Abstract
Cdc42 is a key regulator of the actin cytoskeleton and activator of Wiskott-Aldrich syndrome protein (WASP). Although several studies have separately demonstrated the requirement for both Cdc42 and WASP in Fcγ receptor (FcγR)-mediated phagocytosis, their precise roles in the signal cascade leading to engulfment are still unclear. Reduction of endogenous Cdc42 expression by using RNA-mediated interference (short hairpin RNA [shRNA]) severely impaired the phagocytic capacity of RAW/LR5 macrophages, due to defects in phagocytic cup formation, actin assembly, and pseudopod extension. Addition of wiskostatin, a WASP/neural-WASP (N-WASP) inhibitor showed extensive inhibition of phagocytosis, actin assembly, and cell extension identical to the phenotype seen upon reduction of Cdc42 expression. However, using WASP-deficient bone marrow-derived macrophages or shRNA of WASP or N-WASP indicated a requirement for both WASP and N-WASP in phagocytosis. Cdc42 was necessary for WASP/N-WASP activation, as determined using a conformation-sensitive antibody against WASP/N-WASP and partial restoration of phagocytosis in Cdc42 reduced cells by expression of a constitutively activated WASP. In addition, Cdc42 was required for proper WASP tyrosine phosphorylation, which was also necessary for phagocytosis. These results indicate that Cdc42 is essential for the activation of WASP and N-WASP, leading to actin assembly and phagocytic cup formation by macrophages during FcγR-mediated phagocytosis.
INTRODUCTION
Phagocytosis, the mechanism of internalization of particles >0.5 μm, is used by specialized cells such as macrophages, dendritic cells, and neutrophils for the clearance of foreign particles and cellular debris (Aderem and Underhill, 1999). This process is initiated by the recognition by diverse phagocytic receptors on the cell surface, including the complement receptor (CR) 3 (or αMβ2) and the Fcγ receptor (FcγR), two of the best-characterized phagocytic receptors. Ligation of these receptors initiates a complex series of events, including actin assembly, membrane extension, and fusion, ultimately leading to particle internalization (Swanson and Hoppe, 2004).
The Rho family GTPases (Rac, Rho, and Cdc42) regulate numerous cell functions, many requiring alterations of the cytoskeleton, including FcγR- and complement-mediated phagocytosis (reviewed in Ridley, 2001; Fenteany and Glogauer, 2004). Interestingly, these three GTPases have been shown to differentially regulate the phagocytic process. Phagocytosis mediated by the FcγR has been shown to require both Rac and Cdc42 (Cox et al., 1997; Massol et al., 1998) but not Rho (Caron and Hall, 1998). By contrast, particle uptake mediated by the CR3 requires Rho but not Rac or Cdc42 (Caron and Hall, 1998). Rac1 and Cdc42 function is required for FcγR-mediated phagocytic cup formation (Cox et al., 1997). The differential recruitment, localization, and activation of Rac and Cdc42 during FcγR-mediated phagocytosis suggest a precise and unique function for each Rho GTPase in coordinating this process (Patel et al., 2002; Hoppe and Swanson, 2004). However, all of these studies used the use of overexpression dominant-negative mutants, which may have nonspecific effects; therefore, the role of Rho GTPases in phagocytosis needs to be clarified.
Both Rac and Cdc42 have been shown to be regulators of the Wiskott Aldrich syndrome/WASP family verprolin-homologous (WASP/WAVE) family of proteins. WASP is a hematopoietic, cell-specific protein with distinct functional domains, by which WASP interacts with several cytoplasmic factors involved in signal transduction and actin cytoskeleton (Takenawa and Miki, 2001). The catalytically active domain of WASP lies in its C terminus that is conserved among all WASP/WAVE proteins and contains a VCA (verprolin-homology and cofilin-like and acidic region) domain capable of activating the Arp2/3 complex (reviewed in Takenawa and Suetsugu, 2007). The other domains in WASP either stabilize or regulate the activity of its VCA domain and are found from N to C terminus as follows: a WH1 domain, a basic region, a GTPase-binding domain (GBD), and a proline-rich region that interacts with Src homology 3 domain-containing proteins (Takenawa and Suetsugu, 2007). In resting cells, the hydrophobic core of Cdc42/Rac GBD of WASP is capable of binding the C-terminal VCA region, resulting in autoinhibition of the Arp2/3 stimulatory activity of the protein. However, upon binding of guanosine triphosphate (GTP) loaded-Cdc42 the VCA region is released and the C-terminal region is capable of binding to the Arp2/3 complex, to stimulate actin polymerization (Kim et al., 2000). FcγR-mediated phagocytosis is impaired in peripheral blood monocytes for Wiskott-Aldrich syndrome patients in which formation of the actin cup is also markedly attenuated (Lorenzi et al., 2000; Dewey et al., 2006). Furthermore, the VCA region of WASP was demonstrated to be critical for the phagocytic cup formation (Tsuboi and Meerloo, 2007). However, the mechanism regulating WASP activation during phagocytosis has not been examined.
Because Cdc42 plays a key role in activating WASP both in vitro and in vivo (Aspenstrom et al., 1996; Symons et al., 1996; Takenawa and Miki, 2001), these observations suggest that Cdc42-WASP signaling might necessary for efficient FcγR-mediated phagocytosis by macrophages. Thus, this study focuses on the role of Cdc42–WASP signaling in phagocytosis by reducing endogenous levels of Cdc42 by using small interfering RNA, to exclude nonspecific effects of Cdc42 dominant negative mutants, and both genetic and pharmacological inhibition of WASP.
MATERIALS AND METHODS
Cells, Antibodies, and Reagents
RAW/LR5 cells, were derived from the murine monocyte/macrophage RAW 264.7 cell line (Cox et al., 1997), and COS-7 cells (American Type Culture Collection, Manassas, VA) were cultured in RPMI-1640 medium (Mediatech, Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Murine bone marrow-derived macrophages (BMMs) were isolated and prepared according to Stanley (1997) and were grown in α-minimal essential medium containing 15% fetal bovine serum, 360 ng/ml recombinant human colony stimulating factor-1 (Chiron, Emeryville, CA) and antibiotics. All cells were maintained at 37°C in a 5% CO2 atmosphere. Rabbit anti-sheep erythrocyte immunoglobulin (Ig) G was from Diamedix (Miami, FL). Primary antibodies used in this study were mouse monoclonal anti-Myc (Cell Signaling Technology, Danvers, MA), mouse anti-WASP (B9), rabbit anti-Cdc42 (SC87) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-actin (AC-15) (Sigma-Aldrich), anti-CD16/CD32 (2.4G2) (BD Biosciences Pharmingen, San Diego, CA), mouse horseradish peroxidase (HRP) anti-phosphotyrosine (PY) (PY20) (BD Biosciences), and chicken anti-N-WASP (Isaac, Ishihara, Nusblat, Dovas, Gevrey, Condeelis, and Cox, unpublished data). The conformation-sensitive WASP/N-WASP antibody (CSA) was a generous gift from John Condeelis (Albert Einstein College of Medicine) (Sukumvanich et al., 2004). Alexa Fluor 568 and 641-phalloidin and all secondary antibodies conjugated to Alexa Fluor 488 or 568 used for immunostaining were from Invitrogen (Carlsbad, CA). Human IgG was from Calbiochem (San Diego, CA). Rabbit F(ab′)2 and all secondary antibodies conjugated to HRP (used for Western blotting) were from Jackson ImmunoResearch Laboratories (West Grove, PA). Protein A/G Plus-agarose beads were from Santa Cruz Biotechnology. The SuperFect transfection reagent was from QIAGEN (Valencia, CA), and Wiskostatin was from BIOMOL Research Laboratories (Plymouth Meeting, PA).
