Histone deacetylase inhibitors are a class of anti-cancer agents that induce growth arrest, differentiation, and apoptotic cell death of transformed cells. The authors report three instances of DNA viral reactivation in patients treated with romidepsin on a multicenter phase II trial in patients with cutaneous T-cell lymphoma and peripheral T-cell lymphomas. These observations suggest that vigilance for DNA virus reactivation is needed to quantify the risk in patients treated with histone deacetylase inhibitors.
Keywords: DNA virus, histone deacetylase inhibitor, therapy
Abstract
Histone deacetylase inhibitors are a class of anti-neoplastic agents that induce growth arrest, differentiation, and/or apoptotic cell death of transformed cells in vitro and in vivo. A phase II study exploring the efficacy of romidepsin, an histone deacetylase inhibitor, in patients with cutaneous or peripheral T-cell lymphomas was initiated at the National Cancer Institute. To date, over 120 patients with T-cell lymphoma have been treated on a multi-institutional phase II trial of romidepsin. Reactivation of latent DNA viruses including EBV, HBV, and VZV is well described as a consequence of the immune suppression associated with systemic chemotherapy. The incidence of viral reactivation in patients treated with histone deacetylase inhibitors is not yet known. We report the observation of EBV-associated illnesses in 2 patients and the reactivation of HBV in an additional patient treated with romidepsin. These cases may represent reactivation of DNA viruses due to histone deacetylase inhibitor induced immunosuppression, or direct promotion of viral replication via histone deacetylase inhibitor induced chromatin remodeling, or, alternatively, may be related to the underlying disease process. These observations suggest that vigilance for DNA virus reactivation is needed to quantify the risk in patients treated with histone deacetylase inhibitors.
Introduction
Histone deacetylase inhibitors (HDIs) are a class of anti-cancer agents that induce growth arrest, differentiation, and apoptotic cell death of transformed cells. A range of structurally diverse HDIs have been purified as natural products or have been synthetically produced. Histone deacetylases (HDACs) are enzymes that deacetylate lysine residues of histones and other non-histone proteins; there are at least 18 different HDACs known, grouped into 4 families.1 Romidepsin (depsipeptide, FR-901228, FK-228, NSC-630176) is a bicyclic peptide with antitumor activity that was subsequently shown to be an HDI. Pre-clinical studies have shown that romidepsin has potent in vitro activity against various tumor cell lines including T-cell lymphoma.2 Clinical studies have demonstrated efficacy in cutaneous T-cell lymphoma (CTCL) and peripheral T-cell lymphomas (PTCL) for romidepsin3 and other HDIs such as vorinostat4,5 and panobinostat (LBH589).6 The emergence of virus-associated complications, including Epstein Barr virus (EBV)-associated lymphoproliferative disorders (LPD) and hepatitis B virus (HBV) reactivation is well described following the administration of intensely immunosuppressive chemotherapy regimens.7,8 To date, this association has not been described in patients undergoing HDI therapy.
We report three instances of DNA viral reactivation in patients treated with romidepsin on a multicenter phase II trial in patients with PTCL and CTCL.
Design and Methods
Patients with relapsed or refractory CTCL or relapsed PTCL were enrolled in a phase II trial evaluating the safety and efficacy of romidepsin. The protocol (NCI 01-C-0049 or 1312) was approved by the Institutional Review Boards of the NCI and Peter MacCallum Cancer Centre. All data used in this analysis were obtained from patients who provided written informed consent. Romidepsin was administered as a 4 h infusion on days 1, 8, and 15 of a 28-day cycle, with a starting dose of 14 mg/m2. In cases 1 and 2 the presence of EBV-encoded RNA (EBER) was detected by an iView-Blue detection kit using an in situ hybridization chromogenic assay with fluorescein-labeled oligonucleotide EBER probe on ISH-Red counterstained sections (Benchmark, Ventana, AZ, USA). Appropriate positive controls were incorporated into each run.
