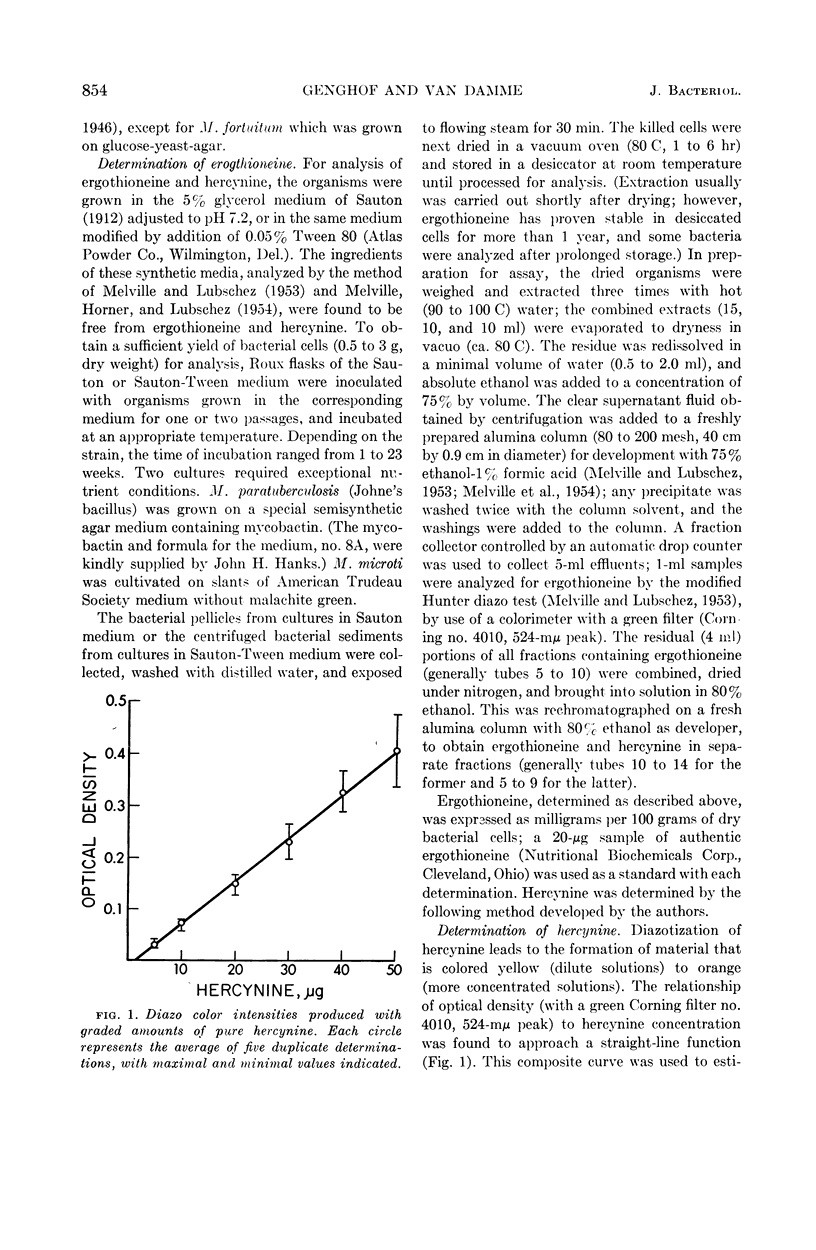
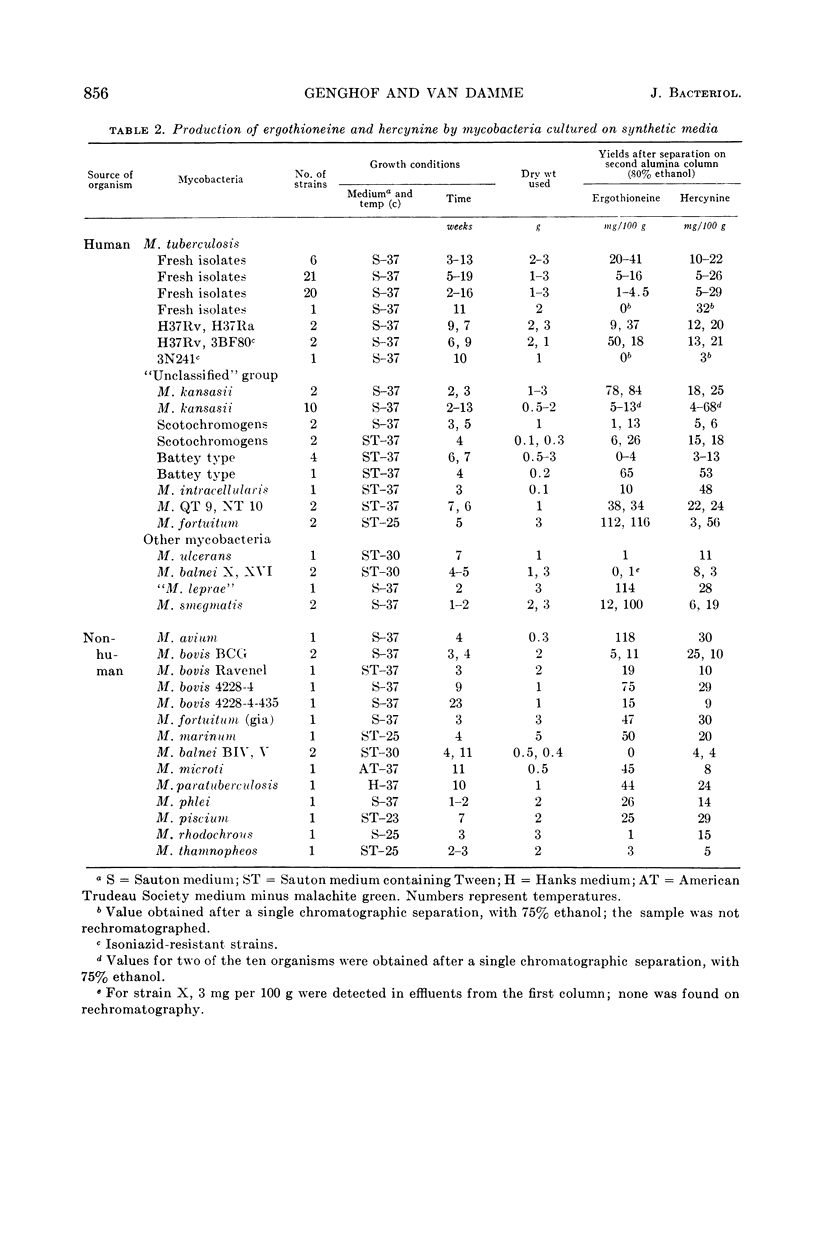
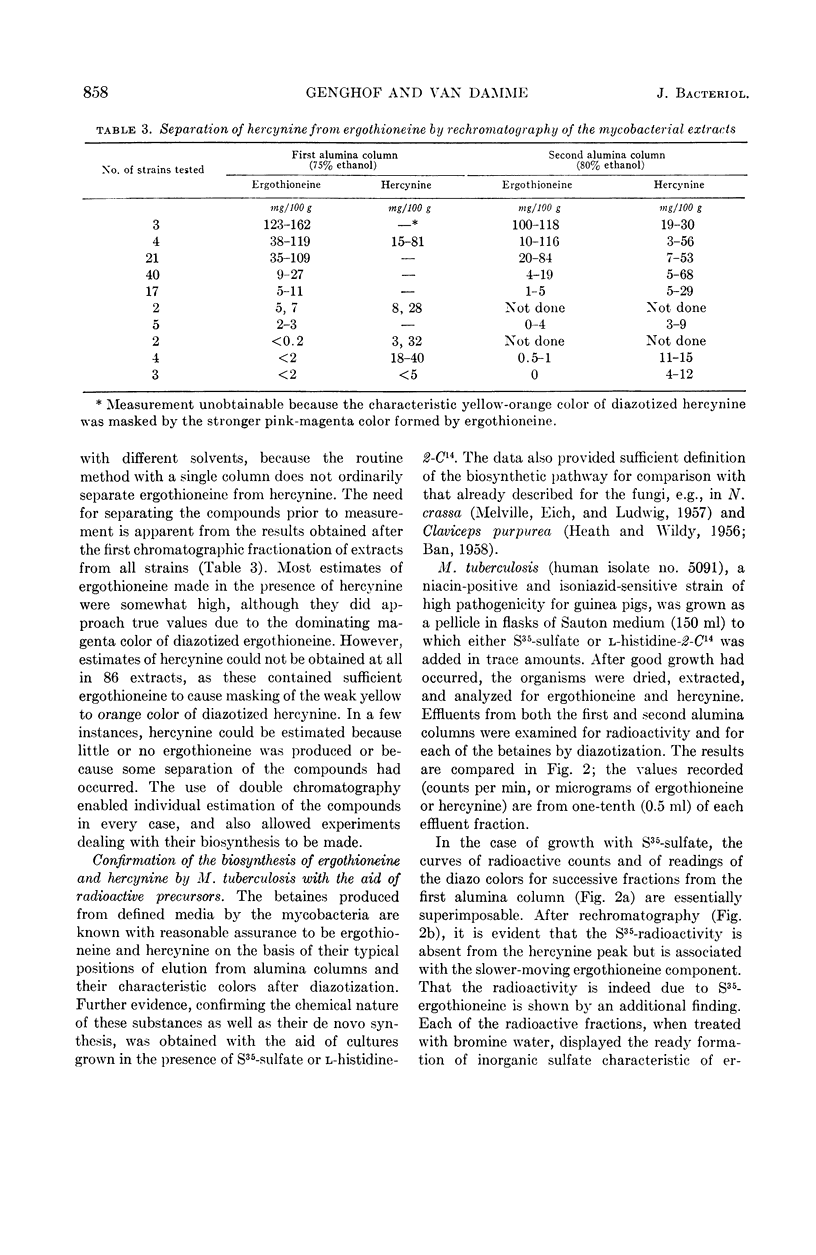
Abstract
Genghof, Dorothy S. (Yeshiva University, New York, N.Y.), and Olga Van Damme. Biosynthesis of ergothioneine and hercynine by mycobacteria. J. Bacteriol. 87:852–862. 1964.—Ergothioneine and hercynine were found to be synthesized by a wide variety of mycobacteria grown in chemically defined media free from these compounds. The cultures examined included 53 recently isolated and laboratory strains of Mycobacterium tuberculosis, 26 “unclassified” mycobacteria (Runyon groups I to IV), and representatives of most other species in the genus. Purification and separation of the betaines was achieved by means of chromatography on two successive alumina columns. Photometric measurement of the diazotized effluents from the second column permitted amounts of each compound to be determined. Measurement of hercynine by this method was made possible for the first time by the development of a standard curve. The pathway of ergothioneine biosynthesis in mycobacteria, as judged by the use S35-sulfate and l-histidine-2-C14 as tracers, appears similar to that found in Neurospora crassa and Claviceps purpurea, that is, from histidine to ergothioneine via hercynine. None of a small group of bacteria other than mycobacteria was found to produce ergothioneine. Two strains of group A streptococci and one of Escherichia coli produced hercyninelike material, as yet unidentified.