Idiopathic thrombocytopenic purpura is no longer seen as a simple antibody mediated disorder and the role of T cells and T-regulatory cytokines such as IFN-Á and IL-18 are now known to play an important role. This information should help better understanding of why some treatments are effective and in this study a down regulation of IL18 is reported in response to high dose dexamethasone.
Keywords: interleukin-18, interleukin-18 binding protein, high-dose dexamethasone, idiopathic thrombocytopenic purpura
Abstract
To evaluate the effects of high-dose dexamethasone (HD-DXM) on the balance of interleukin-18 (IL-18) and its endogenous antagonist IL-18 binding protein (IL-18BP) in ITP patients, IL-18, IL-18BP as well as IFN-γ, IL-4 plasma levels and platelet counts were determined in 17 ITP patients receiving DXM 40 mg/day for four consecutive days and in 24 healthy subjects. Using RT-PCR, the mRNA expression of IL-18, IL-18BP, IFN-γ, IL-4, T-box (T-bet) and GATA-binding protein 3(GATA-3) were studied in all subjects. The in vitro effects of DXM on IL-18BP and IL-18 of peripheral blood mononuclear cells (PBMCs) were studied by ELISA. HD-DXM administration increased IL-18BP and reduced IL-18 expression significantly (p<0.05), which resulted in a downregulation of IL-18/IL-18BP ratio p<0.05). In vitro, DXM had a significant effect on secretion of IL-18BP while diminishing IL-18 release from cultures of PBMCs. These results suggest that downregulation of IL-18/IL-18BP might account for its clinical efficacy of HD-DXM in active ITP.
Introduction
Interleukin-18 (IL-18), a member of IL-1 cytokine family, is a potent interferon-γ (IFN-γ) inducing factor and is synthesized by Kupffer cells in the liver, pancreas, kidney, skeletal muscle, lung, osteoblasts, and keratinocytes.1–3 IL-18 binding protein (IL-18BP) is a constitutively secreted protein able to bind IL-18 with high affinity, providing a potential mechanism whereby IL-18 activity is regulated.4
Idiopathic thrombocytopenic purpura (ITP) is an autoimmune disorder, characterized by early platelet destruction induced by autoantibodies directed against specific glycoproteins of platelet surface.5,6 It has become evident that an increased Th1/Th2 ratio in the peripheral blood has been proposed to correlate with disease activity in ITP.7–9 The expression of IFN-γ, a typical Th1 cytokine, is significantly higher in active ITP patients. Our previous study confirmed that the plasma and mRNA levels of both IFN-γ and IL-18 in active ITP patients were increased significantly compared with the normal controls. And the plasma concentrations of IL-18 correlated with IFN-α. We also observed IL-18BP was not significantly elevated in ITP patients, which resulted in an elevated ratio of IL-18/IL-18BP in patients with active disease. The balance of IL-18/IL-18BP plays a role in progression of ITP.10
Glucocorticoids (GC) have been widely recognized as the most appropriate first-line treatment for ITP, 11 even if the best therapeutic approach is still a matter of debate. Recently, high-dose dexamethasone (HD-DXM) in 4-day cycles has proven its clinical efficacy in the treatment of ITP.12,13 However, the effects of HD-DXM on the balance of IL-18/IL-18BP remains unclear. In the present study, plasma levels as well as mRNA expression of IL-18, IL-18BP in peripheral blood mononuclear cells (PBMCs) and in vitro PBMC cultivation were measured to investigate possible effects of HD-DXM on IL-18 activity in ITP patients.
Design and Methods
Clinical study
Seventeen patients with newly diagnosed ITP (13 females and 4 males, age range 16–58 years, median 38 years) were assigned to receive DXM 40 mg/day for four consecutive days. All of the cases met the diagnosis criteria of ITP as previously described.14 None of them had been treated with GC prior to first sampling. Peripheral blood samples were obtained from all patients prior to and two weeks after HD-DXM therapy (Table 1). A control group consisted of 24 healthy adult volunteers (18 females and 6 males, age range 18–65 years, median 40 years). The study was approved by the Medical Ethical Committee of Qilu Hospital, Shandong University, and informed consent was obtained from all patients.
