There are data to support a role for genetic and immune-related factors in the pathogenesis of lymphomas and plasma cell diseases. In this paper, the AUTHORS review relevant studies in Hodgkin’s and non-Hodgkin’s lymphomas, multiple myeloma, and the precursor condition monoclonal gammopathy of undetermined significance. Taken together, these novel insights raise complex medical considerations and imply ethical dilemmas.
Keywords: Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, Waldenström’s macroglobulinemia, lymphoma, MGUS, multiple myeloma, familial aggregation, susceptibility, immune-related, autoimmune disease, rheumatoid arthritis, monoclonal B-cell lymphocytosis
Abstract
There are data to support a role for genetic and immune-related factors in the pathogenesis of lymphomas and plasma cell diseases. In this paper, we review our published large population-based studies and other relevant studies in Hodgkin’s and non-Hodgkin’s lymphomas, multiple myeloma, and the precursor condition monoclonal gammopathy of undetermined significance. We discuss the overlap in risk factors between related malignancies and explore the underlying mechanisms. Based on these studies, we provide clinical implications and discuss the relevance of these data for patient counseling and clinical follow-up. Finally, we suggest future directions for new studies designed to increase our current knowledge and to define underlying biological mechanisms of our findings.
Introduction
Lymphoproliferative diseases include many different disease entities with distinct cells of origin, pathologies, risk factor profiles, and prognoses.1 Lymphomas have traditionally been separated into Hodgkin’s lymphomas (HL) and non-Hodgkin’s lymphomas (NHL) including multiple subtypes, such as follicular lymphomas (FL), diffuse large B-cell lymphomas (DLBCL), chronic lymphocytic leukemia (CLL), lymphoplasmacytic lymphoma (LPL)/Waldenström’s macroglobulinemia (WM). Multiple myeloma (MM) and monoclonal gammopathy of undetermined significance (MGUS) are grouped together as plasma cell disorders.1
Currently, the causes of these lymphoproliferative diseases are mostly unknown; however, data support a role for genetic and immune-related factors in their pathogenesis. In this paper, we will review the findings from studies on this topic and discuss the implications with respect to the mechanisms and pathways underlying genetic susceptibility, autoimmunity and lymphoproliferative disorders. Furthermore, we discuss the future directions including a combination of population-based and molecular studies, which are needed to better define underlying biological mechanisms. Finally, we address clinical implications and provide perspective on the relevance of these data for patient counseling and clinical follow-up.
Genetic factors
Although many cancers show familial clustering, identifying the predisposing germ-line genes is challenging. Familial aggregation of a disease is a necessary but not sufficient condition to infer a contribution of heredity, and identifying such families has led to elucidation of the genetic basis for numerous conditions. Recent large population-based studies from Scandinavia have provided a comprehensive insight to this phenomenon in lymphoproliferative diseases. While the risk among relatives of a proband with a lymphoproliferative tumor is most prominently increased for the same specific subtype, risks are also increased for other lymphoproliferative diseases, suggesting that certain genes are associated with increased risk for multiple lymphoproliferative diseases. This means that linkage and association studies should look for genes specific to subtypes as well as genes shared among sub-types. The power to detect common susceptibility genes with smaller effects has been facilitated by the ability to conduct genome-wide studies with very densely spaced genetic markers.
Lymphomas
Previous studies have consistently shown significantly increased risks of NHL associated with a family history of lymphoma or other hematopoietic malignancies.2–6 In a study including 70,006 first-degree relatives of 26,089 NHL cases versus 161,352 first-degree relatives of 58,960 matched controls, we found relatives of NHL patients to have significantly increased risk of NHL and HL.6 Furthermore, in a recent study, we evaluated risk of lymphoma subtypes among first-degree relatives of 2,668 FL patients, 2,517 DLBCL patients, and 6,963 HL patients compared to first-degree relatives of controls.7 Relatives were at the highest risk for developing the same lymphoma subtype as the proband. DLBCL was 10-fold increased among relatives of DLBCL patients, FL was 4-fold increased among relatives of FL patients and HL was 4-fold increased among relatives of HL patients, implying that germ-line susceptibility genes are specific to lymphoma subtype. Furthermore, in a study on patients with NHL, a family history of cancer was associated with a non-significantly increased risk of developing certain second cancers (mainly skin and lung cancer), suggesting that there may be some shared (environmental, genetic, or both) susceptibility.8
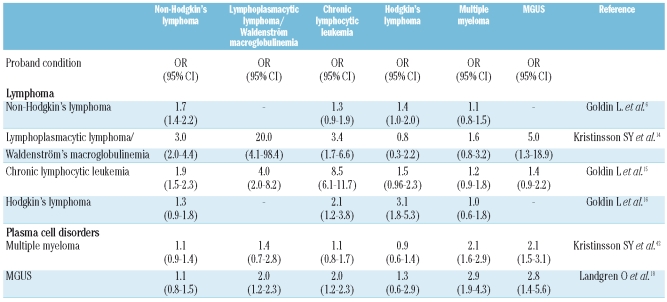
A number of affected families presenting multiple cases, as well as case-control and cohort studies, have been published showing familial clustering of LPL and WM.9–13 Recently, based on data from a referral clinic with particular focus on familial disease, Treon et al. reported that 19% of their WM patients had at least one first-degree relative affected with WM or another B-cell disorder.13 To quantify risk estimates for familial aggregation in the general population, we recently performed a large population-based study, including 2,144 LPL/WM patients, 8,279 population-based matched controls, and linkable first-degree relatives of patients (n=6,177) and controls (n=24,609).14 We found first-degree relatives of LPL/WM patients had a significantly increased risk of developing LPL/WM, other subtypes of NHL (including CLL), and MGUS, compared to first-degree relatives of controls, with the highest risk for LPL/WM (Table 1). However, we found no excess risk of MM or HL. Together with previous studies,6,16,19 these findings support a role for shared common susceptibility genes that predispose to LPL/WM and possibly certain lymphoproliferative disorders. Additionally, a genome-wide linkage analysis of 11 families at high risk for WM found the strongest evidence of linkage on chromosomes 1q and 4q.11
Table 1.
