Abstract
Studies demonstrating recognition deficits with aging often use tasks in which subjects have an incentive to correctly encode or retrieve the experimental stimuli. In contrast to these tasks, which may engage strategic encoding and retrieval processes, the visual paired comparison (VPC) task measures spontaneous eye movements made toward a novel as compared with familiar stimulus. In the present study, seven rhesus macaques aged six to thirty years exhibited a dramatic age-dependent decline in preference for a novel image compared with one presented seconds earlier. The age effect could not be accounted for by memory deficits alone, since it was present even when familiarization preceded test by one second. It also could not be explained by an encoding deficit, since the effect persisted with increased familiarity of the sample stimulus. Reduced novelty preference did correlate with eye movement variables, including reaction time distributions and saccade frequency. At long delay intervals (24 or 48 hours) aging was paradoxically associated with increased novelty preference. Several explanations for the age effect are considered, including the possible role of dopamine.
Keywords: aging, novelty, saccades, recognition memory, attention
Introduction
Memory declines that take place with aging are not homogeneous. Perceptual memory (repetition priming), and skill learning (learning finger tap sequences in the serial reaction time task) decline in a much more variable way, and probably later in life, than does episodic memory as measured, for example, by tasks that require subjects to learn and recall word lists (Howard & Howard, 1992; La Voie & Light, 1994; Prull, Gabrieli, & Bunge, 2000; Verhaeghen, Marcoen, & Goossens, 1993; Verhaeghen & Salthouse, 1997). Because the neural processing mechanisms that are engaged can be complex, it is often difficult or impossible for a single study to fully isolate the cognitive factors that may be sensitive to the aging process. This is particularly apparent in episodic memory tasks, where variables related to attention and motivation can play a large role in determining successful encoding and retrieval.
Experiments designed to examine recognition memory in old and young subjects illustrate how small differences in task design can result in large age effects. While some studies have found age related deficits (Fulton & Bartlett, 1991; Grady et al., 1995; Norman & Schacter, 1997; Park, Puglisi, & Smith, 1986; Parkin & Walter, 1992; Perfect & Dasgupta, 1997; Perfect, Williams, & Anderton-Brown, 1995; Schacter, Koutstaal, Johnson, Gross, & Angell, 1997); others have found no difference between age groups (Grady, McIntosh, Rajah, Beig, & Craik, 1999; Gutchess et al., 2005; Mark & Rugg, 1998; Sekuler, Kahana, McLaughlin, Golomb, & Wingfield, 2005; Sekuler, McLaughlin, Kahana, Wingfield, & Yotsumoto, 2006; Titov & Knight, 1997). Some of these inconsistencies may relate to the specific measure of recognition used (Light, 2000; Prull, Dawes, Martin, Rosenberg, & Light, 2006). Research in non-human primates has yielded similar inconsistencies, with some studies finding an age-related recognition impairment (Herndon, Moss, Rosene, & Killiany, 1997; Moss, Rosene, & Peters, 1988; Presty et al., 1987; Rapp & Amaral, 1991; Shamy et al., 2006), and others not (Rapp, Kansky, & Roberts, 1997). In humans, recognition memory is generally evaluated either by directly asking the subject whether or not a stimulus has been presented before, or by asking the subject to explicitly choose the stimulus that is novel or familiar. It is likely that in these laboratory settings, subjects will tend to find intrinsic or social rewards in being “right,” by correctly identifying familiar stimuli. Thus, such tasks may rely on a supervised learning system, engaging top-down attention and working memory processes in what's often called “strategic” encoding and retrieval. For nonhuman primate studies, delayed match-to-sample (DMS) or delayed non-match-to-sample (DNMS) tests have often been used, in which subjects are selectively rewarded for choosing the familiar (DMS) or novel (DNMS) stimulus. Strategic processing is also likely to be engaged in tasks with these kinds of reward contingencies. Because the systems involved in this type of processing are thought to be affected by age (e.g., see Prull et al., 2000, for review), it can be difficult to disentangle age deficits in processing strategy and motivation from age deficits in recognition memory per se.
An alternative way to measure recognition memory, and one that can be used across species, is to assess the natural changes in behavior a subject exhibits when encountering a novel or familiar stimulus. These types of tasks have long been used to assess recognition memory in pre-verbal infants (Fantz, 1964; for review see Pascalis & de Haan, 2003). The Visual Paired Comparison (VPC) task (also referred to as the “preferential looking task”) tests visual recognition by measuring the amount of time a subject spends looking at a new image (the “novel image”) as compared to a simultaneously presented image that has been seen previously (the “sample image”). The only requirement for acceptable performance in this task is to maintain gaze within the boundaries of the presented images -- human subjects can be instructed explicitly to do so, and non-human subjects can be given juice reward for doing so. Because non-human primates reliably exhibit a natural tendency to prefer novelty in these types of situations (Bachevalier, Brickson, & Hagger, 1993; Pascalis & Bachevalier, 1999; Gothard, Erickson, & Amaral, 2004) recognition can be inferred by the eye movements made toward the novel as compared to the previously viewed image. Data from normal adults and amnesic patients have demonstrated that eye movement measures of recognition tend to co-vary with accuracy measures in conventional, “yes/no,” recognition memory tests (Manns, Stark, & Squire, 2000; McKee & Squire, 1993; Smith, Hopkins, & Squire, 2006). In addition, specific, delay-dependent patterns of novelty preference in the VPC task have been associated with dysfunction of those parts of the temporal lobe commonly associated with normal recognition memory (Buffalo et al., 1999; Nemanic, Alvarado, & Bachevalier, 2004; Pascalis & Bachevalier, 1999; Zola et al., 2000).
The present study uses the VPC task to assess age-related changes in recognition memory in non-human primates, a model that has proven useful in studying age-related memory dysfunction and its neural correlates (Erickson & Barnes, 2003; Gallagher & Rapp, 1997; Peters et al., 1996). One advantage of using the non-human primate model is that it enables experimental control over task variables (familiarity with specific types of images, for example), which can be more difficult with human subjects.
As outlined above, the rationale for using the VPC task is to minimize the influence of top-down, strategic processing in memory encoding and retrieval, so that the cognitive abilities affected by age can be more directly specified. The prefrontal cortex, which is likely an important mediator of strategic encoding and retrieval processes (e.g., Badre & Wagner, 2007), is clearly compromised with age (Raz, 2000). One might expect the influence of the prefrontal cortex to be reduced in conditions that require only spontaneous looking behavior, as in the VPC task. This is consistent with the observation that infants exhibit novelty preference behaviors despite an undeveloped prefrontal cortex. Age-related neurobiological changes have also been observed in the hippocampus, perhaps most saliently in the dentate gyrus region (Barnes & McNaughton, 1985; De Toledo-Morrell, Goncharova, Dickerson, Wilson, & Bennett, 2000; Geinisman, de Toledo-Morrell, Morrell, Persina, & Rossi, 1992; Kramer et al., 2007; Moore, Browning, & Rose, 1993; Yonelinas et al., 2007; for review see Rosenzweig & Barnes, 2003). While there is some debate about the extent to which the hippocampus normally contributes to recognition processes (Squire, Wixted, & Clark, 2007); a large body of evidence suggests that the perirhinal cortex contributes significantly to recognition memory for objects or visual stimuli (Davachi, 2006; Dougal, Phelps, & Davachi, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007; Mayes, Montaldi, & Migo, 2007; Murray, Bussey, Hampton, & Saksida, 2000). A number of experiments have identified dissociations between the effects of hippocampal and rhinal cortex lesions on the degree to which the delay between stimulus presentations affect novelty preference in VPC tasks (Buffalo et al., 1999; Nemanic et al., 2004). The differential effects of hippocampal versus perirhinal cortex lesions on novelty preference may provide a framework for relating the behavioral patterns exhibited by aged versus young adults to the underlying neurophysiological changes responsible for age-related cognitive decline.
Methods
Subjects
Seven rhesus macaque monkeys (Macaca mulatta) of both sexes served as subjects. Individual monkeys will be identified throughout this manuscript as follows: PC- 6 year old male, BZ- 11 year old male, WN- 13 year old female, CL -17 year old female, BL- 25 year old, male, NS- 29 years old male and JN- 30 year old female. The young group included monkeys PC, BZ and WN, whereas the old group included monkeys BL, NS and JN. Because of its intermediate age, monkey CL's data (17 years old) was used for correlation analyses, but not for age group comparisons. Subjects were born, raised and tested for this study at the California National Primate Research Center, at the University of California, at Davis. Animals showing significant visual problems or experimental histories which might confound the results were not included in the present study. The ophthalmologic examinations given to all subjects except the young monkey PC included assessments of corneal curvature (diffractive error), lens clarity, status of maculae, retina, retinal blood vessels, glaucoma and cataract. All subjects were cross-sex pair-housed, except for monkey PC who was singly housed. In order to maintain stable levels of performance during behavioral testing, five subjects (PC, BZ, WN, BL and JN) were kept on a mild fluid restriction (not less than 38ml/kg average daily water intake) and food rations were adjusted. On this regime, animals received a significant amount of their daily water in the form of juice rewards during behavioral testing. For the other two monkeys (CL and NS), water was available ad libitum in their home cage. The diet consisted of standard monkey chow and regular supplements of fresh fruit and vegetables.
