Abstract
Hematopoietic stem cells are believed to reside in a limited number of specialized niches within the bone marrow. In this perspective article, Drs. Forsberg and Smith-Berdan review the prominent role that cell surface receptors play in integrating extrinsic and intrinsic cues to support effective hematopoiesis. See related article on page 1493.
Hematopoietic stem cells (HSC) are responsible for maintaining a functional blood system throughout life. They fulfill this requirement by giving rise both to new HSC and to a cascade of increasingly mature cells, thereby balancing the processes of self-renewal and multilineage differentiation. HSC also have a tremendous capability to respond to stress and rapidly restore hematopoietic homeostasis by giving rise to the appropriate cell types. The mechanisms governing HSC function have been intensely investigated and a long list of molecules has been found to influence the properties of HSC. However, more than 50 years after the first successful hematopoietic transplant and two decades after the prospective isolation of HSC, large gaps in knowledge hamper both our understanding of basic HSC biology and their clinical use in regenerative medicine. Here we review the prominent role that cell surface receptors play in integrating extrinsic and intrinsic cues to support effective hematopoiesis.
HSC are believed to reside in a limited number of specialized niches within the bone marrow. An important role of these niches is to balance HSC self-renewal and differentiation, quiescence and proliferation. Intriguingly, HSC location changes during development, with hematopoiesis shifting from the yolk sac and aorta-gonad-mesonephros region to the placenta, fetal liver and bone marrow.1 In adult life, HSC remain in dynamic contact with bone marrow niches, and can also be found in extramedullary sites such as spleen, liver and blood at various levels in response to stress or experimental stimuli.
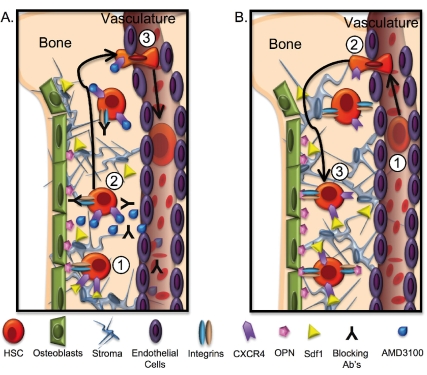
The clinical use of bone marrow and HSC transplantation is well established and has made HSC a paradigm for stem cell therapy. Indeed, hematopoietic transplants are used to treat both hematopoietic and non-hematopoietic disorders and to reconstitute hematopoiesis after cancer therapies of a variety of solid tumors. A prerequisite for proper HSC function upon transplantation is the ability to travel through the blood stream and find these specialized bone marrow niches, a process referred to as homing. Homing and subsequent engraftment are likely accomplished by a combination of passive transport in the blood, active migration through the vascular endothelium, and adhesive interactions with cellular and extracellular components of the niche (Figure 1)2. Once engrafted in the niche, HSC fate decisions are influenced by a combination of cell intrinsic and extrinsic factors.
Figure 1.
Hematopoietic stem cell movement upon mobilization and transplantation. (A) HSC mobilization to the blood stream. HSC residence in bone marrow niches is likely mediated by multiple types of interactions (1). These interactions can be disrupted by addition of blocking antibodies or by inhibitors such as AMD3100 (2), or by indirect stimulus by granulocyte colony-stimulating factor and other cytokines, resulting in relocalization of HSC to the blood stream (3). (B) HSC homing and engraftment. Upon transplantation, HSC travel through the blood (1), relocate from the vasculature to the bone marrow by active extravasation (2), and engraft in bone marrow niches by molecular interaction with numerous niche components (3).