RNA-mediated Interference
Reduction of Cdc42 expression was achieved through the retroviral infection of RAW/LR5 cells with short hairpin RNAs directed against Cdc42 mRNA (sh#1: 5′-TAACTCACCACTGTCCAAA-3′ and sh#2: 5′-AGACTCCTTTCTTGCTTGT-3′), by using pSUPER.reto.puro plasmids according to the manufacturer directions (Oligoengine, Seattle, WA). Stable single clones with reduced Cdc42 expression were obtained after puromycin selection of the infected cells. Control cell lines were also generated using a nonspecific shRNA sequence (Gevrey et al., 2005). Western blotting was used to monitor Cdc42 expression levels. Short hairpin RNAi with sequences that specifically target WASP (Dovas et al., 2009) and N-WASP (Yamaguchi et al., 2005) were also used to reduce endogenous levels of these proteins. Plasmids were transiently transfected using FuGENE HD reagent (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's instructions and selected for 2 d in puromycin.
Constructs and Cell Transfection
Myc-tagged L270P, Y291E, and L270P/Y291F WASP were created from Myc-tagged WASP wid-type (WT) plasmid subcloned in pEF-BOS vector (Suetsugu et al., 1999) or into the WASPbs (CFP-WASP-YFP) (Cammer et al., 2009) by using the QuikChange kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions and confirmed by sequencing. Transient transfections were performed using SuperFect according to the manufacturer's instructions (QIAGEN).
Immunofluorescence Microscopy
RAW/LR5 cells or BMMs were plated on 12-mm glass coverslips overnight. Phagocytic cup formation and red blood cell (RBC) binding were performed as described previously (Cox et al., 1997). Essentially, 5 × 106 sheep RBCs opsonized with rabbit IgG (EIgG) was added to adherent cell cultures for 15 min at 4°C to allow particle attachment, and, after washing with ice-cold buffer, cells were either fixed (binding) or incubated for various times at 37°C before fixation, permeabilization, and staining with the indicated antibodies and/or phalloidin. In some cases, cells were stained with Alexa Fluor 568 anti-rabbit IgG to detect EIgG and monoclonal antibody anti-myc followed by Alexa Fluor 488 anti-mouse IgG to detect transfected cells. Images were taken using the 60× oil/1.40 phase3 objective of an Olympus IX71 microscope coupled to a Sensicam cooled charge-coupled device (CCD) camera (Roper Scientific, Tucson, AZ). Confocal images were obtained using an SP2 AOBS microscope (Leica, Wetzlar, Germany).
To assess the level of surface FcγRs, cells were incubated on ice with anti-CD16/CD32 (2.4G2) antibody and ice-cold 1% bovine serum albumin in buffer with divalent cation (BWD), followed by washing and further incubation on ice with Alexa Fluor 488 in BWD for 30 min. Cells were then fixed for 10 min by using ice-cold 3.7% formaldehyde in BWD and mounted as described above. Images were collected using the 60× oil/1.40 phase3 objective of an Olympus IX71 microscope coupled to a Sensicam cooled CCD camera. Quantitative analysis of staining was performed as described previously in which the intensity of FcγR staining of ∼30 cells/coverslip was measured using ImageJ (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/) (Sukumvanich et al., 2004; Cammer et al., 2009).
Phagocytosis and Spreading Assays
FcγR-mediated phagocytosis was performed as described previously (Cox et al., 1997). In brief, cells were incubated with a suspension of EIgG for 30 min at 37°C in BWD. Noninternalized EIgG was then removed by washes, followed by hypotonic lysis. At least 50 cells were observed by phase contrast microscopy, and the number of RBC in each cell was counted and expressed as the average number of particles ingested per 100 cells (phagocytosis index).
Cells were pretreated with vehicle (dimethyl sulfoxide) or 5 μM wiskostatin for 5 min at 37°C and then allowed to spread on coverslips coated with 1 mg/ml human IgG or F(ab′)2, as described previously (Cox et al., 1999). Adherent surface area and F-actin intensity of ∼30 cells/coverslips were measured using ImageJ.
Immunoprecipitation and Western Blotting
Cells were incubated with EIgG for 5 min at 37°C and then washed by hypotonic lysis. Cells were lysed in ice-cold buffer containing 50 mm Tris, 100 mm NaCl, 1% NP-40, 2 mm EDTA, 1 mm orthovanadate, 1 mm benzamidine, 10 μg/ml aprotinin, and 10 μg/ml leupeptin, pH 7.4. Whole cell lysates were clarified by centrifugation at 16,000 × g for 15 min at 4°C and either used for immunoprecipitation or mixed with 5× Laemmli buffer and boiled for 5 min. Immunoprecipitations were performed by incubating lysates with the CSA antibody and then with protein A/G Plus-agarose beads (Santa Cruz Biotechnology) overnight at 4°C. Total cell lysates and/or immunoprecipitates were resolved by SDS-PAGE, and proteins were transferred onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Billerica, MA) and probed with the indicated antibodies.
To detect WASP tyrosine phosphorylation, cells were left untreated, treated with 12 μM pervanadate (PV) for 30 min or with EIgG for various times at 37°C. COS-7 cells were transient transfected with the indicated WASP constructs and were left untreated or treated with 12 μM PV for 30 min. Cells were lysed as mentioned above, and then WASP was immunoprecipitated using antibodies against WASP, Myc, or green fluorescent protein (GFP). Immunoprecipitates were subjected to Western blotting with HRP-PY, and then reprobed with N/WASP, Myc, or GFP antibodies. Signals were visualized using the SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL), and images were acquired using a Kodak Image Station 440 (Eastman Kodak, Rochester, NY).
Data Analysis
All results were calculated as the mean ± SEM. Data were analyzed using Student's t test, and differences with a p value <0.05 were regarded as significant. Error bars represent SEM.
