The JAK2 (V617F) mutation is found in almost all patients with polycythemia vera and an important fraction of patients with essential thrombocythemia and primary myelofibrosis. This study shows that basophil counts are increased in JAK2 (V617F)-positive patients, and that the basophils contain an increased number of granules. See related article on page 1484.
Keywords: JAK2V617F mutation, basophil, polycythemia vera, pruritus
Abstract
Background
The JAK2V617F mutation has been associated with constitutive and enhanced activation of neutrophils, while no information is available concerning other leukocyte subtypes.
Design and Methods
We evaluated correlations between JAK2V617F mutation and the count of circulating basophils, the number of activated CD63+ basophils, their response in vitro to agonists as well as the effects of a JAK2 inhibitor.
Results
We found that basophil count was increased in patients with JAK2V617F -positive myeloproliferative neoplasms, particularly in those with polycythemia vera, and was correlated with the V617F burden. The burden of V617F allele was similar in neutrophils and basophils from patients with polycythemia vera, while total JAK2 mRNA content was remarkably greater in the basophils; however, the content of JAK2 protein in basophils was not increased. The number of CD63+ basophils was higher in patients with polycythemia vera than in healthy subjects or patients with essential thrombocythemia or primary myelofibrosis and was correlated with the V617F burden. Ultrastructurally, basophils from patients with polycythemia vera contained an increased number of granules, most of which were empty suggesting cell degranulation in vivo. Ex vivo experiments revealed that basophils from patients with polycythemia vera were hypersensitive to the priming effect of interleukin-3 and to f-MLP-induced activation; pre-treatment with a JAK2 inhibitor reduced polycythemia vera basophil activation. Finally, we found that the number of circulating CD63+ basophils was significantly greater in patients suffering from aquagenic pruritus, who also showed a higher V617F allele burden.
Conclusions
These data indicate that the number of constitutively activated and hypersensitive circulating basophils is increased in polycythemia vera, underscoring a role of JAK2V617F in these cells’ abnormal function and, putatively, in the pathogenesis of pruritus.
Introduction
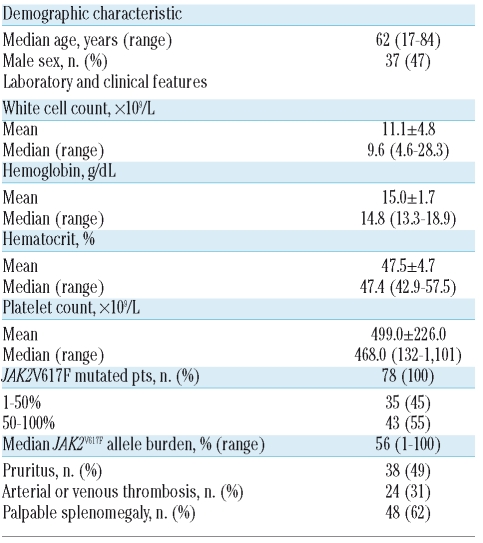
The JAK2V617F mutated allele is present in virtually all patients with polycythemia vera (PV) and in about 60% of those with essential thrombocythemia (ET) and primary myelofibrosis (PMF), which are the other two main clinical entities included within the group of myeloproliferative neoplasms.1 The presence of the mutation, and/or the burden of JAK2V617F allele, have been found to correlate with defined laboratory abnormalities and clinical features in the different myeloproliferative neoplasms.2 In most of the studies performed in PV patients an allele burden greater than 50% was found to correlate with leukocytosis and higher hemoglobin level, lower platelet count, presence and degree of splenomegaly, occurrence of aquagenic pruritus and higher rate of transformation to myelofibrosis.2
JAK2V617F is a constitutively phosphorylated tyro-sine kinase whose expression in cytokine-dependent cell lines confers cytokine independence and cytokine hypersensitivity through the constitutive activation of STAT5, Akt and ERK-dependent pathways.3,4 The adoptive transfer of marrow cells transduced with a retro-virus expressing JAK2V617F in irradiated recipient mice invariably resulted in the development of erythrocytosis,5–9 sometimes accompanied by leukocytosis, splenomegaly and later changes suggestive of myelofibrotic transformation.6–9 The presence and burden of JAK2V617F correlated with endogenous erythroid colony formation in PV patients10,11 and the expression of mutated Jak2 in mice induced erythropoietin-independent growth in vitro.7,9 Modification in the design of gene expression in murine models also resulted in an ET-like phenotype,12,13 indicating overall, that the JAK2V617F mutation is an integral component of the myeloproliferative process that underlies the different myeloproliferative neoplasms.
A unique gene expression profile has been associated with the presence and/or the burden of the V617F allele in neutrophils; among the genes involved, some were associated with neutrophil activation, such as PRV114,17 and the gene encoding for leukocyte alkaline phos-phatase.18 The constitutively activated status of circulating neutrophils associated with the mutated JAK2, together with enhanced activation of platelets and their hyper-responsiveness to agonists,19,20 may contribute to the thrombotic tendency found in patients with PV.21 However, there is a current lack of information concerning the functional relevance of the JAK2V617F mutation in other leukocyte subtypes, such as eosinophils and basophils. In this study, we investigated the features of basophils in patients with PV, other myeloproliferative neoplasms and in control subjects.
Design and Methods
Patients
This study involved a total of 78 patients with PV whose diagnosis satisfied the WHO criteria;22 for comparison, we also included 70 patients with ET and 22 with PMF (all diagnosed according to the WHO criteria), and seven subjects with reactive forms of hypoxic erythrocytosis. Most of the patients with PV were being treated with phlebotomy, but none was receving chemotherapy, at the time of blood sampling. Thirty-four healthy volunteers were included as controls. The study was approved by the local Ethical Committee and informed consent was obtained from all subjects included in the study.