This investigation was conducted as part of an ongoing project of research on the neural basis of age-related memory impairments. With the exception of monkey PC all animals were trained and tested on a standardized battery of learning and memory tasks that included delayed response (visuospatial memory test), the delayed non-match to sample (DNMS), go/no-go and simple object discrimination tasks. Two subjects (CL and NS) underwent a chamber and craniotomy surgery for the implantation of tetrode recording probes for electrophysiology (Skaggs et al., 2007). Though recordings were carried out in parallel to testing for this study, the electrophysiological results are not reported here.
Eye Movement Data Acquisition
All subjects were surgically implanted with head restraint posts that allowed head immobilization during eye movement tracking. While head posts are not required to obtain preferential looking data, head restraint improved the precision of the data obtained with the current technology, which made data quantification easier. Posts were attached to the skull using dental acrylic and titanium screws, under isoflurane anesthesia. For all monkeys except PC, eye position was tracked using the ISCAN infrared pupil monitoring system (ISCAN, Burlington, MA). Monkey PC's eye movements were recorded following surgical implantation of a scleral eye coil, and were data obtained by using the scleral search coil system (as described in Robinson, 1963).
The data acquisition program package CORTEX (NIMH-supported freeware found at http://www.cortex.salk.edu; version 5.9.5) monitored behavioral responses and controlled stimulus display and reward presentation. Eye position was sampled, digitized at 200 or 500 Hz, and recorded as Cartesian coordinates within the visual field of interest. Fields of interest comprised the area within the boundaries that corresponded to the 10 × 10 degrees of visual angle (dva) of the images, plus approximately another degree of space beyond the images. Extending the limits beyond the actual boundaries of the images ensured that if transient noise in the tracker system occurred while subjects scanned within the image boundaries, this would not result in trial abortion.
Stimuli
Over 1000 visually distinct digitized images were gathered and modified from a large number of sources. These include colored photographs, drawings of real objects, abstract drawings, and fractals. Examples of these are shown in Figure 1A. During the final days of training, monkeys were presented with approximately 80 different images repeatedly over two to five days. At the beginning of testing, all subjects had viewed each of these images at least eight times (for 2 s each). These images are referred to throughout this study as “familiar” images.
Figure 1.

A) Examples of images used as stimuli. All images were 300 by 300 pixels (about 10 × 10 dva). B) General procedure used in a trial of the VPC task. The five panels represent the appearance of the display monitor during each phase of the task. The monkey was required to fixate on the spot in the middle of the computer screen within 2 s of its appearance on the monitor (shown in the first upper left panel). The spot disappeared if the monkey looked at the spot for 200 ms and the familiarization phase started (second panel). In the sample phase one image was displayed for 2 s. The third panel shows a black screen representing the delay (1, 5, 10 or 20 s). Next, another fixation spot was displayed. In the final phase of a trial, the test phase, the test pair of images was displayed. In this case the image on the left was seen in the familiarization phase and the image on the right is novel. C) Eye tracker traces superimposed from an example trial upon the sample (left image) and test (middle and right images). In this case, the monkey only looks at the novel image during test, however, monkeys often scanned the relatively familiar image as well.
Training
During both the training and testing phase, monkeys were seated in a primate restraint chair placed inside a sound attenuating booth that reduced other auditory and visual distractions. A computer monitor was placed at about 50cm from the monkey's eyes. At this distance, 1 cm on the monitor corresponded to about 0.88° visual angle. Correct behavioral responses were rewarded with about 0.5ml drops of fruit juice delivered by a juice delivery system (Crist Instrument Co.). The delivery of each drop was paired with an auditory signal that provided animals with an indication that the animal had kept gaze within the required field of view. Errors occurred when the subject moved its gaze outside the required field of view, and were followed by a 2 s time-out period, in which the screen remained black.
Monkeys were initially trained to orient to, and to maintain gaze (fixate) on a white spot presented at the center of the monitor. When fixation spot training was complete, each monkey was then trained to maintain its gaze within the boundaries of an image that subtended about 12 ×12° of visual angle. Performance criterion was met when monkeys fixated for at least 200 ms on the fixation spot and maintained gaze within the boundary of the landscape image for 2 s on 80% of the trials.
After initial shaping of looking behavior, monkeys were trained to look at images arranged in the same way as they appear in most of the actual tests used in this study (Figure 1B), that is, they were presented with one sample image presented in the middle of the screen, followed by two images positioned horizontally next to each other – the test pair. One image in the test pair was the same as the sample image and the other was novel. These images were separated by a blank space (2° of visual angle wide) that was centered in the monitor. Animals were required to fixate for at least 200 ms on the fixation spot and maintain gaze within the boundary of the images for 2 s. Trials were aborted if monkeys looked away from either the fixation spot or the image boundaries. When monkeys were capable of performing 80% of the trials correctly during a session, criterion was met. The time each monkey took to complete training varied between 3 to 8 weeks. Minor variations in the size of the fixation spot and image window boundaries were made for some individuals (young and old) to assist with learning during the training period.
VPC Task Protocol
A diagram of the sequence of a trial in the version of the VPC task used in this study is shown in Figure 1B. A white fixation spot (0.25° of visual angle) appeared in the center of the screen. The monkey was required to fixate its gaze on the spot within 2 s of its appearance on the monitor and maintain fixation for at least 200ms. If these criteria were not met, a time-out period of 2 s was imposed. If fixation occurred, a single image (sample image) was displayed for 2 s (familiarization phase). The monkey was allowed to freely explore the image within its boundaries. If the subject shifted its gaze outside the boundary, the screen went blank for 2s, signaling failure on the trial. These failed (error) trials were repeated later. On the other hand, if the animal maintained its gaze within the boundary for 2s, the image disappeared and a drop of juice was delivered into its mouth as reward.
After a relatively short delay of either 1, 5, 10 or 20 s (intervals randomized between trials) during which monkeys did not receive any stimuli or reward, the small fixation spot reappeared. Unlike the first fixation spot requirements, failure to fixate on this second spot did not abort the trial. If the monkey did not fixate on the spot during the first 1s display period, the spot appeared again until the animal fixated on it for 200ms. When this occurred, two images (test pair) were displayed for 2 s. Once more, the animal was required to maintain its gaze within the boundaries of the two images for the duration of the display. If successful, the monkey received two drops of reward and the trial was considered to be a “correct trial.” As described below, analyses were restricted to correct trials, and the dependent variable of interest was the percentage of time the monkey looked at the novel versus familiar image during the test phase. The inter-trial interval duration was approximately 2 s and the next trial began with the display of a fixation spot.
Experimental Design
Four different experimental procedures were implemented in this study: Novel/Novel, Familiar/Novel, Familiar/Familiar, and Long Delay (described in more detail below). Tests were administered by blocks, each block consisting of 80 or 20 trials to be attained, depending on the procedure. Daily sessions began with an eye-tracker calibration task that allowed the equipment to be calibrated for each animal and also verified the ability for each monkey to correctly saccade to a target stimulus. The order of sessions in a given day generally followed the pattern of: 1) eye-tracker calibration, 2) Familiar/Familiar, 3) Familiar/Novel, 4) Novel/Novel, and finally 5) a session block of either Familiar/Novel or Novel/Novel, or alternatively of the long delay experimental procedure. To avoid possible confounds due to left or right looking biases, the position of the test image was counterbalanced for spatial location in all experimental procedures except for the long delay. This meant that each trial was performed twice, once with the novel image on the right side and once with the novel image on the left side. The sequence of trials within a block was randomized in all experimental procedures. Thus the “novel stimulus” presented on the second randomized exposure was always “relatively novel”.
Eye-tracker calibration block
During a trial of the eye-tracker calibration, a small (0.25 degrees of visual angle) square image or solid color appeared on the computer screen in one of five places: center, up, down, left, or right. Monkeys were rewarded with a drop of juice for correctly saccading to and then fixating on the target stimulus. Although the primary purpose of this task was to calibrate the eyetracker, it also established that monkeys could consistently and accurately direct their eyes toward a target stimulus.
Novel/Novel Blocks
This experimental procedure was implemented in order to determine how novelty-preference patterns across delays differ between age groups. The VPC task protocol is described above. In this condition, both the sample and test images were novel. A different set of images was used for each block. Each set consisted of 80 unique images. Forty images of each set were assigned as sample stimuli and the other forty as test stimuli (i.e., forty test pairs). Because the novel image was presented twice with the relatively familiar image (counterbalanced position), a testing block consisted of 80 trials.
Familiar/Novel Blocks
The magnitude of human adult novelty preference has been shown to increase as familiarization time increases in the VPC task (Richmond, Sowerby, Colombo, & Hayne, 2004). It was anticipated that in the Novel/Novel condition, younger monkeys would exhibit higher novelty preferences than aged monkeys, particularly at longer delays. The aim of this experimental procedure was to determine whether additional viewing experience of the sample stimuli would strengthen memory for those images in older animals, and would bring their novelty preference patterns closer to those observed in young animals. The design of the Familiar/Novel test was the same as in the Novel/Novel test (i.e., it used the VPC protocol) except for one feature: sample images were the same images used during the last 2-5 days of VPC training, which monkeys had been exposed to at least eight times (2 seconds each time), and therefore were operationally defined as familiar. Forty of these familiar images were used as sample images and were paired with forty novel images during the test phase of each trial.
Familiar/Familiar Blocks
In this condition the same VPC task protocol was used, but both stimuli presented during the test phase of each trial were familiar to the subjects, as they had been viewed at least eight times during the last 2-5 days of VPC training. It was expected that by increasing the familiarity of both test stimuli, the young monkeys might begin to show reduced preference for the relatively novel stimuli, or aged monkeys might show increased novelty preference, thus making the age groups more similar.
