The recognition of cyclin D1-negative mantle cell lymphoma is challenging. In this study the authors show that the quantification of cyclin D2 mRNA levels but not its immunohistochemical detection may be a useful tool to distinguish cyclin D1-negative mantle cell lymphoma with cyclin D2 translocations from other small B-cell lymphomas. See related perspective article on page 1488.
Keywords: cyclin D2+, mantle cell lymphoma (MCL), FISH, QRT-PCR
Abstract
Mantle cell lymphoma is characterized by the t(11;14) chromosomal translocation, resulting in the overexpression of cyclin D1 (CycD1). Recently, cases of mantle cell lymphoma negative for cycD1 but positive for cycD2 or cycD3 were identified by gene expression profiling and confirmed by immunohistochemistry. We analyzed 4 cases of cycD2+ mantle cell lymphoma with a translocation involving the CCND2 locus, and its differential diagnosis from 35 mature B-cell non-Hodgkin’s lymphomas based on immunohistochemistry, quantitative RT-PCR and FISH analysis. Bona fide cycD2+ mantle cell lymphoma carried translocations involving the CCND2 gene, and IGH and IGK loci were identified as partners. As a result of this translocation, cycD2 mRNA was highly over-expressed when compared with normal lymphoid tissue and other B-cell non-Hodgkin’s lymphomas, including chronic lymphocytic leukemia, making this technique ideally suited to identify cycD2+mantle cell lymphoma. In contrast, positive immunostaining for cycD2 was found in most B-cell non-Hodgkin’s lymphomas, and therefore, it is not specific for a diagnosis of cycD2+mantle cell lymphoma.
Introduction
Mantle cell lymphoma (MCL) is a distinct subtype of aggressive B-cell non-Hodgkin’s lymphoma (NHL) with specific clinical and pathological features that accounts for approximately 6% of all lymphomas.1 The genetic hallmark of mantle cell lymphoma (MCL) is the t(11;14)(q13;q32) chromosomal translocation that juxtaposes the immunoglobulin heavy chain (IGH) gene on 14q32 to the CCND1 gene on 11q13 resulting in the overexpression of cyclin D1 (cycD1) mRNA and protein.1 Recently, a gene expression profiling study of MCL identified a small subset of tumors negative for cycD1 mRNA expression but morphologically, immunophenotypically, and by global expression profile otherwise undistinguishable from conventional MCL.2 Interestingly, these cases instead expressed cycD2 or cycD3 mRNA, suggesting that any of these cyclins can functionally substitute for cycD1 in MCL. Accordingly, cycD1 negative MCL cases lacked the t(11;14) translocation by fluorescence in situ hybridization (FISH) analysis,2 and were negative for cycD1 protein expression by immunostains.3 However, no evidence of chromosomal translocations involving the corresponding CCND2 and CCND3 gene loci were identified.3 The controversy surrounding cycD1 negative MCL was ended with the demonstration of bona fide cases of cycD2 positive MCL secondary to gene translocations involving the CCND2 locus on chromosome 12p13 with either the IGK locus on chromosome 2p12 t(2;12)(p12;p13),4,5 or a t(12;14)(p13;q32) translocation juxtaposing the CCND2 gene next to the IGH locus.6
The diagnosis of cycD1 negative MCL is challenging because some low-grade B-cell lymphomas, such as chronic lymphocytic leukemia (CLL), marginal zone lymphoma (MZL) and follicular lymphoma (FL), may mimic MCL both morphologically and immunophenotypically. Indeed, the differential diagnosis is important and relevant for patient treatment and prognosis. Until now, the recognition of potential cycD1 negative MCL has been based on microarray analysis,2,3 a technique which is not available in routine practice. Although IHC for cycD2 and cycD3 has been proposed as a surrogate marker for cycD1 negative MCL,3 the need to develop a reliable and accessible technique which is useful in the differential diagnosis is of utmost importance. The aim of this study was to investigate means to differentiate 4 cases of cycD2+ MCL with a CCND2 translocation from low-grade B-cell NHL, based on IHC, quantitative RT-PCR and FISH analysis with special interest on CD5+ B-cell NHL, including CLL and a subset of MZL.
