Abstract
Despite longstanding evidence for increased activation of coagulation in hemolytic anemias, its pathophysiology and its role in the vaso-occlusive crises of sickle disease remain unclear. Here Dr. Ataga reviews the latest evidence on this topic, emphasizing the likely multifactorial origin of the activation and the increasing evidence for the importance of cellular elements or their derived microparticles. See related articles on pages 1513 and 1520
There is increasing evidence that sickle cell disease (SCD), as well as other chronic hemolytic anemias such as β thalassemia, paroxysmal nocturnal hemoglinuria, autoimmune hemolytic anemia and unstable hemoglobinopathies, are characterized by a hypercoagulable state.1 In addition to increased thrombin and fibrin generation, increased tissue factor activity, and increased platelet activation (Figure 1), patients with hemolytic anemias manifest thrombotic complications, including venous thromboembolism, in situ pulmonary thrombosis and stroke.1–7 Furthermore, the risk of thromboembolic complications appears to be higher following splenectomy.1,3,6
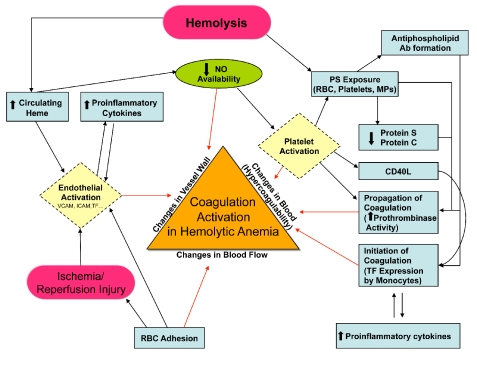
Figure 1.
Schematic representation of pathophysiological mechanisms (described or postulated) leading to coagulation activation in sickle cell disease and other hemolytic anemias. Based on Virchow’s triad, the illustrated pathways contribute to activation of coagulation (and possibly eventual thrombosis) by one of three broad mechanisms, i.e.: (i) changes in the vessel wall; (ii) changes in blood flow; and/or (iii) changes in the composition of blood components (‘hypercoagulability’). Ab: antibody; NO:nitric oxide; PS: phosphatidylserine; RBC: red blood cell; MPs: microparticles; TF: tissue factor. (Adapted from Ataga KI, Key NS. Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology Am Soc Hematol Educ Program. 2007:91–96)
The mechanism of coagulation activation in hemolytic anemias is likely multifactorial. Both SCD and thalassemia are characterized by red blood cell (RBC) membrane abnormalities, with abnormal exposure of phosphatidylserine.1,8 Normally, phosphatidylserine is found in the inner monolayer of the cell membrane, whereas choline-containing phospholipids, such as phosphatidyl-choline and sphingomyelin, are located in the outer monolayer in the plasma membrane.9 Abnormal phosphatidylserine exposure functions as both a recognition signal for cell removal during apoptosis of nucleated cells,10 and a docking site for enzymatic complexes involved in coagulation and anticoagulation pathways.11 External exposure of phosphatidylserine alters the adhesive properties of RBC12 and appears to be involved in the hemostatic changes observed in hemolytic anemias.13–16 The number of phosphatidylserine-positive RBC has been reported to be significantly correlated with plasma markers of thrombin generation, such as prothrombin fragment 1+2 (F1+2), D-dimer and plasmin-antiplasmin complexes in SCD, but no correlation was found between phosphatidylserine-positive platelets and any of these hemostatic markers,13,14 suggesting a role for RBC in coagulation activation.
Circulating microparticles (small membrane-derived vesicles released by cells following activation or apoptosis), derived from RBC, platelets, endothelial cells and monocytes, may also contribute to the hypercoagulable state in hemolytic anemias.17–21 The total number of microparticles, total tissue factor-positive microparticles, monocyte-derived tissue factor-positive microparticles and RBC-derived microparticles are correlated with D-dimer, thrombin-antithrombin complexes and F1+2 levels in SCD patients,17 suggesting that microparticles may contribute to the hypercoagulable state observed in patients with SCD and other hemolytic anemias.
