This report describes the successful use of dose-escalating low molecular weight heparin thrombo-profylaxis in pregnant women with prosthetic heart valves.
Keywords: pregnancy, low molecular weight heparin, mechanical heart valves
Abstract
The use of standard dose low molecular weight heparin (LMWH) to anticoagulate women with mechanical valves in pregnancy is associated with morbidity and mortality. We conducted a prospective audit of the use of adjusted dose high intensity LMWH in 12 pregnancies in 11 women with prosthetic heart valves. LMWH ± low-dose aspirin was started at therapeutic-dose with monitoring of anti-Xa levels to achieve a target level of 1.0–1.2 IU/mL (0.8–1.2 in the first 3/12 pregnancies). This necessitated a mean increase in the dose of LMWH of 54.4% (SD±33.2) over initial dose. Eleven of 12 pregnancies resulted in live births, with one intrauterine fetal death at 37 weeks. One non-fatal valve thrombosis occurred at 26 weeks gestation associated with subtherapeutic anti-Xa levels. Three patients experienced major bleeding. This regime provides a therapeutic option for women with mechanical heart valves during pregnancy, provided anti-Xa levels are kept within the target range. These patients require close surveillance for bleeding and thrombotic complications within a multi-disciplinary setting.
Introduction
Provision of safe and effective anticoagulation for pregnant women with mechanical heart valves is a challenging management problem. All current anticoagulation regimens are associated with maternal thromboembolism (TE) and/or bleeding. Vitamin K antagonist (VKA) therapy throughout pregnancy results in the lowest observed risk of TE (3.9%). However, VKA use is associated with fetal anomaly rates of 6.4%, fetal death of 12% and neurodevelopmental problems.1,2 The rate of TE with unfractionated heparin (UFH) is high at 25% if used throughout pregnancy and 9% if used for the first trimester.1
Therapeutic dose LMWH is an attractive alternative to VKAs and UFH. However, in the HIP-CAT study which compared enoxaparin with sequential UFH and warfarin in pregnant women with mechanical valves, 2/7 women receiving therapeutic dose enoxaparin 1 mg/kg 12 hourly developed fatal valve thrombosis.3 The incidence of TE using LMWH in this setting is not known because of the limited data available, but James et al. found an overall TE rate of 22% and a maternal mortality of 4%.4 In another review, Oran et al. found an overall incidence of valve thrombosis of 8.64% (8/81) and the overall TE rate 12.35% (10/81).5 However, 9 of these 10 patients received a fixed dose of LMWH, and in 2 of these a low fixed dose was used. Among 51 pregnancies where anti-Xa levels were monitored, only one patient was reported to have had TE. The American College of Chest Physicians (ACCP) advises that there are insufficient data for definitive recommendations on how best to anticoagulate women with mechanical valves in pregnancy, in view of concerns for fetal well-being with warfarin therapy and the possible poorer efficacy of subcutaneous UFH and LMWH in preventing maternal TE.6 Recommendations have included adjusted dose LMWH throughout pregnancy to keep a 4 h post-dose anti-Xa level of 1.0–1.2 IU/mL although updated ACCP guidelines recommend adjusted dose LMWH to achieve the manufacturer’s peak anti-Xa level 4 h post subcutaneous injection (approximately 1.0 IU/mL) with consideration of LDA in women with prosthetic heart valves at particularly high risk of thrombosis.7 Against this background, we conducted a prospective audit of our experience with the use of our dose-adjusted regimen of high intensity LMWH in pregnant women with mechanical heart valves, and documented TE and bleeding complications, pregnancy outcome, as well as anti-Xa levels throughout their pregnancies.