Design and Methods
Tissue samples
Formalin-fixed and paraffin-embedded biopsies from 35 well-characterized B-cell lymphomas, including 12 CLL, 8 MZL (5 cases CD5+), 5 FL and 10 cycD1+ MCL were selected from the files of the Institute of Pathology, Technical University of Munich, Germany. All cases were classified according to the guidelines of the World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues.7 Four cases of cycD2+ MCL with a CCND2 translocation were collected from the University Hospital Schleswig-Holstein Campus Kiel, Germany, CHU Sart Tilman, Liege, Belgium, Cleveland Clinic, USA, and Technical University of Munich, Germany. Two of these cases have been the subject of previous publications.4,6 As controls, 9 cases of normal lymph nodes were used.
Immunohistochemistry
All cases were previously studied by paraffin section immunohistochemistry (IHC) to assess lymphoid immunophenotype. The expression of cyclin D1 (SP4 clone, LabVision Corporation) and cyclin D2 (rabbit polyclonal, Cell Signaling Technology) was investigated in paraffin-embedded sections. IHC was performed on an automated immunostainer (Ventana Medical Systems, Inc., Tuczon, AZ, USA) according to the company’s protocol.8
Real-time quantitative RT-PCR
Real-time quantitative RT-PCR analysis was performed using the ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). For the quantification of cycD2 we used the following sequences: 5′-CGCAAGCATGCTCAGACCTT-3′, 5′-TGCGATCATCGACGGTGG-3′, 5′-FAM-TGCCACC-GACTTTAAGTTTGCCATGT-TAMRA-3′. The sequences of cycD1, cycD3 and TBP (TATA box-binding protein), as housekeeping gene have already been described.9,10 The assay and analysis were performed as previously described.11
FISH analysis
Locus-specific interphase FISH was performed on paraffin-embedded tissue sections according to the manufacturer’s instructions (Abbott/Vysis) with minor modifications. The t(11;14) was investigated using commercially available probes (LSI IGH/CCND1; Vysis, Downers Grove, IL) in all MCL and CD5+MZL. Translocations affecting the CCND2 (12p13) and IGK (2p12) loci were investigated using recently described probes.3
Results and Discussion
The 4 cases of cycD1 negative MCL showed clinical, morphological and phenotypic characteristics of MCL. Cases 1 and 2 are 2 male patients aged 71 and 54 years, who presented with stage IV disease. These cases have been previously reported.4,6 Cases 3 and 4 are 2 novel cases that corresponded to an 82-year old female with involvement of the Waldeyer’s ring and cervical lymph nodes (Case 3, Figure 1A–C) and to a 59-year old male with stage IV disease. The lymph nodes in the 4 cases showed a nodular and diffuse growth pattern with a CD20+, CD5+, CD10−, CD23− (4/4), and p27- (3/3) phenotype, but lack of cycD1 expression. Instead, cycD2 was positive. Interphase FISH demonstrated an IGK-CCND2 fusion indicating the presence of a t(2;12)(p12;p13) translocation in Cases 1 and 3. A cytogenetically cryptic translocation t(12;14)(p13;q32) involving the IGH locus in chromosome 14q32 and leading to IGH-CCND2 juxtaposition was present in Case 2.6 In Case 4, interphase FISH demonstrated a clear CCND2 break with normal IGK and IGH, indicating the probability of a novel translocation partner, in addition to the already described translocations with IGK and IGH. Unfortunately, hybridization with an IGL probe failed repeatedly. Immunohistochemical analysis was performed in 35 cases of small B-cell lymphomas for cycD1 and cycD2 proteins. Due to the difficulties in the differential diagnosis of CD5+ small B-cell lymphomas, MZL expressing CD5 were preferentially included in the study. CycD1, as expected, was positive only in the 10 MCL cases, all of which had an IGH-CCND1 juxtaposition indicating t(11;14). In contrast, cycD2 was positive in all normal lymph nodes and lymphomas analyzed. This finding is not completely unexpected since cycD2 is the main cyclin expressed in normal B cells. The percentage of positive cells and intensity of positivity varied from case to case; however, CLL cases showed the strongest reactivity among the lymphomas analyzed (Figure 1D–F). This result clearly indicates that immunohistochemical detection of cycD2 is not helpful in the differential diagnosis of cycD1 negative MCL. On the contrary, reliance on cycD2 IHC may well lead to the overdiagnosis of cycD1 negative MCL in phenotypically and morphologically difficult cases, such as CLL and CD5+ MZL. FISH analysis for the IGH-CCND1 fusion indicating t(11;14) and for chromosomal translocations affecting the CCND2 locus in 12p13 was negative in all the CD5+MZL analyzed.