Long Delay Experimental Procedure
The aim of this test was to assess whether animals remembered stimuli after retention intervals longer than those in the range of seconds used in the Novel/Novel test. Twenty four and 48 h delays were used in this experimental procedure, with the familiarization and the test phases administered in separate blocks on different days. On a given day, sample images were presented one after the other with no testing phase between them. The test phases were administered 24 or 48 hours later, using the same procedure described for the test phase of the VPC protocol. Twenty novel images were used in each block of samples. Another twenty novel images were used as test images. Preliminary data resulting from this experimental procedure suggested that animals showed no novelty preference when the sample image was presented only one time. Therefore, to increase the probability that subjects would show novelty preference (above chance level) after long delays, animals were exposed to the images 10 times (for 2 s each) 24 or 48 h before test.
All data collected from an individual monkey were obtained over about eight days of testing, with each day containing three blocks on average. Each session was separated by at least 24 hours. On any given day of testing, a monkey completed between 80 and 320 trials during the session, distributed among different tests. The sessions differed in length (from around half an hour to two and a half hours) depending on how many blocks the animal completed. A single 80 trial block was usually completed by a monkey in about 25 minutes.
Data Analysis
Analyses were performed using custom-written Matlab (Math Works, Inc., MI) routines, and significance testing was performed both within Matlab and through use of SPSS software (version 13).
Looking preferences analyses
During presentation of the test stimuli, at any given moment of time it was possible for a monkey to be looking at one of three areas: between the two images, where the fixation spot had been presented; at the novel image; and at the familiar image. To first examine the data, each time point (sampled at 200 Hz) on each trial was coded as either 1, indicating the monkey was looking at the novel image, -1, indicating that the monkey was looking at the familiar image, or 0, indicating the monkey was looking between the two images. These coded time-series could then be averaged, according to the condition of interest, to generate curves that indicated the moment-by-moment tendency for a monkey to look at the novel or familiar stimulus. Evaluation of regions of time for which the monkey had a looking preference was done by performing a chi-square analysis on the relative numbers of 1's versus -1's for each time point. Separate analyses were conducted on a series aligned to the cue presentation versus those aligned to the first saccade.
Novelty preference during a given trial could be summarized as the percent of time that the monkey was looking at the novel stimulus as compared to the familiar (the number of 1's as compared to 1's and -1's). These proportions are usually used in the literature as the dependent variable, where 0.5 or 50% is chance, and significantly above or below 50% reflects a novelty and familiarity preference respectively. Commonly overlooked, however, is the fact that proportions limited to the range between zero and one often do not distribute normally. An examination of the distributions in the current study revealed that they were highly variable, and poorly-suited for parametric statistics. Correlations were therefore performed between the dependent variable (percent time looking at the novel image), and independent variables (age, delay, etc.) by calculating the Spearman's rho correlation coefficient. In cases for which pair-wise comparisons were made (e.g., comparing the novel/familiar versus familiar/familiar conditions) an independent sample Mann-Whitney U test was performed. For graphical purposes, error bars were generated using a bootstrapping procedure (generating new distributions by sampling with replacement from the data) where the medians from the original distribution were used to describe the central tendencies, and the standard deviations of the medians from bootstrapping served as confidence intervals.
In addition to novelty preferences, we also made age-comparisons of eye-movement variables, including reaction time (time until first saccade), number of saccades, saccade amplitude (distance of eye movement for each saccade), and center crossings, which was the number of times the animal generated a saccade between the two images. Initial examination of the data revealed that tracker noise varied between monkeys and that this noise had an influence on saccade detection. To identify saccades in a way that was robust against noise, we first resampled the tracker data at 50 Hz. This helped eliminate spurious points. To identify saccade start times, the instantaneous velocity was calculated on the resampled data by calculating the distance from one x-y tracker point to the next. Saccade periods appeared as transient increases in the instantaneous velocity that exceeded the median plus two standard deviations. The first time point at which the velocity exceeded threshold was taken to be the saccade start time. Saccade amplitude was then estimated as the integral of the period that velocity exceeded threshold. Between-subject comparisons of saccade number and saccade amplitude were analyzed using the general linear model. Reaction time analyses were treated with the non-parametric statistics described above. Finally, to analyze correlations with center crossings, which could be described with a Poisson distribution, we used a generalized linear model with a Poisson link function (glmfit command in Matlab).
Results
In order to use the VPC task to test the hypothesis that recognition memory is impaired with aging, it was necessary to establish that the aged subjects were capable of making the eye movements necessary to keep their eyes on the fixation spot or images, and to generate accurate saccades to a target stimulus. Because these abilities were required for task performance, VPC testing sessions did not begin until the monkeys were capable of completing a session with less than 20% error rates. Additionally, VPC testing sessions were preceded by a block of eye-tracker calibration. The VPC blocks did not begin until the monkey consistently made accurate, targeted saccades and fixated on images presented in all five computer screen locations. There were no apparent age effects on latency to reaching the target stimulus, suggesting that age did not affect the accuracy of targeted saccades (median +/- standard deviation; BZ-11: 393 +/- 374 ms; WN-13: 692 +/- 460 ms; CL-17: 192 +/- 160 ms; BL-25: 260 +/- 174 ms; NS-29: 298 +/-281; JN-30: 568 +/- 532).
For each experimental procedure, the total number of blocks, number of correct trials and the mean percent correct trials for each block are shown in Table 1. The number of correct trials refers to the sum of all the correct trials across all blocks; this measure reveals how frequently the monkeys maintained their gaze within the viewing window. On average, each animal completed 5 blocks of the Novel/Novel test, 5 blocks of the Familiar/Novel test, 4 blocks of Familiar/Familiar, and 3 blocks of the long delays test (2 of the 24 h test and 1 of the 48 h test). For all subsequent analyses, only the correct trials were examined: the primary dependent variable of interest was not how well monkeys could look at the images, but the proportion of time that the monkeys viewed the novel versus familiar image.
Table 1.
| Experimental Procedure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novel/Novel | Familiar/Novel | Familiar/Familiar | Long Delay (24h) |
Long Delay (48h) |
||||||||||||
| Monkey | Age (years) | # of sessions | # correct trials | Mean % correct trials | # of sessions | # correct trials | Mean % correct trials | # of sessions | # correct trials | Mean % correct trials | # of sessions | # correct trials | Mean % correct trials | # of sessions | # correct trials | Mean % correct trials |
| PC | 6 | 8 | 546 | 92 | 4 | 320 | 85 | 2 | 160 | 87 | - | - | - | - | - | - |
| BZ | 11 | 5 | 400 | 59 | 6 | 474 | 76 | 3 | 273 | 63 | 2 | 40 | 69 | 1 | 20 | 87 |
| WN | 13 | 3 | 240 | 57 | 5 | 395 | 46 | 3 | 240 | 59 | 2 | 40 | 51 | 1 | 20 | 43 |
| CL | 17 | 4 | 320 | 73 | 6 | 480 | 61 | 7 | 560 | 76 | 2 | 40 | 95 | 1 | 20 | 57 |
| BL | 25 | 5 | 400 | 71 | 4 | 320 | 64 | 3 | 240 | 65 | 1 | 20 | 77 | 1 | 20 | 81 |
| NS | 29 | 4 | 220 | 40 | 3 | 240 | 54 | 4 | 223 | 50 | 1 | 20 | 71 | 1 | 20 | 80 |
| JN | 30 | 4 | 276 | 46 | 5 | 400 | 54 | 3 | 240 | 56 | 2 | 40 | 75 | 1 | 20 | 65 |
| Age group | Young (n=3) | 16 | 1186 | 69 | 15 | 1189 | 69 | 8 | 673 | 70 | 4 | 80 | 60 | 2 | 40 | 65 |
| Old (n=3) | 13 | 896 | 52 | 12 | 960 | 57 | 10 | 703 | 57 | 4 | 80 | 74 | 3 | 60 | 75 | |
Main effect
Our first analyses were directed at determining whether there was a main effect of age on novelty/familiarity preference. From each monkey, completed trials (trials without fixation errors) were examined during the novel/novel task sessions. Figure 2A shows the tendency for monkeys to look at novel images (coded as 1) versus familiar images (coded as -1) by averaging codes across trials at each time point following the presentation of test stimulus pairs. After an initial reaction time delay of about 200ms, younger monkeys showed a robust tendency to start looking at the novel image, which then declined at different rates over the two second viewing time for different individuals. Aged monkeys, however, showed less robust eye movements toward the novel stimulus throughout the two-second test period. There were, however, time periods during which novelty preference responses were significantly above chance for both the 25-year old BL and 30-year-old JN (chi-square test p < 0.05 on each 5ms bin size, comparing the proportion of novel-side eyetracker points to familiar-side points). As the curves in Figure 2A suggest, the periods of time during which monkeys had significant novelty preferences varied between monkeys but generally ranged from about 250ms to 1.5 seconds. The variability in the shape of the averages was reduced when the averages were aligned to the first saccade of each trial (Figure 2B), suggesting that individual differences in reaction times account for much of the variation. In these response-locked aligned averages, the novelty-preference peaks appeared around 200ms, and the height of the peaks reduced almost linearly with increasing age. The percent of time monkeys spent looking at novel images was significantly correlated with age (Spearman's rho = -0.127; p < 0.001; Figure 2B). In both the cue-aligned and response-aligned analyses, all monkeys with the exception of the 29-year-old NS, showed an interval of significant novelty preference. The same pattern can also be observed by comparing the percent of time across trials monkeys spent looking at the novel as compared to familiar images (Figure 2C).