Flow cytometry analysis of activated basophils
Circulating CD63+/CD123+/HLA-DR− basophils were enumerated using 100 μL of heparin-anticoagulated peripheral blood, promptly put on ice after sampling; antibodies were obtained from Becton Dickinson (San Jose, CA, USA). At least 200,000 events were acquired on a FACScan flow cytometer; results are expressed both as the percentage of gated basophils expressing CD63 and as the absolute number of CD63+ basophils by normalizing to total basophil count. CD63 expression level was calculated as the ratio of geometric mean fluorescence intensity (MFI) with isotype control antibody.
Purification of basophils and granulocytes
Basophils were purified from peripheral blood using a negative-depletion immunomagnetic procedure (Miltenyi Biotech; Gladbach, Germany). The purity of the isolated basophil preparations was checked by flow cytometry after labeling with phycoerythrin (PE)-CD123/peridin chlorophyll (PerCP)-HLA-DR monoclonal antibodies (Becton-Dickinson); the median purity was 81% (range, 75 to 86%). Neutrophils were obtained by centrifugation of peripheral blood on a Ficoll density gradient; by visual inspection of cytosmears, neutrophils accounted for 95–97% of the cells while basophils were virtually absent from these cell suspensions.
Analyses involving DNA and RNA
The JAK2V617F burden in density-gradient purified neutrophils and immuno-selected basophils was determined using real-time polymerase chain reaction (PCR) analysis.16 In order to descriminate between unmutated and V617F-mutated JAK2 mRNA in purified neutrophils or basophils, we employed an amplification refractory mutation system (ARMS) PCR technique, as previously described.16 The level of total (mutated plus wild-type) JAK2 mRNA was quantified using TaqMan® gene expression assays (HS-00234567_m1; Applied Biosystems, Foster City, CA, USA) by means of an ABI PRISM 7300 HT Sequence Detection System. Gene expression profiling was performed using the comparative cycle threshold (CT) method of relative quantitation using VIC-labeled RNaseP probe as the housekeeping gene (Applied Biosystems) (ΔCT).
Determination of JAK2 protein content in basophils and granulocytes
Neutrophils and basophils were purified from peripheral blood as described above. In order to obtain enough protein for western blotting analysis, basophils and neutrophils from three PV patients with an V617F allele burden exceeding 50% and from three normal controls were pooled. Cell lysates were resolved on a 10% sodium dodecylsulfate polyacrylamide gel by electrophoresis and blotted onto a polyvinylidene fluoride membrane (Immun-Blot PVDF Membrane, BioRad, Hercules, CA, USA). Blots were probed with antibodies specific for JAK2 (anti-JAK2 rabbit antibody, Cell Signaling Technology, Danvers, MA, USA) and tubulin (β-tubulin mouse monoclonal IgG, Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by peroxidase-labeled secondary antibodies, and revealed with electrochemi-luminescence (Amersham ECL Western Blotting Detection Reagent, Ge Healthcare, Little Chalfont, UK). To measure the cellular content of JAK2 protein we also employed a FACS-based technique. Samples of peripheral blood from PV patients and healthy controls were incubated with CD45 PerCP (Becton Dickinson, USA) and CD11c PE (BD Pharmingen, USA) for neutrophils or CD45 PerCP and CD203c PE for basophils at room temperature for 15 min in the dark. Samples were fixed by mixing one volume of blood with 20 volumes of pre-warmed 1X BD Phosflow Lyse/Fix Buffer (Becton Dickinson, USA) at 37°C for 10 min. After washing twice with BD Pharmingen™ Stain Buffer (Becton Dickinson, USA), cells were permeabilized by adding 1 mL of BD™ Phosflow Perm Buffer III (Becton Dickinson, USA) followed by an anti-JAK2 rabbit antibody at room temperature for 30 min in the dark, washed, incubated with Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen, USA), and finally resuspended in the same buffer prior to flow cytometric analysis using FacsCan (Becton Dickinson, USA). Data were analyzed using WinMDI software (v2.9; http://facs.scripps.edu/soft-ware.html).
Ex vivo stimulation of basophils
Peripheral blood samples were incubated with varying concentrations of recombinant human(h) interleukin(IL)-3 and/or N-formyl-Met-Leu-Phe (fMLP) peptide and the appearance of CD63 on the membrane of CD123+/HLA-DR- gated basophils was measured. In some experiments, the specific JAK2 inhibitor AZD1480 (kindly provided by Astra Zeneca Ltd.) was used. Peripheral blood samples (100 μL volumes) collected in preservative-free heparin were processed immediately after sampling. Samples were equilibrated at 37°C in a water bath in polypropylene tubes for 15 min; then, rhIL-3 (from 0.1 to 10 ng/mL; Peprotech Inc, Rocky Hills, NJ, USA) and fMLP peptide (from 0.01 to 0.04 μM; Sigma, Milan, Italy) were added sequentially, and the mixture incubated for a further 15 min. Control tubes containing no addition (blank), rhIL-3 or fMLP alone (controls) were also prepared. At the end of the incubation, samples were put on ice for 5 min, and basophils were labeled with 20 μL of a fluorescein isoth-iocyanate (FITC)-CD63, PE-CD123 and PerCP anti-HLA-DR antibody cocktail (BD FastImmune, Becton-Dickinson) for 15 min at room temperature. Red blood cells were lysed with 2 mL of 1x FACS™ Lysing solution (Becton-Dickinson) for 15 min at room temperature and nucleated cells were washed twice with 1–2 mL of phosphate-buffered saline.
CD63+ cells were quantified in the basophil gate by acquiring at least 200,000 events; each experiment was performed in duplicate. For inhibition of JAK2-mediated responses, cell samples were pre-incubated for 15 min at 37°C with two different concentrations (400 and 4,000 nM) of the JAK2 inhibitor, AZD1480. Next, optimal amounts of rhIL-3 (10 ng/mL) and f-MLP peptide (0.04 mM) were added, and the cells were analyzed as described above.