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMANN D., LIST P. H., MENSSEN H. G. Uber das Vorkommen von Herzynin neben Ergothionein in der Samenflussigkeit des Ebers sowie in Rinder-Erythrocyten und die biologische Beziehung. Hoppe Seylers Z Physiol Chem. 1959;314(1):33–37. [PubMed] [Google Scholar]
- ACKERMANN D., LIST P. H. Uber das Vorkommen von Herzynin, Ergothionein, Homarin, Trigonellin, Glykokollbetain, Cholin, Trimethylamin, Adenin und fast sämtlicher Aminosäuren des Eiweisses in Limulus polyphemus L. Hoppe Seylers Z Physiol Chem. 1958;313:30–36. doi: 10.1515/bchm2.1958.313.1.30. [DOI] [PubMed] [Google Scholar]
- ASKARI A., MELVILLE D. B. The reaction sequence in ergothioneine biosynthesis: hercynine as an intermediate. J Biol Chem. 1962 May;237:1615–1618. [PubMed] [Google Scholar]
- BAN R. W., STOWELL E. C., Jr Production of ergothioneine by Claviceps purpurea in defined culture. Arch Biochem Biophys. 1956 Jul;63(1):259–260. doi: 10.1016/0003-9861(56)90031-5. [DOI] [PubMed] [Google Scholar]
- BINFORD C. H. Histiocytic granulomatous mycobacterial lesions produced in the golden hamster (Cricetus auratus) inoculated with human leprosy: negative results in experiments using other animals. Lab Invest. 1959 Sep-Oct;8:901–924. [PubMed] [Google Scholar]
