Abstract
Myeloproliferative neoplasms are characterized by overproduction of mature blood cells and increased risk of thromboembolic complications. However, the molecular lesions associated with these disorders also activate circulating blood cells. In this perspective article, Dr. Cervantes and his colleagues examine the role of blood cell activation in the pathophysiology of thrombosis in myeloproliferative neoplasms. See related article on page 1537.
The term classic BCR/ABL-negative myeloproliferative neoplasms encompasses three disorders, polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), which originate in a single pluripotent hematopoietic stem cell and share several clinical, hematologic and histological features. These include bone marrow hypercellularity with variable degrees of fibrosis, overproduction of one or more of the blood cell lines, frequent splenomegaly, increased risk of thrombosis and bleeding, and the tendency to evolve into acute leukemia. Transition from one disorder to another is also occasionally observed. Recently, the discovery of the V617F mutation in the JAK2 gene in the majority of patients with a myeloproliferative neoplasm has provided biological support to the inclusion of the three diseases within the same group. From the clinical standpoint, the tendency to thrombosis is one of the most outstanding characteristics of the myeloproliferative neoplasms, with this especially applying to PV and ET. In addition to non-specific host factors, such as advanced age, a previous history of thrombosis and the possible co-existence of vascular risk factors or inherited thrombophilia, factors contributing to the appearance of this complication include increased red cell mass in the case of PV, thrombocytosis, and the existence of abnormalities of platelet function.1,2 In the last years, evidence on the role of leukocytes in the thrombosis of myeloproliferative neoplasms has been accumulated.
Leukocyte and platelet activation and the thrombosis of myeloproliferative neoplasms
Cellular interactions between leukocytes, platelets and endothelial cells are regulated by complex mechanisms that involve multiple molecules. Overexpression of several of these molecules, resulting in enhanced leukocyte and platelet activation and increased adhesivity of the blood cells to the endothelium, has been reported in PV, ET and PMF. It is currently believed that these alterations play an important role in the pathogenesis of the thrombosis in these diseases.
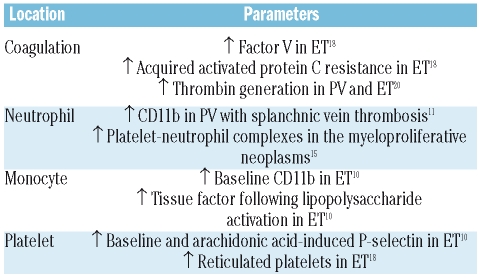
The leukocytes of patients with PV and ET have an activated phenotype, as demonstrated by their increased capacity of phagocytosis and production of reactive oxygen species overexpression of the membrane CD11b antigen and leukocyte alkaline phosphatase, and increased content of plasma and cellular elastase shown in different studies.3–9 However, in none of these studies was a comparison made between patients with myeloproliferative disease with and without thrombosis. Arellano-Rodrigo et al. first analyzed leukocyte and platelet activation in ET patients with and without thrombosis, showing an increased percentage of platelets that expressed P-selectin, as well as overexpression of the monocyte CD11b antigen in patients with thrombosis,10 which suggests that the activation of the platelets and leukocytes (and, notably, the monocytes) could be involved in the genesis of the thrombosis of this disease (Table 1). The CD11b antigen is also overexpressed in the neutrophils of patients with PV, especially in those with Budd-Chiari syndrome or thrombosis of the splenoportal vein tract,11 a finding that would support the role of leukocyte activation in the thrombosis of patients with this neoplasm (Table 1).
Table 1.
Parameters of blood cell and coagulation activation associated with thrombosis in the myeloproliferative neoplasms.