Transmission electron microscopy
The enriched peripheral blood mononuclear cell fraction, obtained after centrifugation over a Ficoll-Hypaque gradient (Lymphoprep, Nycomed Pharma; Oslo, Norway), was processed for transmission electron microscopy by fixation in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.6, for 2 h at 4°C and post-fixing in osmium tetroxide for 60 min at 4°C. The samples were then dehydrated in alcohol at progressively higher concentrations and embedded in Spurr resin (Poliscience, Warrington, PA, USA). Consecutive thin and ultrathin sections were cut using a Reichert ultramicrotome. Ultrathin sections were collected on 200-mesh copper grids, and counterstained with uranyl acetate and lead citrate, as described elsewhere.23 Both the total number of granules per cell, and the number of empty granules, were enumerated in at least ten basophils/sample.
Statistical analysis
Comparisons between groups were performed by the Mann-Whitney U or Fisher’s test as appropriate, using SPSS software (StatSoft, Inc., Tulsa, OK, USA http://www.statsoft.com), GraphPad InStat software (GraphPad Software, Inc., San Diego, USA http://www.graphpad.com) or ORIGIN software (V 7.5, OriginLab Northampton, MA, USA, http://www.origin-lab.com) for the computations. Correlations between JAK2V617F allele burden and hematologic parameters were analyzed using Spearman’s rank non-parametric correlation test. A p value of less than 0.05 was considered to be statistically significant; all tests were two-tailed.
Results
We studied a cohort of 78 PV patients with the JAK2V617F-mutation in whom the mutated allele burden ranged from 1% to 100%, with a median value of 56%; the main hematologic and clinical features of these patients are reported in Table 1. About half of the patients had a history of aquagenic pruritus, which was considered by the referring physician as possibly related to the underlying hematologic disease after careful exclusion of any other known potential cause, and when it was described by the patient as diffuse, non-occasional, itching, exacerbated by contact with water and resistant to common anti-histamine drugs, when used. Given the design and objectives of the study, patients with aquagenic pruritus could have been over-represented and the percentage of such patients does not reflect the overall prevalence of this symptom in an unselected population of PV patients. We also evaluated 70 ET patients, 62% of whom had the JAK2V617F mutation (mean allele burden, 29%) and 17 patients with PMF (13 of whom had the V617F allele; mean allele burden, 49%).
Table 1.
Clinical and hematologic features of the patients with polycythemia vera (n=78) included in the study.
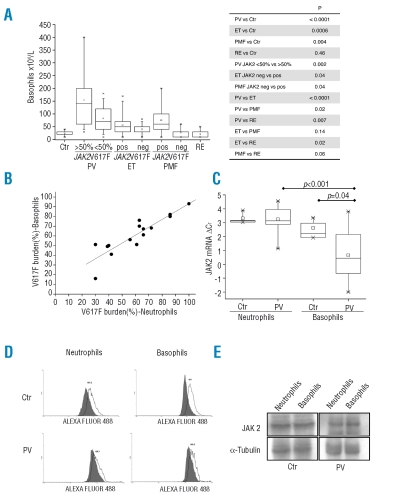
First, we found that the mean absolute counts of circulating basophils, measured routinely by a Coulter counter, were significantly higher in patients with PV (119.8±93.8×106/L; p<0.0001), patients with ET (48.9±30.0×106/L; p=0.0006) and patients with PMF (64.1±59.3×106/L; p=0.0042) than in control subjects (23.5±8.7×106/L); on the other hand, patients with reactive forms of erythrocytosis had a basophil count (20.0±15.3×106/L; p=0.46) similar to that of the control subjects (Figure 1A). The basophil count was significantly higher in patients with PV than in those with ET or PMF (p<0.0001 and p=0.02, respectively). There was also a statistically significant difference in mean basophil count between PV patients with a V617F allele burden of less than or more than 50% (82.8±48.9 and 155.5±109.7×106/L, respectively; p=0.002) and between ET or PMF patients who were V617F mutated or wild-type (55.9±35.8 and 39.6±19.0×106/L, respectively; p=0.04 in the case of ET; 75.4±63.0 and 24.3±18.1×106/L, respectively; p=0.04 in the case of PMF) (Figure 1A).
Figure 1.
(Panel A) The absolute count of peripheral blood basophils in PV patients (n=78) divided according to whether their JAK2V617F allele burden was less than or greater than 50%; results for patients with ET or PMF, control subjects (Ctr) or subjects with reactive forms of erythrocytosis (RE) are also shown. Boxes represent the interquartile range, which contains 50% of the subjects, the horizontal line in the box marks the median, the small square inside indicates the mean value, and bars show the range of values. The p value of the differences among different groups of patiens is shown on the right. (Panel B) Correlation between the burden of JAK2V617F allele concurrently measured in density gradient-purified neutrophils (on the X-axis) and immunomagnetically selected basophils (on the Y-axis) in PV patients (n=15). (Panel C) Level of total JAK2 mRNA in purified neutrophils and basophils from healthy control subjects and PV patients was determined by real time PCR and expressed as ΔCT after normalization to RNAseP as the housekeeping gene. Note that higher ΔCT values indicate lower mRNA content. (Panel D) FACS analysis for intracellular JAK2 staining in neutrophils (on the left) and basophils (on the right) using whole peripheral blood samples; for details, refer to the text. The gray area represents non-specific fluorescence. The Y-axis indicates mean fluorescence intensity (MFI; arbitrary units). (Panel E) Western blot analysis of JAK2 content in neutrophils and basophils pooled from three patients with PV (all with >50% V617F allele) and three healthy subjects. Tubulin was used to normalize protein load.
