Abstract
Background:
Treatment of obstructive sleep apnea (OSA) in outpatients with systolic heart failure improves cardiac function. We evaluated the impact of immediate inpatient diagnosis and treatment of OSA in hospitalized patients with acutely decompensated heart failure (ADHF) on in-hospital cardiac outcomes.
Methods:
A pilot randomized controlled trial was conducted in an academic heart hospital. Patients with ADHF underwent an attended in-hospital sleep study within 2 days of hospital admission to establish the diagnosis of sleep-disordered breathing. The participants were 46 consecutive patients with ADHF who had OSA (apnea-hypopnea index [AHI], ≥ 15 events per hour). Participants were randomly assigned to either the intervention arm (n = 23), with in-hospital treatment of OSA using auto-adjusting positive airway pressure along with standard treatment of ADHF, or to the control arm (n = 23), in which they received only standard treatment for ADHF. The primary outcome was the change in left ventricular ejection fraction (LVEF) 3 nights postrandomization.
Results:
The change in LVEF from baseline to 3 days postrandomization in the intervention arm was significantly superior to that of the control group. The difference in LVEF improvement was 4.6% (p = 0.03). LVEF increased in the intervention group by 4.5% (SE, 1.7%). The LVEF change in the control arm was − 0.3% (SE, 1.5%). The difference in LVEF improvement between the two groups persisted after adjustment for baseline LVEF, type of cardiomyopathy, BMI, AHI, and sex.
Conclusions:
An approach of early identification and in-hospital treatment of OSA in patients with ADHF is feasible and resulted in improvement in systolic function. The impact of this approach on out-of-hospital outcomes requires further investigation.
Trial registration:
ClinicalTrials.gov Identifier: NCT00701038
Heart failure remains one of the most common causes of morbidity and mortality in the United States.1 The human and economic burden of heart failure is largely associated with hospitalizations due to clinical decompensation.1–3 The treatment options for acutely decompensated heart failure (ADHF) remain limited, and have not clearly impacted mortality.4,5 The identification and treatment of highly prevalent comorbidities with known detrimental effects such as obstructive sleep apnea (OSA) may, therefore, have a high potential for a clinically important impact.6
Patients with heart failure have a higher prevalence of OSA than the general population.7–11 OSA, an established cause of hypertension,12,13 also worsens the control of cardiovascular diseases such as coronary disease,14,15 arrhythmia,16–18 and hypertension,19,20 all of which are causes of ADHF.1,21 An association between OSA and poor outcome in patients with heart failure was shown recently.22 The treatment of OSA with positive airway pressure (PAP) improves oxygenation, sympathetic nerve activity,23,24 and afterload,25,26 resulting in improved systolic function in patients with chronic heart failure and OSA.26,27 Therefore, the systematic identification and treatment of OSA in patients with systolic heart failure may provide an important contribution to the management of heart failure. Such an approach is not part of the current standard of care for stable patients with heart failure.28 Moreover, this approach to expedited diagnosis and treatment of OSA during hospital admissions for ADHF, to our knowledge, has not been previously studied in a randomized trial.
Thus, we hypothesized that an approach of expedited in-hospital diagnosis and treatment of OSA in patients with ADHF, to our knowledge, would improve cardiac function prior to hospital discharge relative to the current standard of care. Because, to our knowledge, a trial to test this hypothesis has yet to be reported, we also sought to explore the feasibility of inpatient diagnosis and treatment of OSA in hospitalized patients with ADHF.
Materials and Methods
Participants were consecutive patients hospitalized with ADHF at the Ohio State University Ross Heart Hospital in whom OSA had been recently diagnosed during their admission. ADHF was defined as a chief complaint of dyspnea and left ventricular ejection fraction (LVEF) of ≤ 45%. Additionally, elevated left ventricular pressure, as indicated by at least one sign and one symptom of volume overload, was required (ie, pedal edema, crackles, consistent chest radiograph, increased left ventricular end-diastolic diameter, elevated brain natriuretic peptide [BNP] levels).5 In order to be eligible for randomization, patients had to have a projected length of stay of ≥ 3 days from the morning following the sleep study. The protocol was approved by the Ohio State University Institutional Review Board (2005H0186), and all patients provided informed written consent. The exclusion criteria were as follows: (1) patients who were already using PAP for the treatment of sleep-disordered breathing (SDB) and patients with central sleep apnea (CSA); (2) hemodynamic instability, defined as a mean arterial BP < 55 mm Hg while not receiving vasopressors, or concurrent use of vasopressors; (3) patients with respiratory insufficiency, defined by Pao2/Fio2 ratio < 250 mm Hg; and (4) renal failure requiring renal replacement therapy.
