Abstract
Significant increase in JNK, c-Jun, and Cdk5 activities are reported in Alzheimer's disease (AD). Inhibition of c-Jun prevents neuronal cell death in in vivo AD models, highlighting it as a major JNK effector. Both JNK and Cdk5 promote neurodegeneration upon deregulation; however, Cdk5 has not been mechanistically linked to JNK or c-Jun. This study presents the first mechanism showing Cdk5 as a major regulator of the JNK cascade. Deregulated Cdk5 induces biphasic activation of JNK pathway. The first phase revealed c-Jun as a direct substrate of Cdk5, whose activation is independent of reactive oxygen species (ROS) and JNK. In the second phase, Cdk5 activates c-Jun via ROS-mediated activation of JNK. Rapid c-Jun activation is supported by in vivo data showing c-Jun phosphorylation in cerebral cortex upon p25 induction in transgenic mice. Cdk5-mediated biphasic activation of c-Jun highlights c-Jun, rather than JNK, as an important therapeutic target, which was confirmed in neuronal cells. Finally, Cdk5 inhibition endows superior protection against neurotoxicity, suggesting that Cdk5 is a preferable therapeutic target for AD relative to JNK and c-Jun.
INTRODUCTION
Alzheimer's disease (AD) is the most common neurodegenerative disorder that is characterized by extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles (NFTs) with profound neuronal loss (Dickson, 2001). However, the exact molecular mechanisms underlying disease pathogenesis remain to be fully elucidated.
The c-Jun NH2-terminal kinase (JNK, aka SAPK) belongs to a subfamily of mitogen-activated protein kinases (MAPKs) that are activated by a variety of environmental stressors (Davis, 2000; Borsello and Forloni, 2007). The mechanism of JNK activation involves dual phosphorylation on the motif Thr-ProTyr (Davis, 2000; Borsello and Forloni, 2007). In neuronal cells, JNK is preferentially activated by oxidative stress, one of the earliest contributors to neurodegeneration in many neurodegenerative diseases including AD (Nunomura et al., 2001; Pratico et al., 2001; Lagalwar et al., 2006; Borsello and Forloni, 2007; Petersen et al., 2007). As such, not surprisingly, higher JNK activity is observed in the affected areas of the brain in AD (Shoji et al., 2000; Zhu et al., 2001). JNK is also a key mediator of Aβ and glutamate-induced neurotoxicity, both of which are critical in AD (Yang et al., 1997; Savage et al., 2002; Borsello and Forloni, 2007). JNK's contribution to NFT formation is well documented (Yoshida et al., 2004). Recent studies have further identified a vital role of JNK in neurotoxic Aβ formation by activating γ-secretase upon oxidative stress (Shoji et al., 2000; Zhu et al., 2001; Shen et al., 2008). All these studies suggest the involvement of the JNK cascade in promoting neurodegeneration in AD.
Activated JNK phosphorylates several downstream targets, such as c-Jun, activating transcription factor 2 (ATF2), and Elk-1 (Davis, 2000; Borsello and Forloni, 2007); however, in AD, c-Jun is the major substrate that mediates JNK-induced neurotoxicity. JNK potentiates c-Jun activity by phosphorylation at Ser63 and Ser73 residues in the activation domain (Davis, 2000; Borsello and Forloni, 2007). Pearson et al. (2006) reported that p-c-Jun (Ser63) colocalizes with NFTs in AD brains, whereas Thakur et al. (2007) showed p-c-Jun (Ser73) to be more strongly associated with NFTs and granulovacuolar degeneration in AD brains. In sympathetic neurons, hyperactivation of JNK increases p-c-Jun levels, which is associated with apoptosis and can be blocked by expression of the c-Jun dominant negative mutant (Eilers et al., 1998). Suppression of c-Jun expression protects neonatal hippocampal and sympathetic neurons from cell death in culture, and neurons from c-Jun knockout mice are resistant to Aβ-toxicity (Ham et al., 1995; Kihiko et al., 1999). Importantly, c-Jun is overexpressed in AD patients (Anderson et al., 1994, 1996; Martín et al., 1996), and p-c-Jun staining is only found in affected regions in pathological brain, which is similar to p-JNK (Shoji et al., 2000; Zhu et al., 2001; Pearson et al., 2006; Borsello and Forloni, 2007; Thakur et al., 2007).
Besides JNK, cyclin-dependent kinase 5 (Cdk5) also triggers a number of neurodegenerative pathways in AD upon deregulation. Cdk5 is a proline-directed serine-threonine kinase that is activated by neuron-specific activators (p35 and p39; Lew et al., 1994; Tsai et al., 1994; Humbert et al., 2000). Genetic etiology studies show Cdk5/p35/p39 involvement in early onset AD (Rademakers et al., 2005). Exposure of neurons to neurotoxic stimuli such as Aβ or glutamate disrupts intracellular calcium homeostasis and activates calpain, which cleaves p35 into p25 (Patrick et al., 1999; Lee et al., 2000). The binding of p25 to Cdk5 not only transforms Cdk5 into a hyperactive kinase but also mislocalizes Cdk5 from particulate to cytosolic and nuclear. Deregulated Cdk5 phosphorylates multiple pathological targets and subsequently triggers various pathological events in AD including amyloid-β processing, NFT formation, and neurodegeneration (Patrick et al., 1999; Lee et al., 2000; Cruz et al., 2003, 2006; Monaco, 2004; Saito et al., 2007; Sun et al., 2008a,b). Additionally, our recent studies demonstrated that deregulated Cdk5 not only causes robust Golgi fragmentation in neuronal cells via GM130 phosphorylation (Sun et al., 2008a), but also promotes oxidative stress and mitochondrial dysfunction via inhibitory phosphorylation of antioxidant enzymes (Sun et al., 2008b). As such, deregulated Cdk5 is involved in a number of proximal factors leading to neuronal cell death in AD.
Interestingly, a mechanistic link between Cdk5 and the JNK cascade has not been shown to date. Otth et al. (2003) showed that p-JNK colocalizes with Cdk5 in transgenic (Tg)2576 mice, an animal model for AD; however, the consequences of the association were not analyzed. To address this issue and because deregulated Cdk5 promotes oxidative stress, which is the most prominent stimulus for JNK activation (Borsello and Forloni, 2007; Sun et al., 2008b), in this study, we investigated mechanistic links between Cdk5 and the JNK cascade.
MATERIALS AND METHODS
Materials
Glutamate, 3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and poly-l-lysine were obtained from Sigma (St. Louis, MO). β-amyloid25–35 (Aβ25–35) and 2′,7′-dichlorofluorescein diacetate (DCFDA) were purchased from AnaSpec (San Jose, CA). Roscovitine was purchased from LC Laboratories (Woburn, MA). SP600125 was purchased from Calbiochem (La Jolla, CA). Antibodies for JNK, phospho-JNK (Thr183/Tyr185), phospho-c-Jun (Ser63), and phospho-c-Jun (Ser73) were purchase from Cell Signaling Laboratories (Beverly, MA). Antibodies for Cdk5 (C-8), p35/p25 (C-19), c-Jun (G-4), and actin (C-2) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Culture
HT22 cells were a gift from David Schubert (The Salk Institute for Biological Studies, La Jolla, CA). HT22 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS).