We measured the burden of V617F allele in immuno-magnetically selected basophils and in density-gradient purified neutrophils from 15 PV patients; we found that the two measurements were strongly correlated with each other (r=0.90; Figure 1B), suggesting that the relative proportion of wild-type and mutated JAK2V617 alleles in the two leukocyte subtypes was comparable. On the other hand, we observed that the content of total JAK2 mRNA was significantly greater in PV basophils compared not only to normal basophils (p=0.04) or neutrophils (p=0.01; n=12 subjects) but also to that in concurrently purified PV neutrophils (p=0.00007) (Figure 1C). Such an increase was not due to a preferential transcription or increased stability of mutated JAK2 mRNA in PV basophils, since the relative proportion of the wild-type and V617F-mutated mRNA transcripts was consistent with the results of quantitative genotyping in the same cells and comparable to concurrently purified granulocytes (not shown in detail). To evaluate whether also the content of JAK2 protein was increased in PV basophils, we employed FACS-based analysis in three PV patients and three healthy controls, and western blotting; for the western blotting we pooled purified basophils and granulocytes from three additional PV patients and three healthy controls to overcome the problem of the low protein recovery. However, we were unable to document significantly increased JAK2 content in basophils using either technique (Figure 1D and E). In particular, the mean fluorescence index (MFI) measured in PV basophils was 1,083±1,028, similar to the 1,400±762 measured in the control basophils, and the values of 341±186 and 540±339 found in PV and normal granulocytes, respectively. We, therefore, concluded that the increased JAK2 mRNA levels in PV basophils did not result in increased protein synthesis.
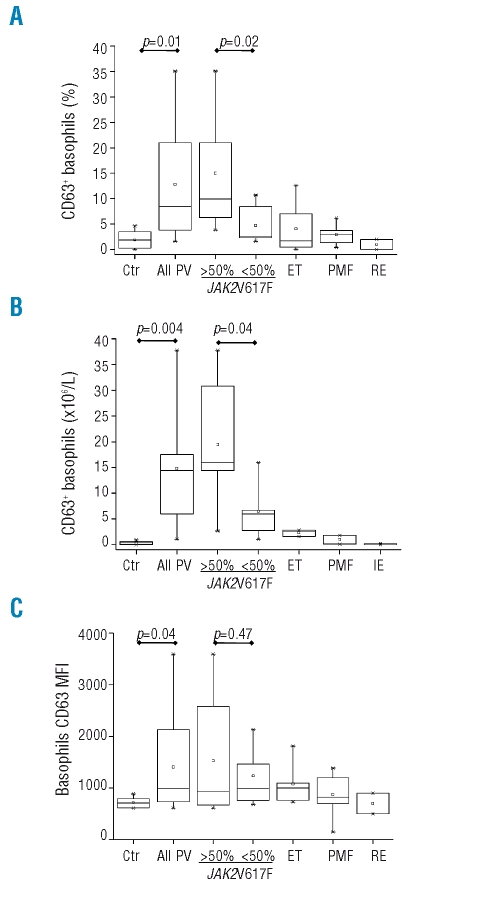
The expression of CD63 on the outer membrane of basophils was used as a marker of their activated status in both in vivo and ex vivo experiments.24 We found that the mean percentage of CD63+ cells in the CD123+/HLA-DR− basophil gate was significantly greater in PV patients than in controls (13.0±10.5% versus 1.8±1.7%; p=0.01), patients with ET or PMF, or patients with reactive erythrocytosis (Figure 2A). The absolute number of activated basophils in the circulation increased from 0.4±0.3×106/L in controls to 15.5±11.6×106/L in PV patients (Figure 2B; p=0.004); the number of CD63+ basophils in ET or PMF patients was similar to that in controls (Figure 2B). Both the relative proportion and the absolute number of CD63+ basophils in the peripheral blood were correlated to the burden of V617F allele; mean values were 15±9.5% and 21.2±10.8×106/L, respectively, in PV patients with a mutated allele burden of more than 50% compared to 4.7±3.8% and 7.1±6.7×106/L in those with a mutated allele burden of less than 50% (p=0.02, and p=0.04, respectively; Figure 2A, 2B). In addition, we found a significant linear regression between the absolute number of circulating CD63+ basophils in PV and the JAK2V617F allele burden (r=0.73, p=0.008; not shown in detail). The CD63 MFI was also significantly higher in PV patients than in control subjects (1,405±856 vs. 740±145.2; p=0.04). patients with ET or PMF, or subjects with reactive erythrocytosis (Figure 2C); on the other hand, although the mean MFI value was greater in patients with a V617F allele burden of more than 50% (1,423±1,112) compared to in those with less than 50% mutated allele (1,152±604), the difference was not statistically significant because of the wide scattering of data (Figure 2C).
Figure 2.
The plots show the fraction of basophils expressing the CD63 activation marker within the basophil gate (Panel A) or the absolute count of CD63+ basophils (Panel B) in the peripheral blood of PV patients, either all together (n=72) or divided according to whether their JAK2V617F allele burden was less than or greater than 50%. Results for patients with ET or PMF, control subjects (Ctr) or subjects with reactive erythrocytosis (RE) are also shown. (Panel C) The mean fluorescence intensity of CD63 on the membrane of gated basophils.
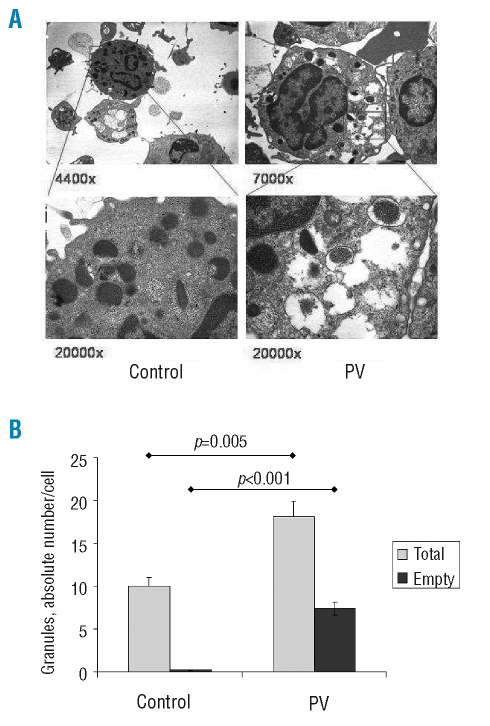
We employed transmission electron microscopy analysis to enumerate the number of granules contained in basophils and to characterize their morphology; a representative image of control and PV basophils is presented in Figure 3A. We found that the number of granules contained in basophils from patients with PV was significantly greater than the number in basophils from healthy controls (18.1±1.4 versus 10.0±1.1, respectively; p=0.005); furthermore, the number of empty granules, most of which also had a disrupted membrane, was significantly higher in PV basophils than in control basophils (7.4±0.9 versus 0.1±0.1, respectively; p<0.001) (Figure 3B). In most instances, the cytoplasm of PV basophils also showed abnormal electron density and extensive vacuolization (Figure 3A).