Role of integrin α9 in hematopoiesis
Integrins are among the factors that regulate HSC function. This family of multifunctional, heterodimeric, transmembrane proteins plays critical roles in the development and homeostasis of many different tissue systems, and hematopoiesis is no exception. Many of the integrins are expressed in distinct patterns during hematopoietic differentiation and play important functional roles in several different processes. In this issue of the month, Schreiber et al. add integrin α9 to the growing number of integrins that are known to influence hematopoietic stem and progenitor cell (HSPC) location, proliferation and differentiation.3
Over time, the view of integrins has expanded from the classical model of relatively static cell-matrix adhesion molecules to incorporate a much more diverse array of functions that includes cell-cell interactions, as well as inside-out and outside-in signaling. Together, these diverse functions help regulate multiple cellular processes. A well-documented example of integrin regulation of hematopoiesis is the control of HSC migration by α4β1. Antibody inhibition of α4β1 induces HSC mobilization to the blood and impairs HSC engraftment upon transplantation.4,5 Until now, however, the expression and functional roles of integrins α7-11 in HSPC had not been examined. Thus, Schreiber et al. began their investigation by showing that α7 and α9, but not α8, α10 or α11, are expressed by human cord blood and bone marrow HSPC. Using flow cytometry, they showed that integrins α9 and β1 are robustly expressed on lineage marker negative (Lin−)CD133+ bone marrow cells and on Lin−CD34+ cord blood cells. Similarly, a concurrent article in Blood demonstrated integrin α9 expression by both mouse and human HSC.6
Schreiber et al. then focused on determining the role of α9, partnering with β1, in HSPC function. They showed that CD34+ HSPC adhere to primary human osteoblasts, and that anti-α9 and anti-β1 antibodies inhibit this interaction. As osteoblasts express multiple proteins capable of mediating this association, HSPC binding to the previously identified α9 ligands vascular cell adhesion molecule-1 (VCAM1),7 tenascin-C8 and osteopontin9 was tested. As expected, HSPC adhered to recombinant VCAM1 and tenascin-C. However, in contrast to the adhesion to osteoblasts, interaction with these recombinant proteins was not affected by HSPC preincubation with anti-α9 antibodies. It is possible that cell-cell interactions are more dynamic than cell adhesion to immobilized targets and, therefore, more susceptible to antibody-mediated inhibition. In addition, the recombinant protein concentrations may be vastly higher than the levels of VCAM1 or tenascin-C on the osteoblast cell surface, and this could explain the apparent discrepancy in α9-mediated interactions. Titration of recombinant protein concentrations may resolve this issue.
Nevertheless, HSPC adhesion to VCAM1 and tenascin-C seems selective for these proteins, as HSPC did not bind to recombinant osteopontin. This is particularly interesting as a parallel study suggests specific binding and functionally relevant interactions between α9-positive HSPC and osteopontin.6 Different assays resulting in conflicting findings make it unclear which molecular interactions are relevant under physiological conditions.
It has been shown previously that integrin α9-deficient mice have a specific decrease in neutrophil development, while numbers of other cell types, as well as in vitro colony formation, were normal.10 In their study, Schreiber et al. showed that the anti-α9 blocking antibody inhibits HSPC proliferation and colony formation in vitro. The authors concluded that integrin α9 influences multiple functions of HSPC, including cell adhesion and differentiation.
In light of the mild phenotype of α9 null mice and the likely redundancies between α9 and other integrins, the profound effects of the α9 blocking antibody are somewhat surprising. Similarly, blocking α4 function with antibodies seemed to have a greater effect on HSC homing and engraftment than genetic deletion of the α4 gene.11,12 If α9 and α4 are functionally redundant, one might expect that inhibiting either molecule alone would have a relatively limited impact on functional interactions. One possible explanation is that rather than simply blocking adhesive interactions, binding of the antibody activates integrin-mediated signaling.13 This could set off a cascade of events not possible when the integrin subunit is absent, and lead to unanticipated outcomes such as apoptosis.14 Induction of signaling could also explain how the anti-α9 antibody has significant effects on cellular function even in suspension cell culture, in which adhesive interactions are likely to be of limited importance. Alternatively, blocking antibodies, having a more acute effect, could preclude compensatory mechanisms that reduce the phenotype in cells with a genetic deletion. The use of α4 and α9 null cells, and testing whether anti-α4 and anti-α9 antibodies induce signaling in wild-type cells would help to distinguish between these possibilities.