Protocol
Screening for Inclusion:
All patients with ADHF at our institution underwent an inpatient attended sleep study on the first or second night of hospital admission. The sleep study (Stardust II; Respironics; Murrysville, PA) measured nasal flow, respiratory effort, oxygen saturation, and body position. The night shift nurses administered and monitored the sleep study. Apnea was scored when complete cessation of flow occurred. Hypopnea was scored when a 50% reduction in the flow signal occurred in association with 3% desaturation. A 10-s duration was required for all events.29 SDB was defined by an apnea-hypopnea index (AHI) of > 15 events per hour. CSA is SDB with at least 50% of the events being central. If > 50% of the events were obstructive, the disorder was considered to be OSA. Hypopneas that occurred in the setting of periodic breathing (crescendo-decrescendo pattern with a cycle time of 50 to 70 s or longer) were scored as central events. Hypopneas that had a flow limitation pattern on the nasal pressure signal were classified as obstructive. A similar testing strategy has been reported previously.11 To be eligible for study enrollment, patients had to have an AHI of ≥ 15 events per hour with at least 5 events per hour being obstructive apneas (obstructive apnea index, ≥ 5).
Enrollment and Randomization:
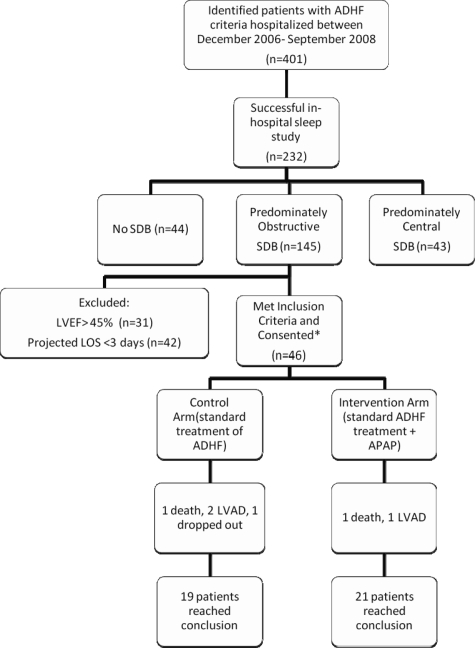
The most common reason for not meeting the inclusion criteria in patients with ADHF who had OSA found on the sleep study was an LVEF ≥ 45% or a projected length of hospital stay of < 3 nights (Fig 1). Randomization was achieved by using the sealed-envelope method with permuted blocks of 10.
Figure 1.
Disposition of participants. The most common reasons for exclusion (64%) were LVEF > 45% and projected length of stay < 3 days on the morning of the sleep study. LOS = length of stay; LVAD = left ventricular assist device; OSDB = obstructive SDB.
Intervention:
Along with the standard treatment for ADHF, patients randomly assigned to the intervention arm (n = 23) were treated with nocturnal auto-adjusting bilevel PAP (APAP) [BIPAP auto; Respironics], with an algorithm that targets only upper airway obstruction (spontaneous and no servo feature). The treatment was started on the night following randomization and continued past hospital discharge. The effectiveness and safety of treatment were evaluated by a daily review of downloads from the device and adjustment of the pressure range. The pressure range was started at 5 to 15 cm H2O on the first night, and the pressure was subsequently fixed whenever possible at the optimal level suggested by the download review on the second or third night. The gradient between inspiratory and expiratory pressures was kept at < 3 cm H2O at all times. Random sleep studies using the same device were performed on treated patients to confirm the correlation between the AHI determined from the device download with the AHI determined by the in-hospital sleep study.
Outcome Measures:
Demographics and baseline outcome measures were collected prior to but on the same day of randomization. The primary outcome measure was as follows: LVEF was evaluated by echocardiography using the biplane Simpson method, and was performed at baseline before randomization (day 0) and on the morning 3 nights after randomization (allowing for 3 nights of APAP in the treatment group). Two blinded sonographers performed all echocardiographic examinations. Secondary outcome measures were collected on the morning 3 nights after randomization as follows: (1) sympathetic activity, as determined by the measurement of plasma norepinephrine levels and 24-h urinary norepinephrine levels at baseline and 3 days postrandomization; (2) measurement of BNP levels; (3) daily measurement of plasma BUN levels; (4) systolic and diastolic BP; and (5) daily weight measurement.