Figure 2.
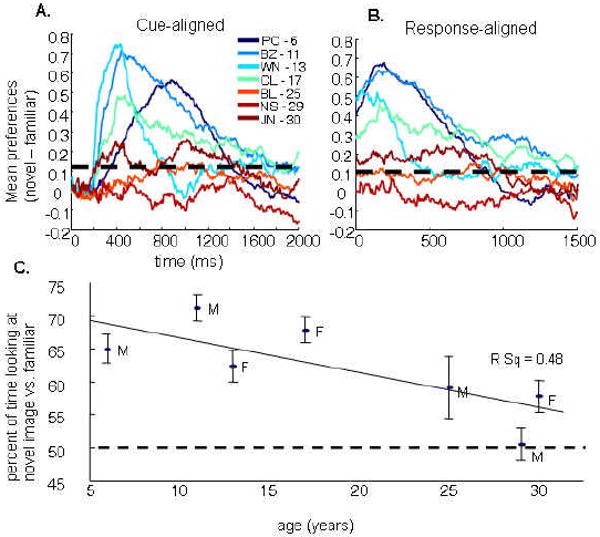
Novelty preference declined with age, but was present in all but one monkey. A) Novelty preferences during the two second test period. Traces represent cross-trial averages of when a monkey's gaze was on the novel image (coded as a 1), familiar image (coded as a -1), or neither (coded as 0) for all tracker points aligned to the presentation of test stimulus pairs. Each trace represents a different monkey, ascending ages plotted from a blue to red color mapping. Periods of significant preference can be evaluated by performing a chi-square goodness of fit test at each point (approximated in the figure by the horizontal, dashed black line). All monkeys show some period of novelty preference except for the 29 year-old NS. B) Novelty preferences during the two second test period following the first saccade. Traces are the same as in “A”, in this case, however, averages are aligned to the first saccade, revealing greater consistency across animals in the time course of novelty preference patterns. Aged monkeys appear to show the most dramatic reduction in novelty preference during the early phase of stimulus viewing. C) The median percent of time that monkeys looked at the novel image as compared to the familiar image across all novel/novel trials are plotted by monkey age. Error bars are confidence intervals estimated by the standard deviation of the medians collected from 100 bootstrapped samples from each animal.
Delay dependence
After determining that novelty preference declines with age, the next step was to investigate the hypothesis that the age-difference may partially be a result of long-term memory changes. Previous studies have shown that damage to medial temporal lobe regions that are important for long-term memory result in novelty-preference declines selectively at longer (above 10-30 seconds) delays (Buffalo et al., 1999; McKee & Squire, 1993; Nemanic et al., 2004; Pascalis & Bachevalier, 1999). Thus, the degree to which the age-related novelty-preference declines were primarily taking place during longer (above 10 or 20 seconds) delays was investigated. Rather than the expected decline in preference over the 1 to 20 second delays, the pattern of viewing was relatively constant (Figure 3; Spearman's rho correlation, p > 0.05 for all monkeys).
Figure 3.
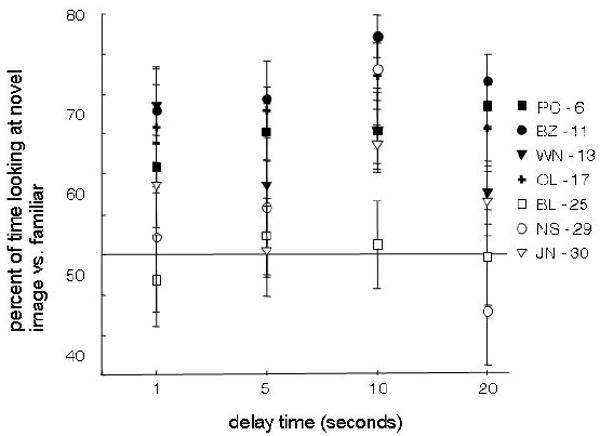
Novelty preference scores did not depend on the delay interval. Trial medians for the percent of time looking at the novel versus the familiar image are plotted for each monkey across delay intervals. Neither young (solid lines) nor aged adult monkeys (dashed lines) were more or less likely to look at the novel image with increasing delay between sample and test stimulus.
Familiarity dependence
The degree to which an animal shows a preference for novelty likely depends on the degree to which it is able to encode the stimuli, which may differ between age groups due to differences in perceptual abilities or general differences in neural responsiveness or plasticity. If this explanation accounts for the novelty preference changes with aging, then it may be possible to overcome the reduced encoding by extensive exposure (or “over-familiarizing” the stimuli). In the familiar/novel condition, sample stimuli had been viewed by the monkeys many times over many days of training and appeared beside novel stimuli during the test phase. In the familiar/familiar condition, monkeys had been familiarized with both test stimuli.
As expected, animals were generally more likely to look at the novel stimulus in the familiar/novel condition (Mann-Whitney U-Test, z = -6.44, p < 0.001; Figure 4). It was not the case, however, that aged animals differentially increased preference for the novel images when they were presented alongside highly familiarized images. Comparisons performed on each monkey individually revealed that the magnitude to which sample familiarization affected novelty preference was greatest for the younger monkeys. Neither the intermediate-aged (17 year-old) monkey CL or the aged, 29-year old monkey NS, were significantly influenced by stimulus familiarization (Mann-Whitney U-Test, alpha = 0.05). The chi-square analysis performed on the time-series revealed a similar effect: the two youngest monkeys, PC and BZ, followed by the oldest monkey, JN, were more likely to look at the novel image in the familiar/novel as compared to the novel/novel condition for the longest periods of time during the 2-second test phase (alpha = 0.05 for each tracker time point).
Figure 4.
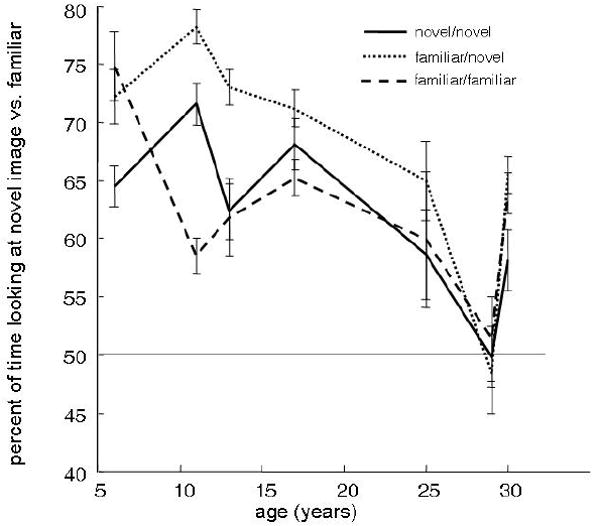
Median percent of time each monkey spent looking at the novel versus familiar image is plotted for all monkeys for three different conditions: novel/novel (solid), familiar/novel (narrow dash), and familiar/familiar (wide dash). Monkeys were more likely to show novelty preference if they had been highly familiarized with the sample stimulus, but not if they were highly familiarized with both stimuli presented during the test phase. The increase in preference with sample familiarization was most robust for the younger monkeys. This is inconsistent with the hypothesis that age-related novelty preference changes are associated with stimulus encoding difficulties.
Correlation with eye movement variables
A number of eye movement variables were examined and correlated with the novelty preference data. Several factors were analyzed with the expectation that they may reveal individual differences in how the monkeys behaved in the task. These included: 1) the average number of saccades made during sample and test phases; 2) the average amplitude of saccades during sample and test phases; 3) the reaction times for the first saccades during the test phase; 4) the number of times the monkey crossed the center to look from one image to the other (center crossings); 5) the stereotypy in direction of the first saccade.
Saccade dynamics
The number of saccades made during sample images and test images distributed normally for each animal, enabling the use of general linear model statistical tests. The mean number of saccades made per trial differed between animals (analysis of variance, p < 0.001 for both sample and test phases) and there was a significant regression across age (r-square = 0.257 for sample phase, 0.208 for test phase, p < 0.001). As Figure 5A and 5B demonstrate, however, two monkeys made consistently fewer saccades than did the others: the 25-year-old BL and the 29-year-old NS.
Figure 5.