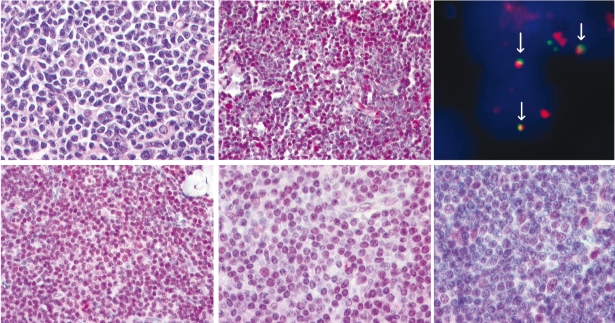
Figure 1.
Histological, immunohistological and FISH analysis in a novel case of cyclin D2 positive MCL with IGK-CCND2 fusion. (A–C) Hematoxylin and eosin-stained lymph node biopsy showing a neoplastic infiltrate composed of small cells with irregular nuclei and scant cytoplasm intermingled with scattered histiocytes (A). Cyclin D2 nuclear expression in the tumor cells detected by a specific polyclonal anti-cyclin D2 antibody (B). Interphase FISH analysis using double-color, double-fusion probes spanning the IGK (green) and CCND2 (red) loci show one fusion (white arrow) signal, indicating IGK-CCND2 juxtaposition, in addition to isolated red and green signals indicating intact CCND2 and IGK loci (C). Cyclin D2 immunohistological analysis in small B-cell NHL (D–F). Cyclin D2 strong nuclear expression in the vast majority of cells in a case of CLL (D). Cyclin D2 moderate nuclear expression in the majority of cells in a case of CD5+MZL (E). CLL case with cyclin D2 nuclear expression in a percentage of tumor cells. Note how some paraimmunoblasts reveal stronger cyclin D2 expression (F). Images were recorded using a Hitachi camera HW/C20 installed in a Zeiss Axioplan microscope with Intellicam software. FISH images were acquired with a 63x/1.40 oil-immersion objective in a Zeiss Axioskop2 fluorescence microscope (Zeiss, Göttingen, Germany) equipped with the appropriate filter sets (AHF, Tübingen, Germany). Image processing was carried out with Zeiss computer software (AIM 3.2). Maximum projection mode was used to produce extended focus image stacks.