Isolation of Primary Cortical Cells
Time-pregnant Sprague Dawley rats were purchased from Charles River (Wilmington, MA). Primary cortical neurons were isolated from Sprague Dawley rat embryos as described previously (Sun et al., 2008a,b). Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere and were used for experiments beginning after 5 d.
Cdk5 Kinase Assay
Glutamate or Aβ25–35-treated cells were rinsed twice with cold PBS and lysed in 1% NP-40 lysis buffer (1% NP-40, 20 mM Tris, pH 8.0, 150 mM NaCl, 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) and cleared by centrifugation at 10,000 rpm for 10 min at 4°C. Cleared lysates were mixed with Cdk5 antibody and protein A-Sepharose beads (Sigma) and incubated at 4°C for 2 h. Immunocomplexes were washed twice with 1% NP-40 lysis buffer and twice with kinase buffer (50 mM Tris, pH 8.0, and 20 mM MgCl2). Immunocomplexes were subjected to in vitro Cdk5 kinase assays using [γ-32P]ATP and Cdk5 substrate peptide (KHHKSPKHR) in a final volume of 30 μl buffered at pH 8.0 containing 50 mM Tris and 20 mM MgCl2 at 30°C. After 20 min, the reactions were terminated by spotting 25 μl of the reaction volume onto p81 phosphocellulose disks (Whatman, Clifton, NJ), which were immersed in 100 ml of 10% acetic acid for 30 min, followed by three washings in 0.5% phosphoric acid (5 min each) and a final rinse with acetone. The radioactivity was measured in a liquid scintillation counter.
Western Blotting
Inhibitor of Cdk5 (roscovitine) or JNK (SP600125) was added 30 min before glutamate or Aβ25–35 treatment. After the indicated treatments, cells were harvested and lysed in a RIPA buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 0.25% deoxycholate, 0.1% SDS, 10 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). The total protein concentration of each cell lysate was determined using the Bradford assay. Equal amounts of cell extracts were separated with SDS-PAGE and transferred to a PVDF membrane. After blocking with 5% skim milk in TBST (20 mM Tris, pH 7.4, 150 mM NaCl, and 0.1% Tween 20), the membrane was probed with a primary antibody overnight at 4°C. The blot was then washed three times with TBST and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. After washing three times with TBST, the blot was developed using a chemiluminescence detection system (Pierce, Rockford, IL).
Expression Plasmids and Constructs
Glutathione S-transferase (GST)-Cdk5 and GST-p25 was a gift from Laurent Meijer (CNRS, Bretagne, France). pET-28b-TAT (V2.1) vector was a gift from Steve Dowdy (University of California, San Diego, La Jolla, CA), mPlum-RFP was a gift from Roger Tsien (University of California, San Diego, La Jolla, CA), GST-c-Jun5–79 was a gift from Janet Cross (University of Virginia, Charlottesville, VA), and TAM67 was a gift from Ze'ev Ronai (Burnham Institute for Medical Research, La Jolla, CA). TAT fusion proteins TAT-Prx-II-T89A, TAT-CIP, and TAT-RFP were generated in our previous studies (Sun et al., 2008a,b). c-Jun5–79 was cloned into pET-28b-TAT (V2.1) vector to generate His-tagged proteins.
Expression and Purification of Recombinant Proteins
GST-Cdk5/p25 was expressed and purified as described previously (Amin et al., 2002). The TAT-fusion proteins (TAT-Prx-II-T89A, TAT-CIP, TAT-RFP) and His-tagged c-Jun5–79 were expressed and purified as described previously (Sun et al., 2008a,b). After purification, protein concentration was determined using the Bradford assay, and the protein purity was assessed by gel electrophoresis. Expressed c-Jun5–79 was also verified by Western blotting using anti-6-His antibody.
Reactive Oxygen Species Measurement
For reactive oxygen species (ROS) measurement, HT22 cells were seeded in a six-well plate for 12 h, and primary cortical neurons were plated for at least 5 d. After indicated treatments, ROS production was measured by DCFDA staining as described previously (Sun et al., 2008b). In brief, DCFDA was added at a final concentration of 30 μM and incubated with cells for an additional 20 min. Cells were detached by scraping gently and were stored on ice. Fluorescence intensity was recorded using a FACScalibur (BD Biosciences, San Jose, CA) with excitation and emission wavelengths of 488 and of 530 nm, respectively.
In Vitro Phosphorylation of c-Jun by Cdk5/p25 Complex
Recombinant c-Jun5–79 (5 μg) were phosphorylated by the purified Cdk5/p25 complex (20 ng) in a final volume of 30 μl containing 50 mM Tris, pH 8.0, 20 mM MgCl2, 100 μM ATP, and 10 μCi [γ-32P]ATP. The reaction mixture was incubated for 30 min at 30°C, after which proteins were separated by SDS-PAGE and transferred to PVDF membrane. The radioactivity associated with protein bands was quantified. Next, phosphorylation of c-Jun5-79 by Cdk5/p25 complex was conducted under the same conditions, but in the absence of [γ-32P]ATP. The reaction mixture was separated by SDS-PAGE and transferred to PVDF membrane, followed by probing using anti-phospho-c-Jun (Ser63) antibody and anti-phospho-c-Jun (Ser73), respectively.
Immunofluorescence for Colocalization of Cdk5 and c-Jun
HT22 cells were plated on poly-l-lysine–coated coverslips at a density of 10,000 cells per well in 24-well plates for 1 d, followed by 5 mM glutamate for 45 min. At the end of the treatment, medium was aspirated, and cells were fixed with 4% formaldehyde for 15 min, rinsed with PBS, and incubated for 20 min with 5% BSA and 0.1% Triton X-100. Cells were then immunostained using antibodies against Cdk5 and c-Jun for 3 h at room temperature. FITC-labeled goat anti-rabbit and Texas red–labeled goat anti-mouse antibodies were used at a 1:1000 dilution, together with DAPI (1 μg/ml). After three washes with PBS and one wash with water, coverslips were mounted on microscope slides with Mowiol mounting medium. Images were taken using a Fluoview laser scanning confocal microscope (Olympus, Melville, NY). The percentage of cells with colocalization of Cdk5 and c-Jun was counted in at least 100 cells from three random frames, in triplicate.
Coimmunoprecipitation of Cdk5 (or p35/p25) and c-Jun
HT22 cells were treated with 5 mM glutamate for 45 min. Cells were then harvested and lysed in RIPA buffer. After centrifugation, whole-cell lysate was mixed with protein A-Sepharose beads (Sigma) and antibody for either Cdk5 or p35/p25. After incubation overnight, immunocomplexes were washed and then subjected to Western blotting using c-Jun antibody.