Relative risk (odds ratio, OR and 95% confidence interval, 95% CI) for lymphoproliferative and plasma cell tumors among first-degree relatives of patients with non-Hodgkin’s lymphoma, lymphoplasmacytic lymphoma/Waldenström’s macroglobulinemia, chronic lymphocytic leukemia, Hodgkin’s lymphoma, multiple myeloma, and MGUS (compared with relatives of matched controls).
We have evaluated the familial risk of CLL in two studies.15,19 Recently, a study including almost 10,000 CLL patients and their close to 27,000 first-degree relatives showed that first degree relatives of CLL patients had an 8.5-fold increased risk for CLL.15 This is similar to that observed in a registry study in Utah.3 Furthermore, we found significantly increased risk for NHL, including LPL/WM, and hairy cell leukemia.15 However, there was no excess of HL, MM or MGUS (Table 1). These studies support the role of germ-line genes underlying risk of CLL and perhaps related malignancies. Regions of the genome likely to contain susceptibility genes have been identified from linkage studies in high-risk families.20 Specific genes have been implicated from candidate gene studies and a genome-wide association study.21,22 However, specific mutations causing susceptibility have not been identified. Additionally, there are reports to suggest an increased risk of second cancer after CLL, mainly lung cancer and skin cancer.23–25 Whether this is caused by CLL therapy, immune dysregulation, shared susceptibility genes, or a combination of these remains to be determined.
The importance of genetic factors in HL is indicated by reports of multiply affected families, case-control studies, and population registry studies.26–30 We analyzed data from registries in Sweden and Denmark, including more than 22,000 HL patients and almost 60,000 controls and found significant familial aggregation of HL and other lymphoproliferative tumors (Table 1).16 The relative risk for HL among first degree relatives of cases compared with controls was 3.1, but relatives were also at increased risk for CLL and NHL. More recently, we found that DLBCL and HL aggregated in families.7 Relative risks were higher in males compared with females, and in siblings of cases compared with parents and offspring. Relatives of earlier onset cases were at higher risk for HL and for all lymphoproliferative tumors and were also at higher risk for developing early onset tumors themselves. In a recent genome-wide scan study, the strongest linkage association was found on chromosome 4p, and the authors found the inheritance pattern most likely to be recessive.32
Plasma cell disorders
There is evidence to support a role for genetic factors, including studies showing familial aggregation of MM3,17,33–38 and familial aggregation of MGUS.18 In addition, racial disparities in incidence patterns for MGUS and MM support a role for germ-line genes in the etiology of MM.39–41 We recently analyzed 13,896 MM patients and 54,365 matched controls, and first-degree relatives of cases (n=37,838) and controls (n=151,068).42 We found that first degree-relatives of MM patients, compared to first degree relatives of controls, had a 2-fold increased risk of MM, MGUS, and acute lymphoblastic leukemia. In a recent study from the Mayo Clinic, first degree relatives of MM patients had a 2-fold increased risk of MGUS, suggesting a familial aggregation of MGUS and MM (see below).43
Similarly, using population-based data from Sweden, we identified 4,458 MGUS patients, 17,505 population-based controls, and first-degree relatives of patients (n=14,621) and controls (n=58,387). Compared to relatives of controls, relatives of MGUS patients had a 2.8-fold increased risk of MGUS, 3-fold risk for MM, 4-fold for LPL/WM, and 3.4-fold risk for CLL.18 Relatives of patients with IgG/IgA MGUS had a significantly increased risk of MGUS, MM, and LPL/WM, whereas relatives of IgM MGUS patients had an increased CLL risk and non-significant excess MM and LPL/WM risks. Risk of NHL or HL was not increased among MGUS relatives. In accordance with our study, the study from the Mayo Clinic, involving relatives of 97 MGUS cases, found a 2.6-fold increased risk of MGUS.43 Additionally, recent studies have suggested that there is a familial aggregation of solid tumors (malignant melanoma and prostate cancer) with MM and MGUS.37,38 In our study on relatives of MM patients, we observed a significantly increased risk for any solid tumor as well as bladder cancer.42 Similarly, relatives of MGUS patients had an increased risk of solid tumors (all tumors grouped together), and specifically bladder cancer and spinal cancer, but not prostate or malignant melanoma.44 These and our results support a role for germ-line susceptibility genes, shared environmental influences, or an interaction between both in MM and MGUS, and possibly even solid tumors.
Immune-related factors
There is convincing evidence that immune dysregulation plays a major role in lymphomagenesis, including studies observing an increased risk of lymphomas following organ transplant, infections, immunodeficiency states, and autoimmune conditions.45 In contrast, much less is known regarding immune-related conditions and risk of development of plasma cell disorders. Autoimmune diseases comprise a broad variety of conditions characterized by dysregulation in various components of the immune response leading to loss of tolerance to auto-antigens. The association of lymphoma and autoimmunity has long been recognized, initially from case reports of autoimmune diseases preceding or co-occurring with lymphoma, and from animal models.46 Over the last three decades, there has been consistent evidence from population-based case-control and cohort studies that certain autoimmune diseases, especially rheumatoid arthritis (RA), Sjögren’s syndrome, and systemic lupus erythematosus (SLE), are associated with lymphoproliferative diseases.47 Explanations for these associations include the role of chronic immune stimulation, treatment for autoimmune disease, and shared genetic and/or environmental factors. Recent studies suggest that chronic antigenic stimulation also plays a role in the causation of plasma cell disorders.48
Lymphoma