Tissue factor, the principal initiator of coagulation, is abnormally expressed on circulating endothelial cells in patients with SCD22 and may contribute to hypercoagulability in hemolytic anemias.23 Microparticles released during hemolysis may be tissue factor-positive.17 Several potential mechanisms for increased tissue factor expression have been described in SCD and other hemolytic anemias, including ischemia-reperfusion injury,24 increased levels of soluble CD40 ligand25 and increased heme levels.26 Heme, an inflammatory mediator and a product of intravascular hemolysis in patients with hemolytic anemia, induces tissue factor expression on the surface of both macrovascular and microvascular endothelial cells, possibly via the transcription factor nuclear factor kappa B.26 Hemolysis also results in the scavenging of nitric oxide (NO) by cell-free hemoglobin27 and releases erythrocyte arginase, an enzyme that converts L-arginine, the substrate for NO synthesis, to ornithine, thus further reducing NO availability.28 In addition to regulating vascular tone and inhibiting endothelial adhesion molecule expression, NO has potent antithrombotic effects. Via cGMP-dependent signaling, NO inhibits platelet activation.29–31 NO has also been shown to inhibit tissue factor expression32 although there are conflicting data regarding this effect.33
In their study published in this issue of the Journal, van Beers and colleagues report that the majority of microparticles in SCD patients originate from platelets and erythrocytes, and that the numbers of these microparticles do not differ significantly between crisis and steady state.34 Unlike a previous study by Shet and colleagues,17 no microparticles originating from monocytes or endothelial cells were detectable and no microparticles expressing tissue factor were identified. Erythrocyte-derived microparticles correlated strongly with plasma levels of hemolytic markers, as well as to von Willebrand factor, D-dimer and F1+2 levels. Furthermore, thrombin generation depended on the total number of microparticles, and anti-human factor XI inhibited thrombin generation by about 50%.
The strong association of erythrocyte-derived microparticles with markers of fibrinolysis and coagulation activation as well as with hemolytic markers further confirms a role for hemolysis in the coagulation activation that is observed in patients with hemolytic anemias. This finding is similar to those in a study by our group in which associations were observed between markers of coagulation activation (F1+2, thrombin-antithrombin complexes and D-dimer) and measures of hemolysis in a cohort of SCD patients.35 In this study we speculated that hemolysis, with resultant scavenging of NO, might play a role in coagulation activation in SCD patients. Platelets are also activated in SCD and other hemolytic anemias.1 Platelets appear to be further activated in SCD patients with pulmonary hypertension and this activation of platelets is directly correlated with measures of hemolysis.29 Furthermore, platelet activation is inhibited by NO donors, although the NO inhibitory effect is abolished by the addition of pathophysiologically relevant levels of cell-free hemoglobin in the platelet-NO donor mixture.
The study by Tripodi and colleagues published in this issue of the Journal evaluated, by means of thromboelastometry and thrombin generation tests, the relative role played by cells and plasma in the hypercoagulability of patients with β thalassemia.36 All the thromboelastometry parameters determined in whole blood, including shortened clotting time and clot formation time, and increased maximum clot firmness, were consistent with hypercoagulability, especially in splenectomized patients. However, thrombin generation determined in platelet-poor plasma was not significantly different from that in healthy individuals. These results suggest that RBC, platelets and possibly other cellular elements play a significant role in the hypercoagulability observed in thalassemic patients.
Although histopathological studies show that SCD-associated pulmonary hypertension is associated with a thrombotic pulmonary arteriopathy,37 we previously observed that while markers of coagulation activation appeared to be higher in SCD patients with pulmonary hypertension than in those without, these differences did not achieve statistical significance.35 The findings from the study by Tripodi and colleagues now appear to confirm our previous suspicion that cellular elements contribute to hypercoagulability in hemolytic anemias and suggest that despite the absence of significant differences in plasma levels of coagulation activation in our study, patients with complications such as SCD-associated pulmonary hypertension may indeed be more hypercoagulable if evaluated using techniques that assess whole blood, rather than just plasma.