Additional support to the pathogenic role of the leukocytes in the thrombosis of myeloproliferative neoplasms is the recently demonstrated association between the presence of leukocytosis at disease presentation and increased risk of thrombosis in these patients.12
Platelet-leukocyte complexes in the myeloproliferative neoplasms
Platelet and leukocyte activation results in an increased percentage of circulating platelet-leukocyte complexes. Thus, in PV patients, 40–50% of leukocytes circulate with platelets adhered on their membrane. This percentage is even higher in ET, with 50–60% of the neutrophils and 80% of the monocytes showing platelet adhesion.10,11,13 Platelet P-selectin and circulating platelet-leukocyte complexes have been reported to correlate with neutrophil degranulation, stabilization of the fibrinogen on the surface of the leukocytes and higher extracellular tissue factor content. All these alterations disappear following treatment with hydroxyurea, probably because of blockade of the interaction between the platelet P-selectin and the neutrophil P-selectin glycoprotein ligand-1.14 Falanga et al. observed a correlation between the percentage of platelet-leukocyte complexes and CD11b expression, indicating that leukocyte activation promotes the formation of these complexes.13 Interestingly, aspirin treatment resulted in a decrease in the formation of platelet-leukocyte complexes.13 A relationship between the percentage of circulating platelet-leukocyte complexes and a history of thrombosis in patients with myeloproliferative neoplasms has been registered in some but not all studies.10,11,13,15 Overall, the abovementioned findings would support the hypothesis that the increased number of circulating platelet-leukocyte complexes could play a role in the thrombosis of myeloproliferative neoplasms. However, the clinical utility of this parameter as a marker of increased risk of thrombosis has not been determined as yet.
Leukocyte activation and coagulation parameters
The link between leukocyte activation and thrombosis in myeloproliferative neoplasms was further strengthened by the finding of increased concentrations of several markers of coagulation and endothelial activation in these patients. Thus, increases in prothrombin fragment 1+2, D-dimer, thrombin-antithrombin complex, von Willebrand factor, and thrombomodulin have been demonstrated in PV and ET patients with increased leukocyte and platelet activation.8,16–19 Trappenburg et al.19 reported that ET patients have higher numbers of microparticles expressing platelet and endothelial markers, which are associated with increased thrombin generation. Moreover, acquired activated protein C resistance has recently been reported in PV and ET, being correlated with reduced concentrations of factor V and free protein S.18,20 It has been hypothesized that, in patients with myeloproliferative neoplasms, continuous leukocyte degranulation due to the leukocyte activation might result in factor V and protein S consumption, with this leading to activated protein C resistance. Of note, acquired activated protein C resistance has recently been found to be independently associated with an increased risk of thrombosis in ET.18
Tissue factor is currently considered as the main trigger of the coagulation cascade in vivo. Although no differences have been found in the baseline plasma concentrations of tissue factor in patients with PV and ET as compared with healthy controls, greater tissue factor production capacity has been observed in activated monocytes from PV patients, whereas a higher concentration of tissue factor molecules has been registered in the membrane of ET monocytes, a feature that was correlated with an increased frequency of thrombosis.10,21
Leukocyte and platelet activation and the JAK2 mutation
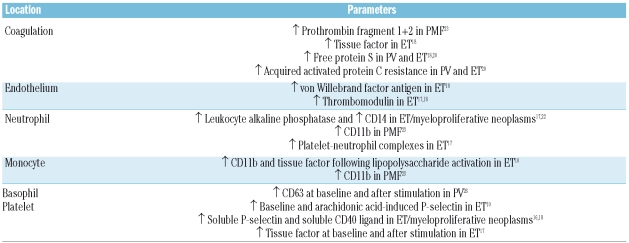
The JAK2 mutation or its increased allele burden has been associated with a higher incidence of thrombosis in patients with myeloproliferative neoplasms in some studies,1,2 but the exact mechanism of this association remains uncertain. Following the discovery of the JAK2 mutation, various studies have tried to correlate leukocyte and platelet activation with the patients’ JAK2 mutational status and allelic burden (Table 2). Thus, platelet P-selectin levels, both at baseline and after arachidonic acid stimulation, were found to be higher in ET patients with the JAK2 mutation than in those with the wild-type allele, indicating that platelet activation is modulated by the JAK2 mutation.10 Since P-selectin induces recruitment and activation of leukocytes in sites of endothelial damage and promotes the formation of platelet-leukocyte complexes, the presence of the JAK2 mutation could result in a prothrombotic state via P-selectin overexpression. The dosage effect of the JAK2 allelic burden on several markers of platelet and endothelial activation, such as soluble P-selectin, soluble CD40 ligand, von Willebrand factor, and the percentage of circulating platelet-leukocyte complexes, recently reported in JAK2-positive ET, would be in keeping with these results.17,18 Increased expression of leukocyte alkaline phosphatase and CD14 has also been found in JAK2-positive myeloproliferative neoplasms.17,22 Furthermore, when ET monocytes were stimulated with lipopolysaccharide, those from JAK2-positive patients expressed a higher number of CD11b and tissue factor molecules in the membrane than those from JAK2-negative patients.10 Neutrophil and monocyte CD11b expression was also found to be higher in JAK2-positive PMF patients than in those with the wild-type allele.23 These data suggest that the JAK2 mutation promotes leukocyte activation, which could result in increased leukocyte adhesivity, endothelial damage, and coagulation activation. In addition, the presence of the JAK2 mutation in circulating endothelial progenitor cells and liver endothelial cells of patients with myeloproliferative neoplasms, especially in those with Budd-Chiari syndrome, further supports a role for defective endothelial cells in the pathogenesis of thrombosis in these diseases.24,25
Table 2.