Over the years, several studies have observed an association with certain autoimmune and chronic inflammatory conditions and risk of NHL.47,49,50 In a recent study, we included almost 25,000 cases of NHL (not including CLL) diagnosed in Denmark and Sweden, over 55,000 population-based matched controls, and their linkable first-degree relatives. We found a personal history of systemic autoimmune conditions to be significantly associated with a 2.6-fold increased risk of developing NHL.51 The risks for specific autoimmune conditions varied; however, a significant increased risk was observed for RA, Sjögren’s syndrome, SLE, and systemic sclerosis. Furthermore, several other autoimmune conditions were also associated with an increased risk of NHL, both conditions with organ involvement (Hashimoto’s thyroiditis, discoid lupus erythematosus, polyarteritis nodosa) and in autoimmune diseases without detectable autoantibodies (Crohn’s disease, psoriasis, and sarcoidosis). In our study, we found that the associations with systemic autoimmune diseases were stronger for aggressive NHL. A few recent studies49,52,53 have examined autoimmune disease associations by specific lymphoma subtypes. These studies have found a strong association with DLBCL and RA, but also SLE, Sjögren’s syndrome, and celiac disease, a finding not observed in FL.49,52,54 Burkitt’s lymphoma (BL) is characterized by a translocation t(8;14) which activates the c-myc oncogene.1 BL is strongly associated with Epstein-Barr virus.55 Other infectious agents are also known to be linked with BL, including plasmodium falciparum and HIV.56,57
Until recently, only a few smaller epidemiological studies had been conducted to assess the role of chronic antigenic stimulatory conditions in relation to risk of developing LPL/WM. In a hospital-based study including 65 WM patients and 213 hospital-based controls, personal history of autoimmune disease was not associated with subsequent risk of developing WM.10 Interestingly, WM patients were more likely than controls to have first-degree relatives with a history of pneumonia, diphtheria, rheumatic fever, and diabetes mellitus. An exploratory evaluation of immunological profiles revealed that relatives of 2 WM cases had IgM MGUS and about 40% had diverse immunological abnormalities.10 In a large case-control study of NHLs, Smedby et al.49 examined 116 LPL patients and found a significant association with RA. More recently, we conducted two large nationwide studies based on U.S. veterans. In a large study including 146,394 patients infected with hepatitis C virus (HCV) and 572,293 controls,58 HCV infection was found to confer a 20–30% increased risk of NHL overall and a 3-fold higher risk of WM. In another large study based on 4 million U.S. veterans, we assessed WM risk in relation to a variety of chronic immune stimulatory conditions. Among 361 patients with WM with up to 27 years of follow-up, we found a 2- to 3-fold elevated risk of WM in individuals with a personal history of an autoimmune disease (RA, Sjögren’s syndrome, immune thrombocytopenic purpura (ITP), and Crohn’s disease) and notably elevated risks associated with hepatitis, human immunodeficiency virus, and rickettsiosis.59 Taken together, these results support a role for chronic immune stimulation in the pathogenesis of WM.
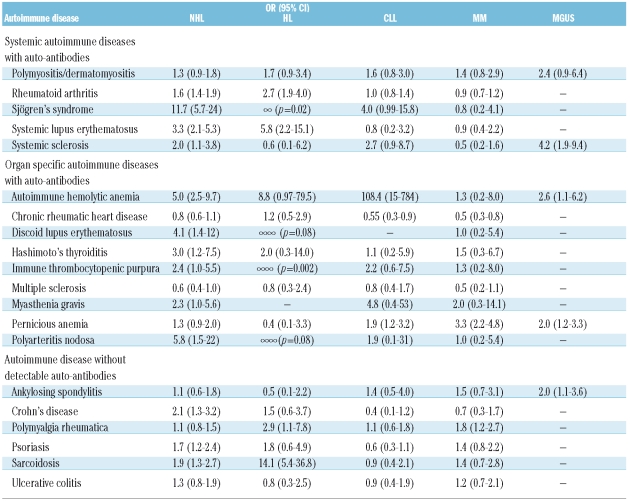
Few studies have been performed to evaluate the risks of autoimmune and chronic immune stimulatory conditions and risk of CLL, with inconsistent results.60–64 Recent large population-based investigations from Scandinavia support the hypothesis that common infectious agents such as encapsulated bacteria65 might play a role in the pathogenesis of CLL, while auto-antigens have not been found to be associated with CLL risk.66 Chronic rheumatic heart disease was associated with a significantly decreased risk of CLL. Smedby et al.49 found type I diabetes associated with CLL/SLL, an association also seen in a pooled InterLymph study.52 In the SEER-Medicare study, other than autoimmune hemolytic anemia, no other condition was associated among 9,171 CLL patients.67 The very strong association for a personal history of autoimmune hemolytic anemia (Table 2) and subsequent CLL observed in the study from Scandinavia was primarily confined to patients diagnosed with autoimmune hemolytic anemia within one year prior to CLL.66 However, since autoimmune hemolytic anemia is a well-known complication of CLL, it is most likely that this association reflects reverse causality (i.e. undetected CLL manifesting with autoimmune hemolytic anemia as an early symptom). If we eliminate the year before diagnosis, the risk was still highly elevated (data not shown). In the SEER-Medicare study, autoimmune hemolytic anemia was significantly associated with lymphoproliferative disease even when analyses were restricted to cases diagnosed with autoimmune hemolytic anemia five years or more prior to CLL.67
Table 2.
Relative risk (odds ratio, OR and 95% confidence interval, 95% CI) for lymphoproliferative and plasma cell tumors in relation to a personal history of selected autoimmune diseases.
In a recent study based on 77,469 healthy adults who were enrolled in the nationwide, population-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial,68 we identified 45 subjects in whom CLL was subsequently diagnosed (up to 6.4 years later) through the collection of peripheral whole-blood. Using six-color flow cytometry and immunoglobulin heavy-chain gene rearrangement (IGHV) by reverse transcriptase polymerase chain reaction (RT-PCR) assay, we found evidence of pre-diagnostic monoclonality among B cells (by either of the two methods) in 44 patients (98%; 95% confidence interval (CI) 88–100%). In 41 patients (91%; 95% CI 79–98%), the clone was confirmed by both methods.68 The distribution of mutated clones, as compared with unmutated clones, was very similar regardless of the time between obtaining the blood sample and the subsequent CLL diagnosis. In addition, among eight unmutated prediagnostic clones, three were present more than three years before the CLL diagnosis, with two being detectable five years before. Thus, this study suggests that virtually all cases of CLL (both with mutated and unmutated IGHV genes) are preceded by MBL.