Design and Methods
All pregnancies in women with mechanical heart valves between 2001 and 2007 who received LMWH were included. The audit was carried out in accordance with United Kingdom regulations.8,9 Since 2004, all women receiving therapeutic dose LMWH were managed in a multi-disciplinary clinic for high-risk obstetric patients. Self-administered LMWH was commenced at full therapeutic dose, based on current weight; dalteparin at 100 units/kg and enoxaparin at 1 mg/kg, 12 hourly subcutaneously (SC) when warfarin had been discontinued. All women received counseling regarding the risks and benefits associated with warfarin, UFH and LMWH as thromboprophylaxis in this setting. Warfarin was stopped before six weeks gestation and LMWH started when the INR was <2.0. LMWH was self-administered in the majority of cases. Aspirin 75 mg (LDA) daily was added in women who were felt to be at particularly high risk of thrombosis (atrial fibrillation, enlarged right atrium and systemic right ventricle). In 6 out of 12 cases, peridelivery anticoagulation was managed according to a regimen using fixed dose UFH 15,000 IU/24 h by continuous intravenous infusion peridelivery, and recommencement of LMWH 24 h post-delivery.10
Anti-Xa levels were measured on samples taken 4 h post-injection using a chromogenic assay (Coamatic Heparin, Chromogenix), with adjustment of the LMWH dose to achieve anti-Xa levels 1.0–1.2 IU/mL (0.8–1.2 in the first 3/12 pregnancies). Platelet counts were monitored weekly for the first three weeks and thereafter every four weeks. From 2004 onwards patients were offered calcium supplements, together with vitamin D (calcichew D3 500mg bd), in those with suboptimal or low vitamin D levels (normal 15–120 IU, optimal >70 IU). The following outcomes were recorded: TE and bleeding complications, and pregnancy outcome.
Results and Discussion
Past obstetric and cardiac history
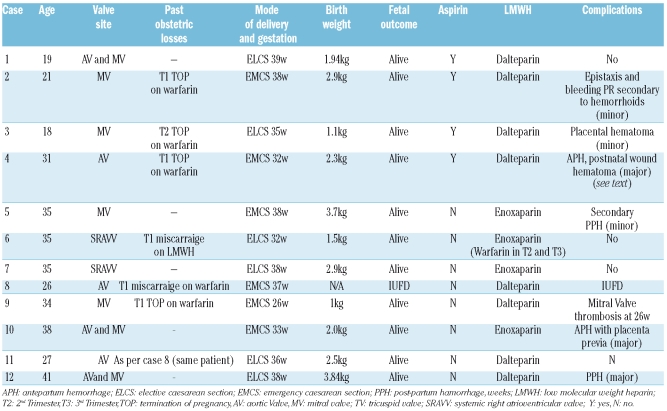
Twelve pregnancies in 11 women (9 Caucasian) who received LMWH during pregnancy were audited. The mean age was 29.8 (SD±7.4) years. Details of the pregnancies are outlined in Table 1. Mechanical valve sites were as follows: mitral (MVR) 4, aortic (AVR) 2, dual aortic and mitral (DVR) 3, and systemic right atrioventricular valve (SRAVV) 2 (Table 1). The median age of the valves in relation to each pregnancy was eight (range 1–19) years. Past obstetric history included 6 fetal losses: 2 first trimester miscarriages and 4 terminations of pregnancy (TOP), including one therapeutic TOP at 22 weeks gestation because of an intracerebral fetal hemorrhage during maternal warfarin therapy.
Table 1.
Details of pregnancies.