Results and Discussion
Case 1
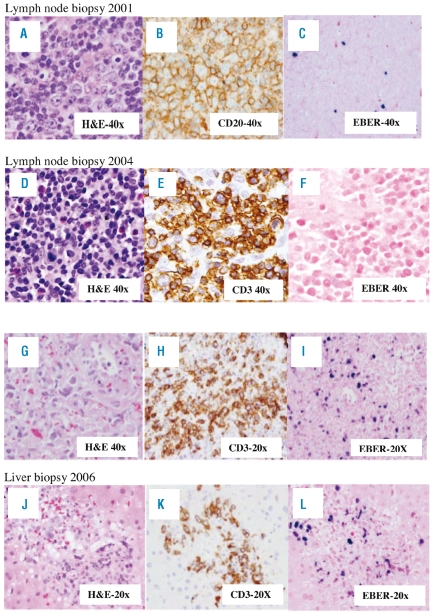
A 40-year old Asian male presented to his physician in 2001 with a neck mass. Biopsy revealed atypical lymphoid hyperplasia, containing rare EBV+ cells by in situ hybridization (EBER1). (Figure 1A–C), and a clonal T-cell population of uncertain significance by PCR analysis. The lymphadenopathy spontaneously regressed. Two years later in 2003, the patient had increasing lymphadenopathy associated with B symptoms. Repeat biopsy was interpreted as a T-cell rich large B-cell lymphoma, the same TCR clone was detected, and the patient was treated to complete remission with cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab (CHOP-R). A biopsy obtained at the time of a recurrence in 2004 (Figure 1 D–F), demonstrated a new morphologically distinct peripheral T-cell lymphoma. TCR gene rearrangement analysis revealed two new discrete bands, not present in earlier biopsies. Flow cytometry analysis of peripheral blood detected an abnormal T-cell population. The patient was then enrolled in the current phase II trial of romidepsin, which resulted in a near complete resolution of lymphadenopathy with the first cycle of therapy and complete resolution after the second cycle. The aberrant T-cell population remained detectable until completion of eight months of therapy, from which time it remained negative until one month prior to disease progression, when an abnormal T-cell population was again noted by flow cytometry.
Figure 1.
Tissue biopsies from Case 1 taken at first presentation (A–C), at first progression post-CHOP+R (D–F), at time of diagnosis of peripheral T-cell lymphoma in 2004 (G–I) and at disease progression in 2006 with NK/T-cell lymphoma from lymph node (D–F) or liver (G–I) biopsies. (A) H & E stain and high power view of lymph node shows atypical lymphoid infiltrate with prominent immunoblastic reaction. (B) CD20 immunostaining reveals numerous B cells including immunoblasts. (C) In situ hybridization studies for Epstein-Barr virus using the EBER probe show scattered positive cells. (D) H&E stain showing atypical lymphoid cells of varied size. Eosinophils are frequent in the background. (E) Tumor cell, some with mitotic figures, are positive for T-cell marker CD3. (F) In situ hybridization studies for Epstein-Barr virus using the EBER probe show no cells positive for EBV. (G) H&E stained section shows highly atypical cells with hyperchromatic, enlarged nuclei with irregular nuclear contours. (H) Tumor cells are positive for T-cell marker CD3 only occasional B cells were identified with the CD20 immunostain (data not shown). (I) In situ hybridization studies for the EBER probe staining the majority of the tumor cells. (J) H&E stained section shows atypical lymphoid infiltrate involving a portal tract. (K) The infiltrate is mainly composed of T cells highlighted by the CD3 immunostain. CD20 stain (data not shown) did not identify B cells at the same area. (L) In situ hybridization with the EBER probe proved to be positive in the tumor cells.
The patient continued to respond to treatment until cycle 15 when, in 2006, he developed high-grade fever, rigors, anemia, thrombocytopenia, and elevated liver enzymes. A bone marrow biopsy revealed hemophagocytosis. The EBV viral load in the peripheral blood was found to be elevated on PCR to 2.9×105 EBV equivalents per 1 million mononuclear cells. Biopsies of lymph node (Figure 1 G–I) and liver (Figure K–L) revealed an aggressive NK-T cell lymphoma positive for EBER-1. Molecular studies performed on lymph node, liver, and bone marrow biopsies and peripheral blood revealed a single band similar to the original one detected in the 2001 and 2003 biopsies. The patient died of multi-organ failure eight days later.