Immunocytochemistry in p25 Transgenic Mice
CaMKII-tTA and tet-o-p25 mice (Cruz et al., 2003) were purchased from the Jackson Laboratory (Bar Harbor, ME) and mated to generate p25-inducible transgenic mice. A doxycycline-containing diet (200 mg/kg; Bio-Serve, Frenchtown, NJ) was provided before weaning (4–6 wk after birth), and, thereafter, p25 expression was induced by replacing the doxycycline diet with a regular diet for 4 wk. After induction, each mouse was perfused by PBS/10% Formalin, and brains were extracted and processed further for the paraffin section (6 μm). Immunocytochemistry was performed by the ABC method according to the manufacturer's protocol (Vector Laboratories, Burlingame, CA). All slides were randomized and blinded with regard to genotype before staining and subsequent analysis. Briefly, slides were immersed in xylene and hydrated through graded ethanol solutions, and endogenous peroxidase activity was eliminated by incubation in 3% hydrogen peroxide for 30 min. For antigen retrieval, each slide was heated in a pressure cooker in rodent decloaker buffer (Biocare Medical, Concord, CA). To reduce nonspecific binding, sections were incubated for 30 min in 10% normal goat serum (NGS) in Tris-buffered saline (TBS; 50 mM Tris-HCl, and 150 mM NaCl, pH 7.6). After rinsing briefly with 1% NGS in TBS, the sections were incubated overnight at 4°C with one of the following primary antibodies: anti-p-c-Jun (Ser63) rabbit polyclonal antibody or anti-p-c-Jun (Ser73) rabbit polyclonal antibody. Antibodies were localized using 3-3′-diaminobenzidine as a chromogen (Dako, Carpinteria, CA) after incubation with a secondary antibody. To exclude the possibility of nonspecific reaction, all the immunocytochemistry experiments contained at least one sample without a primary antibody. Images were acquired through an AxioCam camera on an Axiophot microscope (Zeiss, Thornwood, NY).
MTT Assay
HT22 cells were seeded onto six-well plates and exposed to the following treatments. For Cdk5 inhibition, TAT-CIP was added every 5 h, and TAT-RFP was used as a control. For JNK inhibition, SP600125 was added 30 min before glutamate treatment, and DMSO was used as a control. For c-Jun inhibition, TAM67 was transfected using Lipofectamine (Invitrogen, Carlsbad, CA) 24 h before glutamate treatment, and empty vector and mock transfection were used as controls. After 24 h after 5 mM glutamate treatment, MTT assay was conducted as described previously (Sun et al., 2008a,b). In brief, MTT was added to the final concentration of 0.5 mg/ml and incubated for 30 min. The cells were washed twice with PBS and lysed in DMSO, and the absorbance was measured at 570 nm.
RESULTS
Cdk5 Activation Precedes JNK Activation upon Neurotoxic Stimulation
In HT22 Cells.
To dissect the molecular mechanism of neurodegeneration, HT22 cells (immortalized mouse hippocampus cells) and primary cortical neurons were chosen for the present study. Glutamate toxicity is a major contributor to pathological cell death in the nervous system, which is mediated by oxidative stress (Coyle and Puttfarcken, 1993).
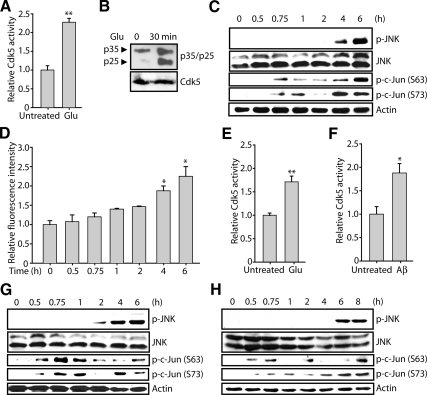
As shown in Figure 1A, Cdk5 was activated within 30 min after glutamate (5 mM) treatment in HT22 cells due to p25 formation (Figure 1B), which is consistent with our previous studies (Sun et al., 2008a,b).
Figure 1.
Neurotoxic stimulation induces Cdk5 deregulation, followed by JNK activation and c-Jun phosphorylation in neuronal cells. (A) Exposure of HT22 cells to glutamate activates Cdk5 activity in 30 min. Cells were seeded for 12 h and treated with 5 mM glutamate. After a 30-min incubation, Cdk5 immunoprecipitation and kinase assay were conducted as described in Materials and Methods. (B) HT22 cells were either untreated (lane 1) or stimulated with 5 mM glutamate for 30 min (lane 2). Whole-cell lysates were separated by SDS-PAGE and transferred to PVDF membrane. Top, p25 and p35 were immunodetected using p35 antibody; bottom, PVDF membrane was then stripped and immunoblotted using Cdk5 antibody as a loading control. (C) Exposure of HT22 cells to glutamate activates the JNK cascade. HT22 cells were treated with 5 mM glutamate for indicated times. Cells were then harvested, lysed, and subjected to Western blot analysis using the indicated antibodies. (D) HT22 cells were treated with 5 mM glutamate for the indicated times, followed by ROS measurement as described in Materials and Methods. (E) Exposure of primary cortical neurons to glutamate activates Cdk5 activity in 30 min. Cells were seeded for 12 h and treated with 5 mM glutamate. After a 30-min incubation, Cdk5 immunoprecipitation and kinase assay were conducted as described in Materials and Methods. (F) Exposure of primary cortical neurons to Aβ25–35 activates Cdk5 activity in 30 min. Cells were seeded and treated with 25 μM Aβ25–35. After a 30-min incubation, Cdk5 immunoprecipitation and kinase assays were conducted as described in Materials and Methods. (G) Exposure of primary cortical neurons to glutamate activates the JNK cascade. Primary cortical neurons were treated with 5 mM glutamate for the indicated times. Cells were then harvested, lysed, and subjected to Western blot analysis using the indicated antibodies. (H) Exposure of primary cortical neurons to Aβ25–35 activates the JNK cascade. Primary cortical neurons were treated with 25 μM Aβ25–35 for indicated times. Cells were then harvested, lysed, and subjected to Western blot analysis using the indicated antibodies. All Western blot analyses were conducted at least three times independently, and representative results are shown. All the bar graphs show the mean; error bars, ±SEM (*p < 0.05, **p < 0.01).
JNK activation was probed in glutamate-treated HT22 cells using a phospho-JNK (Thr183/Tyr185) antibody which recognizes all three isoforms of JNK (JNK1, -2, and -3) upon phosphorylation. As shown in Figure 1C (top two panels), a robust increase in p-JNK level appeared 4–6 h after treatment, whereas the level of total JNK was unchanged. Because oxidative stress has been shown to be a major cause of JNK activation (Davis, 2000; Borsello and Forloni, 2007), intracellular ROS levels were measured in parallel. ROS increased 2–2.5-fold over basal levels 4–6 h after stimulation, which is consistent with the pattern of p-JNK (Figure 1, C and D). These results demonstrate that glutamate-induced Cdk5 activation precedes JNK activation and that glutamate-induced JNK activation is coincident with ROS.
In Primary Cortical Neurons.
Primary cortical neurons were isolated from E17 rat embryos and subjected to glutamate and Aβ25–35 stimulation at 5 d in vitro. Activation of Cdk5 occurs within 30 min after glutamate and Aβ25–35 stimulation in primary cortical neurons (Figure 1, E and F, respectively; Sun et al., 2008a,b). Aβ25–35 is the biologically active and highly toxic core fragment of full-length Aβ (Aβ1–42; Pike et al., 1995) and is produced by enzymatic cleavage of naturally occurring Aβ in brains of AD patients (Kubo et al., 2002). Aβ25–35 induces neurotoxic effects similar to those produced by Aβ1–42 and generates neuropathological signs related to those of early stages of AD (Cheng et al., 2006; Klementiev et al., 2007).