Despite a report showing associations between plasma fibrinolytic activity, as well as platelet procoagulant activity with the frequency of acute pain episodes in SCD, it remains uncertain whether platelet activation, as well as increased thrombin and fibrin generation contribute to the pathophysiology of SCD.38 However, it is becoming increasingly clear that patients with hemolytic anemias are at risk of thromboembolic complications, particularly following splenectomy. The increased frequency of these complications may be a result of the increased number of circulating abnormal RBC and microparticles that express phosphatidylserine following splenectomy. Indeed splenectomized patients with thalassemia intermedia have significantly higher levels of microparticles and thrombin-antithrombin complexes when compared to non-splenectomized patients.39 Furthermore, the levels of phosphatidylserine-positive microparticles and plasma hemoglobin were higher in the splenectomized patients, although this difference did not reach statistical significance.39
The utility of anticoagulation in hemolytic anemias prior to the development of thrombotic complications is uncertain. While it is reasonable to use prophylactic anticoagulants to decrease the risk of deep vein thromboses when these patients are hospitalized, there is a paucity of controlled trials in this setting. In those patients who develop venous thromboembolism, the necessary duration of anticoagulation is also uncertain, as the risk of recurrent episodes is not defined.
As hemolysis is associated with endothelial dysfunction in hemolytic anemias, hypercoagulability may be associated with several hemolysis-associated complications, including pulmonary hypertension. Defining the contribution of platelet activation, as well as increased thrombin and fibrin generation, to the pathophysiology of hemolytic anemias requires further studies. Furthermore, with the increasing evidence that hemolysis plays a crucial role in the development of hypercoagulability, novel approaches, including anti-hemolytic therapies, hemoglobin scavengers and NO donors may decrease the occurrence of thrombotic complications in hemolytic anemias. Finally, well-controlled clinical studies of anticoagulants and/or antiplatelet agents employing appropriate clinical endpoints in hemolytic anemias are warranted.
Footnotes
Dr. Ataga is an Associate Professor of Medicine at the University of North Carolina at Chapel Hill. He is also the Director of the University of North Carolina Comprehensive Sickle Cell Program.
The original studies discussed in this article were supported in part by NIH grants UL1RR025747, HL091265, and HL079915. Support for this work was also provided by an award from the North Carolina State Sickle Cell Program.
References
- 1.Ataga KI, Cappellini MD, Rachmilewitz EA. β-thalassemia and sickle cell anaemia as paradigms of hypercoagulability. Br J Haematol. 2007;139:3–13. doi: 10.1111/j.1365-2141.2007.06740.x. [DOI] [PubMed] [Google Scholar]
- 2.Stein PD, Beemath A, Meyers FA, Skaf E, Olson RE. Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease. Am J Med. 2006;119:897.e7–11. doi: 10.1016/j.amjmed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Taher A, Isma’eel H, Mehio G, Bignamini D, Kattamis A, Rachmilewitz EA, Cappellini MD. Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost. 2006;96:488–91. [PubMed] [Google Scholar]
- 4.Ziakas PD, Poulou LS, Rokas GI, Bartzoudis D, Voulgarelis MJ. Thrombosis in paroxysmal nocturnal hemoglobinuria: sites, risks, outcome. An overview. Thromb Haemost. 2007;5:642–5. doi: 10.1111/j.1538-7836.2007.02379.x. [DOI] [PubMed] [Google Scholar]