Parameters of blood cell and coagulation activation associated with the presence of the JAK2 mutation or its allele burden in the myeloproliferative neoplasms.
Finally, the acquired activated protein C resistance observed in ET and PV is also correlated with the JAK2 mutation, especially in homozygotic patients, supporting a prothrombotic role for the mutation. This hypothesis is reinforced by the high plasma tissue factor concentration and low concentrations of protein S, factor II, factor V, and tissue factor inhibitor found in JAK2-positive patients with myeloproliferative neoplasms.18,20
Basophil and mast cell activation in the myeloproliferative neoplasms
Mast cells and basophils are key mediators of immediate allergic and inflammatory responses, the former being predominant in the tissues and the latter in the circulation. Unlike mast cells, basophils circulate in the blood as mature cells and are thought to be incapable of proliferating. The generation of mast cell-committed progenitors is unclear, since developmental studies in murine models of hematopoiesis have been unable to demonstrate a shared bipotent progenitor cell for mast cells and basophils or whether mast cells are derived directly from a multipotent progenitor cell. In this regard, it must be pointed out that the presence of the KITD816V mutation in the mast cells but not in the basophils of patients with mastocytosis points to a separate origin of the two cells in a different progenitors. Both types of cells have granules that contain histamine, platelet-activating factor, and bioactive proteoglycans, and their degranulation is mediated by the Fc-epsilon receptor, generally triggered by aggregated IgE. In this issue of the journal, Pieri et al.26 report the results of the first study aimed at investigating the function of basophils in myeloproliferative neoplasms according to the patients’ JAK2 mutational status, showing an increased number of activated basophils in PV patients, both at baseline and after stimulation. The fact that the percentage of circulating activated basophils was correlated with the presence of aquagenic pruritus and with the JAK2 allelic burden would indicate that basophil activation is driven by the JAK2 mutation, and, probably mediates aquagenic pruritus. Until now, the origin of aquagenic pruritus in PV was poorly understood. In this context, the findings by Pieri et al.26 support a pathogenic role for basophil activation in the appearance of this symptom. Since the status of the mast cells in JAK2-positive myeloproliferative neoplasms is not well known, their possible activation could be an alternative explanation for the genesis of the pruritus. In this sense, it has recently been shown that mast cells are also involved in the malignant process of patients with myeloproliferative neoplasms and that they have an increased state of activation and release greater levels of pruritogenic factors, which might contribute to the pruritus.27,28 In addition, there is now accumulating evidence that mast cells may play a role in atherothrombosis.29 The observation by Pieri et al. lends additional support to the notion that the JAK2 mutation promotes the activation, and not only the accumulation, of normally functional mature blood cells. The degree of activation is correlated with the mutated allelic burden and would be either responsible for or a contributor to some of the most characteristic clinical manifestations of the myeloproliferative neoplasms, such as aquagenic pruritus and thrombosis. In addition to casting light onto the pathogenesis of the myeloproliferative neoplasms, the results of the study by Pieri et al.26 provide a further rationale for the use of JAK2 inhibitors in these diseases.
Conclusions
The available data demonstrate a state of activation of leukocytes and platelets in patients with the classic Philadelphia chromosome-negative myeloproliferative neoplasms. There is also accumulating evidence indicating that such activation is greater in those patients with thrombosis. However, the data are not robust enough to enable us to consider the finding of a high degree of blood cell activation as an indicator of increased risk of thrombosis in such patients. Finally, a possible link between blood cell activation and the JAK2 mutation or its allele burden is emerging.
Footnotes
Dr. Francisco Cervantes is Senior Consultant at the Hematology Department of the Hospital Clínic of Barcelona and Professor of Hematology at the University of Barcelona, Spain. Dr. Eduardo Arellano-Rodrigo is Consultant at the Hemostasis Department of the Hospital Clínic, in Barcelona, Spain. Dr. Alberto Álvarez-Larrán is Consultant at the Hematology Department of the Hospital del Mar, in Barcelona, Spain.