Redundancies in expression and function of cell surface molecules
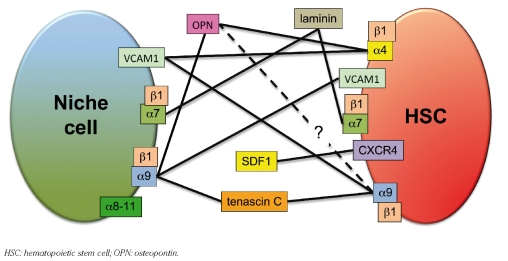
The overlapping expression of molecules that bind the same ligands or share other functions can mask the role of individual molecules (Figure 2). With the addition of α7 and α9, hematopoietic cells express at least ten distinct integrin heterodimers. HSC themselves express many molecules that may have similar functions, such as adhesion to niche components. Redundancies between cell surface molecules seem common, as HSC lacking several molecules with well-established functions in hematopoiesis are nevertheless often capable of overcoming such deficiencies. These include α4, α9 and VCAM1 mentioned above, and CXC chemokine receptor-4 (CXCR4), discussed below. In addition, molecular redundancies are not limited to HSC themselves, but also include cell types that may comprise the niche. For example, both osteoblasts and HSC express α9 in addition to VCAM1.15–17 Does α9 on HSC bind to VCAM1 on osteoblasts, or is it VCAM1 on HSC that binds to α9 on osteoblasts? Maybe two-way binding reinforces the interaction. Alternatively, osteoblasts may use these molecules primarily to adhere to self, to form clusters or layers of osteoblasts. Self-self interactions also open up the exciting possibility that HSC interact directly with other HSC. Such clustering could serve to foster a highly specific environment that supports unique HSC properties such as self-renewal. Testing the potential physiological roles of such cell interactions will require sophisticated experimental approaches, but could lead to important new insights.
Figure 2.
Cartoon of the molecular interactions between HSC and the niche discussed in the text. In this model, a network of interactions with niche cells such as osteoblasts as well as with components of the extracellular matrix reinforces HSC localization to supportive niches. Multiple additional interactions not depicted here influence HSC location.
As one of the likely functions of the niche is to promote HSC quiescence, in vivo links between cell location and proliferation are not surprising. Thus, when manipulating molecules that strictly provide a physical tether to the niche, one would expect more pronounced effects in vivo than in vitro. In the case of integrins, however, the predicted outcome is more complex because of the ability of these proteins to participate in both adhesion and bidirectional signaling. To fully understand integrin regulation of HSC, the function of the integrins themselves has to be determined. Do integrins on HSC engage in cell-cell or cell-matrix interactions or both? What are the molecular targets and where are these targets located? Do integrins participate in signaling in HSC? What factors initiate and propagate such signaling, and what are the functional consequences? For many of the integrins, powerful methods of investigation exist, including specific monoclonal antibodies and germline and conditional gene deletions. These methods will be invaluable to further probe integrin regulation of HSC function.
In addition to molecular and functional redundancies, cellular redundancies also play important roles when considering HSC-specific properties. Even in their predominant in vivo location, the bone marrow, HSC are extremely rare, accounting for only about 0.01% of all nucleated cells. To achieve a competitive advantage for niches specifically supporting HSC properties, HSC most likely take advantage of very selectively expressed cell surface molecules capable of mediating strong niche interactions. Alternatively, HSC may use a unique combination of cell surface receptors (discussed below). Unfortunately, neither Schreiber et al. nor Grassinger et al. determined whether α9 is expressed selectively, or at higher levels, on HSC compared to multi- or oligopotent hematopoietic progenitors. However, Schreiber et al. did show that approximately 70% of Lin−CD34+ bone marrow cells express α9; very few of these are long-term repopulating HSC.19 Integrin α9 is also displayed on neutrophils and on circulating HSPC in cord blood, so it is not specific to HSPC actively engaged in niche interactions. This relatively broad expression pattern makes it unlikely that this molecule, at least by itself, regulates HSC-specific localization to rare HSC niches. Of course, context-dependent integrin activation or downstream signaling may differentiate between outcomes in distinct cell types. Thus, it will be interesting to learn whether integrin α9 expression, activation or signaling is cell-type dependent, or modulated upon HSC mobilization to the blood stream.
Lessons from the chemokine stromal cell-derived factor 1 and its receptor CXCR4
A well-established regulator of hematopoietic migration and location is the seven-transmembrane, G-protein-coupled receptor CXCR4. HSC actively migrate toward the chemokine stromal cell-derived factor 1 (SDF-1 or CXCL12) in what appears to be a direct response to SDF-1 by CXCR4 receptors on HSC.19, 20 Mice deficient in either SDF-1 or CXCR4 die during late embryogenesis and lack bone marrow hematopoiesis,21,22 and CXCR4-blocking antibodies impair HSC engraftment.23 Recent data suggest that HSC specifically localize close to bone marrow cells expressing high levels of SDF-1.24 The SDF-1-CXCR4 interaction can be disrupted by a pharmacological CXCR4 inhibitor, AMD3100, resulting in a rapid increase in HSC in the peripheral blood.25 These experiments have convincingly demonstrated critical roles for SDF-1 and CXCR4 in specifying HSC location.