RESULTS
Reduction of Cdc42 Expression in RAW/LR5 Cells Results in Impaired Phagocytosis
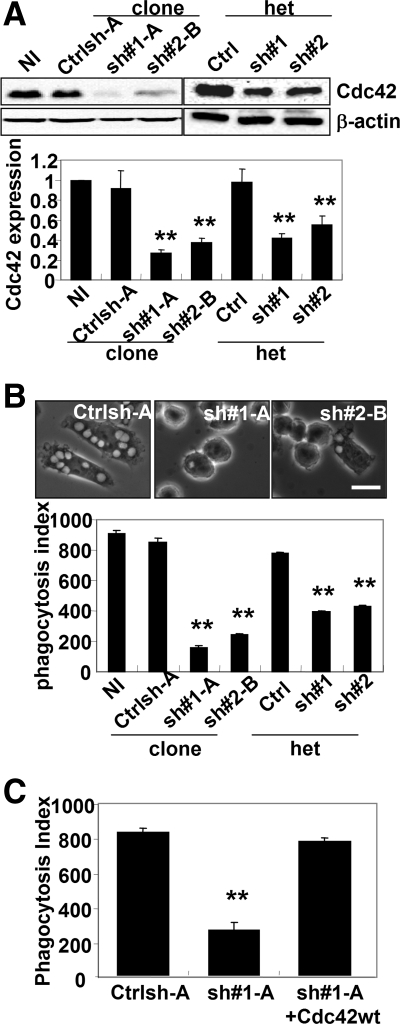
We showed previously that expression of a dominant-negative mutant of Cdc42 (Cdc42 N17) in macrophages dramatically inhibited FcγR-mediated phagocytosis, but the precise role of Cdc42 in this process was not determined (Cox et al., 1997). Because there was a possibility of nonspecific effects when dominant-negative mutants are used, the role of Cdc42 in macrophage phagocytosis was confirmed by reducing endogenous levels of Cdc42 by using small interfering RNA. Cdc42 protein levels were lowered after retroviral infection with pSUPER.retro.puro vectors containing two different sequences encoding a shRNA directed against the coding region of Cdc42 (sh#1 and sh#2). After selection, heterogeneous populations and clonal cell lines (sh#1-A and sh#2-B) were isolated. Cdc42 expression was analyzed by Western blot and compared with noninfected cells or cells infected with a nontargeting control sequence (Ctrlsh-A) (Figure 1A). Both short hairpin sequences were effective at significantly reducing Cdc42 expression, but maximal inhibition of protein levels occurred in isolated clonal cell lines (Figure 1A). The reduction in Cdc42 levels resulted in a significant inhibition of phagocytosis of opsonized red blood cells (EIgG), and the degree of inhibition correlated with the levels of Cdc42 reduction in both heterogeneous populations and clonal cell lines. In contrast, cells treated with a control short hairpin sequence showed similar levels of phagocytosis to noninfected cells (Figure 1B). Because an observed decrease in phagocytosis may be due to altered surface expression of FcγR, which leads to a reduction in the ability of cells to bind EIgG, we assessed both the binding capacity for EIgG and the FcγR surface expression of Cdc42 shRNA-treated cells. These two different methods reflect the number of functional FcγR at the cell surface. No difference was observed in the ability of Cdc42 shRNA-treated cells to bind EIgG compared with control-treated cells (Supplemental Figure 1A); and consistent with this result, similar surface expression levels of FcγR were also observed in Cdc42 shRNA-treated cells compared with control-treated cells (Supplemental Figure 1B). These results clearly indicate that the defect in phagocytosis after reduction of endogenous Cdc42 was not due to a reduced ability of cells to bind EIgG but to a defect downstream of the FcγR.
Figure 1.
Cdc42 was required for the FcγR-mediated phagocytosis. (A) Western blot analysis of Cdc42 and β-actin expression in RAW/LR5 cells noninfected (NI), puromycin-resistant heterogeneous populations (het) retrovirally infected cells with pSUPER.retro/puro plasmids containing a control sequence (Ctrl) or two different shRNA sequences targeting Cdc42 (sh#1 and sh#2), or isolated clones (Ctrlsh-A, sh#1-A, and sh#2-B). Quantification of Cdc42/β-actin signal intensity ratio on three different Western blots is shown for each condition. (B) The ability of cells to undergo FcγR-mediated phagocytosis was determined as described in Materials and Methods. Representative phase contrast microscope images are shown for Ctrlsh-A, sh#1-A, or sh#2-B cell lines after incubation with EIgG for 30 min. Bar, 10 μm. The phagocytic index was calculated as the average number of ingested particles for 100 cells ± SEM (n = 3 independent experiments). **p < 0.01 compared with NI control cells. (C) Cdc42 shRNA-treated cells (sh#1-A) were transiently transfected with human myc-tagged Cdc42 and FcγR-mediated phagocytosis was quantified as described in B. n = 3, **p < 0.01 compared with control sequence infected (Ctrlsh-A) cells.
To determine whether Cdc42 was specifically required for FcγR phagocytosis and not for CR3-mediated phagocytosis as had been suggested previously (Caron and Hall, 1998), we tested the ability of Cdc42 shRNA-treated cells to perform CR3-mediated phagocytosis. Uptake of complement-coated erythrocytes in both control and Cdc42 shRNA-treated cells was comparable (data not shown). Also, to confirm that the observed phagocytosis defect was due specifically to Cdc42 reduction, Cdc42 was re-expressed in Cdc42 shRNA-treated cells by using a human myc-tagged Cdc42 construct. Transient transfection of human Cdc42 in Cdc42 shRNA-treated cells increased the phagocytic ability of Cdc42 shRNA-treated cells to levels comparable to control cells (Figure 1C), and the increase in phagocytosis in the Cdc42 re-expressing cells was not due to enhanced particle binding or FcγR surface expression as determined using quantitative single-cell immunofluorescence (Supplemental Figure 1). Therefore, these data suggested that the phagocytic defect was specific to FcγR in phagocytosis and was due to the loss in Cdc42 expression.
Cdc42 Regulates Actin Polymerization by FcγR
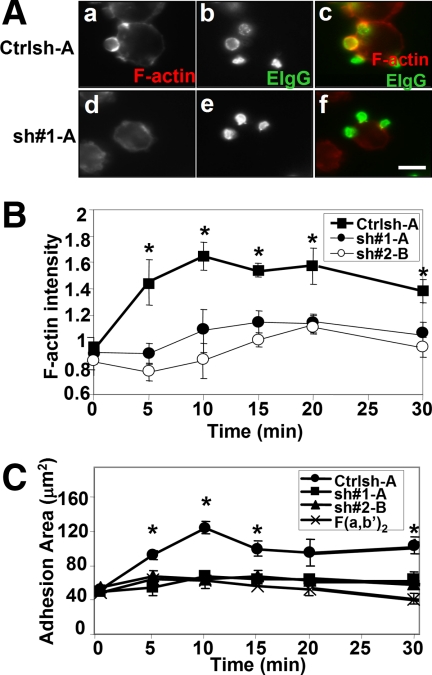
Phagocytic cup formation in Cdc42 shRNA-treated cells was analyzed to determine whether the inhibition of FcγR-mediated phagocytosis correlated with deficient actin polymerization. Control-treated cells formed well defined phagocytic cups (Figure 2A, a–c); and in agreement with results using Cdc42 N17 (Cox et al., 1997), phagocytic cups were absent in Cdc42 shRNA-treated cells (Figure 2A, d and e). Furthermore, Cdc42 shRNA-treated cells did not form phagocytic cups even after 30 min (Supplemental Figure 2). The absence of F-actin staining beneath bound particles indicated that defective phagocytic cup formation was due to reduced actin polymerization during phagocytosis (Figure 2A, d–f). To further demonstrate the involvement of Cdc42 in actin polymerization in response to FcγR ligation, we coated coverslips with human IgG and quantified total cellular F-actin content in a time-dependent manner as described previously (Cox et al., 1999). Control-treated cells and Cdc42 shRNA-treated cells showed a similar level of F-actin at onset of adhesion (basal level). However, after incubation, control-treated cells showed significantly increased actin polymerization that peaked after 10 min, whereas Cdc42 shRNA-treated cells showed only a marginal increase in F-actin content compared with the F(ab′)2 negative control that does not ligate FcγRs (Figure 2B).
Figure 2.