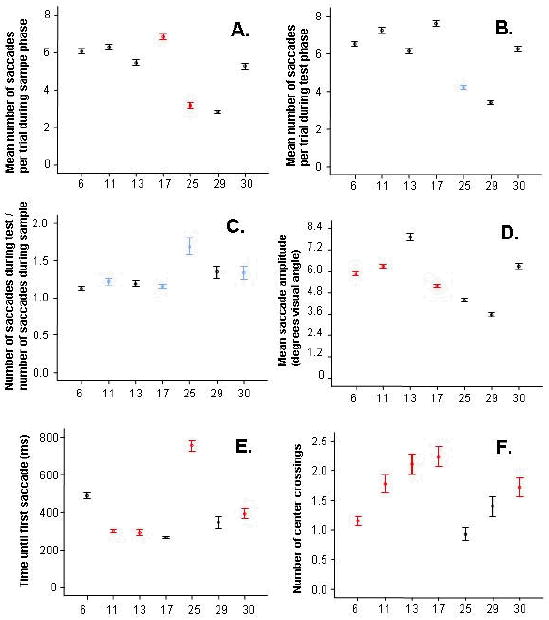
Eye movement variables during the test phase are plotted for each monkey according to age. Significant correlations in individual monkeys between eye-movement factors and novelty preference scores are shown in red (negative correlation) and blue (positive correlation); black points indicate no significant correlation in the individual animal. A) The two male aged monkeys showed fewer saccades during the sample phase. In both the 17-year-old CL and the 25-year-old BL, fewer saccades during this encoding phase tended to correspond to higher novelty preference scores. B) As with the sample phase, the two male aged monkeys made fewer saccades during the test phase. In contrast with the sample phase, the 25-year-old BL tended to make more saccades during trials in which he showed higher novelty preference. C) The ratio of saccades made during test versus sample phases of each trial correlated with novelty preference, suggesting a relationship between more novelty preference with longer fixations during the sample phase, and increased novelty preference with greater image exploration during the test phase. This effect was most robust in the 25-year-old monkey, BL, but was significant in four individual monkeys, and showed a strong trend in two others. D) Saccade amplitude also correlated negatively with age. E) Reaction time was not correlated with age, though there was an indication of greater variance in the aged animals (a finding examined further in Figure 6). F) While the number of center-crossings did not correlate with age, the two aged males were the only monkeys that did not show a negative correlation between center crossings and novelty preference. Error bars represent 95% confidence intervals. Generally, the aged male monkeys, BL and NS, tended to show very different patterns of eye movements, including how those movements correlated with novelty preference, than the other monkeys in the study.
The relationship between saccade number and novelty preference were examined in each monkey. In the 17-year-old monkey CL and the 25-year-old BL, reduced saccades during the sample period correlated with increased novelty preference (CL: Spearman's rho = -0.159 CL, p = 0.004; BL: Spearman's rho = -0.322, p < 0.001; Figure 5A). Trends in the same direction were observed in all monkeys besides the youngest two. During the test condition, however, the same aged monkey, BL, showed the opposite pattern, in which a greater number of saccades correlated with higher novelty preference scores (Spearman's rho = 0.174, p < 0.001; Figure 5B). This observation could be explained, for example, if saccade frequency correlates with interest in the image. A reduced interest during the sample phase may relate to increased preference for the novel test image, just as increased interest during the test phase may be a specific interest for the novel image. To investigate this pattern further, we examined how novelty preference correlated with the ratio of saccades made during the test period versus sample period (Figure 5C). This ratio appeared to be higher and more variable in the aged monkeys (R-square = 0.042, p < 0.001). This was particularly true for the 25-year-old, BL. There was an overall correlation of this ratio with novelty preference (Spearman's rho = 0.143, p < 0.001), and this was significant in four of the seven monkeys, apparently independent of age (Spearman's rho: PC-6: 0.082, p = 0.055; BZ-11: 0.114, p = 0.022; WN-13: 0.049, p = 0.45; CL-17: 0.129, p = 0.021; BL-25: 0.382, p < 0.001; NS-29: 0.112, p = 0.097; JN-30: 0.169, p = 0.005).
Saccade amplitudes during the test session also distributed normally across animals, and, like saccade number, also declined with age (r-square = .087, F = 227.8 p < 0.001; Figure 5D). As with saccade number, the oldest, 30-year-old monkey JN resembled the younger animals, while the 25 and 29 year-old monkeys generally made particularly short saccades. Trials with shorter saccades were correlated with increased novelty preference scores in most of the younger monkeys, but not in the aged monkeys (PC-6: -0.286, p < 0.001; BZ-11: -0.332, p < 0.001; WN-13: -0.075, p = 0.25; CL-17: -0.320, p < 0.001; BL-25: 0.047, p = 0.35; NS: -0.088, p = 0.20; JN-30: -0.116, p = 0.056).
Reaction times
Reaction times to first saccade did not correlate with age (Spearman's rho = -0.013, p = .52; Figure 5e). Examination of reaction time distributions, however, revealed that reaction times tended to be more broadly distributed in the aged monkeys (Figure 6). The most unusual reaction times were observed in the 29-year-old, NS, who tended to move his eyes immediately after the test stimuli appeared, or approximately 400ms later. These predictive saccades were not observed in any of the other monkeys, and suggest that the animal's first eye movements were not made in reaction to the stimuli. The two other aged monkeys, 25-year-old BL and 30-year-old JN, both showed an almost bimodal reaction time response. In these monkeys, as in two of the younger monkeys (BZ-11, WN-13), novelty preference was increased during trials with faster reaction times (Spearman's rho: PC-6: 0.013, p = 0.76; BZ-11: -0.175, p < 0.001; WN-13: -0.204, p = 0.001; CL-17: 0.030, p = 0.60; BL: -0.132, p = 0.008; NS-29: -0.024, p = 0.72; JN-30: -0.136, p = 0.02).
Figure 6.

Histograms of reaction times before the first saccade reveal striking differences between aged versus young adult monkeys. Monkey NS shows what may be a complete absence of cue-evoked saccades, instead showing predictive saccades (though the delay was jittered, potentially making these conducive to high error rates) and a normally-distributed collection of saccades centered around 400ms. Monkey PC and BL show a wider distribution of saccades, though the first peak of BL is delayed to about 400ms. Finally, monkey JN shows a peak matching the young animals in addition to a smaller, much slower reaction time peak. Horizontal axes on all plots are scaled equally, while peaks are normalized to the window.
Center crossings
The number of times monkeys looked from one test image to the other in a trial (the “center crossings”) varied across trials according to a poisson distribution. There was no significant regression between center crossings and age (coefficient = -0.002, p = 0.39; Figure 5F). Younger monkeys were more likely to look at the novel image on trials in which they looked back-and-forth between images less, although this correlation was absent in both the 25-year-old BL and 29-year-old NS (Spearman's rho; PC-6: -0.259, p < 0.001; BZ-11: -0.512, p < 0.001; WN-13: -0.173, p = 0.007; CL-17: -0.360 p < 0.001; BL-25:-0.076, p = 0.13; NS-29: 0.019, p = 0.781; JN-30: -0.177, p = 0.003).
Side biases
There are several explanations for what might guide saccade behavior if not novelty. One possibility was that monkeys were consistently making the same motor actions each trial, or were attracted to images on the same side of the screen. When restricting analysis just to the first saccade, all three aged monkeys showed a significant preference for looking toward a particular side (chi-square left-targeted versus right-targeted saccades; BL-25: 26.9, p < 0.001; NS-29: 38.8, p < 0.001; JN-30: 10.46, p < 0.001; Figure 7). This was not the case for any of the younger animals. After the first saccade, monkey BL had the most robust side bias, often gazing at the same image throughout the remainder of the trial (chi-square for each tracker point, p < 0.05). The other aged monkeys, NS (29) and JN (30), did not tend to maintain their gaze on the side to which they initially looked.
Figure 7.
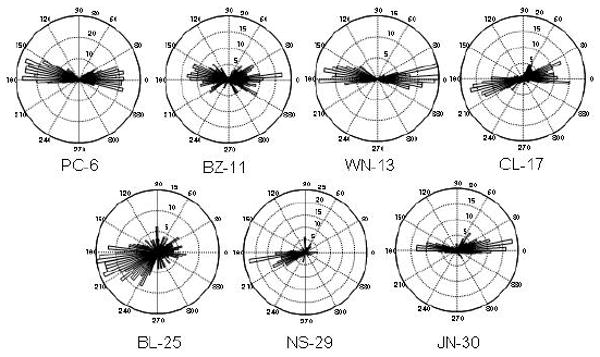
Circular histogram plots show the direction of all first saccades during the test phase. Each 360 degree circle is binned into 100 slices representing the angles of the saccade trajectory from the fixation point. The longer a bar is in the 180 direction, for example, the more times the monkey made its first saccade directly to the left side of the computer screen. Histogram counts are described by the numbers at each ring. All three aged monkeys (BL, NS, JN) exhibited a significant side bias. Side biases with the first saccade were particularly prevalent in monkeys BL (25 years) and NS (29 years). The low variance in the histogram of monkey NS suggests he made particularly stereotyped first saccades. Further analyses revealed that following the first saccade, monkey BL tended to maintain gaze on the image, while monkey NS began shifting gaze between sides following approximately one second of looking time.
Summary of Movement Analyses
Several factors stand out in the eye movement analysis, particularly with respect to monkeys BL (25) and NS (29). Both of these monkeys show a strong reduction in numbers of saccades, saccade amplitudes, and number of center crossings. They also show dramatic differences from the other monkeys in the degree to which their first saccades appear to be evoked by the stimulus presentation, based on the high variance in the timing between stimulus and response. This was particularly the case in the 29-year old NS. Unlike other monkeys, the novelty preference in monkey BL (25-years) appeared to increase during those trials in which he showed fewer saccades during the sample phase and more during the test phase. Also unlike other monkeys, neither BL nor NS were less likely to look at the novel image with more center-crossings. In monkey NS, movements were consistently different from the others, with the first saccade made in a consistent, stereotyped direction toward the left side of the screen. In this monkey, no movement parameters predicted increased novelty preference, and trials of high novelty preference were as probable as trials with low novelty preference. Finally, in monkey JN there was a bimodal reaction time distribution, and her tendency to look at novel images increased with shorter reaction times.