Similar to the results obtained using HT22 cells, p-JNK appeared at a later time point upon glutamate and Aβ25–35 stimulation (Figure 1, F and G, top two panels) when significant ROS were accumulated: ROS increased 1.3-fold over basal levels starting 2 h after glutamate treatment and reached more than 1.5-fold after 4–6 h; ROS increased about 1.5-fold 6 h after Aβ25–35 treatment (data not shown). Together, these results suggest that, upon neurotoxic insult, Cdk5 activation precedes JNK activation and that ROS may be required for JNK activation.
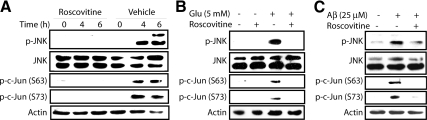
Inhibition of Cdk5 Prevents JNK Activation and c-Jun Phosphorylation upon Neurotoxic Stimulation
JNK phosphorylates c-Jun at Ser63 and Ser73 sites, both of which are relevant in AD pathology (Pearson et al., 2006; Thakur et al., 2007). Given that Cdk5 deregulation leads to an increase in ROS levels in neuronal cells (Sun et al., 2008b) and oxidative stress activates the JNK cascade, we hypothesized that Cdk5 may act as a crucial upstream regulator of JNK and c-Jun. To examine this hypothesis, neuronal cells were pretreated with a Cdk5-specific inhibitor, roscovitine, followed by neurotoxic stimulation. Glutamate was used for HT22 cells, and glutamate and Aβ25–35 were used for primary cortical neurons. Both in HT22 cells and primary cortical neurons, neurotoxic stimulation–induced JNK activation and c-Jun phosphorylation were completely prevented by inhibition of Cdk5 (Figure 2). These results were further verified by transduction of Cdk5-specific inhibitory modulator, TAT-CIP, which was developed in our previous study (data not shown; Sun et al., 2008a). Together, these results revealed that Cdk5 positively regulates JNK activation.
Figure 2.
Inhibition of Cdk5 prevents JNK activation as well as c-Jun phosphorylation upon neurotoxic stimulation in neuronal cells. (A) HT22 cells were exposed to 5 mM glutamate for 6 h in the presence or absence of roscovitine. (B) Primary cortical neurons were exposed to 5 mM glutamate for 6 h in the presence or absence of roscovitine. (C) Primary cortical neurons were exposed to 25 μM Aβ25–35 for 8 h in the presence or absence of roscovitine. In all the cases above, 10 μM roscovitine was added 30 min before neurotoxin treatment. DMSO as vehicle was used as a control. After indicated incubation times, cells were harvested, lysed, and subjected to Western blot analysis using the indicated antibodies. All experiments were conducted at least three independent times, and representative results are shown.
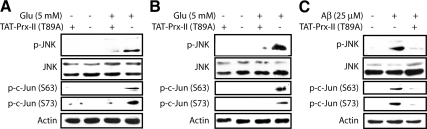
Cdk5-mediated JNK Activation and c-Jun Phosphorylation Are ROS-dependent
Because Cdk5 deregulation promotes oxidative stress (Sun et al., 2008b), one of the major stimulants for JNK activation, and because Cdk5 is an upstream regulator of JNK (Figure 2), we speculated that deregulated Cdk5 mediates JNK activation and subsequent c-Jun phosphorylation by increasing the ROS level. To explore this possibility, “TAT-fused peroxiredoxin-II-T89A” (TAT-Prx-II-T89A; Sun et al., 2008a,b) was transduced into cells to reduce ROS after neurotoxic stimulation in neurons.
Prx-II is a major antioxidant protein that is responsible for eliminating ROS in the cytosol and has been identified as a direct substrate of Cdk5 (Qu et al., 2007; Sun et al., 2008b). Because Prx-II activity is eliminated by phosphorylation at Thr89, Prx-II-T89A is constitutively active (Sun et al., 2008a,b). The fusion of TAT sequence, the minimal Tat transduction domain derived from human immunodeficiency virus 1 (HIV-1), enables efficient protein transduction into cells when directly added to the culture (Nagahara et al., 1998; Becker-Hapak et al., 2001). Transduction of TAT-PrxII-T89A has previously shown to successfully reduce ROS in neuronal cells (Sun et al., 2008a,b). TAT-fusion red fluorescent protein (TAT-RFP) was used as a control, which showed no effect on any biological event investigated (Sun et al., 2008a,b).
HT22 cells and primary cortical neurons were transduced with TAT-PrxII-T89A after neurotoxic stimulation, and phosphorylation of JNK and its target c-Jun was analyzed. Although TAT-RFP addition had no effect on the levels of p-JNK and p-c-Jun, TAT-Prx-II-T89A transduction significantly decreased both p-JNK and p-c-Jun (Figure 3). These results suggest that oxidative stress is the mechanism by which Cdk5 up-regulates JNK activity and c-Jun phosphorylation.
Figure 3.
Transduction of TAT-Prx-II-T89A prevents JNK activation as well as c-Jun phosphorylation upon neurotoxic stimulation in neuronal cells. (A) HT22 cells were exposed to 5 mM glutamate for 6 h with or without sustained addition of TAT-Prx-II-T89A. (B) Primary cortical neurons were exposed to 5 mM glutamate for 6 h with or without sustained addition of TAT-Prx-II-T89A. (C) Primary cortical neurons were exposed to 25 μM Aβ25–35 for 8 h with or without sustained addition of TAT-Prx-II-T89A. In all these cases, 200 nM TAT-Prx-II-T89A was initially added together with the neurotoxin and was readded every 3–4 h. TAT-RFP was used as a control. After indicated incubation times, cells were harvested, lysed, and subjected to Western blot analysis using the indicated antibodies. All experiments were conducted at least three independent times, and representative results are shown.
Biphasic Activation of c-Jun: Rapid c-Jun Phosphorylation Precedes JNK Activation upon Neurotoxic Stimulation
A time course was conducted to examine the levels of p-JNK and p-c-Jun upon neurotoxic stimulation in HT22 cells and primary cortical neurons (Figure 1, C, G, and H), and we observed biphasic phosphorylation of c-Jun. Specifically, p-c-Jun phosphorylation, at both Ser63 and Ser73, increased significantly within 30–45 min and then declined, and later increased again, when JNK became activated (usually 4–6 h after neurotoxic stimulation; Figure 1, C, G, and H). These data suggest that the initial rapid phosphorylation of c-Jun is not JNK-dependent. However, because Cdk5 is rapidly activated upon neurotoxic stimulation (Figure 1, A, E, and F; Sun et al., 2008a,b), we examined whether Cdk5 could directly phosphorylate c-Jun.