- 5.Hendrick AM. Auto-immune haemolytic anaemia – a high-risk disorder for thromboembolism. Hematology. 2003;8:53–6. doi: 10.1080/1024533021000059474. [DOI] [PubMed] [Google Scholar]
- 6.Beutler E, Lang A, Lehmann H. Hemoglobin Duarte: [α2β2 (62) ala leads to pro]: a new unstable hemoglobin with increased oxygen affinity. Blood. 1974;43:527–35. [PubMed] [Google Scholar]
- 7.Au NH, Wong AY, Vickars L, MacGillivray RT, Wadsworth LD. Two new examples of Hb St. Etienne [β92(F8)HisGln] in association with venous thrombosis. Hemoglobin. 2009;33:95–100. doi: 10.1080/03630260902817206. [DOI] [PubMed] [Google Scholar]
- 8.Eldor A, Rachmilewitz EA. The hypercoagulable state in thalassemia. Blood. 2002;99:36–43. doi: 10.1182/blood.v99.1.36. [DOI] [PubMed] [Google Scholar]
- 9.Devaux PF, Zachowski A. Maintenance and consequences of membrane phospholipid asymmetry. Chem Phys Lipids. 1994;73:107–20. [Google Scholar]
- 10.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 11.Zwaal RFA, Schroit AJ. Pathophysiologic implications of membrane phospholipids asymmetry in blood cells: a review. Blood. 1997;89:1121–32. [PubMed] [Google Scholar]
- 12.Setty BN, Kulkani S, Stuart MJ. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood. 2002;99:1564–71. doi: 10.1182/blood.v99.5.1564. [DOI] [PubMed] [Google Scholar]
- 13.Setty BN, Rao AK, Stuart MJ. Thrombophilia in sickle cell disease: the red cell connection. Blood. 2001;98:3228–33. doi: 10.1182/blood.v98.12.3228. [DOI] [PubMed] [Google Scholar]
- 14.Setty BN, Kulkani S, Rao AK, Stuart MJ. Fetal hemoglobin in sickle cell disease: relationship to erythrocyte phosphatidylserine exposure and coagulation activation. Blood. 2000;96:1119–24. [PubMed] [Google Scholar]
- 15.Borenstain-Ben Yashar V, Barenholz Y, Hy-Am E, Rachmilewitz EA, Eldor A. Phosphatidylserine in the outer leaflet of red blood cells from β-thalassemia patients may explain the chronic hypercoagulable state and thrombotic episodes. Am J Hematol. 1993;44:63–5. doi: 10.1002/ajh.2830440114. [DOI] [PubMed] [Google Scholar]
- 16.Wandersee NJ, Lee JC, Deveau SA, Barker JE. Reduced incidence of thrombosis in mice with hereditary spherocytosis following neonatal treatment with normal hematopoietic cells. Blood. 2001;97:3972–5. doi: 10.1182/blood.v97.12.3972. [DOI] [PubMed] [Google Scholar]
- 17.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 18.Habib A, Kunzelmann C, Shamseddeen W, Zobairi F, Freyssinet JM, Taher A. Elevated levels of circulating procoagulant microparticles in patients with β-thalassemia intermedia. Haematologica. 2008;93:941–2. doi: 10.3324/haematol.12460. [DOI] [PubMed] [Google Scholar]
- 19.Pattanapanyasat K, Gonwong S, Chaichompoo P, Noulsri E, Lerdwana S, Sukapirom K, et al. Activated platelet-derived microparticles in thalassaemia. Br J Haematol. 2007;136:462–71. doi: 10.1111/j.1365-2141.2006.06449.x. [DOI] [PubMed] [Google Scholar]
- 20.Simak J, Holada K, Risitano AM, Zivny JH, Young NS, Vostal JG. Elevated circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2004;125:804–13. doi: 10.1111/j.1365-2141.2004.04974.x. [DOI] [PubMed] [Google Scholar]
- 21.Hugel B, Socié G, Vu T, Toti F, Gluckman E, Freyssinet JM, Scrobohaci ML. Elevated levels of circulating procoagulant microparticles in patients with paroxysmal nocturnal hemoglobinuria and aplastic anemia. Blood. 1999;93:3451–6. [PubMed] [Google Scholar]
- 22.Key NS, Slungaard A, Dandelet L, Nelson SC, Moertel C, Styles LA, et al. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood. 1998;91:4216–23. [PubMed] [Google Scholar]