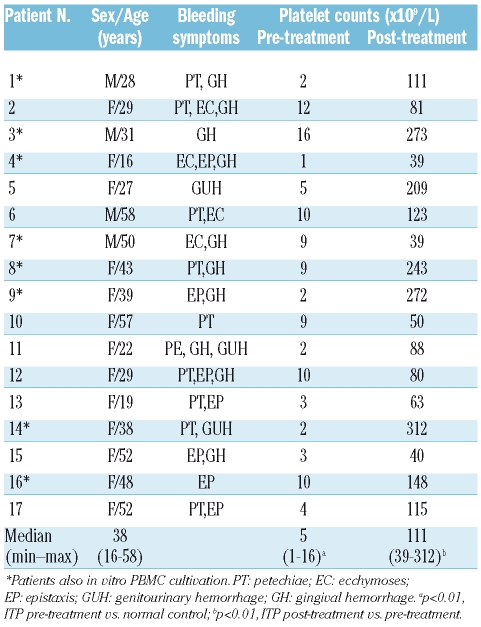
Table 1.
Clinical characteristics and initial responses of ITP patients.
Plasma and PBMC preparation
Plasma obtained from all subjects by centrifugation of heparinized peripheral blood samples were stored at −80°C until determination of cytokines. Mononuclear cells were isolated from heparinized blood by gradient centrifugation on Ficoll-Paque (Pharmacia Diagnostics, Uppsala, Sweden), and were preserved at −80°C in aliquots for examination, and thawed only once to avoid degradation.
PBMC isolated from 8 ITP patients (Table 1) and 11 control subjects (8 women and 3 men) were adjusted to 1×106/mL in RPMI-1640 culture medium (Invitrogen, America), cultured at a density of 5×105 cells/well in a 48-well culture plate and incubated in humidified air in 5% CO2 at 37°C. The cells were treated with 10 μg/mL phytohemagglutinin (PHA) (Sigma, America) and DXM (0 nmol/L, 10 nmol/L, 25 nmol/L, 50 nmol/L, 100 nmol/L, 200 nmol/L) and cell supernatants were collected at 48 h for quantifying the secreted protein of IL-18 and IL-18BP using the ELISA method.
IL-18, IL-18BP, IFN-γ and IL-4 enzyme-linked immunosorbent assay (ELISA)
Cytokine levels in the plasma and culture supernatants were measured using ELISA kits for IL-18 (Uscnlife, Missouri, TX, USA), IL-18BP (R&D Systems, Minneapolis, MN, USA), IFN-γ and IL-4 (Jingmei, Beijing, China) following the protocols recommended by the manufacturers.
Quantitative real time polymerase chain reaction analysis
Total RNA was isolated by Trizol (Invitrogen, America) according to the manufacturer’s instructions. The amount of RNA was determined using the Eppendorf Biophotometer (Brinkmann Instruments, Westbury, NY, USA) and normalized to 1 μg/mL for each subsequent real time quantitative polymerase chain reaction (RT-PCR) process on an ABI PRISM®7500 Sequence Detection System (Applied Biosystems Foster City CA USA) by using SYBRw Green (Toyobo, Osaka, Japan) as a double-strand DNA-specific binding dye. The primers and annealing temperatures used for the amplification were as described before.10 ABI Sequence Detection System software version 1.0 (PE Applied Biosystems, Warrington, UK) was used to determine the cycle number at which fluorescence emission crossed the automatically determined Ct value. All experiments were conducted in triplicate.
Statistical analysis
Data were expressed as mean ± SD. Statistical significance was determined by one-way ANOVA using SPSS Windows version 13.0. The numerical results of RT-PCR results were analyzed using a relative expression software tool (REST©).15
Results and Discussion
In this study, oral HD-DXM was used in a single 4-day course as the initial treatment schedule in previously untreated ITP patients with active disease, and IL-18, IL-18BP as well as Th1, Th2 cytokines and transcription factors were profiled in these patients before and after HD-DXM treatment. All of the patients showed initial responses to four days of HD-DXM treatment, in accordance with previous reports.11–13,16 The platelet counts of ITP patients after HD-DXM treatment (median 111×109/L, range 39–312×109/L) were significantly higher than that of pre-treatment groups (median 5×109/L, range 1–16×109/L, p<0.01) (Table 1). There was no significant association between clinical parameters (age, sex, platelet counts prior to and after HD-DXM) and the level of IL-18 or IL-18BP at any disease stage.