Actin polymerization by FcγR is inhibited by reduced Cdc42 expression. (A) Control (Ctrlsh-A, a–c) and Cdc42 shRNA-treated cells (sh#1-A, d–f) were incubated with EIgG for 5 min at 37°C before fixation and staining for F-actin and EIgG as described in Materials and Methods. Representative confocal images are shown. a and d, F-actin; b and e, EIgG; c and f, merged image (F-actin, red and EIgG, green). Bar, 10 μm. (B) Cells were allowed to spread on human IgG-coated coverslips or F(ab′)2-coated coverslips at 37°C for indicated times, and then cells were fixed and stained for F-actin. Data, expressed as the mean ± SEM, are reported as -fold actin increase compared with F(ab′)2-coated coverslips (n = 3). (C) Data are reported as mean adherent surface areas ± SEM. *p < 0.05 compared with F(ab′)2.
Because cytoskeletal assembly has been coupled to pseudopod extension, impaired actin polymerization by Cdc42 shRNA-treated cells may result in a reduced ability of these cells to extend protrusions in response to FcγR ligation. Therefore, the extent of cell spreading on IgG was also determined. Control-treated cells showed a rapid increase in their adherent surface area, also peaking at 10 min, whereas spreading on IgG was reduced to the level of the F(ab′)2 control in Cdc42 shRNA-treated cells, consistent with the defective actin polymerization observed (Figure 2C). Further incubation did not alter the ability of the Cdc42 shRNA-treated cells polymerize actin or to spread on IgG (Figure 2, B and C, and Supplemental Figure 3). Overall, these data indicate that Cdc42 regulates phagocytic signaling by stimulating actin polymerization, leading to pseudopod extension downstream of FcγR ligation.
WASP/N-WASP, Potential Effectors of Cdc42
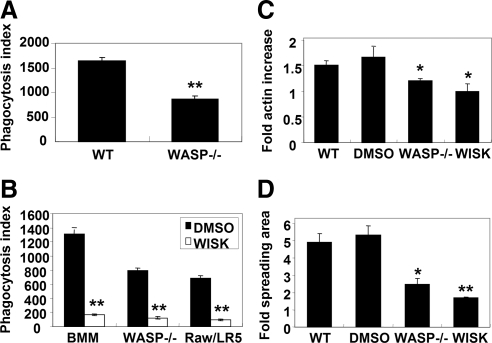
One potential mechanism by which Cdc42 regulates actin assembly is through the activation of WASP and N-WASP; therefore, we sought to determine whether WASP and N-WASP were possible downstream effectors of Cdc42 during phagocytosis. Consistent with results reported using human monocyte-derived macrophages (Lorenzi et al., 2000), BMMs from WASP-deficient mice showed a 50% decrease in phagocytosis (Figure 3A). The residual phagocytosis observed in the absence of WASP may be due to the presence of N-WASP in macrophages (O'Sullivan et al., 1999; Stamm et al., 2005). To address the role of this WASP homologue, the phagocytic ability of cells in the presence of wiskostatin was assessed. Wiskostatin is a selective and reversible inhibitor of WASP and N-WASP by stabilization of the closed, autoinhibited conformation, thereby preventing Arp2/3 complex activation (Peterson et al., 2004). Addition of 5 μM wiskostatin almost completely inhibited phagocytosis of EIgG by BMM and RAW/LR5 cells. In addition, wiskostatin treatment decreased phagocytosis in BMM from WASP-deficient mice (Figure 3B), suggesting that the residual phagocytosis observed in the absence of WASP was due to the presence of N-WASP.
Figure 3.
WASP and N-WASP are required for FcγR-mediated phagocytosis. (A) The ability of WT or WASP−/− BMM cells to undergo FcγR-mediated phagocytosis was determined as described in Figure 1. n = 3, **p < 0.01 compared with the WT BMM cells. (B) WT BMM, WASP−/− BMM, and RAW/LR5 cells were preincubated for 5 min with 5 μM wiskostatin (WASP inhibitor) or DMSO (vehicle) and then subjected to EIgG phagocytosis. **p < 0.01 between DMSO-treated cells and wiskostatin-treated cells. (C and D) WT or WASP−/− BMM cells were either untreated, preincubated with DMSO or 5 μM wiskostatin where indicated, and then allowed to spread on human IgG-coated coverslips or F(ab′)2-coated coverslips at 37°C for 10 min. Cells were fixed and stained for F-actin with Alexa Fluor 568-phalloidin. Data, expressed as the mean ± SEM (n = 3), are reported as -fold actin increase (C) or -fold adherent surface areas (D) compared with cells on F(ab′)2-coated coverslips. n = 3, *p < 0.05, **p < 0.01 compared with the WT BMM cells.
To determine whether this inhibition was correlated with an inhibition of actin polymerization, the effect of wiskostatin treatment on actin polymerization and pseudopod extension was quantified. In WT or dimethyl sulfoxide (DMSO)-treated BMMs, there was a significant increase in F-actin content after FcγR ligation (Figure 3C). However, reduced actin polymerization in response to IgG was observed in WASP-deficient cells or after chemical inhibition (wiskostatin) (Figure 3C). Consistent with this result, cell spreading on IgG-coated substrates was also dramatically decreased in wiskostatin-treated cells (Figure 3D). In addition, F-actin–rich phagocytic cups were rarely detected in cells treated with wiskostatin (data not shown). All in all, the differences observed after wiskostatin treatment were similar to that observed in Cdc42 shRNA-treated cells (Figure 2, B and C).
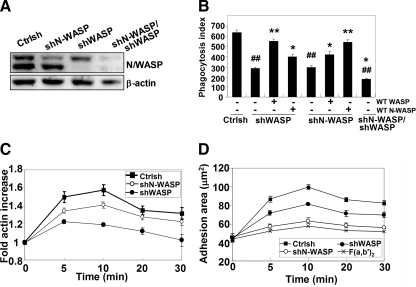
To confirm the results using wiskostatin that suggested that both WASP and N-WASP are required for FcγR-mediated phagocytosis, the levels of endogeneous WASP or N-WASP were reduced using small hairpin shRNA in RAW/LR5 cells. Unfortunately, no stable viable cells were obtained using N-WASP shRNA; thus, we used transient transfection followed by puromycin selection to reduce WASP, N-WASP, or both. Transfection of WASP- or N-WASP-specific shRNA resulted in a 67% reduction of N-WASP or 95% reduction of WASP protein expression compared with control shRNA-treated cells as determined by Western blot. Transfection of both WASP and N-WASP shRNA also resulted in a 72 and 81% reduction of N-WASP and WASP (Figure 4A). Reduction of either WASP or N-WASP expression significantly inhibited phagocytosis compared with control-treated cells. In addition, reduction of both WASP and N-WASP significantly enhanced the inhibition observed with the single reductions and was comparable with that seen after wiskostatin treatment (Figures 3B and 4B). Notably, re-expression of a human myc-WASP or GFP-N-WASP was able to fully rescue the phagocytic ability of shWASP- or shN-WASP–selected cells, respectively, but it was unable to rescue its respective counterpart, e.g., WASP-deficient cells could not be rescued by N-WASP expression (Figure 4B). These results indicated that both WASP and N-WASP are required for FcγR-mediated phagocytosis and that they cannot compensate for each other and therefore may have different functions in phagocytosis.
Figure 4.