LMWH and anti-Xa Levels
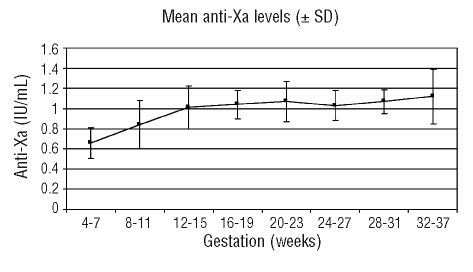
LMWH heparin was commenced at or before six weeks gestation in 11 pregnancies with one remaining patient switching from warfarin to LMWH at eight weeks gestation. Anticoagulation in 8 pregnancies was with dalteparin and 4 patients received enoxaparin. One of twelve patients discontinued LMWH at the end of the first trimester and was anticoagulated with warfarin for the 2nd and 3rd trimesters. Four patients also received low-dose aspirin. Self-administered LMWH was commenced at full therapeutic dose SC based on weight (dalteparin 100 IU/kg or enoxaparin 1 mg/kg 12 hourly). Mean weight at booking was 76.1 kg (range 50–97). Considerable stepwise increases in the doses of LMWH were required to maintain target or near–target 4-hour post-dose anti-Xa levels, with mean LMWH doses pre-delivery of 11,194 IU (range 8,750–15,000) 12 hourly for dalteparin and 90mg (80–95) 12 hourly for enoxaparin, representing a mean increase of 54.4% (SD±33.2) over initial dosage. The mean anti-Xa level between 32 and 37 weeks was 1.09 IU/mL compared to 0.66U/mL between four and seven weeks. Anti-Xa levels (mean and SD) throughout pregnancy are demonstrated in Figure 1. One-hundred and eighty-six anti-Xa levels were measured during a total of 355 weeks gestation (mean duration between measurements, 1.9 weeks). Of the 12 pregnancies, there were 11 live births with a mean birth weight of 2.33kg (SD 0.95) at a mean gestation of 35.3 weeks (SD±3.84), and one IUFD at 37 weeks.
Figure 1.
Mean anti-Xa levels during all pregnancies (±SD)
Thrombotic complications
There was one valve-related thrombosis, which occurred at 26 weeks gestation in a 25-year old patient (Patient 9, Table 1) who had undergone mitral-valve replacement (Bjork-Shirley) at the age of six years for left atrio-ventricular valve regurgitation. She had a past history of subclavian vein thrombosis at the age of 21. Thrombophilia testing showed that she was heterozygous for the G20210A prothrombin gene mutation (PGM). This was her second pregnancy, the first ending in an early spontaneous miscarriage one year previously whilst on warfarin treatment. She switched to therapeutic dose LMWH, dalteparin 5000 IU 12 hourly (weight 50 kg) at eight weeks gestation. Optimal anti-Xa monitoring at our center was not possible due to geographical remoteness. By week 26, her dalteparin dose was 8,750 IU 12 hourly (peak dose during pregnancy) and she developed progressive dyspnea. Pulmonary edema secondary to mitral valve thrombosis was diagnosed. She underwent emergency CS with delivery of a female infant weighing 1.0 kg followed by emergency mitral valve replacement. Importantly, anti-Xa levels at 14 and 20 weeks gestation were subtherapeutic (0.6 and 0.64 IU/mL, respectively). There were no transient ischemic attacks, strokes, cases of heparin-induced-thrombocytopenia or bone fractures. Three patients have undergone bone mineral densitometry (BMD) imaging since delivery and all had normal T-scores.
Bleeding complications
Three patients experienced major bleeding complications. In the antenatal period, there were 2 episodes of antepartum hemorrhage, one with placenta previa (patient 10), associated with a 4 h post-dose anti-Xa level of 1.53 IU/mL. In the other case (patient 4), pregnancy was uneventful until week 27 when she developed bleeding per vagina (PV) which was of small volume (<100 mL). There was no hemodynamic compromise or fall in hemoglobin concentration. At this time the anti-Xa level 4 h post-dose was 1.07 IU/mL and her total daily dalteparin dose was 24,000 IU. Over the next four weeks she continued to have intermittent low-volume PV bleeding which was managed conservatively and low-dose aspirin was discontinued. The anti-Xa range at 4 h post dose was reduced to 0.8–1.0 IU/mL to achieve a balance between adequate thromboprophylaxis and bleeding risk. The peak anti-Xa level was 1.46 IU/mL at 18 weeks gestation. Ultrasound scan demonstrated a 6 cm hematoma over the cervical opening above the cervical os. Persistent vaginal bleeding eventually necessitated caesarian section at 31 weeks with delivery of a female infant (birth weight 2.3 kg). Post-partum she was commenced on intravenous UFH 15,000 IU/24 h by continuous IVI. The dose of UFH was increased after 24 h to attain a therapeutic APTR of 2.0–3.0. Three days post-CS she developed a wound hematoma, which required surgical evacuation and transfusion of 4 units of red cells. The APTR had been labile and more than 5.0 on 2 occasions despite close monitoring.