Case 2
A 57-year old female was diagnosed with stage III CTCL in 2001, which was initially treated with psoralen and ultraviolet A radiation (PUVA), and local radiotherapy for control of head and neck cutaneous tumors. In 2005, she was enrolled in a clinical trial of sipiluzimab (MEDI-507), a monoclonal antibody directed against CD2. Following two courses of sipiluzimab therapy, disease progression complicated by CMV reactivation necessitated withdrawal from study and she was subsequently enrolled in the romidepsin trial in November 2005. Symptomatic improvement and stable disease was observed during the initial five cycles of romidepsin. Following cycle 6, disease progression, evidenced by increased cutaneous tumor masses and worsening lymphadenopathy, prompted removal from study. Further treatment with localized radiotherapy followed by a single dose of gemcitabine was administered; however, clinical deterioration ensued and hydrocortisone 100 mg q8 hourly was initiated. The patient was found to have an EBV viral load of 1.1×106 EBV equivalents per 1 million mononuclear cells in the blood. Lymph node biopsy revealed an EBV-associated lymphoproliferative disorder with a large number of EBV positive cells. Retrospective review of two previous lymph node biopsies, one obtained prior to initiation of romidepsin treatment and the other obtained prior to initiation of gemcitabine demonstrated scant EBV positivity. Single agent rituximab was administered at a dose of 375mg/m2 weekly for five weeks, then every three weeks for 12 weeks. After four months of rituximab therapy, no EBV was detectable on peripheral blood PCR.
Case 3
An Italian-born female with stage IVA PTCL initially presented in 2005 and was treated on the Hyper-CVAD protocol administering cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine. Autologous stem cells were harvested on recovery from cycle 2A. Prior to initiation of treatment, hepatitis B surface antigen (HBsAg) was negative and liver function tests were normal, and remained so throughout the initial chemotherapy regimen. Despite intensive chemotherapy, disease progression occurred at three months post treatment. Autologous peripheral blood stem cell transplantation was performed following conditioning with etoposide (1980 mg), cyclophosphamide (1650 mg) and 12 Gy of total body irradiation (TBI). Further disease progression occurred six months after transplant, for which she was enrolled in the phase II clinical trial of romedepsin. Serology for HBsAg and liver function tests performed as part of clinical trail screening remained negative. Eleven cycles of romedepsin were completed without significant toxicity. Prior to the start of the 12th cycle of romidepsin, new onset liver function abnormalities were noted, with raised alanine transferase (ALT) 226U/L (normal 0–50U/L), aspartate transferase (AST) 95U/L (0–50U/L) and γ-glutamyl transferase (GGT) 62U/L (7–32U/L). Repeat hepatitis B serology revealed detectable HBsAg, hepatitis B e antigen (HBeAg) and hepatitis B core antibody (HBcAb) IgG and IgM. Hepatitis B viral load was 7.941 log 10 copies/mL. Hepatitis A and hepatitis C serology remained negative. A percutaneous liver biopsy showed mild fibrosis and moderate inflammation. Following initiation of entecavir 0.5 mg daily, the liver function tests normalized and the viral load reduced by 3-logs. Retrospective analysis on samples stored prior to all therapy showed seropositivity for HBcAb IgG and HBsAb, indicating latent HBV infection.
Here we describe 3 cases of reactivation of DNA viruses in association with HDI therapy. To our knowledge, these are the first reported instances of DNA virus reactivation in patients treated with any HDI. We postulate several potential explanations for these observations. First, HDIs may reactivate a latent viral infection through transcriptional activation of silenced viral promoters. Second, HDI therapy may have induced immune suppression via the reversible depletion of circulating peripheral blood lymphocytes and/or off target effects on the chemotaxis and effector function of T lymphocytes,9,10 resulting in reduced T cell-mediated immune surveillance of virus infected cells. Lastly, it remains possible that these viral complications may be unrelated to HDAC inhibition but in fact related to concurrent immunodeficiencies related to the underlying disease process or prior therapy, leading to EBV reactivation or, less likely, primary infection.