WASP and N-WASP play unique functions during FcγR-mediated phagocytosis. (A) RAW/LR5 cells were transiently transfected with control shRNA (Ctrlsh), shN-WASP, shWASP, or both plasmids and then selected in puromycin. Efficacy of the shRNA constructs on endogenous protein expression was determined by Western blot with an antibody that recognizes both WASP (bottom band) and N-WASP (top band). Blots were quantified by densitometry and normalized to actin. (B) Control shRNA, shN-WASP-, shWASP-, or shN-WASP/WASP-selected cells were transiently transfected with myc-WASP or GFP-N-WASP, and phagocytic ability was determined. n = 4, *p < 0.05, **p < 0.01 compared with the shWASP or shN-WASP cells. ##p < 0.01 compared with the Ctrlsh cells. (C) Cells were allowed to spread on human IgG or F(ab′)2-coated coverslips at 37°C for indicated times and then stained for F-actin content. Data, expressed as the mean ± SEM, are reported as -fold actin increase compared with Ctrl at 0′ (n = 4). Difference of between Ctrlsh or shN-WASP and shWASP are significant from 5′ to 30′ (p < 0.05). (D) Data are reported as mean adherent surface areas. The differences between Ctrlsh and shN-WASP or shWASP are statistically significant from 5′ to 30′ (p < 0.05). The difference between shN-WASP and shWASP are statistically significant from 5′ to 30′ (p < 0.05).
To determine whether WASP or N-WASP silencing in macrophages correlated with deficient actin polymerization and pseudopod extension, F-actin intensity and the extent of cell spreading on IgG was determined. WASP silencing in RAW/LR5 cells dramatically reduced F-actin increase and spreading compared with control shRNA-treated cells. Surprisingly, cells with reduced N-WASP levels showed F-actin increases similar to control shRNA-treated cells (Figure 4C), whereas spreading area in N-WASP reduced cells was significantly decreased (Figure 4D). These results suggested that WASP was required for actin polymerization, whereas N-WASP was required for pseudopod protrusion. Overall, these data suggested that both WASP and N-WASP are required for FcγR-mediated phagocytosis and may be potential effectors of Cdc42.
Cdc42 Is Required for Activation and Tyrosine Phosphorylation of WASP/N-WASP
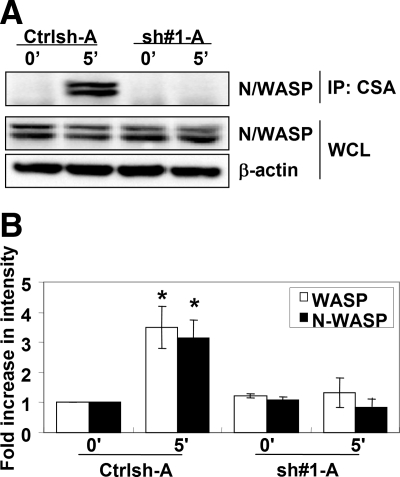
Because Cdc42 is a well characterized activator of WASP and N-WASP the role of Cdc42 in the activation of WASP/N-WASP during phagocytic cup formation was determined. To measure the level of WASP/N-WASP activation, activated WASP/N-WASP was isolated from cell lysates by using a conformation-sensitive antibody that recognizes only the activated, open conformation of WASP/N-WASP (CSA) (Sukumvanich et al., 2004) followed by Western blot analysis with an antibody that recognizes both WASP and N-WASP (Figure 5A, bottom band and top band, respectively). Incubation with EIgG for 5 min resulted in an increased amount of activated WASP (∼3.5-fold) and N-WASP (∼3-fold) in control-treated cells, whereas Cdc42 shRNA-treated cells did not demonstrate any significant increase (Figure 5, A and B), indicating that Cdc42 was required for activation of WASP and N-WASP during FcγR-mediated phagocytosis.
Figure 5.
Cdc42 is required for WASP and N-WASP activation after FcγR ligation. (A) Control (Ctrlsh-A) and Cdc42 shRNA-treated cells (sh#1-A) were unstimulated (0′) or incubated with EIgG for 5 min (5′) at 37°C. Activated WASP or N-WASP was immunoprecipitated using a N/WASP conformation-sensitive antibody, followed by Western blotting with an antibody that recognizes WASP (bottom band) and N-WASP (top band). (A) Representative Western blot is shown. (B) Blots were quantified by densitometry and normalized to the whole cell lysates (WCL). Data are expressed as the -fold increase compared with unstimulated cells ± SEM. n = 3, *p < 0.05 compared with 0′ of Ctrlsh-A.
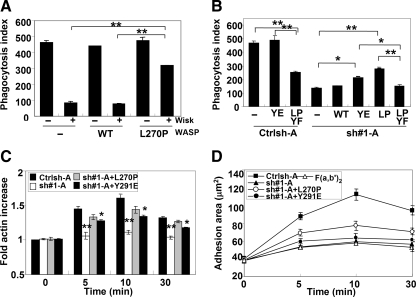
To confirm the requirement for activation of WASP in Cdc42-dependent FcγR phagocytosis, we tested the ability of constitutively activated WASP to bypass the requirement for Cdc42. A mutation in the conserved GBD region of WASP (L270P) has been reported in patients with X-linked Severe Congenital Neutropenia (Ancliff et al., 2006) that results in a decrease in the stability of the autoinhibited structure of WASP (Devriendt et al., 2001). To confirm the activity of this mutation, the phagocytic ability of cells expressing WASP L270P in the presence of wiskostatin was determined. Addition of wiskostatin in nontransfected or WT WASP-transfected cells extensively inhibited FcγR-mediated phagocytosis, similar to Figure 3B (Figure 6A). However, L270P WASP-expressing cells retained some phagocytic capacity in the presence of wiskostatin (Figure 6A). To determine whether phagocytosis was restored by L270P WASP expression in Cdc42 shRNA-treated cells, cells were transiently transfected WT or L270P WASP. WT WASP expression did not enhance the phagocytic ability of Cdc42 shRNA-treated cells, whereas L270P WASP expression showed partial restoration of phagocytosis (Figure 6B). Consistent with this result, cells in which endogenous WASP has been replace by a point mutant of WASP that abolishes Cdc42 binding (H246D WASP) (Kato et al., 1999) showed a significantly decreased phagocytic ability compared with control cells (Supplemental Figure 4A), similar to cells with WASP-deficient macrophages (Figures 3B and 4B). These results suggest that Cdc42 is required to induce an open conformation of WASP during phagocytosis.
Figure 6.
Phagocytosis ability is partial restored by L270P WASP expression in Cdc42-silenced cells. (A) Control shRNA-treated cells (Ctrlsh-A) were transiently transfected with the myc-tagged wild-type WASP or constitutively activated WASP (L270P) and then preincubated with 5 μM wiskostatin (WASP inhibitor) or DMSO (vehicle) followed by a further incubation with EIgG for 30 min at 37°C before fixation. Phagocytic index was determined, and data are expressed as the mean ± SEM for three independent experiments. **p < 0.01 compared with wiskostatin-treated cells. (B) Control (Ctrlsh-A) or Cdc42 shRNA-treated cells (sh#1-A) were transiently transfected with either myc-tagged WT, Y291E (YE), L270P (LP) WASP, or L270P-Y291F (LPYF) WASP, and then phagocytosis was determined. The difference between non- and L270P-Y291F WASP-transfected Ctrlsh-A cells is statistically significant (**p < 0.01). The difference between L270P WASP and non-, WT, or L270P-Y291F WASP-transfected sh#1-A cells is statistically significant (**p < 0.01), n = 3. (C) Indicated cells were allowed to spread on human IgG-coated coverslips or F(ab′)2-coated coverslips at 37°C for indicated times. Data, expressed as the mean ± SEM, are reported as -fold actin increase as compared with F(ab′)2-coated coverslips. n = 3, *p < 0.05; **p < 0.01 compared with Ctrlsh-A. (D) Data are reported as mean adherent surface areas, *p < 0.05; **p < 0.01 compared with sh#1-A.