- 23.Ziakas PD, Poulou LS, Pomoni A. Thrombosis in paroxysmal nocturnal hemoglobinuria at a glance: a clinical review. Curr Vasc Pharmacol. 2008;6:347–53. doi: 10.2174/157016108785909742. [DOI] [PubMed] [Google Scholar]
- 24.Solovey A, Kollander R, Shet A, Milbauer LC, Choong S, Panoskaltsis-Mortari A, et al. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104:840–6. doi: 10.1182/blood-2003-10-3719. [DOI] [PubMed] [Google Scholar]
- 25.Lee SP, Ataga KI, Orringer EP, Parise LV. Biologically active CD40 ligand is elevated in sickle cell disease: potential role for platelet-mediated inflammation. Arterioscler Thromb Vasc. 2006;26:1626–31. doi: 10.1161/01.ATV.0000220374.00602.a2. [DOI] [PubMed] [Google Scholar]
- 26.Setty BN, βl SG, Zhang J, Stuart MJ. Heme induces endothelial tissue factor expression: potential role in hemostatic activation in patients with hemolytic anemia. J Thromb Haemost. 2008;6:2202–9. doi: 10.1111/j.1538-7836.2008.03177.x. [DOI] [PubMed] [Google Scholar]
- 27.Gladwin MT, Lancaster JR, Jr, Freeman BA, Schechter AN. Nitric oxide reactions with hemoglobin: a view through the SNO-storm. Nat Med. 2003;9:496–500. doi: 10.1038/nm0503-496. [DOI] [PubMed] [Google Scholar]
- 28.Morris CR, Morris SM, Jr, Hagar W, Van Warmerdam J, Claster S, Kepka-Lenhart D, et al. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168:63–9. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- 29.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle cell disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–72. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–8. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 31.Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987;92:181–7. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerlach M, Keh D, Bezold G, Spielmann S, Kürer I, Peter RU, et al. Nitric oxide inhibits tissue factor synthesis, expression and activity in human monocytes by prior formation of per-oxynitrite. Intensive Care Med. 1998;24:1199–208. doi: 10.1007/s001340050745. [DOI] [PubMed] [Google Scholar]
- 33.Dusse LM, Cooper AJ, Lwaleed BA. Tissue factor and nitric oxide: a controversial relationship! J Thromb Thrombolysis. 2007;23:129–33. doi: 10.1007/s11239-006-0001-9. [DOI] [PubMed] [Google Scholar]
- 34.van Beers EJ, Schaap MCL, Berckmans RJ, Nieuwland R, Sturk A, van Doormaal FF, et al. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–9. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ataga KI, Moore CG, Hillery CA, Jones S, Whinna HC, Strayhorn D, et al. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica. 2008;93:20–6. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 36.Tripodi A, Cappellini MD, Chantarangkul V, Padovan L, Fasulo MR, Marcon A, Mannucci PM. Hypercoagulability in splenectomized thalassemic patients detected by whole-blood thromboelastometry, but not by thrombin generation in platelet-poor plasma. Haematologica. 2009;94:xxx–xxx. doi: 10.3324/haematol.2009.010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch Pathol Lab Med. 2001;125:1436–41. doi: 10.5858/2001-125-1436-PTAIPW. [DOI] [PubMed] [Google Scholar]
- 38.Tomer A, Harker LA, Kasey S, Eckman JR. Thrombogenesis in sickle cell disease. J Lab Clin Med. 2001;137:398–407. doi: 10.1067/mlc.2001.115450. [DOI] [PubMed] [Google Scholar]
- 39.Westerman M, Pizzey A, Hirschman J, Cerino M, Weil-Weiner Y, Ramotar P, et al. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br J Haematol. 2008;142:126–35. doi: 10.1111/j.1365-2141.2008.07155.x. [DOI] [PubMed] [Google Scholar]