Myocardial fibrosis/necrosis has been documented by histological and Cardiovascular Magnetic Resonance (CMR) studies in patients with hemoglobinopathies.1 The delayed enhancement CMR technique with intravenous administration of gadolinium (Gd) chelates contrast agents is the only validated approach for detecting myocardial fibrosis non-invasively.2
Recently, serious concerns have been raised regarding the safety of Gd chelates. In particular, a link between gadolinium and nephrogenic systemic fibrosis (NSF) has emerged, due to the presence of gadolinium in skin samples of NSF patients.3 However, NSF has also been diagnosed in patients who had not been exposed to gadolinium. NSF is a scleroderma-like disease mainly involving the skin and it is closely related to severe kidney failure (glomerular filtration rate (GFR) < 30 mL/min/1.73 m2).4 No cases have been reported in patients with GFR > 60 mL/min/1.73 m2. Although cause and effect have not been proven for the NSF-gadolinium link, avoidance and care have been strongly recommended.5 In addition, unconfirmed doubts have been raised about the use of Gd-chelate contrast agents in patients with hemoglobinopathies, characterized by heavy co-morbidity due to iron overload, which can also damage the kidneys and could be a co-factor, further enhancing the risk for NSF development.6 To date, there have been no dedicated clinical studies on the safety of the Gd chelates in these patients.
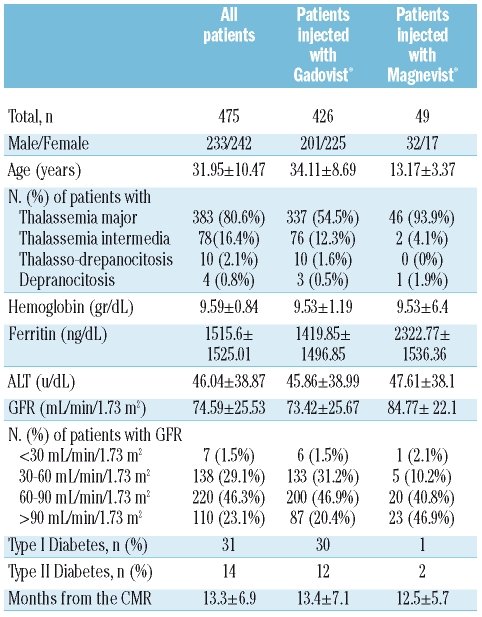
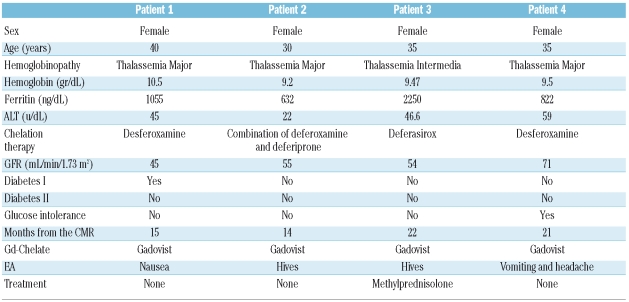
We administered Gd chelates contrast agents in 475 patients with hemoglobinopathies, enrolled in the MIOT (Myocardial Iron Overload in Thalassemia) network.7 The patients older than 18 years were injected with gadobutrolo (Gadovist®; Bayer Schering Pharma; Berlin, Germany) and the patients younger than 18 years were injected with gadopentate dimeglumine (Magnevist®; Bayer Schering Pharma; Berlin, Germany), both at the standard dose of 0.2 mmol/kg. Kidney function was assessed by measuring the GFR, estimated by means of the Modification of Diet in Renal Disease formula8 for patients older than 13 years and by Schwartz formula9 for patients younger than 13 years. The values were normalized to body surface area. Seven patients (6 injected with gadobutrolo and one injected with gadopentate dimeglumine) had severe renal dysfunction. Since diabetes is a leading cause of kidney disease, its presence was also evaluated. The clinical characteristics of all patients are summarized in Table 1. All patients gave written informed consent. They were monitored at the reference thalassemia center every 45 days from the day of CMR up to December 2008 in order to detect the potential insurgence of adverse events (AEs), defined as unfavorable and unintended symptoms associated with the use of gadolinium. In particular, the possible development of first symptoms/signs for NSF, such as hardening of the skin and development of plaques affecting the buttocks, trunk and extremities were controlled. The mean time of the monitoring was 13±7 months; the monitoring time was more than 18 months in 133 patients (28%). We found myocardial fibrosis in 95 (20%) of the 475 thalassemia or sickle cell patients enrolled in the study, suggesting a clinical use of Gd chelates contrast agents in CMR in the