Phosphorylation of WASP on tyrosine 291 has been shown to enhance the ability of WASP to stimulate actin polymerization in vitro (Cory et al., 2002) and to potentiate phagocytic cup formation (Tsuboi and Meerloo, 2007). Thus, the importance of the WASP phosphorylation downstream of Cdc42-mediated conformation change in WASP was evaluated. Expression of an open-conformation of WASP that could not be tyrosine phosphorylated (L270P-Y291F WASP) in control shRNA-treated cells showed a 50% decrease in phagocytosis, and its expression was unable to enhance the phagocytosis of Cdc42 shRNA-treated cells unlike expression of L270P WASP (Figure 6B). Consistent with a role for WASP phosphorylation downstream of Cdc42-mediated activation, Y291E WASP expression in Cdc42 shRNA-treated cells also partially restored phagocytosis in Cdc42 shRNA-treated cells (Figure 6B). Interestingly, cell spreading on IgG-coated substrates was partially rescued, whereas intensity of F-actin was fully rescued by L270P WASP expression in Cdc42 shRNA-treated cells (Figure 6, C and D). This result is consistent with the ability of L270P WASP to restore WASP function and not N-WASP (Figure 4, C and D). Unlike the case with L270P WASP expression, Y291E WASP expression in Cdc42 shRNA-treated cells was unable to rescue cell spreading on IgG-coated substrates, although a partial restoration of F-actin increase was observed (Figure 6, C and D). The unexpected results from Y291E WASP expression suggest that although WASP tyrosine phosphorylation is necessary for phagocytosis it may be insufficient for WASP activation or to mimic phosphorylation.
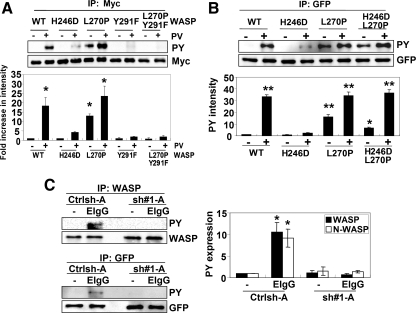
Torres and Rosen (2006) have demonstrated that Cdc42 binding to the GBD domain of WASP is required for the proper phosphorylation of WASP in vitro. To confirm whether this occurs in vivo, we determine ability of different WASP mutations to be tyrosine phosphorylated. Expression of the Cdc42-deficient binding mutant of WASP (H246D) showed reduced PV induced tyrosine phosphorylation compared with wild-type WASP (Figure 7A). As expected, the Y291F and L270P-Y291F mutants were not phosphorylated by PV treatment. Surprisingly, L270P WASP demonstrated higher levels of tyrosine phosphorylation even in the absence of PV treatment. In addition, H246D-L270P mutant fully restored of tyrosine phosphorylation by PV treatment compared with H246D WASP mutant (Figure 7B). Therefore, these results strongly indicated that Cdc42 binding was important for tyrosine phosphorylation of WASP in vivo, potentially by induction of an open conformation.
Figure 7.
Cdc42 regulates phagocytic cup formation via activation and tyrosine phosphorylation of WASP/N-WASP. (A) COS-7 cells were transiently transfected with either myc-tagged WT, H246D, L270P, Y291F, or L270P/Y291F WASP and then left untreated or treated with 12 μM PV for 30 min. Myc-tagged WASP was then immunoprecipitated using myc (monoclonal) antibody, followed by Western blotting with the HRP-PY and myc antibodies. Blots were quantified by densitometry and normalized to myc. Data are expressed as the -fold increase compared with untreated wild-type WASP cells ± SEM. n = 3, *p < 0.05 compared with untreated wild-type WASP. (B) WASPbs WT, H246D, L270P, and H246D/L270P mutants were expressed in COS-7 cells and then left untreated or treated with 12 μM PV for 30 min. Transfected proteins were immunoprecipitated using GFP antibody, followed by Western blotting with the HRP-PY and GFP antibodies. Blots were quantified by densitometry and normalized to GFP. Data are expressed as the -fold increase compared with untreated wild-type WASPbs cells ± SEM. n = 3, *p < 0.05; **p < 0.01 compared with untreated wild-type WASPbs. (C) Control cells (Ctrlsh-A) and clones with reduced Cdc42 expression (sh#1-A) were unstimulated (0′) or incubated with EIgG for 5 min (5′) at 37°C. WASP was immunoprecipitated using WASP antibody, followed by Western blotting with the HRP-PY and WASP antibody (top). Bottom, Crtlsh or sh#1 cells were transfected with GFP-tagged N-WASP before stimulation with EIgG for 5 min and immunoprecipitation with anti-GFP and Western blot analysis as indicated. Representative western blot is shown. Blots were quantified by densitometry and normalized to amount of protein immunoprecipitated. Data are expressed as the -fold increase compared with EIgG-unstimulated Ctrlsh-A. ± SEM. n = 3, *p < 0.05.
Experiments were then performed to determine the role of Cdc42 in WASP or N-WASP tyrosine phosphorylation during FcγR phagocytosis. A significant increase in WASP and N-WASP tyrosine phosphorylation occurred during peak phagocytic cup formation, 3–5 min after stimulation with EIgG (Supplemental Figure 5). To confirm the requirement for Cdc42 in N/WASP tyrosine phosphorylation, we determined the level of tyrosine phosphorylation in either Control-treated cells or Cdc42 shRNA-treated cells during phagocytic cup formation. Incubation with EIgG for 5 min resulted in no significant increase in tyrosine phosphorylation of WASP or N-WASP in Cdc42 shRNA-treated cells compared with control-treated cells (Figure 7C). In addition, H246D mutant also showed no significant increase of tyrosine phosphorylation of WASP after incubation with EIgG (Supplemental Figure 4B). These observations, therefore, strongly indicated that Cdc42 was required for subsequent tyrosine phosphorylation of WASP/NWASP both of which were required for FcγR-mediated phagocytosis.
Together, our data identified a functional role of Cdc42, through the activation and the tyrosine phosphorylation of WASP/N-WASP, leading to FcγR-mediated actin polymerization that is required for phagocytosis.
DISCUSSION
The data presented here demonstrate a requirement for Cdc42-dependent WASP/N-WASP activation and tyrosine phosphorylation in FcγR-mediated phagocytosis. Although previous reports demonstrated the requirements of both Cdc42 and WASP in FcγR-mediated phagocytosis, our data extend these original findings in the following ways: 1) nonspecific effects of Cdc42 dominant-negative mutants are excluded through reduction of endogenous levels of Cdc42 by using shRNA; 2) a role for N-WASP in addition to WASP in phagocytosis was demonstrated; and 3) evidence for molecular mechanism of Cdc42 regulation of phagocytosis in macrophages was provided.