Previous data demonstrate that IL-18 and IL-18BP are constitutively expressed in humans and IL-18 is up-regulated in the active ITP patients.10 IL-18BP has been shown to bind IL-18 with high affinity and effectively inhibit its biological activities by reducing induction of IFN-γ mediated responses in vitro and LPS-induced IFN-γ production in vivo.4 Regulating the balance of IL-18 and IL-18BP in ITP might provide a reasonable therapeutic strategy for ITP. We herein demonstrate that HD-DXM induces IL-18BP production and thereby regulates the balance of IL-18 and IL-18BP.
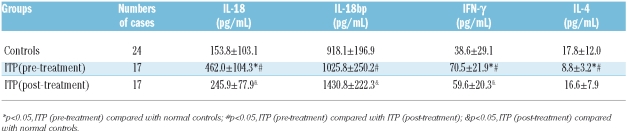
Administration of HD-DXM to ITP patients results in elevation of IL-18BP plasma levels. In biological accordance with this marked augmentation of the antagonist, IL-18 plasma levels steadily declined over the study period, resulting in a 47% decrease after HD-DXM therapy compared to pre-treatment values (Table 2). As demonstrated, IL-18 mRNA levels were reduced and IL-18BP mRNA levels decreased by threefold after HD-DXM treatment. The effect of HD-DXM administration on IL-18 and IL-18BP plasma and mRNA levels consists in a substantial reduction of IL-18/IL-18BP (Figue 1 A–C). However, we did not observe any correlation between IL-18BP and IL-18 levels in the present study.
Table 2.
Cytokine levels in ITP patients and controls (mean±SD).
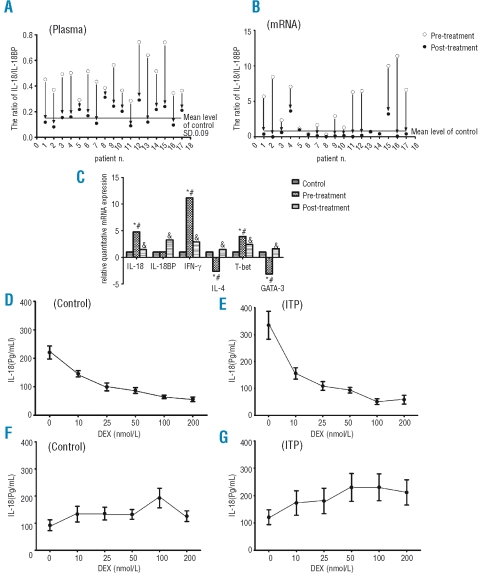
Figure 1.
(A) and (B) Calculated IL-18/IL-18BP level four days after 40 mg/day DXM administration of 17 patients with newly diagnosed ITP. IL-18/IL-18BP ratio was downregulated after HD-DXM therapy. (Data are presented for individual patients). (C) Relative mRNA expressions of IL-18 and IL-18BP and other cytokines in active ITP patients, HD-DXM treated patients and healthy controls. mRNA expressions of IL-18, IL-18BP and other cytokines and transcription factors in freshly isolated human PBMCs were quantified by RT-PCR.*p<0.05, ITP (pre-treatment) compared with normal controls; #p<0.05, ITP (pretreatment) compared with ITP (post-treatment); &p<0.05, ITP (post-treatment) compared with normal controls. (D)–(G) IL-18 and IL-18BP release from DXM-stimulated PBMCs. PBMC derived from 11 healthy volunteers and 8 ITP patients were cultured with or without DXM. IL-18 and IL-18BP release into supernatant were assayed by ELISA. Administration of DXM results in reduction of IL-18 (pg/mL) secretion in a dose-dependent manner (D and E) and elevation of IL-18BP (pg/mL) levels to their highest levels at 100 nmol/L (F and G).
We have demonstrated that DXM down-regulates IL-18 release into the supernatant of cultured PBMCs after 48 hours of culture (Figue 1D and E). DXM-untreated control PBMCs secreted 220.4±72.4 pg/mL IL-18 into the supernatant. Addition of DXM resulted in a 70% decrease (55.9±25.3 pg/mL, p<0.05, 200 nmol/L, Figure 1D). Similar results were obtained in cultures of PBMCs derived from ITP patients, where DXM (200 nmol/L) reduced IL-18 secretion from 335.0±137.7 pg/mL to 58.3±42.7 pg/mL (p<0.05, Figure 1E). The expression of IL-18 in PBMC cultures from ITP patients was higher than those in the normal control group (p<0.05). These in vitro data correspond to the presented in vivo data and might outline the cellular basis of DXM effects on IL-18.