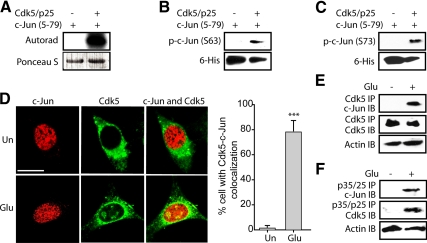
Cdk5 Phosphorylates c-Jun at Ser63 and Ser73 In Vitro
To determine whether c-Jun is a substrate of Cdk5, 6-His-tagged truncated c-Jun recombinant protein (c-Jun5–79) was generated, expressed, purified, and used as a substrate in a kinase assay with the Cdk5–p25 complex. Cdk5/p25 phosphorylated c-Jun in vitro (Figure 4A) at both Ser63 and Ser73 sites (Figure 4, B and C).
Figure 4.
Cdk5 directly phosphorylates c-Jun at both Ser63 and Ser73 residues. (A) Recombinant c-Jun5–79 were phosphorylated by Cdk5–p25 complex using [γ-32P]ATP as described in Materials and Methods. The reaction mixture was incubated for 30 min at 30°C, after which proteins were separated by SDS-PAGE and transferred to PVDF membrane. The radioactivity associated with protein bands was visualized. (B and C) Recombinant c-Jun5–79 were phosphorylated by the Cdk5–p25 complex for 30 min at 30°C as described in Materials and Methods. The reaction mixture was then separated by SDS-PAGE and transferred to PVDF membrane, followed by probing using anti-phospho-c-Jun (Ser63) antibody and anti-phospho-c-Jun (Ser73) antibody, respectively. (D) Left, HT22 cells were treated with 5 mM glutamate for 45 min, followed by immunostaining with c-Jun (red) and Cdk5 (green) as described in Materials and Methods. Representative confocal images are shown. Un, untreated cells; Glu, glutamate. Scale bar, 20 μm. Right, the percentage of cells in which c-Jun and Cdk5 colocalized together was determined as described in Materials and Methods. The bar graphs show the mean; error bars, ±SEM (***p < 0.001). (E) HT22 cells were treated with 5 mM glutamate for 45 min, followed by immunoprecipitation using an antibody for Cdk5 as described in Materials and Methods. Immunocomplexes were then washed and subjected to Western blotting using c-Jun antibody. Ten percent of total cell lysates were used as control by probing with actin. (F) HT22 cells were treated with 5 mM glutamate for 45 min, followed by immunoprecipitation using antibody for p35/p25 as described in Materials and Methods. Immunocomplexes were then washed and subjected to Western blotting using c-Jun antibody. Ten percent of total cell lysates were used as control by probing with actin. All experiments were conducted at least three independent times, and representative results are shown.
Deregulated Cdk5 Binds c-Jun in Cells
To examine the possibility of interaction between Cdk5 and c-Jun in the neuronal cells, localization of both Cdk5 and c-Jun was examined. As shown in Figure 4D (top panel), c-Jun mainly was nuclear-localized, whereas Cdk5 was cytosolic in untreated cells. On glutamate stimulation (45 min), colocalization of Cdk5 and c-Jun was observed in the nucleus (bottom panel), which is consistent with the findings that Cdk5 mislocalizes upon deregulation (Monaco, 2004). To further verify whether Cdk5 directly binds c-Jun, Cdk5 was immunoprecipitated from HT22 after glutamate stimulation (45 min) and probed for c-Jun binding. Although no c-Jun-Cdk5 association was observed in untreated cells, c-Jun coimmunoprecipitated with Cdk5 upon glutamate stimulation (Figure 4E). These data suggest that deregulated Cdk5 directly interacts with c-Jun to mediate the rapid JNK-independent phosphorylation of c-Jun. This association was further verified by specifically isolating p35–p25 immunocomplexes, which revealed c-Jun–Cdk5 association in glutamate-treated, but not in untreated cells (Figure 4F). Moreover, significantly higher amount of Cdk5 was coimmunoprecipitated with p35/p25 from glutamate-treated cells, which is consistent with the previous studies showing that Cdk5 is hyper-activated by forming complexes with p25 (Patrick et al., 1999; Lee et al., 2000).
Cdk5 Mediates Rapid c-Jun Phosphorylation before JNK Activation upon Neurotoxic Stimulation
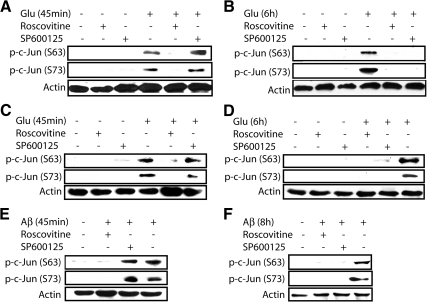
To confirm that Cdk5 directly phosphorylates c-Jun independent of JNK activation, HT22 cells and cortical neurons were pretreated with either Cdk5-specific inhibitor (roscovitine) or a JNK-specific small-molecule inhibitor (SP600125), followed by neurotoxic stimulation for 45 min and 6 h, respectively. As shown in Figure 5, A–F, inhibition of Cdk5 prevents phosphorylation of c-Jun at both times; however, inhibition of JNK only suppressed phosphorylation of c-Jun at 6 h, but not in short-term treatment. Importantly, JNK inhibition largely prevented c-Jun phosphorylation at 6 h, suggesting that phosphorylation of c-Jun at later time is mostly indirect (Cdk5→ROS→JNK→ c-Jun), and is not aided by direct phosphorylation of c-Jun by Cdk5 (Figure 5, B and D). These results were further confirmed using TAT-CIP (Supplementary Figure S1).
Figure 5.
Cdk5 directly mediates a rapid c-Jun phosphorylation before JNK activation in neuronal cells. (A and B) HT22 cells were exposed to 5 mM glutamate for either 45 min or 6 h in the presence or absence of inhibitors. (C and D) Primary cortical neurons were exposed to 5 mM glutamate for either 45 min or 6 h in the presence or absence of inhibitors. (E and F) Primary cortical neurons were exposed to 25 μM Aβ25–35 for either 45 min or 8 h in the presence or absence of inhibitors. In all the cases above, 10 μM roscovitine was used as a Cdk5-specific chemical inhibitor, and 10 μM SP600125 was used as a JNK-specific chemical inhibitor. Both inhibitors were added 30 min before treatment of any stimulus. After indicated incubation times, cells were harvested, lysed, and subjects to Western blot analysis using the indicated antibodies. All experiments were conducted at least three independent times, and representative results are shown.
Overall, these results indicate that the later phosphorylation of c-Jun is due to Cdk5-induced JNK activation, whereas the rapid c-Jun phosphorylation at early time point is directly by Cdk5 in a JNK-independent manner. These results provide the first link between deregulated Cdk5 and two members of JNK cascade: JNK and c-Jun, both of which are involved in promoting neurodegeneration in AD. These results show a novel mechanism by which Cdk5 deregulation may promote neuronal death in AD.