Both EBV and HBV are DNA viruses that exhibit latency following infection. EBV infection occurs in more than 90% of the population, most often without long-term consequences, although a pool of infected B cells remains under tight control of the immune system. In contrast, HBV infection is generally cleared but can result in sub-clinical disease with subsequent chronic hepatitis. The distinction is that, unlike the hepatitis viruses, which are often immunologically cleared, the herpes viruses remain in a lifelong latency phase in healthy individuals, under control of the immune system. It has been estimated that 1–5% of circulating memory T cells react with EBV, implying that the intact immune system is critical in maintaining the latency phase.11
The cellular immune response, including both CD4+ and CD8+ T cells, is essential for controlling both primary infection and latent infection with either EBV or HBV. Indeed, reactivation of another latent DNA virus, Varicella Zoster Virus (VZV), is often observed in patients undergoing chemotherapy. EBV and HBV reactivation is much less common but can occur in patients undergoing a variety of immunosuppressive therapies including treatment with conventional cytotoxic agents,7 stem cell transplantation8 or the monoclonal antibody, infliximab.12 In addition, HBV reactivation has been described following rituximab therapy.13 The degree of immune suppression resulting from HDI therapy including romidepsin has not been defined.
The diverse mechanisms of action of HDIs including induction of apoptosis, and cell cycle arrest14 may potentially alter the number and/or function of lymphocytes involved in the immune surveillance of viral infected cells. Indeed, lymphopenia has been recognized following therapy with romidepsin in phase I studies, with an accumulative incidence of approximately 60% (grade I–III).
Reappearance of HBsAg (sero-reversion) in patients who are initially HBsAg negative and HBcAb positive (as in Case 3) treated with conventional chemotherapeutic agents is less common than clinical flares of hepatitis in those who begin treatment with detectable HBsAg. Moreover, a recent review of reactivation of hepatitis B during anticancer therapy showed that the phenomenon of sero-reversion with the development of clinically detectable hepatitis outside the context of allogeneic bone marrow transplant is likely to be rare.15 Despite receiving highly immunosuppressive conventional cytotoxic chemotherapy and total body irradiation, there was no prior evidence of viral reactivation in our patient with HBV. It seems possible, therefore, that HDI exposure induced direct reactivation of latent virus in this patient.
The acetylation status of the cccDNA bound H3 and H4 histones has been implicated in the control of HBV replication.16 In vitro, the expression of virally transduced genes can be dramatically reactivated in Burkitt’s lymphoma cell lines following treatment with sodium butyrate or TSA,17 and lead to lytic cycle gene expression of EBV after a 4–6 h period of new protein synthesis.18 These results suggest that hypoacetylation of histones is integrally involved in the silencing of virally transduced genes in healthy individuals where latency remains following primary infection. Taken together, it thus seems feasible that the hyperacetylation effect of HDI therapy has the potential to reactivate HBV replication.
The mechanism of development of EBV-associated disease in the other 2 cases is less clear. In the first case, the patient had previously received minimally immunosuppressive therapy and the development of EBV-associated disease occurred on single agent romidepsin more than a year since previous chemotherapy. Notably, there is a higher frequency of EBV-associated NK/T cell lymphomas in patients of Asian ancestry and this process may have been destined to emerge as a second malignancy in this patient. EBV+ NK/T cell lymphomas have not been reported as a complication of immunosuppression. The second patient developed EBV-associated LPD ten months after a trial of sipiluzumab, a known immunosuppressive agent that has been associated with additional cases of EBV LPD.19 It is possible that the subsequent use of romidepsin did not have an initiating role in EBV reactivation, but that, in the context of recent T-cell depleting therapy, it may have either augmented the reactivation of EBV or have limited the T-cell repertoire available to control EBV proliferation following siplizumab therapy.