She was switched to LMWH and subsequently to warfarin, making a full recovery. Post-delivery there was one further major bleeding episode; a major post-partum hemorrhage (PPH) (during LMWH treatment) which necessitated a red cell transfusion of 8 units but no surgical intervention Three patients had minor bleeding: one per-rectum secondary to hemorrhoids and also epistaxis, one secondary PPH and one placental hematoma. The median estimated blood loss was 500 mls (300–900 mls) excluding the deliveries complicated by major hemorrhage (n=2). Median hemoglobin concentrations at day 1 and day 5 post-delivery were 10.7 g/dL (range 8.5–14.5) and 9.8g/dL (range 6.6–12.0), respectively. Median platelet counts at day 1 and day 5-post delivery were 199×109/L (range 145–325) and 225×109/L (150–400×109/L). There were no transient ischemic attacks, strokes, cases of heparin-induced-thrombocytopenia or bone fractures. Three patients have undergone bone mineral densitometry (BMD) imaging since delivery and all had normal T-scores.
Results and Discussion
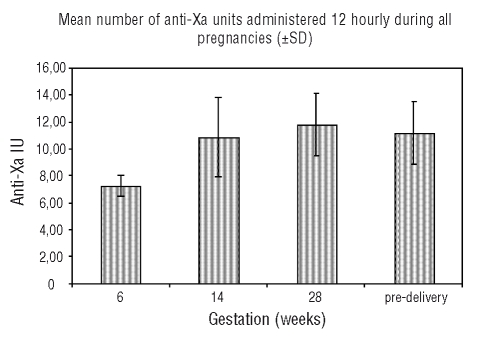
This single-center experience has demonstrated that an adjusted-dose high intensity LMWH regimen in pregnant women with prosthetic heart valves provides effective anticoagulation provided anti-Xa levels are kept within a tight therapeutic range of 1.0–1.2 IU/mL. The importance of meticulous anti-Xa monitoring with appropriate LMWH dose adjustment is underlined by the occurrence of a mitral valve thrombosis in one patient whose monitoring was not well maintained and the anti-Xa level was sub-therapeutic albeit transiently, although there were other contributory factors. There were live births in 11/12 pregnancies and no maternal mortality. Large increases in the doses of LMWH were required to achieve effective anticoagulation during pregnancy, with mean doses pre-delivery showing an approximately 54% increase over initial dose. Interestingly, we did note a minor reduction (which was not statistically significant) in LMWH dose required to maintain target anti-Xa levels in the 3rd trimester in comparison with the 2nd trimester (Figure 2).
Figure 2.
Mean number (±SD) of anti-Xa IU LMWH administered 12 hourly SC during all pregnancies
Our findings that achievement of target anti-Xa levels required stepwise LMWH dose increases during pregnancy concurs with observations in patients with venous thromboembolism treated with dalteparin where 85% of pregnancies (11/13) required upward dose adjustments to maintain anti-Xa levels.11 As LMWHs undergo renal clearance, the increase in glomerular filtration rate (GFR) and expansion of plasma volume during pregnancy may explain the large doses of LMWH required to achieve therapeutic anti-Xa levels.12
The number of TE complications in this audit (one in 12 pregnancies) is lower than that documented by James et al.4 in their review, where they reported 17 TE complications in 72 pregnancies (22%). It is notable that we observed no TE complications in those patients whose anti-Xa levels were well maintained. Major bleeding episodes occurred in 3 patients; however, there was a good maternal and fetal outcome in all cases. In their review, James et al.4 report a 10.9% rate of hemorrhage including one fatal, whereas Rowan et al.13 in their audit, report a rate of 14.3%. Predictably, bleeding occurs; particularly if there are compounding obstetric complications, such as placenta previa.