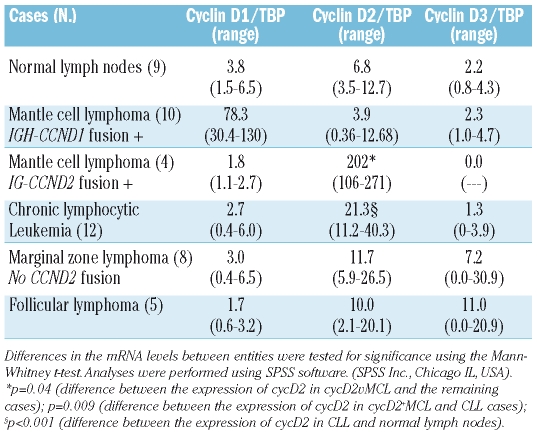
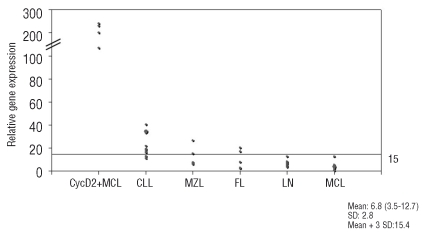
Since the identification of cycD1 negative MCL was based primarily on gene expression profile,2 it seemed logical to consider that quantitative analysis of cycD mRNA levels could be the appropriate method to diagnose cases of cycD1 negative MCL. Therefore, we investigated the levels of the three D-type cyclins in normal lymph nodes and the selected B-NHL cases. The findings are summarized in Table 1. Normal lymph nodes showed, in every case, preferential expression of cycD2 (cycD2/TBP ratio=6.8) with lower expression levels of cycD1 and cycD3. Interestingly, MCL cases with t(11;14) translocation have very low expression of cycD2mRNA. Accordingly, we recently reported that in MCL the low levels of cycD2 are the consequence of downregulation through the abnormally high levels of cyclin D1.12 In general, CycD2 mRNA levels were slightly increased in MZL and FL, moderately increased in CLL and strikingly increased in cycD2+MCL (Figure 2). Although some CLL cases have up to 6 times the amount of cycD2 mRNA found in lymph nodes (mean cycD2/TBP 21 vs. 7, p<0.001), the levels of expression were far below the cycD2 mRNA levels found in cycD2+MCL with a translocation involving the CCND2 locus (cycD2/TBP 21 vs. 202, p=0.004). These results indicate that quantitative RT-PCR and/or FISH are ideal methods to confirm the diagnosis of cycD2+ MCL. Accordingly, our previous studies concerning cycD1 mRNA expression in MCL10 and multiple myeloma,13,14 showed that very high levels of cycD1 mRNA were always associated with translocation involving the CCND1 locus. Importantly, the cases reported of cycD2+MCL without translocation need to be analyzed carefully concerning the amount of cycD2 mRNA expression, and other possible mechanisms of cycD2 deregulation. It is of note that cycD2+ MCL are extremely rare, and in order to avoid overdiagnosis it is mandatory to perform either quantitative analysis of cycD2 mRNA and/or FISH.
Table 1.
D-type cyclins mRNA levels in normal lymphoid tissue and in B-cell NHL.
Figure 2.
Cyclin D2 mRNA expression in mature B-cell NHL. Quantitatitve RT-PCR analysis of cyclin D2 was performed relative to the TBP housekeeping gene and results are depicted as ratio of cyclin D2/TBP transcript numbers. The horizontal line indicates the cut-off value for altered cyclin D2 expression (cycD2/TBP ratio=15.4), which corresponds to the mean value of cyclin D2 in normal lymph nodes (cycD2/TBP ratio=6.8) plus three standard deviations (SD:2.8). The difference in cyclin D2 expression between cyclin D2+ MCL and the remaining cases is statistically significant (p=0.04).
Acknowledgments
the authors would like to thank Karin Bink, Ulrike Bucholz, Jaqueline Müller and Claudia Kloss for their excellent technical assistance.
Footnotes
Authorship and Disclosures
LQ-M was the principal investigator and takes primary responsibility for the paper; LQ-M and FF designed research, coordinated the research, analyzed and interpreted data and drafted the manuscript; JS-H, IK, MK, and SG performed the laboratory work for this study; LdL, EH, RS, and WK, contributed with case material and discussed data.
The authors have no conflict of interests to declare.
Funding: this work was supported in part by a grant from the Mantle Cell Consortium of the Leukemia Research Foundation to LQM, and by the European Union in the “European MCL Lymphoma Network” to RS and WK. LdL is a senior research associate of the Belgian National Fund for Scientific Research.