Novelty preference in trials with short reaction times
The unusual reaction time distributions in the aged monkeys suggested that during certain trials the aged monkeys may have been behaving in a way that was less dependent on the presented stimuli. To explore this further, the data from aged monkeys was reexamined after removing the trials in which reaction times were outside of the 95% confidence interval of young monkeys, BZ (11 years), WN (13 years), and CL (17 years), who reacted to the test stimuli with high temporal consistency. Because 95% of the reaction times in these animals fell between 200ms and 480ms from presentation of test stimuli, trials were removed in the aged monkeys that fell outside this range (yielding 78 of 400 trials in BL, 103 of 220 trials in NS, and 186 of 276 trials in JN).
Figure 8 shows the new between-subjects comparisons after trials had been removed based on reaction time. Both the 25-year-old monkey BL and the 30-year-old monkey JN had higher novelty preference scores. This was expected based on the negative correlation between reaction time and novelty preference presented in Figure 5E. More interesting, however, was how trial removal affected the relative performance across the short (1-20s) delay intervals. Individually, there was still no significant effect of delay on the novelty preference scores in BL or JN (Spearman's rho; JN = -0.061, p = 0.412; BL = -0.177, p = 0.121; Mann-Whitney U-test comparing 20 second delay to 1-second delay: BL: Z = -1.48, p = 0.14; JN: Z = -1.3, p = 0.19). There was, however, a significant negative correlation between age and novelty preference scores during the 20 second delay trials (Spearman's rho = -0.17, p < 0.001) but no correlation between age and novelty preference scores when delays were less than 20 seconds (Spearman's rho = -0.033, p = 0.23). These results can be contrasted with the same analyses performed with all trials included, which yield significant age effects on novelty preference even in the shortest (1 second) delay trials (Spearman's rho = -0.085, p < 0.05).
Figure 8.
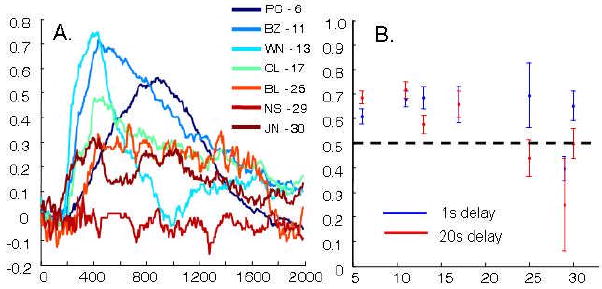
Novelty preference in aged animals was investigated after excluding trials with particularly fast (under 200ms) or slow (above 480ms) reaction times. A) Same as Figure 2A. The exclusion of abnormal reaction time trials increased the novelty preference for most of the test phase for both 24-year-old BL and the 30-year-old JN, but not the 29-year-old NS. B) Medians of the percent of time looking at the novel image at one-second delays (blue) and twenty-second delays (red) are plotted across monkey ages. Young monkeys show similar novelty preference scores for both delay periods. Both BL and JN have similar novelty preference scores as young animals in trials with shorter, one-second delays, while at the longer, twenty-second delays scores approximate chance. There is no correlation between novelty preference and age for trials with delays under 20 seconds, but there is for trials with twenty second delays, consistent with the hypothesis that aged monkeys have recognition impairments at longer delays.
No consistent pattern emerged from trial removal in the 29-year-old monkey NS. This appeared to be true independent of the eye-movement variable used to select trials. For example, as Figure 7 shows, NS was highly consistent, or stereotyped, in the direction of first saccade. Trials in which NS made first saccades in this direction had no greater or lesser novelty preference than trials in which NS began by looking in a different direction (median = 51% toward the novel image when looking toward the stereotyped direction, 48% when looking toward another direction).
Novelty preferences at long delays
The test phase of long-delay sessions took place 24 and 48 hours after exposure to the sample phase. In both conditions, there was a positive correlation between novelty preference scores and age (Spearman's rho; 24 hour delay = 0.148, p = 0.05; 48 hour delay = 0.211, p = 0.04; combined = 0.166, p = 0.005). This pattern was exactly opposite to that of the novelty preference patterns at short delays, and could have resulted from a greater tendency in younger animals toward familiarity preferences on the one hand, and increased novelty preference in the aged monkeys on the other.
Eye movement variables were also analyzed for the long delay condition. Although reaction time distributions were shaped similarly as in the short-delay conditions, they tended to be much longer in the long delay condition (Mann-Whitney U test for all monkeys: p < 0.001; BZ-11: p <0.001; WN-13: p < 0.001; CL-17: p = 0.25; BL-25: p < 0.001; NS-29: p = 0.94; JN-30: p < 0.001). Between the novel/novel short delay condition and the long delay conditions, in the two younger monkeys, BZ (11 years), and WN (13 years), median reaction time went up from 280ms and 240ms to 800ms and 810ms respectively. In the aged monkeys BL (25 years) and JN (30 years), median reaction time increased from 740ms and 300ms to 960ms and 800ms respectively. As in the short delay condition, there was still a significant negative correlation between age and saccade number (r-square = 0.174, p < 0.001), and saccade number was not significantly lower for any monkey in the long delay test condition as compared with the novel/novel short-delay condition (independent samples t-test, alpha = 0.05).
Discussion
The most important overall finding in these experiments was that aged animals exhibit reduced novelty preference in the visual paired comparison (VPC) task at delay intervals out to 20 seconds. This finding is consistent with the hypothesis that age-dependent recognition impairments exist even in the absence of reward contingencies that might otherwise engage strategic encoding and retrieval processes. The use of high-precision eyetracking methods (which in this case required implantation of head posts) made it possible to examine the novelty preference time course within the two second looking interval. These analyses revealed that monkeys were most likely to view the novel image with earlier saccades, and that that this novelty preference during initial viewing appeared to be the component most sensitive to age. Investigation of novelty preference for different delay conditions revealed that these age-related changes were not only an effect of compromised memory systems. Different memory systems at both the cellular and systems levels display unique time courses, and impairment of these systems would be expected to affect memory selectively on tests given at relevant delays. The observed age-effect, however, was present even in the shortest interval between familiarization and test. This encourages additional explanations to be entertained. One possibility is that the aged monkeys suffered a perceptual or encoding deficit (compromised input to the system). An alternative explanation is that the aged adults were particularly sensitive to influences other than novelty, such as distractions in the room, the images depicted in the stimuli, or even instrumentally-learned “superstitious” behaviors. It can be argued, however, that the data are most consistent with another alternative, namely, that aging is associated with a reduction in the degree to which novelty drives the generation of eye movements. Each of these hypotheses is addressed in turn.
Perceptual and Encoding Impairments
The hypothesis must be entertained that perceptual deficits could contribute to the observed age-related impairment in novelty preference. Eye exams and the eye-tracker calibration tasks suggest reasonable visual acuity and tracking ability in the old monkeys compared with the young. A minor visual deficit could lead to reduced novelty preference if the monkeys could not discriminate between the two images. This seems unlikely, however, because the randomness of image selection meant that there was an infinite possible range of shape, edge frequency, color, objects, and luminance; thus, the probability of high enough similarity between two arbitrary image pairings was minute. Overall, the present data provide no conclusive support for the idea that visual differences between age groups significantly contribute to behavioral differences.
The hypothesis that encoding impairments account for the reduced novelty-preference scores was tested in the familiar/novel experimental condition, in which monkeys were familiarized with the sample stimulus by multiple days of training. If old monkeys have an encoding deficit, then they should benefit more from the familiarity treatments than the younger monkeys. At least one of the aged monkeys, the 30-year-old JN, did benefit from increased familiarity of the sample stimulus. This effect, however, was not greater in magnitude than the increases observed in the younger monkeys. If anything, older monkeys were less influenced by the increased familiarity of the sample stimuli than were the young monkeys. Although encoding processes may be changed in the aged animals, any encoding differences can not completely explain the dramatic decrease in novelty preference with age.
Interference
Among the factors, besides novelty, that may have influenced the saccade-generation system, include the possibility that aged animals are sensitive to sounds outside of the testing chamber, or more interested in the pictures depicted in the task stimuli. Both of these explanations seem unlikely. Environmental distractions would likely have caused monkeys to look away from the computer screen entirely, but the training protocol ensured that all monkeys were capable of fixating and keeping eye movements within the viewing window, and trials in which monkeys failed to do so were removed from analysis. If aged monkeys were differentially more interested in specific images than were young monkeys, they should not have exhibited the strong side biases that were observed because each image was repeated during the test session on both the left and right side of the screen.
Another factor that could have influenced novelty preference scores in the older monkeys is if the saccades themselves became controlled as an instrumental behavior. Eye movements within the image window are assumed to be flexibly directed by the features of the images themselves; however, if the monkey makes a saccade away from the images, the trial is aborted and reward is omitted. A higher frequency of aborted trials might lead to the reinforcement of less flexible, “superstitious,” eye-movement patterns. The highly stereotyped first-saccades observed in the 29-year-old NS may be an example of this. However, monkey NS had equally low novelty preference scores on trials in which he looked toward the stereotyped direction as on trials in which he looked in an irregular direction, indicating that controlled action processes could not account for his reduced novelty preference.
Together, these data suggest that the age-dependent reduction in novelty preference is likely not due to interference factors. It should be noted that, in contrast to traditional memory test paradigms in which interference might refer to the disruption of goal-directed encoding and retrieval processes, in the present paradigm there is no reason for encoding and retrieval to be directed by top-down goals. Rather, the effects of interfering factors in this situation may reflect how the relative intrinsic values of ambient stimuli, task stimuli, or actions themselves might override the value of novelty in directing the animal's behavior.