- BOJALIL L. F., CERBON J., TRUJILLO A. Adansonian classification of mycobacteria. J Gen Microbiol. 1962 Jun;28:333–346. doi: 10.1099/00221287-28-2-333. [DOI] [PubMed] [Google Scholar]
- BUHLER V. B., POLLAK A. Human infection with atypical acid-fast organisms; report of two cases with pathologic findings. Am J Clin Pathol. 1953 Apr;23(4):363–374. doi: 10.1093/ajcp/23.4.363. [DOI] [PubMed] [Google Scholar]
- Barger G., Ewins A. J. The Identity of Trimethylhistidine (Histidine-Betaine) from Various Sources. Biochem J. 1913 Mar;7(2):204–206. doi: 10.1042/bj0070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Gillman W., Hottle G. A., Pappenheimer A. M. An Improved Medium for the Cultivation of Hemolytic Streptococcus. J Bacteriol. 1942 Apr;43(4):495–498. doi: 10.1128/jb.43.4.495-498.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUTTINO J. T., McCABE A. M. Pure granulomatous nocardiosis, a new fungus disease distinguished by intracellular parasitism; a description of a new disease in man due to a hitherto undescribed organism, Nocardia intracellularis, n. sp., including a study of the biologic and pathogenic properties of this species. Am J Pathol. 1949 Jan;25(1):1–47. [PMC free article] [PubMed] [Google Scholar]
- DARZINS E. The epizootic of tuberculosis among the Gias in Bahia. Acta Tuberc Scand. 1952;26(1-2):170–174. [PubMed] [Google Scholar]