HL has been associated with some autoimmune diseases. In our analyses of personal history of autoimmune conditions in 7,476 HL patients compared to controls,69 we found several autoimmune conditions to be strongly associated with HL including RA, SLE, sarcoidosis, and ITP. We found the overall 2.7-fold increased risks for systemic autoimmune diseases. Furthermore, a family history of sarcoidosis and ulcerative colitis were also associated with HL risk, suggesting a role for shared susceptibility factors between the two diseases. Anderson et al.53 found increased risk for RA, SLE, localized scleroderma, and discoid lupus erythematosus in HL patients. Consistent with our results from Scandinavia, sarcoidosis was associated with a 3-fold risk with borderline significance.53
Plasma cell disorders
The associations between chronic antigen stimulation and risk of MM have been evaluated in several studies.17,70–73 Based on small numbers there are some suggestions that certain types of infectious agents could play a role71,73 while the role of autoimmunity in relation to MM risk remains less clear.17,72 In a large population-based study from Sweden, we found pernicious anemia and polymyalgia rheumatica to be associated with MM, with the most prominent risks being within a year of autoimmune disease diagnosis.74 Similar to CLL (see above), we found chronic rheumatic heart disease to be associated with a decreased risk of MM. Anderson et al. also found pernicious anemia to be associated with MM but no other conditions were associated.53 Some studies have found increased risk of MM in RA patients75,76 but others have not.74,77 Furthermore, in a recent study of 4,641 U.S. veterans with MM, we found MM to be associated with a prior history of all autoimmune disorders combined, all systemic autoimmune disorder diseases combined, and separate conditions, polymyositis/dermatomyositis, systemic sclerosis, autoimmune hemolytic anemia, pernicious anemia, and ankylosing spondylitis.48 In the study based on U.S. veterans, 2,046 patients with MGUS were evaluated with respect to a prior medical history of immune-related diseases. Associations similar to those observed in MM were found among MGUS patients. For autoimmune conditions overall, there was a 1.7-fold increased risk of developing MGUS. This suggests that some autoimmune conditions may act as an early trigger in the pathway to MM. Alternatively, these associations could be a reflection of an underlying immune disruption linked with MGUS. Future work is needed to confirm and expand on these observations.
Proposed disease mechanisms
There are several possible mechanisms by which autoimmunity could be related to risk of developing lymphoma.78 One hypothesis is that both autoimmune and lymphoproliferative diseases result as a consequence of multistep processes that eliminate the checkpoints that inhibit uncontrolled B-cell growth, including uncontrolled growth of autoimmune lymphocytes.79 If this is true, such multistep processes likely involve both inherited and somatic mutations of genes in relevant molecular pathways. Proposed mechanisms for progression of autoimmune diseases into lymphomas include dysregulation and hyperactivity of B cells associated with autoimmune diseases and impaired T-cell function that may lead to lymphoma. For example, B-cell activating factor of the TNF family (BAFF) enhances survival of B cells, and is found to be over-expressed in Sjögren’s syndrome, RA, SLE, and lymphomas.80–82
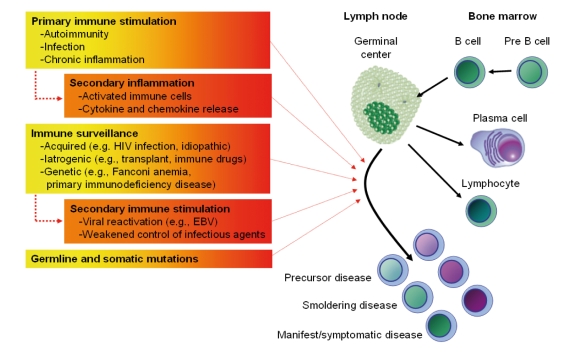
Figure 1 illustrates major immune-related factors thought to contribute to lymphomagenesis. Autoimmunity may lead to both overstimulation and defective apoptosis of B cells. Secondary inflammation because of autoimmune stimulation can also promote these processes which are evident in the continuum of histological changes associated with Sjögren’s syndrome.83 Several infections have been associated with development of lymphoproliferative disease and are likely to operate through some of the same pathways.45,58,65,71,84,85 In addition to various types of extrinsic factors, genetic factors likely play a role in lymphomagenesis and autoimmunity. It has been suggested that there could be some inherited mutations causing susceptibility to both autoimmune diseases and lymphomas. One way to test for this hypothesis would be to look for familial aggregation of both autoimmune diseases and lymphomas in families. However, our previous large population-based case-control studies found that family history of autoimmune disease was generally not a predictor of lymphoma risk.66,17,51,69 Similarly, a prior study of relatives of RA patients did not find an increased risk of lymphoma.86 It is also possible that autoimmune disease therapy plays a role in the development of subsequent lymphoma. However, the literature of lymphoma risk following autoimmune therapies (such as methotrexate and TNF-α blocking agents) is inconclusive, as discussed below.
Figure 1.
Proposed model regarding the role of immune-related and genetic factors in the pathogenesis of lymphoproliferative and plasma cell malignancies.
Clinical implications
It is important to consider the clinical implications of the above discussed associations. First-degree relatives of patients with lymphoproliferative disease are at an increased relative risk of developing various subtypes of lymphoproliferative diseases, with the highest risk for the same disease as the proband. However, because of the low baseline risk of most of these lymphoproliferative malignancies in the general population, the absolute risk for a first-degree relative to develop the same lymphoma or another lymphoproliferative malignancy is still very low. Furthermore, early detection of indolent lymphomas in particular is not likely to affect outcome since most asymptomatic patients typically are not treated. Additionally, although relatives of patients with a lymphoproliferative disease are at increased risk for developing MGUS,14 the average transformation rate from MGUS to MM or WM is only about 1–1.5% per year.87 Relatives of patients with lymphoproliferative diseases can be informed that they have a higher relative risk of developing lymphoproliferative disease (compared to family members of unaffected individuals). It should be emphasized that the absolute risk is very low, there is no treatment for early lesions, and there is no available therapy to prevent potential progression. Consequently, at this time, it is not recommended to screen for lymphoproliferative or plasma cell disease among family members outside clinical research studies.
The observation that immune-related and inflammatory conditions are associated with an excess risk of developing lymphomas may also have clinical implications regarding the treatment of those conditions. Indeed, it has been suggested in some, but not all, studies that the treatment of autoimmune diseases (with methotrexate and TNF-α blocking agents) might play a role in the development of subsequent lymphoma.88–90 Some studies have reported spontaneous lymphoma regression in RA patients following removal of methotrexate therapy.91,92 Others have found that lymphomas occurring following transplantation are more likely to be of aggressive subtypes and are thought to be due to immunosuppression and subsequent re-activation of Epstein-Barr virus (EBV).93 New therapeutic agents blocking TNF-α clearly benefit patients with RA and other autoimmune conditions. From a clinical perspective, we need to conduct long-term surveillance of our patients receiving therapy for autoimmune diseases while keeping in mind that the absolute lifetime risk of lymphoproliferative disease in the general population is very low.