Figure 3.
(Panel A) Representative transmission electron microscopy analysis of circulating basophils in a control subject (images on the left) and a PV patient (on the right; V617F allele burden =70%). Thin (upper panels) and ultrathin (lower panels) sections were observed under vacuum with an EM 109 Zeiss microscope equipped with built-in electromagnetic objective lenses and camera (Oberkochen, Germany). Photographs were taken with Kodak Technical Pan film (Kodak, Rochester, NY, USA), developed with Kodak D 19 1+4 automatic developer and scanned with an EPSON Perfection 3200 photoscanner (Seiko EPSON, Nagano-ken, Japan). Original magnification was 4,400× and 7,000× for the upper left and right panel, respectively, and 20,000× for the lower panels. (Panel B) The absolute numbers of granules contained in basophils from PV patients (n=5) and healthy subjects (n=4) (gray columns) after enumerating at least ten basophils/subject; the numbers of those granules devoid of their electron-dense content (empty granules) are also presented (black columns). Statistically significant differences are reported in the plot.
Altogether, these data suggest that the number of activated basophils circulating in PV patients is higher than that in control subjects and correlated to the V617F allele burden; furthermore, these cells have morphological abnormalities compatible with ongoing in vivo activation and degranulation.
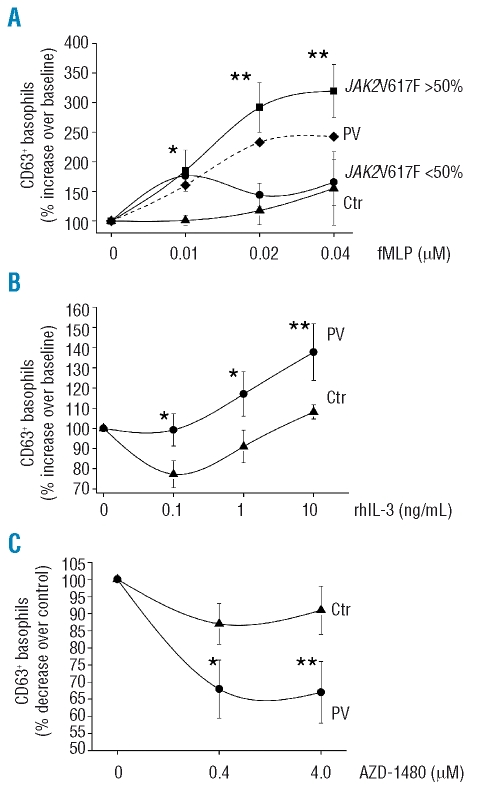
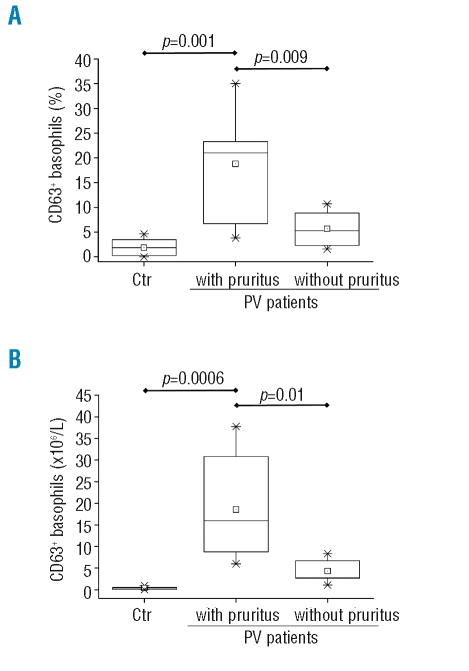
To evaluate possible correlations between the JAK2V617F mutation and basophil function, we evaluated cell ex vivo activation by monitoring the expression of the activation marker CD63 on the cell surface; to this end, cells were first incubated with rhIL-3, known to exert a potent priming effect mainly through the JAK2/STAT5 pathway,25 then challenged with the f-MLP peptide, with acts by binding to a heterotrimeric G-protein coupled receptor. We found that at any of the three fMLP concentrations employed (from 0.01 to 0.04 μM) the fraction of basophils induced to express CD63 was significantly greater in PV patients than in controls, particularly in those PV patients with a mutated allele burden of more than 50% (Figure 4A). At the highest dose of 0.04 μM fMLP, there were 2.44±0.6-fold more basophils expressing CD63 in PV compared to control samples (1.38±0.3 fold increase over baseline); in patients with more than 50% mutated allele the increase of CD63+ basophils compared to baseline was 3.3±0.2 fold (p<0.01; Figure 4A). Similarly, when basophils were primed with varying amounts of rhIL-3 (range, 0.1 to 10 ng/mL) and then challenged with an optimal amount of fMLP, the response of PV cells was significantly greater than that of control cells at any IL-3 dose (Figure 4B). Overall, these data suggest that the response of PV basophils to the priming effect of IL-3 is abnormally enhanced compared to that of control cells. To address the role of mutated JAK2, we employed the potent and selective JAK2 inhibitor AZD1480. This agent was shown to be able to significantly reduce the ex vivo activation of PV basophils in response to optimal amounts of fMLP and rhIL-3 (Figure 4C); at a 4.0 μM dose of AZD-1480, there was a 66% reduction in the fraction of PV basophils expressing CD63. Notably, the inhibitory effect was more pronounced in PV basophils than in control basophils, which were not appreciably affected by the drug. We found no meaningful correlation between number of circulating CD63+ basophils and hematologic or clinical characteristics of PV patients, including splenomegaly, thrombosis, or need for chemotherapy (not shown in detail); on the other hand, we found that both the relative proportion and the absolute count of circulating CD63+ basophils were significantly higher in patients suffering from aquagenic pruritus than in those who did not have this symptom (Figure 5). Also, in agreement with previous reports,26,27 we found that the V617F allele burden was significantly greater in patients with pruritus (71±18%) than in those without (48±19%; p=0.002).