Disconnect between novelty detection and saccade generation: a case made for dopamine
In addition to reduced novelty-preference scores with age, saccade latencies were often longer or more broadly distributed in the aged adults. The same effect of age on saccade latencies has been found in humans for a variety of eye-movement tasks (Abel & Douglas, 2007). Aging was also associated with a reduction in saccade number and amplitude, particularly in two of the aged animals, 25-year-old BL and 29-year-old NS. These bare a striking resemblance to the effects of reduced dopamine on eye movements. Saccade frequency and amplitudes are reduced in 1-methyl-4-phenyl1,2,5,6-tetrahydropyridine (MPTP) dopamine-depleted humans (Hotson, Langston, & Langston, 1986), and rhesus macaque monkeys (Brooks, Fuchs, & Finocchio, 1986; Kato et al., 1995; Schultz et al., 1989). Saccade latencies toward visual targets also increase with reduced dopamine in the striatum (Hikosaka, 2007; Kori et al., 1995; Schultz et al., 1989). In addition to the role of midbrain dopamine activity in directing saccades, the activity of dopamine neurons is increased in response to novelty (Bunzeck & Duzel, 2006; Ljungberg, Apicella, & Schultz, 1992; Steinfels, Heym, Strecker, & Jacobs, 1983; Zhu, Bardo, Bruntz, Stairs, & Dwoskin, 2007), and dopamine antagonists can dramatically reduce novelty-seeking behaviors (Peters, Vallie, Difronzo, & Donaldson, 2007). Based on these parallels, the hypothesis emerges that the reduced novelty preference scores might result from an altered dopaminergic contribution to saccade generation with age. This hypothesis is also supported by studies showing that the midbrain dopaminergic system is negatively affected by age (Goldman-Rakic & Brown, 1981; Hemby, Trojanowski, & Ginsberg, 2003; Ma et al., 1999; Wang et al., 1998). Further studies relating age to novelty preference, saccade dynamics, and dopamine will be necessary to confirm or reject this explanation.
Memory deficit? Novelty preference in trials with short reaction times
When the aged monkeys were reexamined after removing trials with abnormally long reaction times, novelty preference scores were much higher in two of the three old monkeys. Age was no longer negatively correlated with novelty preference when the delay between familiarization and test was between 1-10 seconds. However, on trials with a twenty second delay between familiarization and test, age remained correlated with novelty preference scores. This is consistent with the hypothesis that age is associated with recognition memory impairment by comparison with the patterns observed in studies following hippocampal lesions (Nemanic et al., 2004; Pascalis & Bachevalier, 1999), or, given the short time-course, lesions to the perirhinal cortex (Buffalo et al., 1999; Nemanic et al., 2004). The data are also congruent with the hypothesis that age-related changes in plasticity processes (Deupree, Bradley, & Turner, 1993; Moore, Browning, & Rose, 1993; Rosenzweig, Rao, McNaughton, & Barnes, 1997), network activity (Barnes, Suster, Shen, & McNaughton, 1997; Shen, Barnes, McNaughton, Skaggs, & Weaver, 1997; Tanila, Shapiro, Gallagher, & Eichenbaum, 1997; Wilson et al., 2004), and structural changes in the temporal lobe (De Toledo-Morrell, Goncharova, Dickerson, Wilson, & Bennett, 2000; Geinisman, de Toledo-Morrell, Morrell, Persina, & Rossi, 1992; Kramer et al., 2007; Yonelinas et al., 2007) have important functional consequences for cognition in older animals.
Increased novelty preference with aging when tested at 24 and 48 hour delays
In contrast to the shorter (1-20 second) delay conditions, at longer (24 and 48 hour) delays, aging was associated with higher novelty preference scores. This relationship was robust in both the 24 and 48 hour conditions, but appears paradoxical in light of the observed reduction in novelty preference at the shorter delays. Table 1 shows that aged monkeys were more successful completing trials during the long delay conditions (75% in long delay condition versus 52% in the novel/novel short delay condition). The analysis of eye movement variables also revealed that saccade latencies were increased in long delay trials as compared to the short-delay, novel/novel condition. Saccade number, on the other hand, was not significantly changed between conditions. The longer saccade latencies may provide a partial explanation for the paradoxical reversal of novelty preference at long delays. Such slow reaction times imply that the integration times were longer, and therefore that the underlying processing taking place during behavior in the long-delay conditions may be qualitatively different.
The reduced novelty preference in the younger monkeys during long delay does not necessarily indicate that the young monkeys did not recognize the stimuli. Previous work on infants has shown that there can be a shift between novelty to familiarity preference depending on the interval between exposure and re-exposure to the stimulus (reviewed by Pascalis & de Haan, 2003). The same is true for normal adults, and the direction of preference is not predictive of whether or not the subject recognizes the stimulus (Richmond, Colombo, & Hayne, 2007; Richmond et al., 2004). One possibility is that this process is changed with age, due either to different plasticity dynamics or reliance on different memory systems.
Although a number of mechanistic explanations are possible, aging is clearly associated with abnormal looking behavior compared with the younger animals in the long delay conditions. Additionally, if age-dependent changes to the dopamine system are responsible for the reduction in novelty preference at short delays, this might imply that novelty-preference responses at longer delays is less dependent on the dopamine system. The longer reaction times of young and old monkeys at the 24 and 48 hour delays may be consistent with the view that novelty preference in this case is not based on a quick burst of dopamine in the caudate.
Implications
The goal of the present study was to test the hypothesis that aging affects recognition memory even when the animal is not motivated to engage strategic encoding and retrieval processes. The prediction that aged monkeys would have reduced novelty preference as compared to younger monkeys in the longer (20 second) delay intervals, was validated, but was accompanied by a reduced novelty preference at even shorter (1-10 second) intervals, particularly on trials with longer reaction times. If the amount of time a monkey spends looking at a novel image in comparison to a familiar image is thought of as the amount of relative attention devoted to novelty, then these results might appear to have more implications for the effect of aging on attention to novelty, rather than memory for the familiar stimulus. On the other hand, reduced attention to a stimulus will undoubtedly reduce the degree to which a stimulus is encoded, and memory failures may then appear at a later time point. If aged animals show reduced spontaneous attention to novelty, then incidental novel stimuli are less likely to be encoded, and may therefore result in subsequent memory deficits. This has important implications for real-world settings, in which memory deficits might often not be for the stimuli or events that at first appeared important, but those that were incidental features of the environment. Ironically, removing age-effects on motivated, strategic memory processing reveals a strong age-effect on the innate tendency to encode novelty. If the attentional deficit is mediated by dopamine dysfunction, as presently hypothesized, then drugs that affect the dopaminergic system may help alleviate some of the memory impairments observed in old age.
Figure 9.
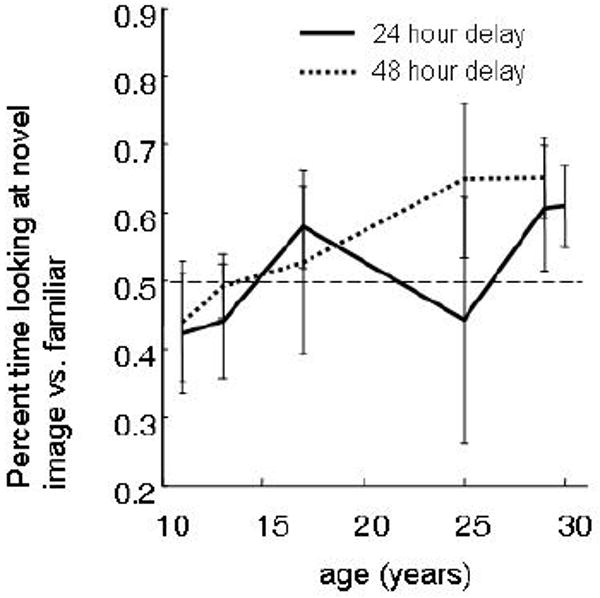
When novelty preference was tested 24 or 48 hours following stimulus encoding (solid and dashed lines respectively), aged monkeys showed relatively higher novelty preference scores than did young adults. This pattern was the opposite to the predicted pattern, and was consistent for both the 24 and 48 hour delays sessions.