References
- 1.Jares P, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–62. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 2.Rosenwald A, Wiestner A, Chan WC, Connors JM, Campo E, Gascoyne RD, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–97. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 3.Fu K, Greiner TC, Dave S, Wright G, Rosenwald A, Chiorazzi M, et al. Lymphoma/Leukemia Molecular Profiling Project. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106:4315–21. doi: 10.1182/blood-2005-04-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gesk S, Martín-Subero JI, Nagel I, Harder L, Fu K, Bernd HW, et al. A chromosomal translocation in cyclin D1-negative/cyclin D2-positive mantle cell lymphoma fuses the CCND2 gene to the IGK locus. Blood. 2006;108:1109–10. doi: 10.1182/blood-2006-01-0015. [DOI] [PubMed] [Google Scholar]
- 5.Wlodarska I, Vanhentenrijk V, Van Roosbroeck K, Pospísilová H, Minnei F, Verhoef G, et al. Translocations targeting CCND2, CCND3, and MYCN do occur in t(11;14)-negative mantle cell lymphomas. Blood. 2008;12:5683–90. doi: 10.1182/blood-2007-10-118794. [DOI] [PubMed] [Google Scholar]
- 6.Herens C, Lambert F, Quintanilla-Martinez L, Bisig B, Deusings C, de Leval L. Cyclin D1-negative mantle cell lymphoma with cryptic t(12;14) (p13;q32) and cyclin D2 overexpression. Blood. 2008;111:1745–6. doi: 10.1182/blood-2007-10-120824. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 8.Quintanilla-Martinez L, Davies-Hill T, Fend F, Calzada-Wack J, Sorbara L, Campo E, et al. Sequestration of p27Kip1 protein by cyclin D1 in typical and blastic variants of mantle cell lymphoma (MCL): implications for pathogenesis. Blood. 2003;101:3181–7. doi: 10.1182/blood-2002-01-0263. [DOI] [PubMed] [Google Scholar]
- 9.Koch I, Slotta-Huspenina J, Hollweck R, Anastasov N, Hofler H, Quintanilla-Martinez L, Fend F. Real-time quantitative RT-PCR shows variable, assay-dependent sensitivity to formalin fixation: implications for direct comparison of transcript levels in paraffin-embedded tissues. Diagn Mol Pathol. 2006;15:149–56. doi: 10.1097/01.pdm.0000213450.99655.54. [DOI] [PubMed] [Google Scholar]
- 10.Specht K, Kremer M, Müller U, Dirnhofer S, Rosemann M, Höfler H, et al. Identification of cyclin D1 mRNA overexpression in B-cell neoplasias by real-time reverse transcription-PCR of microdissected paraffin sections. Clin Cancer Res. 2002;8:2902–11. [PubMed] [Google Scholar]
- 11.Quintanilla-Martinez L, Miething C, Klier M, Rudelius M, Davies-Hill T, Anastasov N, et al. NPM-ALK-dependent expression of the transcription factor CCAAT/enhancer binding protein β in ALK-positive anaplastic large cell lymphoma. Blood. 2006;108:2029–36. doi: 10.1182/blood-2005-10-014258. [DOI] [PubMed] [Google Scholar]
- 12.Klier M, Anastasov N, Hermann A, Meindl T, Angermeier D, Raffeld M, et al. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 2008;11:2097–105. doi: 10.1038/leu.2008.213. [DOI] [PubMed] [Google Scholar]
- 13.Slotta-Huspenina J, Koch I, Richter M, Bink K, Kremer M, Specht K, et al. Cyclin D1 positive multiple myeloma: predominance of the short, 3′UTR-deficient transcript is associated with high cyclin D1 mRNA levels in cases with t(11;14) translocation, but does not correlate with proliferation rate or genomic deletions. Leuk Res. 2008;32:79–88. doi: 10.1016/j.leukres.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Specht K, Haralambieva E, Bink K, Kremer M, Mandl-Weber S, Koch I, et al. Different mechanisms of cyclin D1 overexpression in multiple myeloma revealed by fluorescence in situ hybridization and quantitative analysis of mRNA levels. Blood. 2004;104:1120–6. doi: 10.1182/blood-2003-11-3837. [DOI] [PubMed] [Google Scholar]