Figure 4.
(Panel A) Expression of the activation marker CD63 in peripheral blood cells after being incubated ex vivo with increasing amounts of fMLP peptide (0 to 0.04 μM) in the presence of an optimal amount of rhIL-3 (10 ng/mL). Results are expressed as per cent increase of CD63+ basophils over unstimulated cells. The mean (±SD) values measured in control subjects (n=5; triangles) and PV patients (n=10), either all together (dashed line; for clarity, SD is not presented) or divided according to their V617F allele burden [>50% (squares) or <50% (dots), n=5 each], is presented. (Panel B) Experiments as above were performed using increasing amounts of rhIL-3 in the presence of a fixed dose of fMLP peptide (0.02μM). Only PV patients with more than 50% mutated V617F allele were included in these experiments and compared to controls (n=5 each). Results are expressed as per cent increase of CD63+ basophils over cultures containing fMLP only. (Panel C) Peripheral blood cells from PV patients and control subjects (n=5 each) were pre-incubated with the specific JAK2 inhibitor AZD1480 at two different concentrations, and then challenged with fMLP peptide (0.04 μM) and IL-3 (10 ng/mL). The fraction of cells in the basophil gate expressing CD63 was measured by FACS; results are expressed as per cent decrease of CD63+ basophils in wells containing the drug compared to cells without inhibitor. Only PV patients with more than 50% mutated V617F allele were used in these experiments. *p<0.05; **p<0.01.
Figure 5.
Plots show the percentage of basophils expressing the CD63 activation marker (Panel A) or their absolute count (Panel B) in the peripheral blood of PV patients according to whether they did or did not have aquagenic pruritus.
Discussion
To the best of our knowledge, this is the first study addressing possible effects of the JAK2V617F mutation in basophils from patients with PV and other myeloproliferative neoplasms. The data presented here suggest that: (i) the basophil count in the peripheral blood of patients with myeloproliferative neoplasms, but particularly in those with PV and in JAK2V617F-mutated cases of ET or PMF, is significantly higher than the normal basophil count. The design of this study does not allow us to conclude whether this is due to an increased output from JAK2V617F mutated basophil progenitors, increased size of the early progenitor pool, increased survival of the mature cells, or a combination of these. In this regard, it is intriguing that IL-3 was recently shown to protect normal basophils from apoptosis through the activation of BCL-XL and a Pim-1 dependent pathway;28 (ii) the count of constitutively activated basophils in the circulation, as measured by their expression of the activation marker CD63, is significantly increased in PV patients; intriguingly, the number of activated basophils is associated with higher allele burden and with the complaint of aquagenic pruritus. Of note, the activated basophil count of patients with ET or PMF did not differ significantly from that of healthy subjects. Indirect support for an in vivo activated status of PV basophils was also provided by the findings of an increased number of empty granules in these cells according to electron microscopy analysis; (iii) in vitro, PV basophils showed hypersensitivity to IL-3 and were hyper-responsive to the fMPL agonist compared to normal cells; (iv) abnormal in vitro activation was largely prevented by treatment with a JAK2 inhibitor. One additional finding of this study is that the content of total JAK2 mRNA in PV basophils was significantly increased compared to that in either PV granulocytes or control basophils, without evidence of preferential transcription or accumulation of V617F mutated RNA. To ascertain whether also the content of JAK2 protein was increased in PV basophils, we performed FACS analysis and western blotting; results obtained with both techniques indicated that the protein content was similar in PV basophils, PV granulocytes and normal cells. Given the low number of basophils that could be recovered after immunomagnetic purification we were unable to perform experiments aiming at distinguishing between increased JAK2 mRNA transcription from increased mRNA stability as the mechanism(s) for the higher levels of JAK2 mRNA measured in basophils. However, it is of interest that these findings are reminiscent of those related to the PRV1 gene, whose expression was found to be enhanced in PV granulocytes without being associated with increased protein content.29
To evaluate the activation status of circulating basophils and their response in vitro to agonists, we measured the expression of CD63 on the basophil cell membrane. CD63 is a tetraspanin contained in the inner granule surface in resting basophils; its expression on the outer cell surface correlates with basophil degranulation and histamine release, and serves as a reliable marker of allergen-induced basophil activation.24 The effector functions of basophils are potently enhanced by several cytokines, including IL-5, granulocyte-monocyte colony-stimulating factor, and nerve growth factor; however, IL-3 is the most potent priming cytokine for human basophils, enhancing mediator secretion, the production of IL-4 and IL-3, the de novo synthesis of leukotriene C4 and granzyme B.25,30,31 IL-3, as well as IL-5, induces JAK2 and STAT5 phosphorylation as a non-redundant mechanism for basophil activation.25 Data from ex vivo experiments indicated that PV basophils are hypersensitive to functional activators, possibly through constitutive signaling from mutated JAK2, as revealed by the priming effects of low-dose IL-3 and enhanced response to fMLP peptide, and by the inhibition produced by a potent JAK2 inhibitor.