We also observed an increased induction of IL-18BP by DXM-treated PBMCs in vitro. As shown in Figure 1F and G, DXM had a significant effect on secretion of IL-18BP from healthy and ITP patients PBMC cultures. DXM appeared to induce an increase of IL-18BP in all conditions. However, the amount of IL-18BP released in the cell supernatants was comparable between the two sample groups and, generally, much lower than those observed in the plasma. However, stimulation with DEX did not result in changes on IL-18 and IL-18BP mRNA levels (data not shown).
To exclude the possibility that induction of IL-18BP is secondary to DXM-induced IFN-γ release, we determined IFN-γ levels after DXM treatment in vitro. IFN-γ levels were just at the detection limit of the assay (data not shown). IFN-γ levels were not elevated during the course of HD-DXM treatment in vivo, thereby excluding the possibility that induction of IL-18BP is secondary to IFN-γ. From this point of view, suppression of the Th1 cytokine IL-18 and induction of IL-18BP by HD-DXM is favorable for the resolution of disease.
Evidence exists showing that an imbalance of Th1 versus Th2 polarization in favor of Th1 cell subsets appears to be a key pathogenic mechanism in ITP and the clinical efficacy of HD-DXM in ITP depends on the correction of Th1 polarization. In this study we have also demonstrated other Th1 and Th2 cytokines and transcription factors that regulate the differentiations of Th1 and Th2 cells (Figure 1C). In accordance with previous reports,7–9 elevated IFN-γ/IL-4, IL-18/IL-4 and T-bet/GATA-3 ratio were observed in patients with active ITP compared with the control group, which further implied a Th1-dominated cytokine profile in ITP with active disease. After HD-DXM treatment all fell to the normal range. HD-DXM therapy for ITP could cause a shift in the Th1/Th2 cytokine balance, leading to a more balanced Th1/Th2 cytokine profile response in vivo.
GC was known to affect cytokine synthesis in T cells by binding to and activating cytoplasmic GC receptors. The receptor-corticosteroid complex then translocates to the nucleus, where it regulates the transcription of target genes through several mechanisms. GC may directly inhibit Th1 cytokine production in T cells and potentially enhance Th2 cytokine synthesis by inhibiting IL-12 production in antigen-presenting cells.17–19 The present observation that IL-18 and IFN-γ plasma and mRNA levels were reduced after HD-DXM treatment is in agreement with these data. The HD-DXM-mediated Th1/Th2 cytokine profile alterations observed in this study might be the results of a downregulation of IL-18 and other Th1 cytokines by induction of IL-18BP while permitting the production of Th2 cytokines. As ITP is a heterogeneous and complex autoimmune disease, several abnormalities involving the cellular mechanisms of immune modulation have been described.5,6 The precise mechanisms of HD-DXM await further clarification.
Taken together our findings suggest that the reversal of IL-18/IL-18BP in ITP is driven by IL-18BP upregulation after HD-DXM treatment. The role of IL-18 and IL-18BP remains to be determined, but they are possibly involved in the clinical efficacy of HD-DXM in ITP treatment.
Footnotes
The authors reported no potential conflicts of interest.
Funding: this work was supported by grants from National Natural Science Foundation of China (30600259, 30600680, 30770922, 30800491, and 30801258), 973 Program (N. 2006 CB 503803), Foundation for the Author of National Excellent Doctoral Dissertation of PR China (200561), Program for New Century Excellent Talents in University (NCET-07-0514), Key Project of Chinese Ministry of Education (109097), Key Clinical Research Project of Public Health Ministry of China 2007–2009, Commonweal Trade for Scientific Research (200802031), and Taishan scholar project funding.
Authorship and Disclosures
SN: experimental design, data analysis and manuscript writing; ZX: contributed to data analysis and writing of the manuscript; WQ, WC contributed to the statistical analysis; QP: data analysis; PJ: data analysis and writing of the manuscript; HM: obtained funding, experimental design and writing of the manuscript. All authors revised the manuscript critically and approved the final version to be published.
