In this study, the authors have investigated the anti-leukemic action of alloreactive single KIR positive natural killer cells on CD34+CD38− AML cells, showing that the HDAC inhibitor valproic acid augments this activity.
Keywords: acute leukemia, stem cells, natural killer, immunotherapy
Abstract
The concept of tumor immunosurveillance has raised prospects for natural killer cell-based immunotherapy of human cancer. The cure of acute myeloid leukemia may depend on eradication of leukemic stem cells, the self-renewing component of leukemia. Whether natural killer cells can recognize and lyse leukemic stem cells is not known. To develop strategies that effectively target acute myeloid leukemia-leukemic stem cells, we investigated anti-leukemic effects of human alloreactive single KIR+ natural killer cells. Natural killer effectors with KIR specificity mismatched with respect to HLA class I allotype of target cells effectively recognized acute myeloid leukemia-leukemic stem cells defined phenotypically as CD34+CD38−, while healthy bone marrow-derived CD34+CD38− hematopoietic stem cells were spared, as demonstrated by cytotoxicity and hematopoietic colony-forming assays. The HDAC inhibitor valproic acid increased the activating NKG2D ligand-dependent lysis of acute myeloid leukemia-CD34+CD38− leukemic stem cells. These results show that alloreactive natural killer cells have the potential to detect and target leukemic stem cells, and thus to improve the treatment outcome in acute myeloid leukemia.
Introduction
Leukemia-initiating cells, also termed leukemic stem cells (LSCs), are implicated in sustaining the malignancy and thus a poor treatment outcome.1 Relapse may occur because of the resistance of quiescent LSCs to cell cycle-dependent cytotoxic chemotherapies. Consequently, specific targeting of LSCs has emerged as a novel therapeutic goal.2 Natural killer (NK) cells are the innate immunity lymphocytes designated to recognize and kill malignant cells.3 This property has been clinically verified in acute myeloid leukemia (AML) by graft-versus-leukemia effect improving the outcome of recipients of stem cells from haploidentical donors.4,5 The alloreactivity of NK cells is based on the absence of inhibitory killer immunoglobulin-like receptors (KIRs) engagement with human leukocyte antigen (HLA) class I molecules, and is triggered by cognate recognition of cell surface ligands by activating NK cell receptors.6 NKG2D ligands (NKG2D-L) serve as tumor-specific antigens initiating NKG2D receptor-dependent activation of NK cells.7,8 While numerous studies have characterized the cytolytic potential of human NK cells against leukemic blasts,9–12 their ability to target LSCs has not been examined. Although the precise phenotypic identity of human LSCs remains elusive, AML-initiating LSCs were shown to reside within the CD34+CD38− population, the phenotype of which corresponds to a healthy bone marrow population containing the hematopoietic stem cells (HSCs).13–15 Here we demonstrate that AML-CD34+CD38− LSCs are efficiently recognized and destroyed by single KIR+ NK cells with predicted mismatch with respect to HLA class I specificity of the AML patient. This study provides arguments for exploiting immunotherapy with alloreactive NK cells to target LSCs.
Design and Methods
Patients and healthy controls
Peripheral blood (PB) from AML patients (n=8) and normal bone marrow (N-BM) or normal G-CSF mobilized PB (N-mPB) from healthy donors (n=8) were obtained with informed consent, in agreement with the guidelines of the Ethical Committee of the University Hospital Basel. Patients’ selection criteria were: primary untreated AML, high blast content (79%±16%) with predominantly CD34+ phenotype (76%±20%), and HLA class I allotype enabling a KIR mismatch (Online Supplementary Table S1).
Flow cytometry (FACS)
Fluorochrome-conjugated monoclonal antibodies (mAbs) against human CD45, CD34, CD38, and control IgG1 (BD Biosciences, San Jose, CA) were used. Unconjugated mAbs against ULBP1 (M295), ULBP2 (M311), ULBP3 (M551) (D Cosman, Amgen, WA, USA), and MICA/B (BD Biosciences), all at 10 μg/mL, were revealed with goat α-mouse IgG-FITC (Jackson ImmunoResearch, West Grove, PA): 100,000 events were acquired using a CyAn ADP Flow Cytometer (DAKO Cytomation, Glostrup, Denmark) and analyzed with FlowJo software (Tree Star, Standford, CA, USA).