An intriguing finding of this study was the association between an increased number of activated basophils in the circulation of PV patients and the complaint of aquagenic pruritus. Pruritus, exacerbated by contact with water during warm baths or showers, is a typical feature of PV, being reported by up to 65% of patients at diagnosis.32 This symptom can antedate diagnosis or appear during the course of the disease; it is poorly responsive to phlebotomy or myelosuppressive therapy, while interferon-α,33.34 or selective serotonin reuptake inhibitors35 can be successful. Pruritus has been associated with iron deficiency,36 high leukocyte count,36 platelet activation,35 histamine release,37,38 infiltration of the derma by mononuclear cells and eosinophils,39 and degranulation of dermal mast cells,39,40 but the underlying mechanisms remain substantially obscure. Furthermore, we, like others, found that pruritus was more common among patients harboring a greater than 50% V617F allele burden;26,27,41 accordingly, we found that the number of circulating activated basophils, measured by their expression of CD63, was significantly increased in this category of patients. Basophils are implicated in immediate hypersensitivity reactions and anaphylaxis, and their granules contain several biogenic amines, including histamine, which might be involved in the pathogenesis of pruritus although no clear correlation of pruritus with plasma histamine levels in PV has been found. However, it is also possible that other ‘non-canonical’ mediators such as leukotriene C4 or granzyme B, to name a few,30,31 might also be involved in the pathogenesis of pruritus. Furthermore, basophils can produce and release a vast array of cytokines, such as IL-4, IL-13 and IL-33, which facilitate recruitment and activation of other inflammatory cells (including neutrophils, eosinophils, and mast cells); therefore, PV basophils might not necessarily act as effector cells by themselves in causing pruritus.31,42 The experimental design of our study does not allow us to distinguish these several possibilities and the mechanistic link between basophils and pruritus requires additional investigation. In this regard, there is recent evidence that an increased output of CD34+ cell-derived mast cells in patients with myeloproliferative neoplasms plays a role in the etiogenesis of pruritus, possibly through the release of prostaglandin D2 and increased levels of IL-31.43
Overall, the results of this study indicate that PV basophils are constitutively activated and hypersensitive to IL-3, favoring a direct role of JAK2V617F mutation. They also lend support to the hypothesis that activated basophils contribute to pruritus in PV patients and that JAK2 inhibitors might be effective in countering this agonizing and usually treatment-insensitive symptom.
Footnotes
Authorship and Disclosures
LP, CB and PG performed research, analyzed data, and contributed to writing the manuscript; MZ performed research and analyzed data; RAR analyzed data and contributed to writing the manuscript; NB performed research; AB collected clinical samples and contributed to writing the manuscript; AMV designed research, collected clinical samples, analyzed data, and wrote the manuscript.
The authors reported no potential conflicts of interest.
Funding: this work was supported by Ministero della Università e Ricerca (COFIN 2006067001_003), Istituto Toscano Tumori, and Associazione Italiana per la Ricerca sul Cancro, Milano.
References
- 1.Vannucchi AM, Guglielmelli P, Tefferi A. Advances in understanding and management of myeloproliferative neoplasms. CA Cancer J Clin. 2009;59:171–91. doi: 10.3322/caac.20009. [DOI] [PubMed] [Google Scholar]
- 2.Vannucchi AM, Antonioli E, Guglielmelli P, Pardanani A, Tefferi A. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia. 2008;22:1299–307. doi: 10.1038/leu.2008.113. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–83. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 4.Vainchenker W, Constantinescu SN. A unique activating mutation in JAK2 (V617F) is at the origin of polycythemia vera and allows a new classification of myeloproliferative diseases. Hematology Am Soc Hematol Educ Program. 2005:195–200. doi: 10.1182/asheducation-2005.1.195. [DOI] [PubMed] [Google Scholar]
- 5.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 6.Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–81. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–60. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 8.Bumm TG, Elsea C, Corbin AS, Loriaux M, Sherbenou D, Wood L, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006;66:11156–65. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- 9.Zaleskas VM, Krause DS, Lazarides K, Patel N, Hu Y, Li S, et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS One. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 11.Dupont S, Masse A, James C, Teyssandier I, Lecluse Y, Larbret F, et al. The JAK2 V617F mutation triggers erythropoietin hypersensitivity and terminal erythroid amplification in primary cells from patients with polycythemia vera. Blood. 2007;110:1013–21. doi: 10.1182/blood-2006-10-054940. [DOI] [PubMed] [Google Scholar]
- 12.Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–40. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 13.Shide K, Shimoda HK, Kumano T, Karube K, Kameda T, Takenaka K, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22:87–95. doi: 10.1038/sj.leu.2405043. [DOI] [PubMed] [Google Scholar]
- 14.Kralovics R, Teo SS, Buser AS, Brutsche M, Tiedt R, Tichelli A, et al. Altered gene expression in myeloproliferative disorders correlates with activation of signaling by the V617F mutation of Jak2. Blood. 2005;106:3374–6. doi: 10.1182/blood-2005-05-1889. [DOI] [PubMed] [Google Scholar]
- 15.Vannucchi AM, Guglielmelli P, Antonioli E, Mappa S, Pancrazzi A, Bogani C, et al. Inconsistencies in the association between the JAK2(V617F) mutation and PRV-1 over-expression among the chronic myeloproliferative diseases. Br J Haematol. 2006;132:652–4. doi: 10.1111/j.1365-2141.2005.05951.x. [DOI] [PubMed] [Google Scholar]
- 16.Vannucchi AM, Pancrazzi A, Bogani C, Antonioli E, Guglielmelli P. A quantitative assay for JAK2(V617F) mutation in myeloproliferative disorders by ARMS-PCR and capillary electrophoresis. Leukemia. 2006;20:1055–60. doi: 10.1038/sj.leu.2404209. [DOI] [PubMed] [Google Scholar]