hemoglobinopathies. Of the patients injected with gadopentate dimeglumine, none manifested AEs. Of the 426 patients injected with gadobutrolo, only 4 (0.94%) manifested AEs, all classifiable as mild. All patients were female with a mean age of 35±4.1 years and had thalassemia. Of 4 patients with AEs, 3 showed a moderate reduction (60–30 mL/min/1.73 m2) of GFR. All AEs were resolved before the patients left the hospital and in only one case was it necessary to administer drugs. Table 2 shows the types of AEs observed and the characteristics of the patients who manifested them. The incidence of mild AEs resulted comparable with the data reported in literature for patients without hemoglobinopathies. Although 150 patients (32%) injected with gadobutrolo or gadopentate dimeglumine had moderate to severe kidney dysfunction, no cases of NFS have been detected. Although a potential risk factor for the development of NSF, such as iron overload, was significantly present in our population, it did not seem to play a relevant role.
Table 1.
Patients’ characteristics.
Table 2.
Characteristics of the patients who manifested adverse events.
In conclusion, in a large cohort of thalassemia and sickle cell patients the use of gadopentate dimeglumine and gadobutrolo in CMR seems to be safe and well tolerated, with a risk comparable to the general population. These data support the routine use of Gd chelates contrast agents in MR and especially in CMR to detect myocardial fibrosis/necrosis for diagnostic and clinical management of thalassemia or sickle cell patients. However, gadolinium should be used with caution in patients with hemoglobinopathies who have severe renal dysfunction.
Footnotes
Funding: the MIOT project has received “no-profit support” from Chiesi, Bayer-Schering and GE Healthcare. It is also supported by the Italian Foundation “Leonardo Giambrone” and was undertaken on behalf of the Society for Thalassemia and Hemoglobinopathies (SOSTE).
References
- 1.Meloni A, Pepe A, Positano V, Favilli B, Maggio A, Capra M, et al. Influence of myocardial fibrosis and blood oxygenation on heart T2* values in thalassemia patients. J Magn Reson Imaging. 2009;29:832–7. doi: 10.1002/jmri.21704. [DOI] [PubMed] [Google Scholar]
- 2.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357:21–8. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 3.Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2:264–7. doi: 10.2215/CJN.03921106. [DOI] [PubMed] [Google Scholar]
- 4.DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19:191–4. doi: 10.1111/j.1525-139X.2006.00152.x. [DOI] [PubMed] [Google Scholar]
- 5.Perazella MA, Rodby RA. Gadolinium use in patients with kidney disease: a cause for concern. Semin Dial. 2007;20:179–85. doi: 10.1111/j.1525-139X.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 6.Perazella MA. Gadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosis. Curr Drug Saf. 2008;3:67–75. doi: 10.2174/157488608783333989. [DOI] [PubMed] [Google Scholar]
- 7.Meloni A, Ramazzotti A, Positano V, Salvatori C, Mangione M, Marcheschi P, et al. Evaluation of a web-based network for reproducible T2* MRI assessment of iron overload in thalassemia. Int J Med Inform. 2009;78:503–12. doi: 10.1016/j.ijmedinf.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–63. [PubMed] [Google Scholar]