Purification and culture of AML and N-BM cells
Mononuclear cells (MNCs) from AML PB and N-BM were prepared and CD34+CD38− and CD34+CD38+ sub-populations were purified by FACS-sorting (Cytopeia Influx and Spigot 6.1.4 software; Seattle, WA; Online Supplementary Figure S1). MNCs (1×106/mL) or purified cell populations (1–2×105/200 μL) were cultured for two days in serum-free X-vivo 10 medium (Lonza, Basel, Switzerland), 20% BIT9500 (Stem Cell Technologies, Vancouver, Canada) and growth factors.12 Valproic acid (VA) was at 1 mM (Orfiril; Desitin, Liestal, Switzerland).
Natural killer cell lines
Single KIR+ NK cell lines were obtained from PB CD56+CD3− NK cells by FACS-sorting of CD158a+, CD158b+, or CD158e+ cells and culture for 14–21 days in IL-2 containing medium.12,16 NK cell lines were 95–99% pure with respect to CD158a, b or e expression (Online Supplementary Figure 2S and Table S1).
Cellular cytotoxicity and colony-forming unit (CFU) assays
The cytotoxic activity of single KIR+ NK cells against K562 erythroleukemia, and FACS-sorted AML PB, N-BM and N-mPB cell subpopulations was tested by chromium-release assay with 2–5×103 targets/well at indicated effector to target (E:T) ratios.12 For blocking experiments, NK effectors were preincubated with anti-NKG2D mAb (M585; D. Cosman) or mouse IgG1k (BD Biosciences) at 10 mg/mL for one hour at 37°C. For CFU assays, FACS-sorted AML (1×105) and N-BM (1×103) cell subpopulations were seeded into 1% methylcellulose,17 either immediately or after 2-day culture in medium or with VA, and additional 4 h incubation without or with NK cells at E:T ratio of 5:1. Primary CFUs were counted after 14 days in an inverted microscope, harvested, and all cells were replated into secondary methylcellulose cultures.
Statistical analysis
Expression of NKG2D-L and cytolysis by NK cells were analyzed using Student’s t test.
Results and Discussion
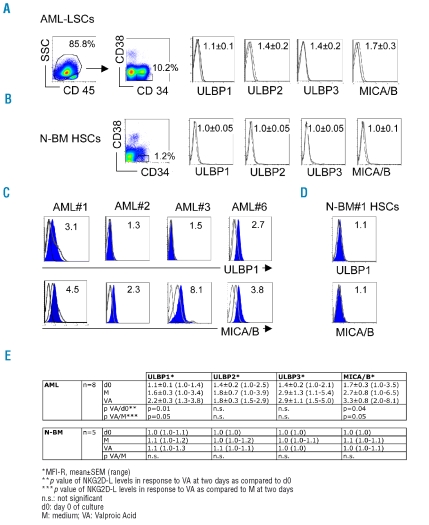
The AML-LSCs, defined phenotypically as the CD45dimCD34+CD38− population and analysed in de novo untreated AML, expressed low/undetectable levels of NKG2D-L, including UL-16 binding proteins (ULBP1-3) and MHC-related MICA/B molecules (Figure 1A). Ligands were also at background levels on N-BM CD34+CD38− HSCs (Figure 1B). The paucity of NKG2D-L on AML-LSCs supports earlier findings with leukemic myeloblasts.12 The absence of NKG2D receptor-dependent interactions is known to accelerate cancer progression,18 and conversely, tumors which up-regulate cell surface NKG2D-L in response to cellular stress, DNA damage or pharmacological treatment are rendered susceptible to killing by NK cells.19,20 We have recently demonstrated that NKG2D-L levels increased in response to histone deacetylase (HDAC)-inhibitor VA, a drug with anti-neoplastic activities, and this increase enhanced the cytolysis of AML blasts.12 Here, we observed a VA-dependent upregulation of NKG2D-L on CD34+CD38− LSCs (Figure 1C), whereas no response to VA was seen with N-BM HSCs (Figure 1D). The VA effect on AML-LSCs was modest, but apparent with ULBP1 and MICA/B, the expression of which increased 2.0±0.8 and 1.9±1.5 fold, respectively (p<0.05; Figure 1E).
Figure 1.
Expression of NKG2D-L by AML-LSCs and N-BM HSCs. (A) FACS analysis of AML-LSCs. Gating was performed sequentially on AML cells identified as CD45-dim and on the CD34+CD38− cells. (B) FACS analysis of N-BM HSCs gated on CD34+CD38− cells. Expression levels of ULBP1-3 and MICA/B were defined as the mean fluorescence intensity ratio (MFI-R) of values obtained with specific mAbs divided by values given by secondary or control mAbs; MFI-R±SEM for n=8 in A, and n=6 in B are given. NKG2D-L, black line; isotype control, grey line. (C) FACS analysis of the effect of VA on ULBP1 and MICA/B expression levels by AML-CD34+CD38− LSCs from 4 individual AML patients. (D) FACS analysis of the effect of VA on ULBP1 and MICA/B expression levels by N-BM CD34+CD38− HSCs from a representative donor. Cells were cultured for two days with VA, filled histograms; in medium alone, grey line; staining with isotype control, black line. MFI-R of ULBP1 and MICA/B levels after VA treatment are given. (E) Summary of ULBP1-3 and MICA/B expression levels by AML (n=8) and N-BM (n=6) CD34+CD38− cells before (d0) and after two days exposure to medium alone (M) or VA; *p<0.05, statistical significance of NKG2D-L expression in response to VA, as compared to d0, and to M.