Increased p-c-Jun in p25 Transgenic Mice
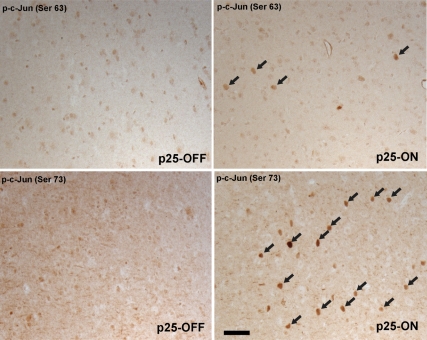
We further advanced our studies into an animal model to verify the direct interaction between Cdk5 and c-Jun. In a number of studies, the expression of p25, which induce Cdk5 activity, has been shown to cause profound neurodegeneration, cognitive dysfunction, and tau-associated pathology in forebrain neuron–specific p25-inducible mice (Cruz et al., 2003; Fischer et al., 2005; Muyllaert et al., 2008). To examine whether levels of p-c-Jun are also induced by Cdk5 in vivo, we examined the level of p-c-Jun in p25-inducible transgenic mice. After a 4-wk induction of p25, the number of both p-c-Jun (Ser63)– and p-c-Jun (Ser73)–positive cells was significantly increased in the cerebral cortical area compared with control mice, which have the same genotype but no induction of p25 (Figure 6). These data strongly confirm our in vitro studies and suggest that the activation of Cdk5 in p25-inducible mice induces the phosphorylation of c-Jun in cortical neurons. Importantly, no neuronal death was observed after 4 wk, which is consistent with the previous study showing neuronal cell death after 8 wk of induction (Cruz et al., 2003). Thus, our data also indicate that the induction of c-Jun phosphorylation is an early event after Cdk5 activation and precedes neuronal cell death in this mouse model.
Figure 6.
Induction of c-Jun phosphorylation by Cdk5 activation in vivo. The phosphorylation of c-Jun is significantly induced in the nuclei of cortical neurons 4 wk after induction of p25 (arrows). Although, in noninduced mice (left panels), the immunoreactivity for both anti-p-c-Jun (Ser 63) and p-c-Jun (Ser 73) is weak and has a diffuse staining pattern, the number of positive cells for both antibodies is strongly increased after p25 induction (right panels). Scale bar, 50 μm. All experiments were conducted at least three independent times, and representative results are shown.
Inhibition of c-Jun Activity Confers a Higher Degree of Neuroprotection than JNK Inhibition
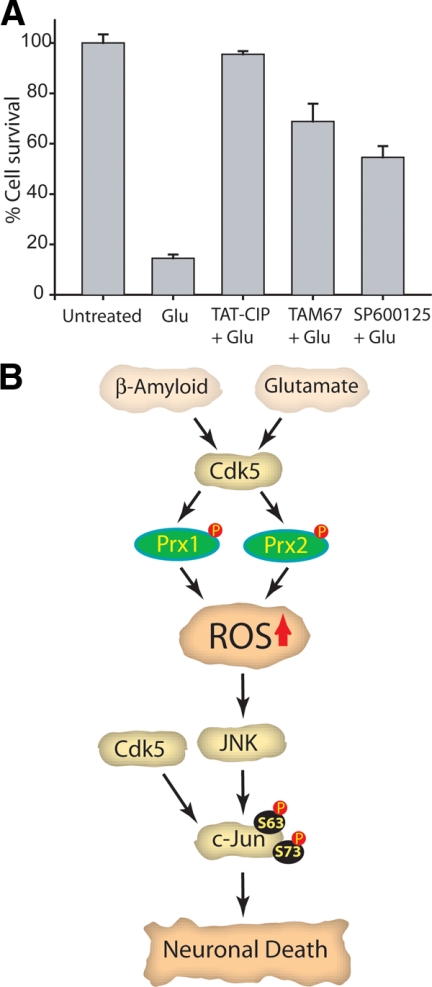
To investigate the exact contribution of Cdk5, JNK and c-Jun in promoting neurotoxicity in neuronal cells, each of these were independently inhibited in glutamate-treated cells, and cell viability was quantified. TAT-CIP was used for Cdk5 inhibition, because roscovitine causes appreciable toxicity upon longer exposure (>24 h; Sun et al., 2008a,b), presumably because of Cdk1 and Cdk2 inhibition. JNK was inhibited using SP600125 and c-Jun using TAM67. TAM67 is a dominant-negative truncated mutant of c-Jun, which inhibits the c-Jun–mediated signaling pathway (Alani et al., 1991). Cdk5 inhibition protected glutamate-treated HT22 cells significantly from cell death (>80%), whereas JNK or c-Jun inhibition was only partially protective (Figure 7A). Importantly, c-Jun inhibition rescued a higher percentage of cells than JNK inhibition (>60 and 40%, respectively), suggesting that c-Jun is not only downstream of JNK but also receives other inputs in neuronal cells. Because Cdk5 deregulation activates many neurodegenerative pathways, it is not surprising that JNK and c-Jun inhibition were not as neuroprotective as Cdk5 inhibition.
Figure 7.
Role of Cdk5 in regulating the JNK cascade in neurons upon neurotoxic insults. (A) Inhibition of Cdk5 or c-Jun activity confers higher degree of neuroprotection compared with JNK inhibition. Inhibition of Cdk5, JNK, or c-Jun was performed in HT22 cells as described in Materials and Methods, followed by 5 mM glutamate stimulation. After an additional 24-h incubation, cell viability was analyzed by MTT assay. This assay was conducted three independent times. Representative results are shown. (B) Proposed model of Cdk5 in regulation of the JNK cascade. Cdk5 regulates JNK pathway both directly and indirectly. Yellow squares highlight three therapeutic targets in AD. Inhibition/ablation of any of these targets confers neuroprotection from neurotoxic signals in neuronal cells. Cdk5 deregulation increases oxidative stress via inactivation of two antioxidant enzymes, Prx1 and Prx2, which lead to ROS-mediated JNK and c-Jun activation. In addition, deregulated Cdk5 also directly phosphorylates c-Jun, suggesting that Cdk5 inhibition is expected to be more neuroprotective than JNK or c-Jun in AD. Cdk5 in yellow square denotes deregulated Cdk5.
DISCUSSION
Numerous studies have shown the vital role of Cdk5/p25, JNK, and c-Jun in mediating Aβ- and glutamate-induced toxicity in neuronal cells; however, no evidence has linked them functionally. Interestingly, one study has reported the colocalization of Cdk5 and p-JNK in an animal model of AD, although the consequence of this association was not analyzed (Otth et al., 2003). In this study, we provide a novel mechanism linking deregulation of Cdk5 and activation of two members of the JNK cascade (Figure 7B): JNK and c-Jun, both of which are involved in promoting neuronal cell death in AD both in concert and independently. Specifically, we demonstrate that Cdk5 deregulation downstream of Aβ or glutamate triggers JNK activation by increasing the ROS level. Inhibition of Cdk5 activity or removal of ROS fully prevents JNK activation, as indicated by c-Jun phosphorylation, suggesting that Cdk5 is a major upstream regulator of the JNK cascade upon neurotoxic stimulation (Figure 7B). In addition, our studies further reveal c-Jun as a direct substrate of Cdk5, which was also confirmed in vivo in the p25-inducible transgenic mouse (Figure 7B). This is the first report that shows Cdk5 deregulation initiates c-Jun phosphorylation at both Ser63 and Ser73, both of which have been shown to be relevant in AD (Pearson et al., 2006; Thakur et al., 2007). Therefore, we postulate that deregulated Cdk5 induces a biphasic activation of c-Jun. Initial deregulation of Cdk5 causes it to associate directly with c-Jun, resulting in its phosphorylation, which is independent of both JNK and ROS. At a later stage, Cdk5 induces p-c-Jun levels through activation of JNK by promoting oxidative stress (Figure 7B).