Statistical Analysis:
The study was designed as a pilot for the planning of a larger trial, which included several important inpatient and outpatient outcomes. In this pilot trial, we could not expect significant changes in long-term outcomes except for LVEF. For LVEF, our expectation was based on a prior outpatient trial27 with a longer intervention time in which a large impact of 3 SDs was found.
The primary outcome measurement was LVEF. We used a longitudinal mixed model with LVEF measurement at baseline and 3 days postrandomization.29 The longitudinal mixed model allowed us to use the missing data as random assumptions in a statistical software package (Proc Mixed, in SAS, version 9.3; SAS Institute; Cary, NC), but our results were almost identical to those of an analysis of covariance approach30,31 (baseline LVEF used as the covariate) because of limited missing data and the similarity of conditional and unconditional models in this case. We tested the primary hypothesis about LVEF with α = 0.05, and all other reported p values were provided for sensitivity analysis or secondary outcomes and not for primary hypothesis testing. A comparison between the two groups was based on the intention-to-treat principle, but we also explored the relationship between adherence and LVEF improvement in the treatment group. This pilot trial was large enough to have adequate power for the primary outcome of LVEF, only if we assume that the effect was as large as that seen in a previous outpatient trial.27 We expected a smaller effect size for other outcomes.
For the primary outcome, we were missing two postrandomization LVEF measurements in the treatment group because of one left ventricular assist device implantation and one death. In the control group, one death, two left ventricular assist device implantations, and one drop out accounted for the four missing postrandomization LVEF measurements.
Results
Characteristics of Participants
During the study period between December 2006 and September 2008, 46 patients with ADHF and newly diagnosed OSA were enrolled into the study and randomly assigned to either the intervention arm (n = 23) or the control arm (n = 23). The baseline characteristics of the participants are summarized in Table 1. There was no apparent imbalance between the two groups. Importantly, 19 patients in each group were receiving IV diuretics during randomization. One patient in each group received ultrafiltration for volume removal. The use of β-blockers and angiotensin-converting enzyme inhibitors was balanced between the two groups (Table 1).
Table 1.
Baseline Characteristics and Other Characteristics of Participants
| Baseline Characteristics | Control Group (n = 23) | Intervention Group(n = 23) | 95% CI |
|---|---|---|---|
| LVEF, % | 25.4 (1.8) | 26.3 (1.8) | −6.1 to 4.3 |
| AHI, events/h | 33 (3.0) | 36 (2.0) | −11 to 5 |
| BNP level | 1,154 (261)* | 1,117 (259)† | −708 to 780 |
| SBP, mm Hg | 110 (4.0) | 107 (4.0) | −8 to 14 |
| DBP, mm Hg | 67 (2.0) | 66 (2.0) | −5 to 8 |
| Heart rate,beats/min | 88 (4.0) | 81 (3.0) | −3 to 17 |
| Creatinine level | 1.3 (0.08) | 1.4 (0.13) | −0.4 to 0.2 |
| LVEDD, mm | 62 (2.0)† | 64 (2.0)* | −8 to 2 |
| LVEDV, mL | 227 (18)‡ | 235 (20)§ | −63 to 47 |
| LVESD, mm | 54 (2)† | 56 (2)‡ | −8 to 4 |
| LVESV, mL | 169 (15)‡ | 171 (17)§ | −48 to 44 |
| Other characteristics | |||
| Age, yr | 58 (3.0) | 55 (3.0) | −5 to 11 |
| BMI, kg/m2 | 32 (2.0) | 35 (3.0) | −10 to 3 |
| Male gender, % | 83 (8.0) | 65 (10) | −9 to 44 |
| Ischemic cardiomyopathy, % | 78 (9.0) | 87 (7.0) | −32 to 14 |
| Receivingβ-blocker therapy (carvedilol/metoprolol), % | 83 (8.0) | 74 (9.0) | −16 to 34 |
| Receiving angiotensin-converting enzyme inhibitor therapy, % | 65 (10) | 48 (11) | −12 to 47 |
| Diabetes, % | 54 (11) | 61 (10) | −41 to 20 |
Values are given as the mean (SE), unless otherwise indicated. DBP = diastolic BP; LVEDD = left ventricular end-diastolic diameter; LVESD = left ventricular end systolic diameter; SBP = systolic BP.