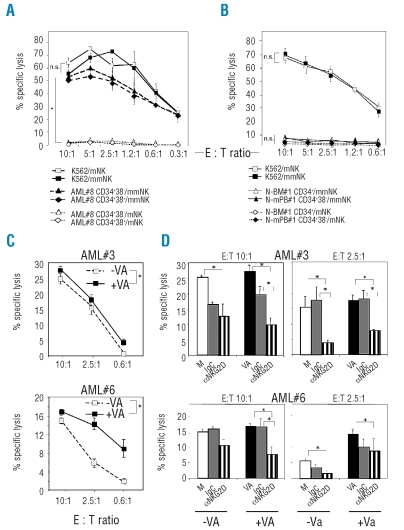
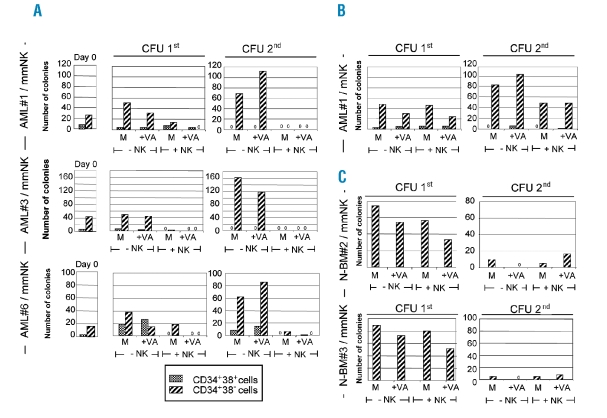
To test whether AML-LSCs are susceptible to NK cell-mediated lysis, NK effectors carrying single KIR specificities were selected according to patients’ HLA class I allotypes (Online Supplementary Table S1). HLA-mismatched, but not HLA-matched, single KIR+ NK cells were able to lyse purified AML-CD34+CD38− LSCs, with an efficiency comparable to killing of leukemic blasts, phenotypically defined as CD34+CD38+ (Figure 2A). Matched NK cells effectively lysed control HLA class I-negative K562 cells, confirming the requirement for HLA-KIR mismatch in LSC detection (Figure 2A). There were interindividual differences in susceptibility of purified AML-CD34+CD38− cells from individual patients (n=3, range 15–55% at E:T ratio of 10:1) but lysis was always seen using HLA-mismatched and not HLA-matched effectors, in accordance with our earlier studies on unfractionated leukemic blasts.12 Single KIR+ NK cells expressed also the inhibitory receptor NKG2A on 30–60% of cells, but NKG2A ligand HLA-E on AML targets was low or absent (MFI-R<10), in comparison with high levels of KIR ligands HLA-ABC (MFI-R 100–300). Unlike with AML targets, neither HLA-mismatched nor HLA-matched NK cells lysed purified N-BM CD34+ cells or N-mPB CD34+CD38− cells (Figure 2B), indicating that normal progenitors are protected, and underlining a specificity of alloreactive single KIR+ NK cells towards leukemic targets. Treatment of AML-CD34+CD38− LSCs with VA resulted in an increase in susceptibility to alloreactive single KIR+ NK cells which was consistently observed at 10:1 to 0.6:1 E:T ratios, but the extent of which varied dependent on AML targets (Figure 2C). This was likely linked to VA-mediated upregulation of cell-surface NKG2D-L (Figure 1C). The cytolysis of LSCs was indeed partly NKG2D-dependent, as it was specifically reduced in the presence of anti-NKG2D mAbs, the blocking effect of which was particularly pronounced with VA-treated AML cells (Figure 2D). To define the effect of NK cells on the colony-forming properties of LSCs,21 serial replating CFU assays were performed and colony numbers generated from purified AML and N-BM subpopulations in response to VA and single-KIR+ NK cells were monitored (Figure 3A–C). AML-CD34+CD38− LSCs displayed higher clonogenicity than AML-CD34+CD38+ blasts when plated directly (day 0) or after 2-day incubation with VA in primary (1st) CFU, and efficiently gave rise to colonies in secondary (2nd) CFU assays (Figure 3A) due to aberrant self-renewal. With all 3 tested AML patients’ LSCs, exposure to HLA class I-KIR mismatched NK cells strongly reduced the capacity to form 1st and 2nd CFU. Preincubation of AML-LSCs with VA potentiated this effect, since 1st CFUs were fully eradicated (Figure 3A). Matched NK cells did not affect 1st and 2nd CFU numbers generated from AML-LSCs (Figure 3B), in agreement with absent killing in cytotoxicity assays (Figure 2A). To test whether NK cells can discriminate between normal and leukemic CFUs, the N-BM CD34+CD38− HSCs were used; in contrast to AML, HSC-derived colonies do not support serial replatings (Figure 3C). The 1st CFU numbers from N-BM HSCs were unaffected by exposure to single-KIR+ mismatched NK cells, and were preserved after VA treatment,22 indicating that allorecognition by single KIR+ NK cells is specific towards malignant colony-forming LSCs.