Importantly, after the initial phosphorylation by Cdk5, c-Jun phosphorylation subsides briefly and then increases with JNK activation (Figure 1, C, G, and H). Similar to the c-Jun phosphorylation pattern, Cdk5 activity also peaks at 1 h after glutamate or Aβ stimulation in HT22 cells and the primary cortical neurons, return to basal levels at 2–3 h, and subsequently increase again at 5–6 h (data not shown). Down-regulation of Cdk5 activity occurs in the presence of increased p25 and Cdk5, suggesting that other mechanisms also contribute to Cdk5 activity. This result is consistent with a previous study showing a similar down-regulation of Cdk5 activity 2–3 h after excitotoxic stimulation (O'Hare et al., 2005). The mechanism remains unclear. Thus, drop in c-Jun phosphorylation, just before JNK activation, could be due to the temporary loss of Cdk5 activity.
Notably, there is one study that shows Cdk5/p35 can confer neuroprotection upon UV irradiation by inhibiting JNK3 activity via phosphorylation (Li et al., 2002). However, because of different cellular localization of Cdk5/p35 and Cdk5/p25 (particulate vs. cytosolic and nuclear localization), aberrant Cdk5 activation via p25 formation upon neurotoxic stimulation is expected to reveal a different outcome. To test this possibility, we conducted a kinase assay using immunoprecipitated JNK, Cdk5/p25, and [γ-32P]ATP. No significant phosphorylation on JNK isoforms was observed (data not shown), suggesting that unlike Cdk5/p35, JNK is not a direct substrate of Cdk5/p25. This result is consistent with the findings showing cytosolic Cdk5/p35 as neuroprotective, whereas nuclear Cdk5/p25 as neurotoxic (O'Hare et al., 2005).
Previous studies have revealed that inhibition of the JNK cascade using JNK-specific inhibitors, CEP-1347 (Cephalon) or SP600125, prevents neuronal cell death in in vivo models of AD and Parkinson's disease (PD). Furthermore, c-Jun phosphorylation has been implicated in promoting AD pathogenesis in several instances. Because JNK is the only known upstream regulator of c-Jun discovered to date, its inhibition is a viable therapeutic approach for AD. These results have prompted pharmaceutical companies to direct their attention toward treatment of AD and PD using specific JNK inhibitors. Therefore, our findings revealing the mechanistic link between Cdk5 and two crucial members of JNK pathway have important therapeutic significance. Inhibition of Cdk5 endows superior protection against neurotoxicity, which further confirms that Cdk5 acts upstream to regulate the JNK cascade along with other unidentified Cdk5-mediated downstream effectors. In conclusion, deregulation of Cdk5 triggers diverse events that are fatal to neurons. One such event is mediated via the JNK cascade. Therefore, our study suggests that Cdk5 is a preferable therapeutic target for AD relative to JNK and c-Jun.
Supplementary Material
ACKNOWLEDGMENTS
We thank Laurent Meijer for GST-Cdk5 and GST-p25, Steve Dowdy for pET-28b-TAT (V2.1) vector, Roger Tsien for mPlum-RFP, Janet Cross for GST-c-Jun5–79, Ze'ev Ronai for TAM67, David Schubert for HT22 cells, and Philip Low (Purdue University) for the confocal microscope. This work was supported by Purdue Research Foundation (K.S.) and National Institutes of Health Grant R01AG028679 (M.A.S. and H.L.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0433) on September 23, 2009.
REFERENCES
- Alani R., Brown P., Binétruy B., Dosaka H., Rosenberg R. K., Angel P., Karin M., Birrer M. J. The transactivating domain of the c-Jun proto-oncoprotein is required for cotransformation of rat embryo cells. Mol. Cell. Biol. 1991;11:6286–6295. doi: 10.1128/mcb.11.12.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin N. D., Albers W., Pant H. C. Cyclin-dependent kinase 5 (cdk5) activation requires interaction with three domains of p35. J. Neurosci. Res. 2002;67:354–362. doi: 10.1002/jnr.10116. [DOI] [PubMed] [Google Scholar]
- Anderson A. J., Cummings B. J., Cotman C. W. Increased immunoreactivity for Jun- and Fos-related proteins in Alzheimer's disease. Exp. Neurol. 1994;125:286–295. doi: 10.1006/exnr.1994.1031. [DOI] [PubMed] [Google Scholar]
- Anderson A. J., Su J. H., Cotman C. W. DNA damage and apoptosis in Alzheimer's disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J. Neurosci. 1996;16:1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Hapak M., McAllister S. S., Dowdy S. F. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24:247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- Borsello T., Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr. Pharmaceut. Design. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- Cheng G., Whitehead S. N., Hachinski V., Cechetto D. F. Effects of pyrrolidine dithiocarbamate on beta-amyloid (25–35)-induced inflammatory responses and memory deficits in the rat. Neurobiol. Dis. 2006;23:140–151. doi: 10.1016/j.nbd.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Cruz J. C., Tseng H. C., Goldman J. A., Shih H., Tsai L. H. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Cruz J. C., Kim D., Moy L. Y., Dobbin M. M., Sun X., Bronson R. T., Tsai L. H. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J. Neurosci. 2006;26:10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Dickson D. W. Neuropathology of Alzheimer's disease and other dementias. Clin. Geriatr. Med. 2001;17:209–228. doi: 10.1016/s0749-0690(05)70066-5. [DOI] [PubMed] [Google Scholar]
- Eilers A., Whitfield J., Babij C., Rubin L. L., Ham J. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J. Neurosci. 1998;18:1713–1724. doi: 10.1523/JNEUROSCI.18-05-01713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Sananbenesi F., Pang P. T., Lu B., Tsai L. H. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Ham J., Babij C., Whitfield J., Pfarr C. M., Lallemand D., Yaniv M., Rubin L. L. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Humbert S., Lanier L. M., Tsai L. H. Synaptic localization of p39, a neuronal activator of cdk5. Neuroreport. 2000;11:2213–2216. doi: 10.1097/00001756-200007140-00030. [DOI] [PubMed] [Google Scholar]
- Kihiko M. E., Tucker H. M., Rydel R. E., Estus S. c-Jun contributes to amyloid beta-induced neuronal apoptosis but is not necessary for amyloid beta-induced c-jun induction. J. Neurochem. 1999;73((6)):2609–2612. doi: 10.1046/j.1471-4159.1999.0732609.x. [DOI] [PubMed] [Google Scholar]
- Klementiev B., Novikova T., Novitskaya V., Walmod P. S., Dmytriyeva O., Pakkenberg B., Berezin V., Bock E. A neural cell adhesion molecule-derived peptide reduces neuropathological signs and cognitive impairment induced by A beta 25–35. Neuroscience. 2007;145:209–224. doi: 10.1016/j.neuroscience.2006.11.060. [DOI] [PubMed] [Google Scholar]