- 17.Goerttler PS, Steimle C, Marz E, Johansson PL, Andreasson B, Griesshammer M, et al. The Jak2V617F mutation, PRV-1 overexpression, and EEC formation define a similar cohort of MPD patients. Blood. 2005;106:2862–4. doi: 10.1182/blood-2005-04-1515. [DOI] [PubMed] [Google Scholar]
- 18.Passamonti F, Rumi E, Pietra D, Della Porta MG, Boveri E, Pascutto C, et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107:3676–82. doi: 10.1182/blood-2005-09-3826. [DOI] [PubMed] [Google Scholar]
- 19.Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC, Villamor N, Colomer D, Cervantes F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;91:169–75. [PubMed] [Google Scholar]
- 20.Alvarez-Larran A, Arellano-Rodrigo E, Reverter JC, Domingo A, Villamor N, Colomer D, et al. Increased platelet, leukocyte, and coagulation activation in primary myelofibrosis. Ann Hematol. 2008;87:269–76. doi: 10.1007/s00277-007-0386-3. [DOI] [PubMed] [Google Scholar]
- 21.Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia. 2008;22:2020–8. doi: 10.1038/leu.2008.253. [DOI] [PubMed] [Google Scholar]
- 22.Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110:1092–7. doi: 10.1182/blood-2007-04-083501. [DOI] [PubMed] [Google Scholar]
- 23.Centurione L, Di Baldassarre A, Zingariello M, Bosco D, Gatta V, Rana RA, et al. Increased and pathologic emperipolesis of neutrophils within megakaryocytes associated with marrow fibrosis in GATA-1(low) mice. Blood. 2004;104:3573–80. doi: 10.1182/blood-2004-01-0193. [DOI] [PubMed] [Google Scholar]
- 24.de Weck AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, et al. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. Int Arch Allergy Immunol. 2008;146:177–89. doi: 10.1159/000115885. [DOI] [PubMed] [Google Scholar]
- 25.Miura K, Saini SS, Gauvreau G, MacGlashan DW., Jr Differences in functional consequences and signal transduction induced by IL-3, IL-5, and nerve growth factor in human basophils. J Immunol. 2001;167:2282–91. doi: 10.4049/jimmunol.167.4.2282. [DOI] [PubMed] [Google Scholar]
- 26.Tefferi A, Lasho TL, Schwager SM, Strand JS, Elliott M, Mesa R, et al. The clinical phenotype of wild-type, heterozygous, and homozygous JAK2V617F in polycythemia vera. Cancer. 2006;106:631–5. doi: 10.1002/cncr.21645. [DOI] [PubMed] [Google Scholar]
- 27.Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–9. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 28.Didichenko SA, Spiegl N, Brunner T, Dahinden CA. IL-3 induces a Pim1-dependent antiapoptotic pathway in primary human basophils. Blood. 2008;112:3949–58. doi: 10.1182/blood-2008-04-149419. [DOI] [PubMed] [Google Scholar]
- 29.Temerinac S, Klippel S, Strunck E, Roder S, Lubbert M, Lange W, et al. Cloning of PRV-1, a novel member of the uPAR receptor superfamily, which is overexpressed in polycythemia rubra vera. Blood. 2000;95:2569–76. [PubMed] [Google Scholar]
- 30.Tschopp CM, Spiegl N, Didichenko S, Lutmann W, Julius P, Virchow JC, et al. Granzyme B, a novel mediator of allergic inflammation: its induction and release in blood basophils and human asthma. Blood. 2006;108:2290–9. doi: 10.1182/blood-2006-03-010348. [DOI] [PubMed] [Google Scholar]
- 31.Min B. Basophils: what they ‘can do’ versus what they ‘actually do’. Nat Immunol. 2008;9:1333–9. doi: 10.1038/ni.f.217. [DOI] [PubMed] [Google Scholar]
- 32.Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international internet-based survey of 1179 MPD patients. Cancer. 2007;109:68–76. doi: 10.1002/cncr.22365. [DOI] [PubMed] [Google Scholar]
- 33.Muller EW, de Wolf JT, Egger R, Wijermans PW, Huijgens PC, Halie MR, et al. Long-term treatment with interferon-α 2b for severe pruritus in patients with polycythaemia vera. Br J Haematol. 1995;89:313–8. doi: 10.1111/j.1365-2141.1995.tb03306.x. [DOI] [PubMed] [Google Scholar]
- 34.de Wolf JT, Hendriks DW, Egger RC, Esselink MT, Halie MR, Vellenga E. α-interferon for intractable pruritus in polycythaemia vera. Lancet. 1991;337:241. doi: 10.1016/0140-6736(91)92206-h. [DOI] [PubMed] [Google Scholar]
- 35.Tefferi A, Fonseca R. Selective serotonin reuptake inhibitors are effective in the treatment of polycythemia vera-associated pruritus. Blood. 2002;99:2627. doi: 10.1182/blood.v99.7.2627. [DOI] [PubMed] [Google Scholar]
- 36.Diehn F, Tefferi A. Pruritus in polycythaemia vera: prevalence, laboratory correlates and management. Br J Haematol. 2001;115:619–21. doi: 10.1046/j.1365-2141.2001.03161.x. [DOI] [PubMed] [Google Scholar]
- 37.Steinman HK, Kobza-Black A, Lotti TM, Brunetti L, Panconesi E, Greaves MW. Polycythaemia rubra vera and water-induced pruritus: blood histamine levels and cutaneous fibrinolytic activity before and after water challenge. Br J Dermatol. 1987;116:329–33. doi: 10.1111/j.1365-2133.1987.tb05846.x. [DOI] [PubMed] [Google Scholar]
- 38.Westin J, Granerus G, Weinfeld A, Wetterquist H. Histamine metabolism in polycythaemia vera. Scand J Haematol. 1975;15:45–57. doi: 10.1111/j.1600-0609.1975.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Naser MB, Zaki MS, Mousa MH, Hamed H, Rizk MS, Mohamed HE, et al. Cutaneous mononuclear cells and eosinophils are significantly increased after warm water challenge in pruritic areas of polycythemia vera. J Cutan Pathol. 2007;34:924–9. doi: 10.1111/j.1600-0560.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 40.Buchanan JG, Ameratunga RV, Hawkins RC. Polycythemia vera and water-induced pruritus: evidence against mast cell involvement. Pathology. 1994;26:43–5. doi: 10.1080/00313029400169091. [DOI] [PubMed] [Google Scholar]
- 41.Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, Marchioli R, et al. Clinical profile of homozygous JAK2V617F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110:840–6. doi: 10.1182/blood-2006-12-064287. [DOI] [PubMed] [Google Scholar]
- 42.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9:9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 43.Ishii T, Wang J, Zhang W, Mascarenhas J, Hoffman R, Dai Y, et al. Pivotal role of mast cells in prurito-genesis in patients with myeloproliferative disorders. Blood. 2009;113:5942–50. doi: 10.1182/blood-2008-09-179416. [DOI] [PubMed] [Google Scholar]