The anti-Xa assay is the most informative available assay for monitoring LMWH treatment, even though it is recognized that the assay has its limitations.14 For example, Leizorovicz et al. found a weak correlation between thrombosis and anti-Xa levels and no significant correlation with hemorrhage in a large cohort of patients receiving LMWH thromboprophylaxis for general surgery.15 However, another study has shown that most major bleeds occurred in those patients receiving the highest doses of LMWH16 and those with mean anti-Xa levels greater than 0.8 IU/mL. Other studies have shown that the pharmacokinetics of LMWH are altered during pregnancy.17,18 In 2002, the Control of Anticoagulation Subcommittee of the Scientific and Standardisation Committee of the International Society for Thrombosis and Haemostasis made the following recommendation on monitoring LMWH: Use of anti-Xa assays may provide some clue to the pharmacokinetics of LMWH when used to treat thrombosis in those in whom standard or weight-adjusted dosing is likely to be unreliable, especially subjects with severe renal failure, the obese, the pregnant, neonates and infants.19 Therefore, given the very high doses of LMWH prescribed to our patients, added to the altered LMWH pharmacokinetics in pregnancy and the ACCP recommendation regarding dose adjustments of LMWH according to anti-Xa levels when treating pregnant women with mechanical heart valves, it would seem that anti-Xa monitoring is necessary in these high-risk patients.7
The risk of osteoporotic fracture associated with long-term use of LMWH in pregnancy is very low, estimated at 0.04%.20 Rodger et al. reported normal bone-density six weeks after completion of dalteparin thromboprophylaxis during pregnancy in a cohort of 62 patients.21 However, our patients received higher doses of LMWH in comparison with patients in these studies, and the risk of osteopenia in this situation remains to be defined.
This regime provides a therapeutic option for women with mechanical heart valves during pregnancy, who should be considered highly complex when antenatal care is being planned. They require specialist multi-disciplinary care with early intervention to overcome complications.
Footnotes
Authorship and Disclosures
JQ, KVK, and RB analyzed the data and wrote the paper; DP, FW and MC were responsible for the clinical management of patients; all authors were involved in the final revision of the paper.
The authors declare no potential conflict of interest.
References
- 1.Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: A systematic review of the literature. Arch Intern Med. 2000;160:191–65. doi: 10.1001/archinte.160.2.191. [DOI] [PubMed] [Google Scholar]
- 2.Wesseling J, Van Driel D, Heymans HS. Coumarins during pregnancy: long-term effects on growth and development of school-age children. Thromb Haemost. 2001;85:609–13. [PubMed] [Google Scholar]
- 3.Ginsberg JS, Chan WS, Bates SM, Kaatz S. Anticoagulation of pregnant women with mechanical heart valves. Arch Intern Med. 2003;163:694–8. doi: 10.1001/archinte.163.6.694. [DOI] [PubMed] [Google Scholar]
- 4.James AH, Brancazio LR, Gehrig TR. Low-molecular-weight heparin for thromboprophylaxis in pregnant women with mechanical heart valves. J Matern Fetal Neonatal Med. 2006;19:543–9. doi: 10.1080/14767050600886666. [DOI] [PubMed] [Google Scholar]
- 5.Oran B, Lee-Parritz A, Ansell J. Low-molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb Haemost. 2004;92:747–51. doi: 10.1160/TH04-06-0337. [DOI] [PubMed] [Google Scholar]
- 6.Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:627S–44S. doi: 10.1378/chest.126.3_suppl.627S. [DOI] [PubMed] [Google Scholar]
- 7.Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:844S–86S. doi: 10.1378/chest.08-0761. [DOI] [PubMed] [Google Scholar]
- 8.Principles for best practice in clinical audit National Institute for Clinical Excellence (NICE) 2008. http://www.nice.org.uk/media/796/23/BestPracticeClinicalAudit.pdf.