Acknowledgments
Supported by: AG003376, the state of Arizona and ADHS, California National Primate Research Center Grant P51 RR000169, the McKnight Brain Research Foundation.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
References
- Abel LA, Douglas J. Effects of age on latency and error generation in internally mediated saccades. Neurobiol Aging. 2007;28(4):627–637. doi: 10.1016/j.neurobiolaging.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4(1):77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99(6):1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388(6639):272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Brooks BA, Fuchs AF, Finocchio D. Saccadic eye movement deficits in the MPTP monkey model of Parkinson's disease. Brain Res. 1986;383(1-2):402–407. doi: 10.1016/0006-8993(86)90049-1. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem. 1999;6(6):572–599. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51(3):369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- De Toledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: in vivo detection of entorhinal cortex atrophy. Ann N Y Acad Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14(3):249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Dougal S, Phelps EA, Davachi L. The role of medial temporal lobe in item recognition and source recollection of emotional stimuli. Cogn Affect Behav Neurosci. 2007;7(3):233–242. doi: 10.3758/cabn.7.3.233. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38(1-2):61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Fantz RL. Visual Experience In Infants: Decreased Attention To Familiar Patterns Relative To Novel Ones. Science. 1964;146:668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Fulton A, Bartlett JC. Young and old faces in young and old heads: the factor of age in face recognition. Psychol Aging. 1991;6(4):623–630. doi: 10.1037//0882-7974.6.4.623. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2(4):437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6(2):177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Anim Cogn. 2004;7(1):25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269(5221):218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FI. The effects of age on the neural correlates of episodic encoding. Cereb Cortex. 1999;9(8):805–814. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, et al. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Trojanowski JQ, Ginsberg SD. Neuron-specific age-related decreases in dopamine receptor subtype mRNAs. J Comp Neurol. 2003;456(2):176–183. doi: 10.1002/cne.10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87(1):25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Ann N Y Acad Sci. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- Hotson JR, Langston EB, Langston JW. Saccade responses to dopamine in human MPTP-induced parkinsonism. Ann Neurol. 1986;20(4):456–463. doi: 10.1002/ana.410200404. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH., Jr Adult age differences in the rate of learning serial patterns: evidence from direct and indirect tests. Psychol Aging. 1992;7(2):232–241. doi: 10.1037//0882-7974.7.2.232. [DOI] [PubMed] [Google Scholar]
- Kato M, Miyashita N, Hikosaka O, Matsumura M, Usui S, Kori A. Eye movements in monkeys with local dopamine depletion in the caudate nucleus. I. Deficits in spontaneous saccades. J Neurosci. 1995;15(1 Pt 2):912–927. doi: 10.1523/JNEUROSCI.15-01-00912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kori A, Miyashita N, Kato M, Hikosaka O, Usui S, Matsumura M. Eye movements in monkeys with local dopamine depletion in the caudate nucleus. II. Deficits in voluntary saccades. J Neurosci. 1995;15(1 Pt 2):928–941. doi: 10.1523/JNEUROSCI.15-01-00928.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21(4):412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Voie D, Light LL. Adult age differences in repetition priming: a meta-analysis. Psychol Aging. 1994;9(4):539–553. doi: 10.1037//0882-7974.9.4.539. [DOI] [PubMed] [Google Scholar]
- Light LL, Prull MW, LaVoie DJ, Healy MR. Dual process theories of memory in old age. In: Perfect EAMTJ, editor. Models of cognitive aging. Oxford, England: Oxford University Press; 2000. [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67(1):145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Ma SY, Ciliax BJ, Stebbins G, Jaffar S, Joyce JN, Cochran EJ, et al. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J Comp Neurol. 1999;409(1):25–37. doi: 10.1002/(sici)1096-9861(19990621)409:1<25::aid-cne3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Manns JR, Stark CE, Squire LR. The visual paired-comparison task as a measure of declarative memory. Proc Natl Acad Sci U S A. 2000;97(22):12375–12379. doi: 10.1073/pnas.220398097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RE, Rugg MD. Age effects on brain activity associated with episodic memory retrieval. An electrophysiological study. Brain. 1998;121(Pt 5):861–873. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11(3):126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McKee RD, Squire LR. On the development of declarative memory. J Exp Psychol Learn Mem Cogn. 1993;19(2):397–404. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- Moore CI, Browning MD, Rose GM. Hippocampal plasticity induced by primed burst, but not long-term potentiation, stimulation is impaired in area CA1 of aged Fischer 344 rats. Hippocampus. 1993;3(1):57–66. doi: 10.1002/hipo.450030106. [DOI] [PubMed] [Google Scholar]
- Moss MB, Rosene DL, Peters A. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging. 1988;9(5-6):495–502. doi: 10.1016/s0197-4580(88)80103-9. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Hampton RR, Saksida LM. The parahippocampal region and object identification. Ann N Y Acad Sci. 2000;911:166–174. doi: 10.1111/j.1749-6632.2000.tb06725.x. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24(8):2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Schacter DL. False recognition in younger and older adults: exploring the characteristics of illusory memories. Mem Cognit. 1997;25(6):838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Park DC, Puglisi JT, Smith AD. Memory for pictures: does an age-related decline exist? Psychol Aging. 1986;1(1):11–17. doi: 10.1037//0882-7974.1.1.11. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychol Aging. 1992;7(2):290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9(6):609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M. Recognition memory and novelty preference; What model? In: Fagan JW, Hayne H, editors. Progress in infancy research. Vol. 3. Mahwah, N.J.: Lawrence Erlbaum; 2003. pp. 95–119. [Google Scholar]
- Perfect TJ, Dasgupta ZR. What underlies the deficit in reported recollective experience in old age? Mem Cognit. 1997;25(6):849–858. doi: 10.3758/bf03211329. [DOI] [PubMed] [Google Scholar]
- Perfect TJ, Williams RB, Anderton-Brown C. Age differences in reported recollective experience are due to encoding effects, not response bias. Memory. 1995;3(2):169–186. doi: 10.1080/09658219508258964. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, et al. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55(8):861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Peters JR, Vallie B, Difronzo M, Donaldson ST. Role of dopamine D1 receptors in novelty seeking in adult female Long-Evans rats. Brain Res Bull. 2007;74(4):232–236. doi: 10.1016/j.brainresbull.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Presty SK, Bachevalier J, Walker LC, Struble RG, Price DL, Mishkin M, et al. Age differences in recognition memory of the rhesus monkey (Macaca mulatta) Neurobiol Aging. 1987;8(5):435–440. doi: 10.1016/0197-4580(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, Martin AM, 3rd, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: adult age differences and neuropsychological test correlates. Psychol Aging. 2006;21(1):107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Prull MW, Gabrieli JDE, Bunge SA. Age-related changes in memory: a cognitive neuroscience perspective. In: Craik FIM, Salthouse, Timothy A, editors. The Handbook of Aging and Cognition. 2nd. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2000. pp. 91–153. [Google Scholar]
- Rapp PR, Amaral DG. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging. 1991;12(5):481–486. doi: 10.1016/0197-4580(91)90077-w. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8(8):1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse, Timothy A, editors. The Handbook of Aging and Cognition. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2000. pp. 1–90. [Google Scholar]
- Richmond J, Colombo M, Hayne H. Interpreting visual preferences in the visual paired-comparison task. J Exp Psychol Learn Mem Cogn. 2007;33(5):823–831. doi: 10.1037/0278-7393.33.5.823. [DOI] [PubMed] [Google Scholar]
- Richmond J, Sowerby P, Colombo M, Hayne H. The effect of familiarization time, retention interval, and context change on adults' performance in the visual paired-comparison task. Dev Psychobiol. 2004;44(2):146–155. doi: 10.1002/dev.10161. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A Method Of Measuring Eye Movement Using A Scleral Search Coil In A Magnetic Field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7(5):549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Koutstaal W, Johnson MK, Gross MS, Angell KE. False recollection induced by photographs: a comparison of older and younger adults. Psychol Aging. 1997;12(2):203–215. doi: 10.1037//0882-7974.12.2.203. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R, Scarnati E, Sundstrom E, Jonsson G, Studer A. Saccadic reaction times, eye-arm coordination and spontaneous eye movements in normal and MPTP-treated monkeys. Exp Brain Res. 1989;78(2):253–267. doi: 10.1007/BF00228897. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Kahana MJ, McLaughlin C, Golomb J, Wingfield A. Preservation of episodic visual recognition memory in aging. Exp Aging Res. 2005;31(1):1–13. doi: 10.1080/03610730590882800. [DOI] [PubMed] [Google Scholar]
- Sekuler R, McLaughlin C, Kahana MJ, Wingfield A, Yotsumoto Y. Short-term visual recognition and temporal order memory are both well-preserved in aging. Psychol Aging. 2006;21(3):632–637. doi: 10.1037/0882-7974.21.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamy JL, Buonocore MH, Makaron LM, Amaral DG, Barnes CA, Rapp PR. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol Aging. 2006;27(10):1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA, McNaughton BL, Skaggs WE, Weaver KL. The effect of aging on experience-dependent plasticity of hippocampal place cells. J Neurosci. 1997;17(17):6769–6782. doi: 10.1523/JNEUROSCI.17-17-06769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Permenter M, Archibeque M, Vogt J, Amaral DG, et al. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol. 2007;98(2):898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- Smith CN, Hopkins RO, Squire LR. Experience-dependent eye movements, awareness, and hippocampus-dependent memory. J Neurosci. 2006;26(44):11304–11312. doi: 10.1523/JNEUROSCI.3071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Strecker RE, Jacobs BL. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258(2):217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- Tanila H, Shapiro M, Gallagher M, Eichenbaum H. Brain aging: changes in the nature of information coding by the hippocampus. J Neurosci. 1997;17(13):5155–5166. doi: 10.1523/JNEUROSCI.17-13-05155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titov N, Knight RG. Adult age differences in controlled and automatic memory processing. Psychol Aging. 1997;12(4):565–573. doi: 10.1037//0882-7974.12.4.565. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: a quantitative integration of research findings. J Gerontol. 1993;48(4):P157–171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997;122(3):231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, et al. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998;30(1):56–61. doi: 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gureviciene I, McMahan RW, Gallagher M, Eichenbaum H, et al. Cognitive aging and the hippocampus: how old rats represent new environments. J Neurosci. 2004;24(15):3870–3878. doi: 10.1523/JNEUROSCI.5205-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1134–1140. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Bruntz RC, Stairs DJ, Dwoskin LP. Individual differences in response to novelty predict prefrontal cortex dopamine transporter function and cell surface expression. Eur J Neurosci. 2007;26(3):717–728. doi: 10.1111/j.1460-9568.2007.05690.x. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20(1):451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