Surprisingly, however, two groups have independently shown that CXCR4−/− HSC, isolated from the bone marrow of mice in which CXCR4 was deleted in adulthood, home and engraft in the bone marrow upon transplantation.24,26 Prior to these reports, it was conceivable that mice lacking CXCR4 fail to establish hematopoiesis because CXCR4 null HSC are incapable of migrating from the fetal liver to seed the bone marrow. Clearly, however, CXCR4 null HSC are capable of migration and engraftment, although less efficiently. Why, then, do the CXCR4 null HSC in the fetal liver fail to seed the bone marrow and initiate adult hematopoiesis?
Considering the differences between temporal and spatial elements may provide some clues. First, it is possible that CXCR4 is required not only by HSC, but also by cells in the niche. Homotypic interactions between CXCR4 expressed on two different cell types have not been reported, but other CXCR4-dependent cells may be important components of the niche. Alternatively, CXCR4 may promote downstream events necessary for HSC function, but after such maturation CXCR4 is no longer required. This type of scenario has been described for the transcription factor SCL, which is necessary for fetal specification of HSC, but dispensible after that.27 In the case of CXCR4, the migratory abilities conveyed to cells expressing this molecule may be a key factor. Perhaps a certain number of HSC are required to establish a supportive niche environment in synergy with other factors, but once established, the niche is capable of also supporting CXCR4-deficient HSC. This is an intriguing possibility that can be tested by reciprocal transplantation using wild-type and CXCR4-deficient mice as both donors and recipients.
Amazingly, it is still unclear exactly how adult bone marrow hematopoiesis is established. As described above, seeding of the bone marrow by fetal liver HSC is one possibility. It is well established that fetal liver HSC are capable of reconstituting bone marrow hematopoiesis in transplantation models, and HSC are present in the blood stream at increased levels just prior to the initiation of bone marrow hematopoiesis.28 Alternatively, bone marrow hematopoiesis may not be dependent on migration and engraftment of fetal liver HSC, as there is considerable evidence for HSC arising de novo in the bone marrow. In this case, the main role of CXCR4 may shift from its ability to direct long-range migratory events to its properties promoting retention of HSC in bone marrow niches. Residence time in the bone marrow would allow HSC to gain CXCR4 independence, perhaps by upregulating expression of integrins or other molecules fulfilling similar functions. Some of these factors may be lacking in fetal liver HSC, making them unable to engraft under the suboptimal conditions of CXCR4 deletion. Indeed, there are several examples of differential requirements for factors in fetal versus adult HSC.
How is hematopoietic stem cell-specific location regulated?
Osteoblasts, expressing integrin-binding partners, and SDF-1-expressing cells are among the cell types proposed to create an HSC-supportive environment (Figure 1). However, both CXCR4 and integrin α9 are expressed by numerous other bone marrow cells, making it unlikely that either molecule by itself dictates HSC location. How then is HSC location specified?
We find it likely that a matrix of interactions provided by multiple different molecules regulate HSC location to the niche (Figure 2). Such a network of additive forces may be necessary to achieve sufficiently strong interactions and enable rare HSC to outcompete more numerous cells expressing some, but not all, of the same adhesion molecules. Although overlapping in adhesive function, each molecular association may be regulated by distinct mechanisms, allowing diverse stimuli to modify HSC location. Positive and negative forces, such as adhesion and cytokines, would thus regulate dynamic HSC interactions with the niche in a combinatorial manner and allow HSC to respond to the varying demands of the blood system. The proposed scenario is reminiscent of the histone code hypothesis, by which several types of protein modifications promoting transcriptional activation or repression work together to dictate the final transcriptional outcome. Likewise, combinatorial expression of adhesion molecules may create a niche code that ultimately determines HSC location.