References
- 1.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, et al. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–23. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 2.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–9. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 3.Reddy P. Interleukin-18: recent advances. Curr Opin Hematol. 2004;11:405–10. doi: 10.1097/01.moh.0000141926.95319.42. [DOI] [PubMed] [Google Scholar]
- 4.Hurgin V, Novick D, Rubinstein M. The promoter of IL-18 binding protein: activation by an IFN-γ-induced complex of IFN regulatory factor 1 and CCAAT/enhancer binding protein β. Proc Natl Acad Sci USA. 2002;99:16957–62. doi: 10.1073/pnas.262663399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coopamah MD, Garvey MB, Freedman J, Semple JW. Cellular immune mechanisms in autoimmune thrombocytopenic purpura: An update. Transfus Med Rev. 2003;17:69–80. doi: 10.1053/tmrv.2003.50004. [DOI] [PubMed] [Google Scholar]
- 6.McMillan R. Antigen-specific assays in immune thrombocytopenia. Transfus Med Rev. 1990;4:136–43. doi: 10.1016/s0887-7963(90)70258-1. [DOI] [PubMed] [Google Scholar]
- 7.Semple JW, Freedman J. Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood. 1991;78:2619–25. [PubMed] [Google Scholar]
- 8.Semple JW, Milev Y, Cosgrave D, Mody M, Hornstein A, Blanchette V, et al. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–54. [PubMed] [Google Scholar]
- 9.Panitsas FP, Theodoropoulou M, Kouraklis A, Karakantza M, Theodorou GL, Zoumbos NC, et al. Adult chronic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood. 2004;103:2645–7. doi: 10.1182/blood-2003-07-2268. [DOI] [PubMed] [Google Scholar]
- 10.Shan NN, Zhu XJ, Peng J, Qin P, Zhuang XW, Wang HC, et al. Interleukin 18 and interleukin 18 binding protein in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2009;144:755–61. doi: 10.1111/j.1365-2141.2008.07520.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodeghiero F. First-line therapies for immune thrombocytopenic purpura: re-evaluating the need to treat. Eur J Haematol Suppl. 2008;69:19–26. doi: 10.1111/j.1600-0609.2007.01000.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y, Wong RS, Soo YO, Chui CH, Lau FY, Chan NP, et al. Initial treatment of immune thrombocytopenic purpura with highdose dexamethasone. N Engl J Med. 2003;349:831–6. doi: 10.1056/NEJMoa030254. [DOI] [PubMed] [Google Scholar]
- 13.Mazzucconi MG, Fazi P, Bernasconi S, De Rossi G, Leone G, Gugliotta L, et al. Therapy with high-dose dexamethasone (HDDXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura. A GIMEMA experience. Blood. 2007;109:1401–7. doi: 10.1182/blood-2005-12-015222. [DOI] [PubMed] [Google Scholar]
- 14.British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. British Journal of Haematology. 2003;120:574–96. doi: 10.1046/j.1365-2141.2003.04131.x. [DOI] [PubMed] [Google Scholar]
- 15.Michael W, Graham W, Dempfle HL. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research. 2002;30:1–10. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo CS, Chu XX, Shi Y, He W, Li L, Wang L, et al. Correction of Th1-dominant cytokine profiles by high-dose dexamethasone in patients with chronic Idiopathic thrombocytopenic purpura. J Clin Immunol. 2007;27:557–62. doi: 10.1007/s10875-007-9111-1. [DOI] [PubMed] [Google Scholar]
- 17.DeKruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160:2231–7. [PubMed] [Google Scholar]
- 18.Peng J, Liu CF, Liu D, Ren C, Li W, Wang Z, et al. Effects of B7- blocking agent and/or CsA on induction of platelet-specific T-cell anergy in chronic autoimmune thrombocytopenic purpura. Blood. 2003;101:2721–6. doi: 10.1182/blood-2002-06-1666. [DOI] [PubMed] [Google Scholar]
- 19.Adcock IM, Brown CR, Gelder CM, Shirasaki H, Peters MJ, Barnes PJ. The effects of glucocorticoids on transcription factor activation in human peripheral blood mononuclear cells. Am J Physiol. 1995;268:331–8. doi: 10.1152/ajpcell.1995.268.2.C331. [DOI] [PubMed] [Google Scholar]