- Kubo T., Nishimura S., Kumagae Y., Kaneko I. In vivo conversion of racemized beta-amyloid ([D-Ser 26]A beta 1–40) to truncated and toxic fragments ([D-Ser 26]A beta 25–35/40) and fragment presence in the brains of Alzheimer's patients. J. Neurosci. Res. 2002;70:474–483. doi: 10.1002/jnr.10391. [DOI] [PubMed] [Google Scholar]
- Lagalwar S., Guillozet-Bongaarts A. L., Berry R. W., Binder L. I. Formation of phospho-SAPK/JNK granules in the hippocampus is an early event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006;65:455–464. doi: 10.1097/01.jnen.0000229236.98124.d8. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Kwon Y. T., Li M., Peng J., Friedlander R. M., Tsai L. H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Lew J., Huang Q. Q., Qi Z., Winkfein R. J., Aebersold R., Hunt T., Wang J. H. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- Li B. S., Zhang L., Takahashi S., Ma W., Jaffe H., Kulkarni A. B., Pant H. C. Cyclin-dependent kinase 5 prevents neuronal apoptosis by negative regulation of c-Jun N-terminal kinase 3. EMBO J. 2002;21:324–333. doi: 10.1093/emboj/21.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín G., Seguí J., Díaz-Villoslada P., Montalbán X., Planas A. M., Ferrer I. Jun expression is found in neurons located in the vicinity of subacute plaques in patients with multiple sclerosis. Neurosci. Lett. 1996;212:95–98. doi: 10.1016/0304-3940(96)12776-2. [DOI] [PubMed] [Google Scholar]
- Monaco E. A., 3rd. Recent evidence regarding a role for Cdk5 dysregulation in Alzheimer's disease. Curr. Alzheimer Res. 2004;1:33–38. doi: 10.2174/1567205043480519. [DOI] [PubMed] [Google Scholar]
- Muyllaert D., Terwel D., Kremer A., Sennvik K., Borghgraef P., Devijver H., Dewachter I., Van Leuven F. Neurodegeneration and neuroinflammation in cdk5/p25-inducible mice: a model for hippocampal sclerosis and neocortical degeneration. Am. J. Pathol. 2008;172:470–485. doi: 10.2353/ajpath.2008.070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara H., Vocero-Akbani A. M., Snyder E. L., Ho A., Latham D. G., Lissy N. A., Becker-Hapak M., Ezhevsky S. A., Dowdy S. F. Transduction of full-length TAT fusion proteins into mammalian cells: TATp27Kip1 induces cell migration. Nat. Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- Nunomura A., et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- O'Hare M. J., Kushwaha N., Zhang Y., Aleyasin H., Callaghan S. M., Slack R. S., Albert P. R., Vincent I., Park D. S. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J. Neurosci. 2005;25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otth C., Mendoza-Naranjo A., Mujica L., Zambrano A., Concha I. I., Maccioni R. B. Modulation of the JNK and p38 pathways by cdk5 protein kinase in a transgenic mouse model of Alzheimer's disease. Neuroreport. 2003;14:2403–2409. doi: 10.1097/00001756-200312190-00023. [DOI] [PubMed] [Google Scholar]
- Patrick G. N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L. H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Pearson A. G., Byrne U. T., MacGibbon G. A., Faull R. L., Dragunow M. Activated c-Jun is present in neurofibrillary tangles in Alzheimer's disease brains. Neurosci. Lett. 2006;398:246–250. doi: 10.1016/j.neulet.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Petersen R. B., Nunomura A., Lee H. G., Casadesus G., Perry G., Smith M. A., Zhu X. Signal transduction cascades associated with oxidative stress in Alzheimer's disease. J. Alzheimers Dis. 2007;11:143–152. doi: 10.3233/jad-2007-11202. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz-Wasserman A. J., Kosmoski J., Cribbs D. H., Glabe C. G., Cotman C. W. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25–35 region to aggregation and neurotoxicity. J. Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- Pratico D., Uryu K., Leight S., Trojanoswki J. Q., Lee V. M. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D., et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Rademakers R., Sleegers K., Theuns J., Van den Broeck M., Bel Kacem S., Nilsson L. G., Adolfsson R., van Duijn C. M., Van Broeckhoven C., Cruts M. Association of cyclin-dependent kinase 5 and neuronal activators p35 and p39 complex in early-onset Alzheimer's disease. Neurobiol. Aging. 2005;26:1145–1151. doi: 10.1016/j.neurobiolaging.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Saito T., Konno T., Hosokawa T., Asada A., Ishiguro K., Hisanaga S. p25/cyclin-dependent kinase 5 promotes the progression of cell death in nucleus of endoplasmic reticulum-stressed neurons. J. Neurochem. 2007;102:133–140. doi: 10.1111/j.1471-4159.2007.04540.x. [DOI] [PubMed] [Google Scholar]
- Savage M. J., Lin Y. G., Ciallella J. R., Flood D. G., Scott R. W. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer's disease model is associated with amyloid deposition. J. Neurosci. 2002;22:3376–3385. doi: 10.1523/JNEUROSCI.22-09-03376.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Chen Y., Liu H., Zhang K., Zhang T., Lin A., Jing N. Hydrogen peroxide promotes Abeta production through JNK-dependent activation of gamma-secretase. J. Biol. Chem. 2008;283:17721–17730. doi: 10.1074/jbc.M800013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Iwakami N., Takeuchi S., Waragai M., Suzuki M., Kanazawa I., Lippa C. F., Ono S., Okazawa H. JNK activation is associated with intracellular beta amyloid accumulation. Brain Res. Mol. Brain Res. 2000;85:221–233. doi: 10.1016/s0169-328x(00)00245-x. [DOI] [PubMed] [Google Scholar]
- Sun K. H., de Pablo Y., Vincent F., Johnson E. O., Chavers A. K., Shah K. Novel genetic tools reveal Cdk5's major role in golgi fragmentation in Alzheimer's disease. Mol. Biol. Cell. 2008a;19:3052–3069. doi: 10.1091/mbc.E07-11-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K. H., de Pablo Y., Vincent F., Shah K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J. Neurochem. 2008b;107:265–278. doi: 10.1111/j.1471-4159.2008.05616.x. [DOI] [PubMed] [Google Scholar]
- Thakur A., Wang X., Siedlak S. L., Perry G., Smith M. A., Zhu X. c-Jun phosphorylation in Alzheimer disease. J. Neurosci. Res. 2007;85:1668–1673. doi: 10.1002/jnr.21298. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Delalle I., Caviness V. S., Jr., Chae T., Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Yang D. D., Kuan C. Y., Whitmarsh A. J., Rincón M., Zheng T. S., Davis R. J., Rakic P., Flavell R. A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Hastie C. J., McLauchlan H., Cohen P., Goedert M. Phosphorylation of microtubule-associated protein tau by isoforms of c-Jun N-terminal kinase (JNK) J. Neurochem. 2004;90:352–358. doi: 10.1111/j.1471-4159.2004.02479.x. [DOI] [PubMed] [Google Scholar]
- Zhu X., Raina A. K., Rottkamp C. A., Aliev G., Perry G., Boux H., Smith M. A. Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer's disease. J. Neurochem. 2001;76:435–441. doi: 10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.