Taken together, these novel insights raise complex medical considerations and imply ethical dilemmas. As treating physicians, we need to handle this information with care, and provide and council our patients with clinically relevant information. More knowledge about the risks will help make the treatment decision more informed.
Future directions
Future studies are needed to identify susceptibility gene(s) contributing to lymphoproliferative and plasma cell diseases, and define the role of immune-related conditions and their interaction in the etiology of lymphomas. Furthermore, studies need to incorporate molecular components to validate diagnoses and evaluate more biologically homogeneous groupings of autoimmune disorders and lymphoproliferative tumors. Other areas of interest might be to investigate prospectively the natural history and pathogenesis of autoimmune disorders in individuals who subsequently develop lymphoproliferative diseases. Also we need to better define the role of immune-related conditions (and their treatment) on prognosis and survival in lymphoproliferative diseases as this may have clinical implications for the treatment of patients with autoimmune conditions. Future studies designed to assess the underlying mechanisms of our recently defined associations might not only improve our understanding of the pathogenesis of lymphoproliferative diseases. They could also have a direct influence on clinical management by leading to more informed risk assessments of novel autoimmune drugs that might be involved in lymphomagenesis.
Footnotes
Authorship and Disclosures
SY Kristinsson and O Landgren initiated this work and wrote the report. All authors read, gave comments, and approved the final version of the manuscript.
The authors reported no potential conflicts of interest.
Funding: this research was supported by grants from the Swedish Cancer Society, Stockholm County Council, the Karolinska Institutet Foundations, and the Intramural Research Program of the NIH, NCI.
References
- 1.Swerdlov SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Swerdlov SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. Lyon: IARC; 2008. [Google Scholar]
- 2.Altieri A, Bermejo JL, Hemminki K. Familial risk for non-Hodgkin lymphoma and other lymphoproliferative malignancies by histopathologic subtype: the Swedish Family-Cancer Database. Blood. 2005;106:668–72. doi: 10.1182/blood-2005-01-0140. [DOI] [PubMed] [Google Scholar]
- 3.Cannon-Albright LA, Thomas A, Goldgar DE, Gholami K, Rowe K, Jacobsen M, et al. Familiality of cancer in Utah. Cancer Res. 1994;54:2378–85. [PubMed] [Google Scholar]
- 4.Cartwright RA, McKinney PA, O’Brien C, Richards ID, Roberts B, Lauder I, et al. Non-Hodgkin’s lymphoma: case control epidemiological study in Yorkshire. Leuk Res. 1988;12:81–8. doi: 10.1016/s0145-2126(98)80012-x. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee N, Hartge P, Cerhan JR, Cozen W, Davis S, Ishibe N, et al. Risk of non-Hodgkin’s lymphoma and family history of lymphatic, hematologic, and other cancers. Cancer Epidemiol Biomarkers Prev. 2004;13:1415–21. [PubMed] [Google Scholar]
- 6.Goldin LR, Landgren O, McMaster ML, Gridley G, Hemminki K, Li X, et al. Familial aggregation and heterogeneity of non-Hodgkin lymphoma in population-based samples. Cancer Epidemiol Biomarkers Prev. 2005;14:2402–6. doi: 10.1158/1055-9965.EPI-05-0346. [DOI] [PubMed] [Google Scholar]
- 7.Goldin L, Björkholm M, Kristinsson SY, Turesson I, Landgren O. Highly increased familial risks for specific lymphoma subtypes. Br J Haematol. 2009;146:91–4. doi: 10.1111/j.1365-2141.2009.07721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landgren O, Pfeiffer RM, Stewart L, Gridley G, Mellemkjaer L, Hemminki K, et al. Risk of second malignant neoplasms among lymphoma patients with a family history of cancer. Int J Cancer. 2007;120:1099–102. doi: 10.1002/ijc.22414. [DOI] [PubMed] [Google Scholar]
- 9.Fine JM, Lambin P, Massari M, Leroux P. Malignant evolution of asymptomatic monoclonal IgM after seven and fifteen years in two siblings of a patient with Waldenstrom’s macroglobulinemia. Acta Med Scand. 1982;211:237–9. doi: 10.1111/j.0954-6820.1982.tb01935.x. [DOI] [PubMed] [Google Scholar]
- 10.Linet MS, Humphrey RL, Mehl ES, Brown LM, Pottern LM, Bias WB, et al. A case-control and family study of Waldenstrom’s macroglobulinemia. Leukemia. 1993;7:1363–9. [PubMed] [Google Scholar]
- 11.McMaster ML, Goldin LR, Bai Y, Ter-Minassian M, Boehringer S, Giambarresi TR, et al. Genomewide linkage screen for Waldenstrom macroglobulinemia susceptibility loci in high-risk families. Am J Hum Genet. 2006;79:695–701. doi: 10.1086/507687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogmundsdottir HM, Johannesson GM, Sveinsdottir S, Einarsdottir S, Hegeman A, Jensson O, et al. Familial macroglobulinaemia: hyperactive B-cells but normal natural killer function. Scand J Immunol. 1994;40:195–200. doi: 10.1111/j.1365-3083.1994.tb03450.x. [DOI] [PubMed] [Google Scholar]
- 13.Treon SP, Hunter ZR, Aggarwal A, Ewen EP, Masota S, Lee C, et al. Characterization of familial Waldenstrom’s macroglobulinemia. Ann Oncol. 2006;17:488–94. doi: 10.1093/annonc/mdj111. [DOI] [PubMed] [Google Scholar]