References
- 1.Cervantes F, Passamonti F, Barosi G. Life expectancy and prognostic factors in the classic BCR/ABL-negative myeloproliferative disorders. Leukemia. 2008;22:905–14. doi: 10.1038/leu.2008.72. [DOI] [PubMed] [Google Scholar]
- 2.De Stefano V, Za T, Rossi E, Fiorini A, Ciminello A, Luzzi C, et al. Influence of the JAK2 V617F mutation and inherited thrombophilia on the thrombotic risk among patients with essential thrombocythemia. Haematologica. 2009;94:733–7. doi: 10.3324/haematol.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuelsson J, Berg A. Further studies of the defective stimulus-response coupling for the oxidative burst in neutrophils in polycythemia vera. Eur J Haematol. 1991;47:239–45. doi: 10.1111/j.1600-0609.1991.tb01565.x. [DOI] [PubMed] [Google Scholar]
- 4.Westwood NB, Copson ER, Page LA, Mire-Sluis AR, Brown KA, Pearson TC. Activated phenotype in neutrophils and monocytes from patients with primary proliferative polycythaemia. J Clin Pathol. 1995;48:525–30. doi: 10.1136/jcp.48.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carulli G, Minnucci S, Azzara A, Gianfaldoni ML, Angiolini C, Sagripanti A, et al. Neutrophil functions in essential thrombocythemia. Hematol Pathol. 1995;9:37–47. [PubMed] [Google Scholar]
- 6.Iki S, Yuo A, Yagisawa M, Inuo EK, Inoue Y, Usuki K, et al. Increased neutrophil respiratory burst in myeloproliferative disorders: selective enhancement of superoxide release triggered by receptor-mediated agonists and low responsiveness to in vitro cytokine stimulation. Exp Hematol. 1997;25:26–33. [PubMed] [Google Scholar]
- 7.Wolach B, Gavrieli R, Manor Y, Lishner M. Leukocyte function in chronic myeloproliferative disorders. Blood Cells Mol Dis. 1998;24:544–51. doi: 10.1006/bcmd.1998.0218. [DOI] [PubMed] [Google Scholar]
- 8.Falanga A, Marchetti M, Evangelista V, Vignoli A, Licini M, Balicco M, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96:4261–6. [PubMed] [Google Scholar]
- 9.Burgaleta C, González N, César J. Increased CD11/CD18 expression and altered metabolic activity on polymorphonuclear leukocytes from patients with polycythemia vera and essential thrombocythemia. Acta Haematol. 2002;108:23–8. doi: 10.1159/000063063. [DOI] [PubMed] [Google Scholar]
- 10.Arellano-Rodrigo E, Alvarez-Larrán A, Reverter JC, Villamor N, Colomer D, Cervantes F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;91:169–75. [PubMed] [Google Scholar]
- 11.Alvarez-Larrán A, García-Pagán JC, Abraldes JG, Arellano E, Reverter JC, Bosch J, et al. Increased CD11b neutrophil expression in Budd-Chiari syndrome or portal vein thrombosis secondary to polycythaemia vera. Br J Haematol. 2004;124:329–35. doi: 10.1046/j.1365-2141.2003.04770.x. [DOI] [PubMed] [Google Scholar]
- 12.Carobbio A, Antonioli E, Guglielmelli P, Vannucchi AM, Delaini F, Guerini V, et al. Leukocytosis and risk stratification assessment in essential thrombocythemia. J Clin Oncol. 2008;26:2732–6. doi: 10.1200/JCO.2007.15.3569. [DOI] [PubMed] [Google Scholar]
- 13.Falanga A, Marchetti M, Vignoli A, Balducci D, Barbui T. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp Hematol. 2005;33:523–30. doi: 10.1016/j.exphem.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Maugeri N, Giordano G, Petrilli MP, Fraticelli V, de Gaetano G, Cerletti C, et al. Inhibition of tissue factor expression by hydroxyurea in polymorphonuclear leukocytes from patients with myeloproliferative disorders: a new effect for an old drug? J Thromb Haemost. 2006;4:2593–8. doi: 10.1111/j.1538-7836.2006.02194.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MK, de Nully BP, Lund BV, Nielsen OJ, Hasselbalch HC. Increased circulating platelet-leukocyte aggregates in myeloproliferative disorders is correlated to previous thrombosis, platelet activation and platelet count. Eur J Haematol. 2001;66:143–51. doi: 10.1034/j.1600-0609.2001.00359.x. [DOI] [PubMed] [Google Scholar]