After romidepsin was discontinued, the administration of gemcitabine and hydrocortisone may have contributed to increased viral titers. Only scant EBV+ cells were noted on the biopsy obtained prior to the gemcitabine dose. Interestingly, gemcitabine has been used in a strategy to induce lytic EBV replication.20
Why some patients experience viral reactivation, or LPD in the case of EBV reactivation, is unknown. Aside from treatment related immunodeficiencies, other factors may include altered histone acetylation status at the site of viral DNA integration, as reported with hepatitis B.21 In the case of EBV, additional factors may include the epigenetic regulation of episomal origin recognition and replication.22 Indeed romidepsin has shown activity against EBV-positive lymphoblastoid cell lines expressing Lat-III genes but not those expressing a Lat-I profile.23
Finally, it should be noted that some patients with T-cell lymphomas appear to have a pre-existing constitutional immunodeficiency making them more prone to DNA virus reactivation during the course of their disease process. Indeed, we and others have previously described LPD in patients with CTCL24 or peripheral T-cell lymphoma.25 Ultimately, it is likely that the DNA virus reactivation in these patients was multi-factorial and epigenetic therapy can be considered one more factor in predisposing a patient to development of this complication.
Based on our experiences, as epigenetic therapies enter the anticancer armamentarium, a better understanding of the incidence and risks for viral reactivation with HDI is needed. It is possible that we will see new associations between these therapies and reactivation of latent viruses including hepatitis B. Although the current recommendations of the American Association for the Study of Liver Diseases do not include the use of antiviral prophylaxis in those patients undergoing immunosuppressive therapy who are HBsAg negative and HBcAb positive, we recommend that patients be screened for HepBsAg and HepBcAb before commencing HDI. Similarly, as more widespread use of epigenetically active agents occurs, monitoring CD4+ and CD8+ T-cell counts before, during and after treatment with simultaneous monitoring of EBV DNA in plasma by PCR, at least in the context of clinical development, may be an effective way to better quantify the risk of EBV-associated disorders and should be considered as part of future studies.
Acknowledgments
we would like to thank Jeff Cohen for his expert advice and review of the manuscript, and Kamal Sharma for collating some of the initial case information.
Footnotes
Authorship and Disclosures
DSR analyzed data and wrote the paper. RLP wrote the clinical trial, analyzed data, and wrote the paper. PB wrote the paper. LJK reviewed all pathology and provided data. SP and ESJ reviewed all pathology and approved the paper. MR analyzed data. JEJ provided expert opinion and approved the paper. HMP and SEB performed research and wrote the paper.
The authors reported no potential conflicts of interest.
Funding: this research was supported, in part, by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Rasheed WK, Johnstone RW, Prince HM. Histone deacetylase inhibitors in cancer therapy. Expert Opin Investig Drugs. 2007;16:659–78. doi: 10.1517/13543784.16.5.659. [DOI] [PubMed] [Google Scholar]
- 2.Piekarz RL, Robey RW, Zhan Z, Kayastha G, Sayah A, Abdeldaim AH, et al. T-cell lymphoma as a model for the use of histone deacetylase inhibitors in cancer therapy: impact of depsipeptide on molecular markers, therapeutic targets, and mechanisms of resistance. Blood. 2004;103:4636–43. doi: 10.1182/blood-2003-09-3068. [DOI] [PubMed] [Google Scholar]
- 3.Piekarz RL, Robey R, Sandor V, Bakke S, Wilson WH, Dahmoush L, et al. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood. 2001;98:2865–8. doi: 10.1182/blood.v98.9.2865. [DOI] [PubMed] [Google Scholar]
- 4.Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2008;26:332–3. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 5.Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–9. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prince HM, Geroge D, Patnaik A, Mita M, Dugan M, Butterfoss D, et al. Phase I study of oral LBH589, a Novel Deacetylase (DAC) Inhibitor in Advanced Solid Tumors and Non-Hodgkin’s Lymphoma. J Clin Oncol. 2007;25 (Suppl 18):3500. [Google Scholar]