*n = 20 (No. is smaller than the group size because of missing values).
†n = 22 (No. is smaller than the group size because of missing values).
‡n = 19 (No. is smaller than the group size because of missing values).
§n = 17 (No. is smaller than the group size because of missing values).
Effect of In-Hospital Treatment of OSA on LVEF
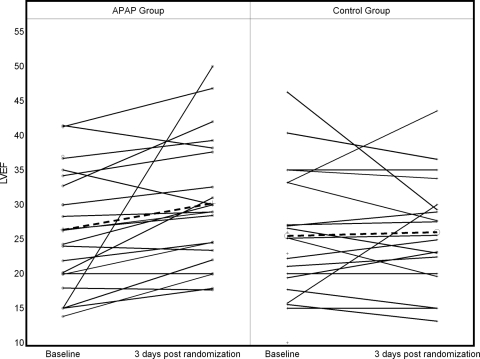
The difference in LVEF improvement between the two groups was 4.6% (p = 0.031) in favor of the APAP intervention. At study conclusion, the LVEF was 30.4% in the intervention group and 25.8% in the control group. The change in LVEF after 3 nights of treatment in the intervention group was an increase of 4.5% (SE = 1.7), while the change in LVEF in the control group was a nonsignificant decrease of − 0.3% (SE = 1.5). These changes and other echocardiographic parameters are detailed in Table 2. Note that changes in left ventricular end-systolic volume (LVESV) and left ventricular end-diastolic volume (LVEDV) were also significantly superior in the treatment group. The difference in the change of LVESV between the two groups was 24.8 mL (p = 0.0007), while the difference in the change of LVEDV was 23.9 mL (p < 0.03). Figure 2 provides the change in LVEF from baseline to 3 days postrandomization for each patient. The trends make apparent the consistency in improvement for the treatment group.
Table 2.
Effect of In-Hospital APAP on Cardiac Function 3 Days Postrandomization
| Variables | Control | Treatment | Difference Between APAP Effects/p Value |
|---|---|---|---|
| LVEF, % | 4.6/0.03 | ||
| Final, 3 d postrandomization | 25.8 | 30.4 | |
| Baseline | 25.4 | 26.3 | |
| Change from baseline (final to baseline) | − 0.2 | 4.4 | |
| LVESV, mL | −24.8/0.0007 | ||
| Final, 3 d postrandomization | 169 | 144 | |
| Baseline | 169 | 171 | |
| Change from baseline (final to baseline) | 3.2 | − 22.1 | |
| LVEDV, mL | −23.9/0.03 | ||
| Final, 3 d postrandomization | 228 | 204 | |
| Baseline | 227 | 235 | |
| Change from baseline (final to baseline) | 2.1 | -22 |
Figure 2.
Individual change of LVEF in control and intervention arms. Dotted lines represent the average change for each group. Note that the patient in the treatment group with a 35% change in LVEF was removed in the sensitivity analysis, and the significance of the treatment advantage persisted (LVEF difference, 3.3%; p = 0.03).
In a sensitivity analysis, we removed the one patient with the large LVEF improvement of 35% (Fig 2). The superiority of APAP treatment remained significant (difference in LVEF change was 3.3% in favor of the treatment group; p = 0.03). In addition, the difference in LVEF improvement was not affected by adjustment for baseline AHI, sex, BMI, and underlying type of cardiomyopathy. The results of adjustment to selected variables are listed in Table 3.
Table 3.
Sensitivity Analysis of APAP Effect on LVEF
| Covariate Adjustments | APAP Effect on LVEF | p Value |
|---|---|---|
| AHI | 5.1 | 0.02 |
| BMI | 4.5 | 0.04 |
| Cardiomyopathy type | 4.5 | 0.04 |
| Gender | 4.3 | 0.04 |
Effect of APAP Treatment on Other In-Hospital Measures
Table 4 provides other changes from baseline to day 3 for the two groups. No significant differences between the groups were found except for weight decrease. However, these were not planned analyses.
Table 4.