- 16.Robertson B, Urquhart C, Ford I, Townend J, Watson HG, Vickers MA, et al. Platelet and coagulation activation markers in myeloproliferative diseases: relationships with JAK2 V617 F status, clonality, and antiphospholipid antibodies. J Thromb Haemost. 2007;5:1679–85. doi: 10.1111/j.1538-7836.2007.02626.x. [DOI] [PubMed] [Google Scholar]
- 17.Falanga A, Marchetti M, Vignoli A, Balducci D, Russo L, Guerini V, et al. V617F JAK-2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp Hematol. 2007;35:702–11. doi: 10.1016/j.exphem.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 18.Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC, Colomer D, Villamor N, Bellosillo B, et al. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: relationship with thrombosis occurrence and JAK2 V617F allele burden. Am J Hematol. 2009;84:102–8. doi: 10.1002/ajh.21338. [DOI] [PubMed] [Google Scholar]
- 19.Trappenburg MC, van Schilfgaarde M, Marchetti M, Spronk HM, ten Cate H, Leyte A, et al. Elevated procoagulant microparticles expressing endothelial and platelet markers in essential thrombocythemia. Haematologica. 2009;94:911–8. doi: 10.3324/haematol.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchetti M, Castoldi E, Spronk HM, van OR, Balducci D, Barbui T, et al. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood. 2008;112:4061–8. doi: 10.1182/blood-2008-06-164087. [DOI] [PubMed] [Google Scholar]
- 21.Kornberg A, Rahimi-Levene N, Yona R, Mor A, Rachmilewitz EA. Enhanced generation of monocyte tissue factor and increased plasma prothrombin fragment 1+2 levels in patients with polycythemia vera: mechanism of activation of blood coagulation. Am J Hematol. 1997;56:5–11. doi: 10.1002/(sici)1096-8652(199709)56:1<5::aid-ajh2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Passamonti F, Rumi E, Pietra D, la Porta MG, Boveri E, Pascutto C, et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107:3676–82. doi: 10.1182/blood-2005-09-3826. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Larrán A, Arellano-Rodrigo E, Reverter JC, Domingo A, Villamor N, Colomer D, et al. Increased platelet, leukocyte, and coagulation activation in primary myelofibrosis. Ann Hematol. 2008;87:269–76. doi: 10.1007/s00277-007-0386-3. [DOI] [PubMed] [Google Scholar]
- 24.Leibundgut EO, Horn MP, Brunold C, Pfanner-Meyer B, Marti D, Hirsiger H, et al. Hematopoietic and endothelial progenitor cell trafficking in patients with myeloproliferative diseases. Haematologica. 2006;91:1465–72. [PubMed] [Google Scholar]
- 25.Sozer S, Fiel MI, Schiano T, Xu M, Mascarenhas J, Hoffman R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009;113:5246–9. doi: 10.1182/blood-2008-11-191544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pieri L, Bogani C, Guglielmelli P, Zingariello M, Rana RA, Bartalucci N, et al. The JAK2V617F mutation induces constitutive activation and agonist hypersensitivity in basophils of polycythemia vera. Haematologica. 2009;94:1537–475. doi: 10.3324/haematol.2009.007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Ishii T, Zhang W, Sozer S, Dai Y, Mascarenhas J, et al. Involvement of mast cells by the malignant process in patients with Philadelphia chromosome negative myeloproliferative neoplasms. Leukemia. 23:1577–86. doi: 10.1038/leu.2009.85. [DOI] [PubMed] [Google Scholar]
- 28.Ishii T, Wang J, Zhang W, Mascarenhas J, Hoffman R, Dai Y, et al. Pivotal role of mast cells in pruritogenesis in patients with myeloproliferative disorders. Blood. 2009;113:5942–50. doi: 10.1182/blood-2008-09-179416. [DOI] [PubMed] [Google Scholar]
- 29.Kovanen PT. Mast cells: multipotent local effector cells in atherothrombosis. Immunol Rev. 2007;217:105–22. doi: 10.1111/j.1600-065X.2007.00515.x. [DOI] [PubMed] [Google Scholar]