The Rho GTPases play a key role in phagocytosis by linking membrane receptors to the actin cytoskeleton (Etienne-Manneville and Hall, 2002). Previous reports showed that inhibition of Cdc42 blocks FcγR-mediated phagocytosis (Cox et al., 1997) and that Cdc42 was activated and recruited to forming phagosomes in response to IgG-coated particles (Hoppe and Swanson, 2004). Our data confirm the role of Cdc42 in FcγR-mediated phagocytosis through the reduction of endogenous protein levels by using shRNA. Use of dominant-negative or constitutively active mutants of Cdc42 had some limitations. Dominant-negative or constitutively active forms of Rho GTPases might have caused nonspecific effects owing to extensive overlapping of Rho GTPase substrates (Wang and Zheng, 2007). For example, Rac1, Cdc42, and TC10 could all bind p21-activated kinases (Bishop and Hall, 2000). Previously, expression of a dominant-negative mutant of Cdc42 led to decreased phagocytosis; however, cells expressing this mutant also showed a reduction in particle binding (Cox et al., 1997). In contrast, cells depleted of Cdc42 endogenous protein showed no difference of particle binding (Supplemental Figure 1). Furthermore, expression of dominant-negative Rac or Cdc42 individually inhibited FcγR-mediated phagocytosis, but they did not completely block F-actin accumulation at nascent phagosomes (Cox et al., 1997; Massol et al., 1998). However, in this report, reduction of endogenous Cdc42 by using shRNA resulted in an absence of F-actin accumulation in cells challenged with EIgG (Figure 2). These data suggest that nonspecific effects occur when dominant-negative mutants are used.
Although both Cdc42 and Rac induce actin filament assembly, several lines of evidence suggest that they play distinct roles in the phagocytic process. Cdc42, Rac1, and Rac2 are differentially recruited to the phagosome (Hoppe and Swanson, 2004). Activation of Cdc42 occurred early and preferentially at the tips of extending pseudopodia, activation of Rac1 occurred in phagocytic cups and during closure, and Rac2 was primarily active during phagosome closure (Hoppe and Swanson, 2004). Also based on the timing of and localization of Rho GTPases, Rac activity may be needed at later times in the phagocytic process than Cdc42 activity. Our data are consistent with a role for Cdc42 in initiating actin polymerization during phagocytosis (Figure 2). Artificial clustering of constitutively active Cdc42 or WASP near cell-bound particles induced the polymerization of actin (Castellano et al., 1999). Yet, Rac was also shown to be required for FcγR-mediated F-actin accumulation (Cox et al., 1997; Massol et al., 1998), leading to the possibility that Cdc42 may be upstream of Rac during FcγR-mediated phagocytosis. Others have shown that Cdc42 is upstream of Rac during the regulation of focal complex assembly (Allen et al., 1997) and during N-formyl-methionyl-leucyl-phenylalanine tripeptide stimulated actin nucleation in human neutrophils (Glogauer et al., 2000). Further experiments are required to address these issues.
WASP is activated by Cdc42 through binding in GTP-loaded Cdc42 to its CRIB domain in vitro (reviewed in Miki and Takenawa, 2003). Recent studies indicated that WASP plays critical roles in the phagocytic cup formation and phagocytosis (Lorenzi et al., 2000; Tsuboi and Meerloo, 2007). However, the signaling pathways linking phagocytic receptors to WASP activation are still undefined. Our results demonstrate that inhibition of WASP/N-WASP by wiskostatin correlated with inhibition of phagocytosis and phagocytic cup formation. Also, addition of wiskostatin extensively blocked both actin polymerization and cell spreading similar to the phenotype observed after reduced expression of Cdc42. Indeed, Cdc42 was required for activation of WASP/N-WASP during phagocytic cup formation and expression of a constitutively activated form of WASP (L270P) was partially able to restore the phagocytic ability in Cdc42 shRNA-treated cells.
Our data also suggest an independent requirement for N-WASP in phagocytosis. Expression of an activated form of WASP (L270P) did not fully recover phagocytosis in the presence of wiskostatin (Figure 6A) or in Cdc42 shRNA-treated cells (Figure 6B). This is also consistent with the data demonstrating the more dramatic reduction in phagocytosis with wiskostatin treatment, an inhibitor of both WASP and N-WASP, than that seen with WASP deficient macrophages (Figure 3A). Furthermore, both WASP and N-WASP were activated during phagocytosis in a Cdc42-dependent manner (Figure 5, B and C). N-WASP is a ubiquitously expressed homologue of WASP that is expressed in macrophages in addition to WASP (Stamm et al., 2005). A role for N-WASP in the internalization of pathogens in nonhematopoietic cells has been reported recently (McGee et al., 2001; Bierne et al., 2005). Consistent with this, our data showed that both N-WASP and WASP are required for FcγR-mediated phagocytosis (Figure 4B). In addition, each protein could not compensate the function of other in phagocytosis suggesting that WASP and N-WASP seem to play unique roles in phagocytosis. Our data indicated that WASP function contributes to actin polymerization, whereas N-WASP was required for the extension of protrusions in FcγR-mediated phagocytosis (Figure 4). Previous work has demonstrated a role for membrane insertion during FcγR-mediated phagocytosis and spreading (Cox et al., 1999, 2000) and Cdc42 and N-WASP have been shown to regulate exocytosis of neuroendocrine cells (Gasman et al., 2004), suggesting that N-WASP may play a role in membrane insertion during phagocytosis. However, further experiments are required to elucidate the precise role of N-WASP in phagocytosis.
Data presented by Torres and Rosen (2006) suggest that Cdc42 is required to induce a conformational change in WASP that permits its subsequent phosphorylation in vitro, and WASP phosphorylation has been shown to regulate its activity in vitro (Cory et al., 2002). In addition, WASP phosphorylation is important for the phagocytic cup formation (Tsuboi and Meerloo, 2007). Our data clearly showed Cdc42 binding is a necessary step for the activation and tyrosine phosphorylation of WASP (Figure 7), both of which are required for phagocytosis. Together, these observations support the idea of a pathway from FcγR ligation to Cdc42-mediated activation and subsequent tyrosine phosphorylation of WASP leading to actin assembly that is required for efficient phagocytosis.
In conclusion, we have demonstrated Cdc42 as a key signaling molecule in FcγR-mediated phagocytosis of macrophages, indicating for the first time a requirement of Cdc42-mediated WASP/N-WASP activation and have uncovered an independent role for N-WASP in FcγR-mediated phagocytosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the John Condeelis, Jeffrey Segall, and E. Richard Stanley laboratories for helpful discussions. Confocal images were generated at the Analytical Imaging Facility of the Albert Einstein College of Medicine. This work was supported by the Korea Research Foundation grant KRF-2006-352-C00072 (to H. P.) and National Institute Health National Institute of General Medical Sciences grant GM-071828 (to D. C.).
Abbreviations used:
- BMM
bone marrow-derived macrophage
- CSA
conformation-sensitive WASP/N-WASP antibody
- EigG
erythrocytes opsonized with immunoglobulin G
- FcγR
Fcγ receptor
- shRNA
short hairpin RNA
- WASP
Wiskott-Aldrich syndrome protein
- N-WASP
neural Wiskott-Aldrich syndrome protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-03-0230) on September 9, 2009.