- 14.Kristinsson SY, Bjorkholm M, Goldin LR, McMaster ML, Turesson I, Landgren O. Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: a population-based study in Sweden. Blood. 2008;112:3052–6. doi: 10.1182/blood-2008-06-162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldin LR, Bjorkholm M, Kristinsson SY, Turesson I, Landgren O. Elevated risk of chronic lymphocytic leukemia and other indolent non-Hodgkin’s lymphomas among relatives of patients with chronic lymphocytic leukemia. Haematologica. 2009;94:647–53. doi: 10.3324/haematol.2008.003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldin LR, Pfeiffer RM, Gridley G, Gail MH, Li X, Mellemkjaer L, et al. Familial aggregation of Hodgkin lymphoma and related tumors. Cancer. 2004;100:1902–8. doi: 10.1002/cncr.20189. [DOI] [PubMed] [Google Scholar]
- 17.Landgren O, Linet MS, McMaster ML, Gridley G, Hemminki K, Goldin LR. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: a population-based case-control study. Int J Cancer. 2006;118:3095–8. doi: 10.1002/ijc.21745. [DOI] [PubMed] [Google Scholar]
- 18.Landgren O, Kristinsson SY, Goldin LR, Caporaso NE, Blimark C, Mellqvist UH, et al. Risk of plasma-cell and lymphoproliferative disorders among 14,621 first-degree relatives of 4,458 patients with monoclonal gammopathy of undetermined significance (MGUS) in Sweden. Blood. 2009;114:791–5. doi: 10.1182/blood-2008-12-191676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldin LR, Pfeiffer RM, Li X, Hemminki K. Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: results from the Swedish Family-Cancer Database. Blood. 2004;104:1850–4. doi: 10.1182/blood-2004-01-0341. [DOI] [PubMed] [Google Scholar]
- 20.Sellick GS, Goldin LR, Wild RW, Slager SL, Ressenti L, Strom SS, et al. A high-density SNP genome-wide linkage search of 206 families identifies susceptibility loci for chronic lymphocytic leukemia. Blood. 2007;110:3326–33. doi: 10.1182/blood-2007-05-091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Bernardo MC, Crowther-Swanepoel D, Broderick P, Webb E, Sellick G, Wild R, et al. A genome-wide association study identifies six susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2008;40:1204–10. doi: 10.1038/ng.219. [DOI] [PubMed] [Google Scholar]
- 22.Slager SL, Kay NE, Fredericksen ZS, Wang AH, Liebow M, Cunningham JM, et al. Susceptibility genes and B-chronic lymphocytic leukaemia. Br J Haematol. 2007;139:762–71. doi: 10.1111/j.1365-2141.2007.06872.x. [DOI] [PubMed] [Google Scholar]
- 23.Hisada M, Biggar RJ, Greene MH, Fraumeni JF, Jr, Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–81. doi: 10.1182/blood.v98.6.1979. [DOI] [PubMed] [Google Scholar]
- 24.Travis LB, Curtis RE, Hankey BF, Fraumeni JF., Jr Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst. 1992;84:1422–7. doi: 10.1093/jnci/84.18.1422. [DOI] [PubMed] [Google Scholar]
- 25.Tsimberidou AM, Wen S, McLaughlin P, O’Brien S, Wierda WG, Lerner S, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–10. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjorkholm M, Holm G, De Faire U, Mellsted H. Immunological defects in healthy twin siblings to patients with Hodgkin’s disease. Scand J Haematol. 1977;19:396–404. doi: 10.1111/j.1600-0609.1977.tb01491.x. [DOI] [PubMed] [Google Scholar]
- 27.Grufferman S, Cole P, Smith PG, Lukes RJ. Hodgkin’s disease in siblings. N Engl J Med. 1977;296:248–50. doi: 10.1056/NEJM197702032960504. [DOI] [PubMed] [Google Scholar]
- 28.Bernard SM, Cartwright RA, Darwin CM, Richards ID, Roberts B, O’Brien C, et al. Hodgkin’s disease: case control epidemiological study in Yorkshire. Br J Cancer. 1987;55:85–90. doi: 10.1038/bjc.1987.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86:1600–8. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 30.Shugart YY, Hemminki K, Vaittinen P, Kingman A, Dong C. A genetic study of Hodgkin’s lymphoma: an estimate of heritability and anticipation based on the familial cancer database in Sweden. Hum Genet. 2000;106:553–6. doi: 10.1007/s004390000291. [DOI] [PubMed] [Google Scholar]
- 31.Ji J, Hemminki K. Familial blood vessel tumors and subsequent cancers. Ann Oncol. 2007;18:1260–7. doi: 10.1093/annonc/mdm092. [DOI] [PubMed] [Google Scholar]
- 32.Goldin LR, McMaster ML, Ter-Minassian M, Saddlemire S, Harmsen B, Lalonde G, et al. A genome screen of families at high risk for Hodgkin lymphoma: evidence for a susceptibility gene on chromosome 4. J Med Genet. 2005;42:595–601. doi: 10.1136/jmg.2004.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksson M, Hallberg B. Familial occurrence of hematologic malignancies and other diseases in multiple myeloma: a case-control study. Cancer Causes Control. 1992;3:63–7. doi: 10.1007/BF00051914. [DOI] [PubMed] [Google Scholar]
- 34.Judson IR, Wiltshaw E, Newland AC. Multiple myeloma in a pair of monozygotic twins: the first reported case. Br J Haematol. 1985;60:551–4. doi: 10.1111/j.1365-2141.1985.tb07452.x. [DOI] [PubMed] [Google Scholar]
- 35.Zawadzki ZA, Aizawa Y, Kraj MA, Haradin AR, Fisher B. Familial immunopathies: report of nine families and survey of literature. Cancer. 1977;40:2094–101. doi: 10.1002/1097-0142(197711)40:5<2094::aid-cncr2820400518>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Ogmundsdottir HM, Haraldsdottirm V, Johannesson GM, Olafsdottir G, Bjarnadottir K, Sigvaldason H, et al. Familiality of benign and malignant paraproteinemias. A population-based cancer-registry study of multiple myeloma families. Haematologica. 2005;90:66–71. [PubMed] [Google Scholar]
- 37.Lynch HT, Ferrara K, Barlogie B, Coleman EA, Lynch JF, Weisenburger D, et al. Familial myeloma. N Engl J Med. 2008;359:152–7. doi: 10.1056/NEJMoa0708704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camp NJ, Werner TL, Cannon-Albright LA. Familial myeloma. N Engl J Med. 2008;359:1734–5. doi: 10.1056/NEJMc081677. [DOI] [PubMed] [Google Scholar]