Figure 2.
(left). Cytotoxicity of NK cells against AML-LSCs and N-HSCs. (A-D) Single-KIR+ NK cells were used as effectors in chromium-release assays. (A) AML-CD34+CD38− LSCs and CD34+CD38+ blasts from patient AML#8 or control K562 cells were exposed to NK cells, matched (mNK, KIRe+ NK-1) or mismatched (mmNK, KIRa+ NK-1), at the indicated E:T ratios. (B) N-BM CD34+ and N-mPB CD34+CD38− HSCs from 2 healthy donors, or control K562 cells were exposed to NK cells, mNK (KIRe+ NK-2 for N-BM; KIRa+ NK-3 for N-mPB#1) or mmNK (KIRa+ NK-2 for N-BM; KIRb+ NK-3 for N-mPB), at the indicated E:T ratios. Availability of N-BM samples was limited and this did not allow selection for CD34+CD38− cells in numbers sufficient for the cytotoxicity assay. (C) AML-CD34+CD38− LSCs from patients AML#3 and AML#6, either non-treated (−VA) or incubated for two days with VA (+VA) were exposed to mmNK cells (KIRe+ NK-1) at the indicated E:T ratios. (D) AML-CD34+D38− LSCs from patients AML#3 and AML#6 were incubated for two days with medium alone (M; ○), or with VA (▪) and exposed to mmNK cells (as in C) which were blocked by preincubation with control mouse IgG ( ) or aNKG2D mAb (▥) for 1 h at 37°C, at 10:1 E:T ratio. All experiments were performed in triplicates; mean±SEM values are shown. *p<0.05. The NK cell lines are defined in Online Supplementary Table S1.
) or aNKG2D mAb (▥) for 1 h at 37°C, at 10:1 E:T ratio. All experiments were performed in triplicates; mean±SEM values are shown. *p<0.05. The NK cell lines are defined in Online Supplementary Table S1.
Figure 3.
Effect of NK cells on hematopoietic colony formation by AML-LSCs and N-BM HSCs. (A–C) Single KIR+ NK cells were used as effectors and their effect on growth of CFU from AML-LSCs and N-BM HSCs was tested in 1% methylcellulose cultures containing erythropoietin (3U/mL), interleukin (IL)-3 and -6, granulocyte- and granulocyte-macrophage-colony stimulating factor (20 ng/mL, each), stem cell factor and flt3 ligand (100 ng/mL, each). AML-CD34+CD38− LSCs (1×105) and CD34+CD38+ blasts (1×105) from 3 patients (AML#1, AML#3 and AML#6), or N-BM CD34+CD38− HSCs (1×103) from 2 healthy donors (N-BM#2 and #3) were incubated for two days with medium alone (M) or VA (+VA) and plated in methylcellulose directly (−NK) or after exposure to NK cells (+NK), either matched (mNK) or mismatched (mmNK), for 4 h at E:T ratio of 5:1. (A) CFU numbers by AML cells prior to incubation (Day 0). A–C Numbers of CFU, primary 1st or secondary 2nd. (A) mmNK were KIRe+ NK-1. (B) mNK were KIRa+ NK-1. In C, mmNK were KIRa+ NK-1. The NK cell lines are defined in Online Supplementary Table S1. Results are shown as mean of duplicate analyses. Cultures in which no CFU-derived colonies were seen are indicated with “0”.