To fully appreciate the complex regulation of HSC location and fate, we must begin to assess the relationships between the many factors that influence HSC function. Is there a hierarchy between different adhesion molecules, both in terms of the sequence in which they act and in signaling cascades? Do integrins serve their expected function as integrators of multiple signals with context-dependent specificity? Combining experimental evidence from the vast number of studies performed to date with computational-based network mapping would likely reveal pathway hierarchies, as well as relevant convergence on common components. Novel and sophisticated approaches will be necessary to unravel the complex networks that make stem cells unique and to exploit their full clinical potential.
Footnotes
Dr. Camilla Forsberg is an Assistant Professor at the Institute for Biology of Stem Cells in the Department of Biomolecular Engineering, University of California Santa Cruz, USA. She is supported by a New Faculty Award from the California Institute of Regenerative Medicine (CIRM). Stephanie Smith-Berdan is a Research Specialist at the University of California Santa Cruz, USA. Her professional energies are focused on understanding the molecular cues regulating hematopoietic stem cell migration and differentiation.
The authors thank Drs. David Alexander, Fernando Ugarte, Amy Wagers and Emmanuelle Passegué for insightful comments on the manuscript.
References
- 1.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–44. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 2.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–30. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber TD, Steinl C, Essl M, Abele H, Geiger K, Muller CA, et al. The integrin α9β1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica . 2009 Jul 16; doi: 10.3324/haematol.2009.006072. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis SJ, Tanentzapf G. Integrin-mediated adhesion and stem-cell-niche interactions. Cell Tissue Res . 2009 Jul 9; doi: 10.1007/s00441-009-0828-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Wagers AJ, Allsopp RC, Weissman IL. Changes in integrin expression are associated with altered homing properties of Lin(−/lo)Thy1.1(lo)Sca-1(+)c-kit(+) hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Exp Hematol. 2002;30:176–85. doi: 10.1016/s0301-472x(01)00777-9. [DOI] [PubMed] [Google Scholar]
- 6.Grassinger J, Haylock DN, Storan MJ, Haines GO, Williams B, Whitty GA, et al. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with α9β1 and α4β1 integrins. Blood. 2009;114:49–59. doi: 10.1182/blood-2009-01-197988. [DOI] [PubMed] [Google Scholar]
- 7.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin α9β1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–20. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokosaki Y, Palmer EL, Prieto AL, Crossin KL, Bourdon MA, Pytela R, et al. The integrin α9β1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–6. [PubMed] [Google Scholar]
- 9.Smith LL, Cheung HK, Ling LE, Chen J, Sheppard D, Pytela R, et al. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by α9β1 integrin. J Biol Chem. 1996;271:28485–91. [PubMed] [Google Scholar]
- 10.Chen C, Huang X, Atakilit A, Zhu QS, Corey SJ, Sheppard D. The integrin α9β1 contributes to granulopoiesis by enhancing granulocyte colony-stimulating factor receptor signaling. Immunity. 2006;25:895–906. doi: 10.1016/j.immuni.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Arroyo AG, Yang JT, Rayburn H, Hynes RO. α4 integrins regulate the proliferation/differentiation balance of multi-lineage hematopoietic progenitors in vivo. Immunity. 1999;11:555–66. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- 12.Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90:4779–88. [PubMed] [Google Scholar]
- 13.Humphries JD, Schofield NR, Mostafavi-Pour Z, Green LJ, Garratt AN, Mould AP, et al. Dual functionality of the anti-β1 integrin antibody, 12G10, exemplifies agonistic signalling from the ligand binding pocket of integrin adhesion receptors. J Biol Chem. 2005;280:10234–43. doi: 10.1074/jbc.M411102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:459–70. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka Y, Morimoto I, Nakano Y, Okada Y, Hirota S, Nomura S, et al. Osteoblasts are regulated by the cellular adhesion through ICAM-1 and VCAM-1. J Bone Miner Res. 1995;10:1462–9. doi: 10.1002/jbmr.5650101006. [DOI] [PubMed] [Google Scholar]
- 16.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92:9647–51. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–45. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions. Ann NY Acad Sci. 2001;938:83–95. doi: 10.1111/j.1749-6632.2001.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 20.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–54. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 22.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 23.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–83. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, et al. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature. 2003;421:547–51. doi: 10.1038/nature01345. [DOI] [PubMed] [Google Scholar]
- 28.Christensen JL, Wright DE, Wagers AJ, Weissman IL. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2:e75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]