- 39.Landgren O, Gridley G, Turesson I, Caporaso NE, Goldin LR, Baris D, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904–6. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landgren O, Katzmann JA, Hsing AW, Pfeiffer RM, Kyle RA, Yeboah ED, et al. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin Proc. 2007;82:1468–73. doi: 10.1016/S0025-6196(11)61089-6. [DOI] [PubMed] [Google Scholar]
- 41.Landgren O, Kyle RA. Multiple myeloma, chronic lymphocytic leukaemia and associated precursor diseases. Br J Haematol. 2007;139:717–23. doi: 10.1111/j.1365-2141.2007.06866.x. [DOI] [PubMed] [Google Scholar]
- 42.Kristinsson SY, Björkholm M, Goldin LR, Blimark C, Mellqvist UH, Wahlin A, et al. Patterns of hematologic malignancies and solid tumors among 37,838 first-degree relatives of 13,896 multiple myeloma patients in Sweden. Int J Cancer. 2009;125:2147–50. doi: 10.1002/ijc.24514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vachon CM, Kyle RA, Therneau TM, Foreman BJ, Larson DR, Colby CL, et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood. 2009;114:785–90. doi: 10.1182/blood-2008-12-192575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kristinsson SY, Goldin LR, Bjorkholm M, Turesson I, Landgren O. Risk of solid tumors and myeloid hematologic malignancies among first-degree relatives of monoclonal gammopathy of undetermined significance (MGUS) patients. Haematologica. 2009;94:1179–81. doi: 10.3324/haematol.2009.006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexander DD, Mink PJ, Adami HO, Chang ET, Cole P, Mandel JS, et al. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120 (Suppl 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 46.Mellors RC. Autoimmune disease in NZB-Bl mice. II. Autoimmunity and malignant lymphoma. Blood. 1966;27:435–48. [PubMed] [Google Scholar]
- 47.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165:2337–44. doi: 10.1001/archinte.165.20.2337. [DOI] [PubMed] [Google Scholar]
- 48.Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood. 20081;111:3388–94. doi: 10.1182/blood-2007-10-121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 50.Engels EA, Cerhan JR, Linet MS, Cozen W, Colt JS, Davis S, et al. Immune-related conditions and immune-modulating medications as risk factors for non-Hodgkin’s lymphoma: a case-control study. Am J Epidemiol. 2005;162:1153–61. doi: 10.1093/aje/kwi341. [DOI] [PubMed] [Google Scholar]
- 51.Mellemkjaer L, Pfeiffer RM, Engels EA, Gridley G, Wheeler W, Hemminki K, et al. Autoimmune disease in individuals and close family members and susceptibility to non-Hodgkin’s lymphoma. Arthritis Rheum. 2008;58:657–66. doi: 10.1002/art.23267. [DOI] [PubMed] [Google Scholar]
- 52.Ekstrom Smedby K, Vajdic CM, Falster M, Engels EA, Martinez-Maza O, Turner J, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029–38. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson LA, Gadalla S, Morton LM, Landgren O, Pfeiffer R, Warren JL, et al. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer. 2009;125:398–405. doi: 10.1002/ijc.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baecklund E, Backlin C, Iliadou A, Granath F, Ekbom A, Amini RM, et al. Characteristics of diffuse large B cell lymphomas in rheumatoid arthritis. Arthritis Rheum. 2006;54:3774–81. doi: 10.1002/art.22277. [DOI] [PubMed] [Google Scholar]
- 55.zur Hausen H, Schulte-Holthausen H, Klein G, Henle W, Henle G, Clifford P, et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228:1056–8. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]
- 56.Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt’s lymphoma: a polymicrobial disease? Nat Rev Microbiol. 2005;3:182–7. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 57.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. Aids. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 58.Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–7. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 59.Koshiol J, Gridley G, Engels EA, McMaster ML, Landgren O. Chronic immune stimulation and subsequent Waldenstrom macroglobulinemia. Arch Intern Med. 2008;168:1903–9. doi: 10.1001/archinternmed.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng W, Linet MS, Shu XO, Pan RP, Gao YT, Fraumeni JF., Jr Prior medical conditions and the risk of adult leukemia in Shanghai, People’s Republic of China. Cancer Causes Control. 1993;4:361–8. doi: 10.1007/BF00051339. [DOI] [PubMed] [Google Scholar]
- 61.Doody MM, Linet MS, Glass AG, Friedman GD, Pottern LM, Boice JD, Jr, et al. Leukemia, lymphoma, and multiple myeloma following selected medical conditions. Cancer Causes Control. 1992;3:449–56. doi: 10.1007/BF00051358. [DOI] [PubMed] [Google Scholar]
- 62.Linet MS, Cartwright RA. Chronic lymphocytic leukemia: epidemiology and etiologic findings. Nouv Rev Fr Hematol. 1988;30:353–7. [PubMed] [Google Scholar]
- 63.Linet MS, McCaffrey LD, Humphrey RL, Brookmeyer R, Van Natta ML, Tielsch JM, et al. Chronic lymphocytic leukemia and acquired disorders affecting the immune system: a case-control study. J Natl Cancer Inst. 1986;77:371–8. [PubMed] [Google Scholar]
- 64.Rosenblatt KA, Koepsell TD, Daling JR, Lyon JL, Swanson GM, Greenberg RS, et al. Antigenic stimulation and the occurrence of chronic lymphocytic leukemia. Am J Epidemiol. 1991;134:22–8. doi: 10.1093/oxfordjournals.aje.a115989. [DOI] [PubMed] [Google Scholar]