- FRASER R. S. Blood ergothioneine levels in disease. J Lab Clin Med. 1951 Feb;37(2):199–206. [PubMed] [Google Scholar]
- GENGHOF D. S., INAMINE E., KOVALENKO V., MELVILLE D. B. Ergothioneine in microorganisms. J Biol Chem. 1956 Nov;223(1):9–17. [PubMed] [Google Scholar]
- GORDON R. E., MIHM J. M. A comparative study of some strains received as nocardiae. J Bacteriol. 1957 Jan;73(1):15–27. doi: 10.1128/jb.73.1.15-27.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEATH H., WILDY J. The biosynthesis of ergothioneine and histidine by Claviceps purpurea. I. The incorporation of [2-14C]acetate. Biochem J. 1956 Dec;64(4):612–620. doi: 10.1042/bj0640612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELVILLE D. B., HORNER W. H., LUBSCHEZ R. Tissue ergothioneine. J Biol Chem. 1954 Jan;206(1):221–228. [PubMed] [Google Scholar]
- MELVILLE D. B., LUBSCHEZ R. A method for the determination of ergothioneine in blood. J Biol Chem. 1953 Jan;200(1):275–285. [PubMed] [Google Scholar]
- MELVILLE D. B., LUDWIG M. L., INAMINE E., RACHELE J. R. Transmethylation in the biosynthesis of ergothionelne. J Biol Chem. 1959 May;234(5):1195–1198. [PubMed] [Google Scholar]
- RUNYON E. H. Anonymous mycobacteria in pulmonary disease. Med Clin North Am. 1959 Jan;43(1):273–290. doi: 10.1016/s0025-7125(16)34193-1. [DOI] [PubMed] [Google Scholar]