- 7.Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299–307. doi: 10.1002/1096-9071(200011)62:3<299::aid-jmv1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Locasciulli A, Bruno B, Alessandrino EP, Meloni G, Arcese W, Bandini G, et al. Hepatitis reactivation and liver failure in haemopoietic stem cell transplants for hepatitis B virus (HBV)/hepatitis C virus (HCV) positive recipients: a retrospective study by the Italian group for blood and marrow transplantation. Bone Marrow Transplant. 2003;31:295–300. doi: 10.1038/sj.bmt.1703826. [DOI] [PubMed] [Google Scholar]
- 9.Cabrero JR, Serrador JM, Barreiro O, Mittelbrunn M, Naranjo-Suárez S, Martín-Cófreces N, et al. Lymphocyte chemotaxis is regulated by histone deacetylase 6, independently of its deacetylase activity. Mol Biol Cell. 2006;17:3435–45. doi: 10.1091/mbc.E06-01-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosley AJ, Meekings KN, McCarthy C, Shepherd D, Cerundolo V, Mazitschek R, et al. Histone deacetylase inhibitors increase virus gene expression but decrease CD8+ cell antiviral function in HTLV-1 infection. Blood. 2006;108:3801–7. doi: 10.1182/blood-2006-03-013235. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–92. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 12.Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53:1363–5. doi: 10.1136/gut.2004.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutsumi Y, Kawamura T, Saitoh S, Yamada M, Obara S, Miura T, et al. Hepatitis B virus reactivation in a case of non-Hodgkin’s lymphoma treated with chemotherapy and rituximab: necessity of prophylaxis for hepatitis B virus reactivation in rituximab therapy. Leuk Lymphoma. 2004;45:627–9. doi: 10.1080/1042819031000151923. [DOI] [PubMed] [Google Scholar]
- 14.Robbins AR, Jablonski SA, Yen TJ, Yoda K, Robey R, Bates SE, et al. Inhibitors of histone deacetylases alter kinetochore assembly by disrupting pericentromeric heterochromatin. Cell Cycle. 2005;4:717–26. doi: 10.4161/cc.4.5.1690. [DOI] [PubMed] [Google Scholar]
- 15.Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anti-cancer therapy. Hepatology. 2006;43:209–20. doi: 10.1002/hep.21051. [DOI] [PubMed] [Google Scholar]
- 16.Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, et al. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology. 2006;130:823–37. doi: 10.1053/j.gastro.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Chen WY, Bailey EC, McCune SL, Dong JY, Townes TM. Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc Natl Acad Sci USA. 1997;94:5798–803. doi: 10.1073/pnas.94.11.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye J, Gradoville L, Daigle D, Miller G. De novo protein synthesis is required for lytic cycle reactivation of Epstein-Barr Virus, but not Kaposi’s sarcoma-associated herpesvirus, in response to histone deacetylase inhibitors and protein kinase C agonist. J Virol. 2007;81:9279–91. doi: 10.1128/JVI.00982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Mahony D, Morris JC, Stetler-Stevenson M, Matthews H, Pittaluga S, Albert P, et al. EBV-Related lymphoproliferative Disease complicating Therapy with Siplizumab, a novel Anti-CD2 mediated T- and NK-cell depleting agent, in patients with T-cell malignancies. Blood. 2007;110 doi: 10.1158/1078-0432.CCR-08-1254. Abstract 3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng WH, Hong G, Delecluse HJ, Kenney SC. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol. 2004;78:1893–902. doi: 10.1128/JVI.78.4.1893-1902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieberman PM. Chromatin regulation of virus infection. Trends Microbiol. 2006;14:132–40. doi: 10.1016/j.tim.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Yates J, Warren N, Reisman D, Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci USA. 1984;81:3806–10. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roychowdhury S, Baiocchi RA, Vourganti S, Bhatt D, Blaser BW, Freud AG, et al. Selective efficacy of depsipeptide in a xenograft model of Epstein-Barr virus-positive lymphoproliferative disorder. J Natl Cancer Inst. 2004;96:1447–57. doi: 10.1093/jnci/djh271. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues M, Westerman D, Lade S, McCormack C, Prince HM. Methotrexate-induced lymphoproliferative disorder in a patient with Sézary syndrome. Leuk Lymphoma. 2006;47:2257–9. doi: 10.1080/10428190600799961. [DOI] [PubMed] [Google Scholar]
- 25.Zettl A, Lee SS, Rüdiger T, Starostik P, Marino M, Kirchner T, et al. Epstein-Barr virus-associated B-cell lymphoproliferative disorders in anglo-immunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol. 2002;117:368–79. doi: 10.1309/6UTX-GVC0-12ND-JJEU. [DOI] [PubMed] [Google Scholar]