REFERENCES
- Aderem A., Underhill D. M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Allen W. E., Jones G. E., Pollard J. W., Ridley A. J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- Ancliff P. J., et al. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood. 2006;108:2182–2189. doi: 10.1182/blood-2006-01-010249. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P., Lindberg U., Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr. Biol. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Bierne H., Miki H., Innocenti M., Scita G., Gertler F. B., Takenawa T., Cossart P. WASP-related proteins, Abi1 and Ena/VASP are required for Listeria invasion induced by the Met receptor. J. Cell Sci. 2005;118:1537–1547. doi: 10.1242/jcs.02285. [DOI] [PubMed] [Google Scholar]
- Bishop A. L., Hall A. Rho GTPases and their effector proteins. Biochem. J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- Cammer M., Gevrey J. C., Lorenz M., Dovas A., Condeelis J., Cox D. The mechanism of CSF-1 induced wasp activation in vivo: A role for PI 3-kinase and CDC42. J. Biol. Chem. 2009;284:23302–23311. doi: 10.1074/jbc.M109.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E., Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Castellano F., Montcourrier P., Guillemot J. C., Gouin E., Machesky L., Cossart P., Chavrier P. Inducible recruitment of Cdc42 or WASP to a cell-surface receptor triggers actin polymerization and filopodium formation. Curr. Biol. 1999;9:351–360. doi: 10.1016/s0960-9822(99)80161-4. [DOI] [PubMed] [Google Scholar]
- Cory G. O., Garg R., Cramer R., Ridley A. J. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. J. Biol. Chem. 2002;277:45115–45121. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- Cox D., Chang P., Zhang Q., Reddy P. G., Bokoch G. M., Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D., Lee D. J., Dale B. M., Calafat J., Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc. Natl. Acad. Sci. USA. 2000;97:680–685. doi: 10.1073/pnas.97.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D., Tseng C. C., Bjekic G., Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- Devriendt K., et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat. Genet. 2001;27:313–317. doi: 10.1038/85886. [DOI] [PubMed] [Google Scholar]
- Dewey R. A., et al. Retroviral WASP gene transfer into human hematopoietic stem cells reconstitutes the actin cytoskeleton in myeloid progeny cells differentiated in vitro. Exp. Hematol. 2006;34:1161–1169. doi: 10.1016/j.exphem.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Dovas A., Gevrey J. C., Grossi A., Park H., Abou-Kheir W., Cox D. Regulation of podosome dynamics by Wiskott-Aldrich Syndrome protein (WASP) phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J. Cell Sci. 2009 doi: 10.1242/jcs.051755. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Fenteany G., Glogauer M. Cytoskeletal remodeling in leukocyte function. Curr. Opin. Hematol. 2004;11:15–24. doi: 10.1097/00062752-200401000-00004. [DOI] [PubMed] [Google Scholar]
- Gasman S., Chasserot-Golaz S., Malacombe M., Way M., Bader M. F. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol. Biol. Cell. 2004;15:520–531. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevrey J. C., Isaac B. M., Cox D. Syk is required for monocyte/macrophage chemotaxis to CX3CL1 (Fractalkine) J. Immunol. 2005;175:3737–3745. doi: 10.4049/jimmunol.175.6.3737. [DOI] [PubMed] [Google Scholar]
- Glogauer M., Hartwig J., Stossel T. Two pathways through Cdc42 couple the N-formyl receptor to actin nucleation in permeabilized human neutrophils. J. Cell Biol. 2000;150:785–796. doi: 10.1083/jcb.150.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe A. D., Swanson J. A. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol. Biol. Cell. 2004;15:3509–3519. doi: 10.1091/mbc.E03-11-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Miki H., Imai K., Nonoyama S., Suzuki T., Sasakawa C., Takenawa T. Wiskott-Aldrich syndrome protein induces actin clustering without direct binding to Cdc42. J. Biol. Chem. 1999;274:27225–27230. doi: 10.1074/jbc.274.38.27225. [DOI] [PubMed] [Google Scholar]
- Kim A. S., Kakalis L. T., Abdul-Manan N., Liu G. A., Rosen M. K. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- Lorenzi R., Brickell P. M., Katz D. R., Kinnon C., Thrasher A. J. Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood. 2000;95:2943–2946. [PubMed] [Google Scholar]
- Massol P., Montcourrier P., Guillemot J. C., Chavrier P. Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J. 1998;17:6219–6229. doi: 10.1093/emboj/17.21.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee K., Zettl M., Way M., Fallman M. A role for N-WASP in invasin-promoted internalisation. FEBS Lett. 2001;509:59–65. doi: 10.1016/s0014-5793(01)03139-8. [DOI] [PubMed] [Google Scholar]
- Miki H., Takenawa T. Regulation of actin dynamics by WASP family proteins. J. Biochem. 2003;134:309–313. doi: 10.1093/jb/mvg146. [DOI] [PubMed] [Google Scholar]
- O'Sullivan E., Kinnon C., Brickell P. Wiskott-Aldrich syndrome protein, WASP. Int. J. Biochem. Cell Biol. 1999;31:383–387. doi: 10.1016/s1357-2725(98)00118-6. [DOI] [PubMed] [Google Scholar]
- Patel J. C., Hall A., Caron E. Vav regulates activation of Rac but not Cdc42 during FcgammaR-mediated phagocytosis. Mol. Biol. Cell. 2002;13:1215–1226. doi: 10.1091/mbc.02-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. R., Bickford L. C., Morgan D., Kim A. S., Ouerfelli O., Kirschner M. W., Rosen M. K. Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nat. Struct. Mol. Biol. 2004;11:747–755. doi: 10.1038/nsmb796. [DOI] [PubMed] [Google Scholar]
- Ridley A. J. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2:303–310. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- Stamm L. M., Pak M. A., Morisaki J. H., Snapper S. B., Rottner K., Lommel S., Brown E. J. Role of the WASP family proteins for Mycobacterium marinum actin tail formation. Proc. Natl. Acad. Sci. USA. 2005;102:14837–14842. doi: 10.1073/pnas.0504663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R. Murine bone marrow-derived macrophages. Methods Mol. Biol. 1997;75:301–304. doi: 10.1385/0-89603-441-0:301. [DOI] [PubMed] [Google Scholar]
- Suetsugu S., Miki H., Takenawa T. Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex. Biochem. Biophys. Res. Commun. 1999;260:296–302. doi: 10.1006/bbrc.1999.0894. [DOI] [PubMed] [Google Scholar]
- Sukumvanich P., DesMarais V., Sarmiento C. V., Wang Y., Ichetovkin I., Mouneimne G., Almo S., Condeelis J. Cellular localization of activated N-WASP using a conformation-sensitive antibody. Cell Motil. Cytoskeleton. 2004;59:141–152. doi: 10.1002/cm.20030. [DOI] [PubMed] [Google Scholar]
- Swanson J. A., Hoppe A. D. The coordination of signaling during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 2004;76:1093–1103. doi: 10.1189/jlb.0804439. [DOI] [PubMed] [Google Scholar]
- Symons M., Derry J. M., Karlak B., Jiang S., Lemahieu V., McCormick F., Francke U., Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Torres E., Rosen M. K. Protein-tyrosine kinase and GTPase signals cooperate to phosphorylate and activate Wiskott-Aldrich syndrome protein (WASP)/neuronal WASP. J. Biol. Chem. 2006;281:3513–3520. doi: 10.1074/jbc.M509416200. [DOI] [PubMed] [Google Scholar]
- Tsuboi S., Meerloo J. Wiskott-Aldrich syndrome protein is a key regulator of the phagocytic cup formation in macrophages. J. Biol. Chem. 2007;282:34194–34203. doi: 10.1074/jbc.M705999200. [DOI] [PubMed] [Google Scholar]
- Wang L., Zheng Y. Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 2007;17:58–64. doi: 10.1016/j.tcb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Lorenz M., Kempiak S., Sarmiento C., Coniglio S., Symons M., Segall J., Eddy R., Miki H., Takenawa T., Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J. Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.