Effect of APAP on Hemodynamic and Neurohumoral Measures
| Control Group |
Treatment Group |
Change From Baseline |
|||||
|---|---|---|---|---|---|---|---|
| Parameters | Baseline | Final | Baseline | Final | Control Group | Treatment Group | p Value |
| 24-h urinary norepinephrine, μg/24 h | 0.041 | 0.038 | 0.034 | 0.023 | − 0.003 | − 0.011 | − 0.0008 |
| (0.18) | |||||||
| BNP level, pg/mL | 1,223 | 1,240 | 1,121 | 664 | 17 | − 457 | − 474 |
| (0.13) | |||||||
| BUN level, mg/dL | 24.9 | 22.5 | 28.7 | 30.3 | − 2.4 | 1.6 | 4.1 |
| (0.18) | |||||||
| Creatinine level, mg/dL | 1.30 | 1.27 | 1.36 | 1.57 | − 0.03 | 0.21 | − 0.24 |
| (0.19) | |||||||
| Systolic BP, mm Hg | 110 | 105 | 107 | 100 | − 5.2 | − 6.5 | − 1.3 |
| (0.78) | |||||||
| Diastolic BP, mm Hg | 66 | 65 | 66 | 63 | − 1.2 | − 3 | − 1.9 |
| (0.6) | |||||||
| Weight, kg | 97 | 96 | 102 | 100 | − 0.5 | − 2 | − 1.5 |
| (0.048) | |||||||
Respiratory Parameters
The mean (± SD) duration of in-hospital APAP use in the treatment group was 3.2 ± 0.5 hours per night of hospital stay in the 23 patients randomly assigned to APAP. The mean inspiratory positive airway pressure was 9.8 ± 2 cm H2O, and the mean expiratory positive airway pressure was 7 ± 2 cm H2O. All patients in the treatment group had an average AHI of < 4 events per hour as determined from the device download. Adjustment to the pressure level and the number of hours of nightly use did not explain the significant LVEF improvement in the treatment group.
Discussion
To our knowledge, this is the first trial to evaluate the effect of inpatient identification and treatment of OSA on cardiac function in patients with ADHF. In this randomized controlled trial, 46 patients with ADHF and newly diagnosed OSA were randomly assigned to either a group receiving in-hospital treatment of OSA with APAP or to a control group receiving no treatment for OSA. There was no difference in the treatment of ADHF between the two groups. Patients in the intervention arm experienced a significantly greater increase in their LVEF after 3 nights of nocturnal APAP than control patients. The difference in LVEF improvement persisted after adjustment for type of cardiomyopathy, AHI, and BMI.
The mechanism of the effect of inpatient PAP treatment in the setting of ADHF and OSA was not part of the design of this trial. The relatively fast improvement suggests that the change is probably related to improved cardiac volumes and hemodynamics including preload with APAP treatment. Patients who received treatment had a significant reduction in the LVEDV and LVESV. Additionally, in our exploratory analysis, there was an advantage of more weight loss in the APAP group, which may be consistent with improved preload. PAP is also suggested to reduce afterload by increasing the intrathoracic pressure and to improve the myocardiac energy requirement.32,33 Left ventricular wall stress is mainly determined by the systolic BP and LVESV. While not directly tested in this study, the lack of change in the BP may suggest a reduction in left ventricular wall stress through the decrease in LVESV.
Whether in-hospital PAP treatment, independent of underlying OSA, has a role in the broader setting of ADHF management will require further evaluation. In cases of ADHF and respiratory insufficiency, PAP is already established as an effective method of ventilatory support.34 Several studies demonstrated that noninvasive ventilation was safe in this setting, and resulted in improved gas exchange, increased LVEF, and reduced left ventricular filling pressure in patients with ADHF.34,35,36 To our knowledge, this is the first study to evaluate the use of PAP designed only to maintain the patency of the upper airway during sleep and not as a form of ventilation in the setting of ADHF. We excluded patients with significant hypoxia or hemodynamic instability. The decision to use APAP over continuous PAP was based on the potential for more tolerance, on the recognition of the frequency of changes in the sleep-wake state and upper airway tone in ADHF patients, and on the need to administer the least possible pressure to differentiate the treatment from its use for noninvasive ventilation.
A limitation of this trial is the use of an inpatient cardiopulmonary sleep study rather than polysomnography for the diagnosis of OSA. We have validated our approach against polysomnography.37 Additionally, an identical device was already shown to have excellent ability in the unattended setting to discriminate between OSA and CSA in patients with heart failure when compared with polysomnography.38 The sensitivity of these methods is further enhanced by the attended setting of our sleep studies. The use of the inpatient study was not intended for definitive diagnosis; rather, it was to identify patients who manifest an obstructive pattern of SDB during the episode of ADHF and are candidates for PAP, so treatment could begin during hospital admission.