In this first report addressing the susceptibility of AML-LSCs to NK cells, we demonstrate that selection of NK effectors with a predicted KIR-HLA class I mismatch is prerequisite for targeting AML-LSCs. Allorecognition can be increased by VA which up-regulates NKG2D-L, thus priming the AML for the cytotoxic effectors and underlying the importance of interventions which enhance the NKG2D axis for tumor recognition. VA-induced epigenetic modifications may also promote the entry of LSCs into the cell cycle rendering them more accessible to chemo- and immunotherapies.23 Importantly, we show that healthy CD34+CD38− cells containing the HSCs are not targeted by HLA-mismatched NK cells and do not respond to VA, indicating that normal hematopoietic functions will be spared and arguing for the specificity in eradication of malignant CD34+CD38− cells containing the LSCs. Our data reinforce the concept of alloreactive NK cell-based adoptive immunotherapy,24 in combination with antineoplastic drugs to enhance the tumor reactivity, as a rational strategy towards curing leukemia.
Acknowledgments
we thank Amgen for aULBP1,2,3 and aNKG2D mAbs, and V Jäggin and E Traunecker for cell sorting.
Footnotes
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
UL and AW-F were the principal investigators and take primary responsability for the paper. UL, US, SJ, and SD performed research, analyzed data, and edited the paper. AG provided clinical data. AW-F and CPK designed research, analyzed data, wrote and edited the paper.
The authors reported no potential conflicts of interest.
Funding: this work was supported by grants from the Swiss National Science Foundation 3100-110511, Oncosuisse 01664-02-05 and 02175-02-2008, Freie Akademische Gesellschaft, and Stiftung für Hämatologische Forschung.
References
- 1.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 2.Krause DS, Van Etten RA. Right on target: eradicating leukemic stem cells. Trends Mol Med. 2007;13:470–81. doi: 10.1016/j.molmed.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 4.Velardi A, Ruggeri L, Moretta A, Moretta L. NK cells: a lesson from mismatched hematopoietic transplantation. Trends Immunol. 2002;23:438–44. doi: 10.1016/s1471-4906(02)02284-6. [DOI] [PubMed] [Google Scholar]
- 5.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–40. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parham P, McQueen KL. Alloreactive killer cells: hindrance and help for haematopoietic transplants. Nat Rev Immunol. 2003;3:108–22. doi: 10.1038/nri999. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL. On guard--activating NK cell receptors. Nat Immunol. 2001;2:23–7. doi: 10.1038/83130. [DOI] [PubMed] [Google Scholar]
- 8.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 9.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–96. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 10.Nowbakht P, Ionescu MC, Rohner A, Kalberer CP, Rossy E, Mori L, et al. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105:3615–22. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 11.Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–73. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 12.Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008;111:1428–36. doi: 10.1182/blood-2007-07-101311. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 14.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–43. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–21. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 16.Siegler U, Kalberer CP, Nowbakht P, Sendelov S, Meyer-Monard S, Wodnar-Filipowicz A. Activated natural killer cells from patients with acute myeloid leukemia are cytotoxic against autologous leukemic blasts in NOD/SCID mice. Leukemia. 2005;19:2215–22. doi: 10.1038/sj.leu.2403985. [DOI] [PubMed] [Google Scholar]
- 17.Bridenbaugh S, Kenins L, Bouliong-Pillai E, Kalberer CP, Shklovskaya E, Gratwohl A, et al. Clinical stem-cell sources contain CD8+CD3+ T-cell receptor-negative cells that facilitate bone marrow repopulation with hematopoietic stem cells. Blood. 2008;111:1735–8. doi: 10.1182/blood-2007-02-076000. [DOI] [PubMed] [Google Scholar]
- 18.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasser S, Raulet DH. The DNA damage response arouses the immune system. Cancer Res. 2006;66:3959–62. doi: 10.1158/0008-5472.CAN-05-4603. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Larrea C, Suarez-Alvarez B, Lopez-Soto A, Lopez-Vazquez A, Gonzalez S. The NKG2D receptor: sensing stressed cells. Trends Mol Med. 2008;14:179–89. doi: 10.1016/j.molmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–68. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Bug G, Gul H, Schwarz K, Pfeifer H, Kampfmann M, Zheng X, et al. Valproic acid stimulates proliferation and self-renewal of hematopoietic stem cells. Cancer Res. 2005;65:2537–41. doi: 10.1158/0008-5472.CAN-04-3011. [DOI] [PubMed] [Google Scholar]
- 23.De Felice L, Tatarelli C, Mascolo MG, Gregorj C, Agostini F, Fiorini R, et al. Histone deacetylase inhibitor valproic acid enhances the cytokine-induced expansion of human hematopoietic stem cells. Cancer Res. 2005;65:1505–13. doi: 10.1158/0008-5472.CAN-04-3063. [DOI] [PubMed] [Google Scholar]
- 24.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–39. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]