- 65.Landgren O, Rapkin JS, Caporaso NE, Mellemkjaer L, Gridley G, Goldin LR, et al. Respiratory tract infections and subsequent risk of chronic lymphocytic leukemia. Blood. 2007;109:2198–201. doi: 10.1182/blood-2006-08-044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landgren O, Engels EA, Caporaso NE, Gridley G, Mellemkjaer L, Hemminki K, et al. Patterns of autoimmunity and subsequent chronic lymphocytic leukemia in Nordic countries. Blood. 2006;108:292–6. doi: 10.1182/blood-2005-11-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson LA, Gadalla S, Morton LM, Landgren O, Pfeiffer RM, Warren JL, et al. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer. 2009;125:398–405. doi: 10.1002/ijc.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Landgren O, Albitar M, Ma W, Abbasi F, Hayes RB, Ghia P, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360:659–67. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landgren O, Engels EA, Pfeiffer RM, Gridley G, Mellemkjaer L, Olsen JH, et al. Autoimmunity and susceptibility to Hodgkin lymphoma: a population-based case-control study in Scandinavia. J Natl Cancer Inst. 2006;98:1321–30. doi: 10.1093/jnci/djj361. [DOI] [PubMed] [Google Scholar]
- 70.Alexander DD, Mink PJ, Adami HO, Cole P, Mandel JS, Oken MM, et al. Multiple myeloma: a review of the epidemiologic literature. Int J Cancer. 2007;120 (Suppl 12):40–61. doi: 10.1002/ijc.22718. [DOI] [PubMed] [Google Scholar]
- 71.Landgren O, Rapkin JS, Mellemkjaer L, Gridley G, Goldin LR, Engels EA. Respiratory tract infections in the pathway to multiple myeloma: a population-based study in Scandinavia. Haematologica. 2006;91:1697–700. [PubMed] [Google Scholar]
- 72.Linet MS, Harlow SD, McLaughlin JK. A case-control study of multiple myeloma in whites: chronic antigenic stimulation, occupation, and drug use. Cancer Res. 1987;47:2978–81. [PubMed] [Google Scholar]
- 73.Gregersen H, Pedersen G, Svendsen N, Thulstrup AM, Sorensen HT, Schonheyder HC. Multiple myeloma following an episode of community-acquired pneumococcal bacteraemia or meningitis. Apmis. 2001;109:797–800. [PubMed] [Google Scholar]
- 74.Landgren O, Linet MS, McMaster ML, Gridley G, Hemminki K, Goldin LR. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: a population-based case-control study. Int J Cancer. 2006;118:3095–8. doi: 10.1002/ijc.21745. [DOI] [PubMed] [Google Scholar]
- 75.Thomas E, Brewster DH, Black RJ, Macfarlane GJ. Risk of malignancy among patients with rheumatic conditions. Int J Cancer. 2000;88:497–502. [PubMed] [Google Scholar]
- 76.Mellemkjaer L, Linet MS, Gridley G, Frisch M, Moller H, Olsen JH. Rheumatoid arthritis and cancer risk. Eur J Cancer. 1996;32A:1753–7. doi: 10.1016/0959-8049(96)00210-9. [DOI] [PubMed] [Google Scholar]
- 77.Gridley G, McLaughlin JK, Ekbom A, Klareskog L, Adami HO, Hacker DG, et al. Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst. 1993;85:307–11. doi: 10.1093/jnci/85.4.307. [DOI] [PubMed] [Google Scholar]
- 78.Mackay IR, Rose NR. Autoimmunity and lymphoma: tribulations of B cells. Nat Immunol. 2001;2:793–5. doi: 10.1038/ni0901-793. [DOI] [PubMed] [Google Scholar]
- 79.Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 80.Youinou P. Is BAFF the murderer in lupus? Lupus. 2008;17:613–4. doi: 10.1177/0961203308092164. [DOI] [PubMed] [Google Scholar]
- 81.Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Semin Immunol. 2006;18:305–17. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Mackay F, Tangye SG. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr Opin Pharmacol. 2004;4:347–54. doi: 10.1016/j.coph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 83.Hansen A, Lipsky PE, Dorner T. B-cell lymphoproliferation in chronic inflammatory rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:561–9. doi: 10.1038/ncprheum0620. [DOI] [PubMed] [Google Scholar]
- 84.Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:401–4. doi: 10.1158/1055-9965.EPI-06-1056. [DOI] [PubMed] [Google Scholar]
- 85.Engels EA, Chatterjee N, Cerhan JR, Davis S, Cozen W, Severson RK, et al. Hepatitis C virus infection and non-Hodgkin lymphoma: results of the NCI-SEER multi-center case-control study. Int J Cancer. 2004;10:76–80. doi: 10.1002/ijc.20021. [DOI] [PubMed] [Google Scholar]
- 86.Ekstrom K, Hjalgrim H, Brandt L, Baecklund E, Klareskog L, Ekbom A, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003;48:963–70. doi: 10.1002/art.10939. [DOI] [PubMed] [Google Scholar]
- 87.Kyle RA, Therneau TM, Rajkumar SV, Remstein ED, Offord JR, Larson DR, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759–64. doi: 10.1182/blood-2003-03-0801. [DOI] [PubMed] [Google Scholar]
- 88.Hakulinen T, Isomaki H, Knekt P. Rheumatoid arthritis and cancer studies based on linking nationwide registries in Finland. Am J Med. 1985;78:29–32. doi: 10.1016/0002-9343(85)90242-6. [DOI] [PubMed] [Google Scholar]
- 89.Mariette X, Cazals-Hatem D, Warszawki J, Liote F, Balandraud N, Sibilia J. Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3-year prospective study in France. Blood. 2002;99:3909–15. doi: 10.1182/blood.v99.11.3909. [DOI] [PubMed] [Google Scholar]
- 90.Toussirot E, Wendling D. The use of TNF-α blocking agents in rheumatoid arthritis: an update. Expert Opin Pharmacother. 2007;8:2089–107. doi: 10.1517/14656566.8.13.2089. [DOI] [PubMed] [Google Scholar]
- 91.Salloum E, Cooper DL, Howe G, Lacy J, Tallini G, Crouch J, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14:1943–9. doi: 10.1200/JCO.1996.14.6.1943. [DOI] [PubMed] [Google Scholar]
- 92.Usman AR, Yunus MB. Non-Hodgkin’s lymphoma in patients with rheumatoid arthritis treated with low dose methotrexate. J Rheumatol. 1996;23:1095–7. [PubMed] [Google Scholar]
- 93.Nalesnik MA, Jaffe R, Starzl TE, Demetris AJ, Porter K, Burnham JA, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173–92. [PMC free article] [PubMed] [Google Scholar]