig. 2d, Supplementary Fig. 2c online).
A second study used synthetic skin that was prepared by first populating dehydrated collagen matrix, itself derived from human skin grafts, with primary human keratinocytes. Next, several dermal layers were formed by culturing over an extended period. Following incubation with robotnikinin, the primary human synthetic skin tissue was analyzed by qPCR for levels of Gli1 and Gli2 transcripts. These experiments revealed that the tissue displayed reduced levels of Gli1 and Gli2 mRNA while remaining histologically normal (Fig. 2e, Supplementary Fig. 2e online). Robotnikinin was thus found to retain its activity in human derived tissue.
We have described the first example of a small molecule that binds to purified ShhN protein. Robotnikinin inhibits Shh signaling in a concentration-dependant manner but exhibits no inhibitory activity in a cell line lacking the Ptc1 receptor, does not compete with cyclopamine/Smo interactions, and does not exhibit an inhibitory effect in the presence of the well-characterized Smo agonists, purmorphamine and SAG. Robotnikinin displays significant repression of Shh-induced Gli1/Gli2 in primary human skin cells and human-derived skin tissue. In light of the ShhN-binding properties of the macrocycle and the results of our epistasis analyses (its lack of significant Shh pathway inhibition, using Gli activity as a surrogate for pathway activity in the Ptc1−/− cell line, and the ability of two agonists of the downstream Smo to override its effects), we propose a novel mechanism of action involving direct targeting of the ShhN protein complex. Recent evidence has indicated that Hh signaling is facilitated by HhN binding partners Ihog, Boi, and heparin in Drosophila, and Shh binding partners (Ihog orthologs) Cdo and Boc in vertebrates.24,25 The data presented herein suggest that robotnikinin interferes with the ability of the ShhN protein complex to relay its signal efficiently to Ptc1. Small-molecule-mediated disruption of protein/protein interactions involving extracellular growth and differentiation factors with their receptors is in general exceedingly challenging, yet the process described here led directly to a success. The discovery of robotnikinin has provided a powerful small-molecule probe of an important signaling pathway involving a step in the pathway not previously accessible to small-molecule modulation. We believe that robotnikinin will be especially valuable as a probe of diseases associated with aberrant Shh-pathway activity.
Supplementary Material
Acknowledgments
We thank Michael A. Foley for suggesting the use of robotnikinin in primary cell and tissue models in the Mandinova laboratory; Andrew M. Stern for his insightful guidance and critique; Greg Copeland, Olivia McPherson, Damian Young, and Tim Lewis for their helpful suggestions. This work was funded by the NIGMS (GM-38627 awarded to S.L.S) and the National Pancreas Foundation, American Gastroenterological Association and American Liver Foundation (L.F.P.), and, in part, with funds from the National Cancer Institute’s Initiative for Chemical Genetics (Contract No. N01-CO-12400). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Service, nor does the mention of trade names, commercial products or organizations imply endorsement by the US government. S.L.S. is a Howard Hughes Medical Institute Investigator.
Footnotes
COMPETING INTEREST STATEMENT S.L.S. is a shareholder of Infinity Pharmaceuticals, a company to which reference is made in the text. All other authors declare that they have no competing financial interests.
References
- 1.Ingham PW, McMahon AP. Genes and Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 2.Rubin L, de Sauvage FJ. Nat Rev Drug Disc. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 3.Nusslein-Volhard C, Wieschaus E. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 4.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. PNAS. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JK, Taipale J, Cooper MK, Beachy PA. Genes and Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams JA, et al. PNAS. 2003;100:4616–4621. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding S, Schultz PG. Nat Biotechnol. 2004;7:833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, et al. Chem Biol. 2004;11:1229–1238. doi: 10.1016/j.chembiol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Fallon JF, Beachy PA. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 10.Chiang C, et al. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 11.Thayer SP, et al. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Hoff DD, et al. Proc 99th Annu Meeting Amer Assoc Cancer Res; 2008. Abstract LB-138. [Google Scholar]
- 13.Epstein EH. Nat Rev Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacBeath G, Koehler AN, Schreiber SL. J Am Chem Soc. 1999;121:7967–7968. [Google Scholar]
- 15.Kuruvilla FG, et al. Nature. 2002;416:653–657. doi: 10.1038/416653a. [DOI] [PubMed] [Google Scholar]
- 16.Tan DS. Nat Biotech. 2002;20:561–563. doi: 10.1038/nbt0602-561. [DOI] [PubMed] [Google Scholar]
- 17.Burke MD, Berger EM, Schreiber SL. Science. 2003;302:613–618. doi: 10.1126/science.1089946. [DOI] [PubMed] [Google Scholar]
- 18.Wong JC, et al. Chem Biol. 2004;11:1279–1291. doi: 10.1016/j.chembiol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Bradner JE, et al. Chem Biol. 2006;13:493–504. doi: 10.1016/j.chembiol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Taipale J, et al. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 21.Surajit S, Chen JK. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- 22.Chen JK, Taipale J, Cooper MK, Beachy PA. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinella-Jaegle S, et al. J Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 24.Yao S, Lum L, Beachy PA. Cell. 2006;125:343–357. doi: 10.1016/j.cell.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 25.Tenzen T, et al. Dev Cell. 2006;10:647–656. doi: 10.1016/j.devcel.2006.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.