- 9.University College London Hospitals Clinical Audit Guidebook. 2007. http://insight/department/CorporateBoard/Governance/ClinicalAuditEffectiveness/Documents/Clinical%20Audit%20Guidebook%20January%202008.doc.
- 10.Lambert JR, Austin SK, Peebles D, Cohen H. Audit of the peri-delivery use of unfractionated heparin in women on therapeutic low-molecular weight heparin. Br J Haematol. 2008;142:453–6. doi: 10.1111/j.1365-2141.2008.07198.x. [DOI] [PubMed] [Google Scholar]
- 11.Barbour LA, Oja JL, Schultz LK. A prospective trial that demonstrates that dalteparin requirements increase in pregnancy to maintain therapeutic levels of anticoagulation. Am J Obstet Gynecol. 2004;191:1024–9. doi: 10.1016/j.ajog.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 12.Jeyabalan A, Conrad KP. Renal function during normal pregnancy and preeclampsia. Front Biosci. 2007;12:2425–37. doi: 10.2741/2244. [DOI] [PubMed] [Google Scholar]
- 13.Rowan JA, McCowan LM, Raudkivi PJ, North RA. Enoxaparin treatment in women with mechanical heart valves during pregnancy. Am J Obstet Gynecol. 2001;185:633–7. doi: 10.1067/mob.2001.117657. [DOI] [PubMed] [Google Scholar]
- 14.Baglin T, Barrowcliffe TW, Cohen A, Greaves M. Guidelines on the use and monitoring of heparin. British Committee for Standards in Haematology. Br J Haematol. 2006;133:19–34. doi: 10.1111/j.1365-2141.2005.05953.x. [DOI] [PubMed] [Google Scholar]
- 15.Leizoroviecz A, Bara L, Samama MM, Haugh MC. Factor Xa inhibition: correlation between the plasma levels of anti-Xa activity and occurrence of thrombosis and haemorrhage. Haemostasis. 1993;23:89–98. doi: 10.1159/000216915. [DOI] [PubMed] [Google Scholar]
- 16.Niewenhuis HK, Albada J, Banga JD, Sixma JJ. Identification of risk factors for bleeding during treatment of acute venous thromboembolism with heparin or low molecular weight heparin. Blood. 1991;78:2337–43. [PubMed] [Google Scholar]
- 17.Hunt BJ, Doughty HA, Majumdar G, Copplestone A, Kerslake S, Buchanan N, et al. Thromboprophylaxis with low molecular weight heparin (Fragmin) in high risk pregnancies. Thromb Haemost. 1997;77:39–43. [PubMed] [Google Scholar]
- 18.Crowther MA, Spitzer K, Julian J, Ginsberg J, Johnston M, Crowther R, Laskin C. Pharmacokinetic profile of a low-molecular weight heparin (reviparin) in pregnant patients. A prospective cohort study. Thromb Res. 2002;98:133–8. doi: 10.1016/s0049-3848(99)00228-5. [DOI] [PubMed] [Google Scholar]
- 19.Greaves M. Limitations of the laboratory monitoring of heparin therapy. Thromb Haemost. 2002;87:163–4. [PubMed] [Google Scholar]
- 20.Greer I, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Greer IA, Nelson-Piercy C. Blood. 2005;106:401–7. doi: 10.1182/blood-2005-02-0626. [DOI] [PubMed] [Google Scholar]
- 21.Rodger MA, Kahn SR, Cranney A. Long-term dalteparin in pregnancy is not associated with a decrease in bone mineral density: substudy of a randomized controlled trial. J Thromb Haemost. 2007;5:1600–6. doi: 10.1111/j.1538-7836.2007.02634.x. [DOI] [PubMed] [Google Scholar]