The trend toward improvement in all exploratory parameters in this underpowered study, despite the short treatment duration, should be interpreted as being consistent with a strong treatment effect rather than with lack of significance. This pilot randomized controlled trial demonstrated that the early identification of OSA in hospitalized patients with ADHF and the immediate initiation of PAP treatment during the decompensation episode improved systolic function. This improvement in LVEF was small, but occurred within 3 days of treatment in this pilot study, and persisted after adjustment to multiple covariates. Improvement in several clinical parameters was also observed, including improvement in cardiac filling pressures and more weight loss in the treatment group, further supporting a consistent effect for in-hospital treatment. The decrease in LVESV may denote reduced systolic workload and predict future remodeling with continuous treatment. The findings of this study should be confirmed in a larger trial that addresses the impact of this approach on other inpatient and out-of-hospital outcomes. Overall, the positive findings of this study, taken together with previous studies in the area of OSA and heart failure,27 support the consideration of a change in clinical practice in favor of the expedited identification of OSA in hospitalized patients with heart failure.
Acknowledgments
Author contributions: All authors contributed to the research for and writing of the article. Drs. Khayat, Abraham, and Jarjoura designed the research. Drs. Khayat and Pu, and Mr. Patt conducted and coordinated the research. Dr. Jarjoura performed the analysis and modeling of the data. Dr. Khayat drafted the article. Dr. Jarjoura reviewed, revised, and edited the article. Mr. Patt contributed to the “Material and Methods” section and the figures. Drs. Pu and Abraham reviewed, contributed to, and edited the introduction, the “Materials and Methods” section, and the “Discussion” section. Dr. Khayat is the principal investigator on the research grant supporting this project, had full access to the data, and assumes responsibility for the integrity of the data and the accuracy of the analysis.
Financial/nonfinancial disclosures: Drs. Khayat and Abraham have received research support from Respironics, Inc. Drs. Pu and Jarjoura, and Mr. Patt have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations:
- ADHF
acutely decompensated heart failure
- AHI
apnea-hypopnea index
- APAP
auto-adjusting bilevel positive airway pressure
- BNP
brain natriuretic peptide
- CSA
central sleep apnea
- LVEDV
left ventricular end-diastolic volume
- LVEF
left ventricular ejection fraction
- LVESV
left ventricular end-systolic volume
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- SDB
sleep-disordered breathing
Footnotes
This research was supported by National Institutes of Health grant R21HL092480-01 (to Drs. Khayat, Abraham, Pu, and Jarjoura). The research was also supported by a research grant from Respironics, Inc.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
For editorial comment see page 953
References
- 1.Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics: 2008 update; a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.O'Connell JB. The economic burden of heart failure. Clin Cardiol. 2000;23:III6–III10. doi: 10.1002/clc.4960231503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieminen MS, Bohm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 5.Elkayam U, Tasissa G, Binanay C, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98–104. doi: 10.1016/j.ahj.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Naughton MT. The link between obstructive sleep apnea and heart failure: underappreciated opportunity for treatment. Curr Heart Fail Rep. 2006;3:183–188. doi: 10.1007/s11897-006-0020-z. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Oldenburg O, Lamp B, Faber L, et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Oldenburg O, Lamp B, Topfer V, et al. Prevalence of sleep-related breathing disorders in ischemic and non-ischemic heart failure. Dtsch Med Wochenschr. 2007;132:661–666. doi: 10.1055/s-2007-973599. [DOI] [PubMed] [Google Scholar]
- 10.Ferrier K, Campbell A, Yee B, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128:2116–2122. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 11.Schulz R, Blau A, Borgel J, et al. Sleep apnoea in heart failure. Eur Respir J. 2007;29:1201–1205. doi: 10.1183/09031936.00037106. [DOI] [PubMed] [Google Scholar]
- 12.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 13.Brooks D, Horner RL, Kozar LF, et al. Obstructive sleep apnea as a cause of systemic hypertension: evidence from a canine model. J Clin Invest. 1997;99:106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassar A, Morgenthaler TI, Lennon RJ, et al. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:1310–1314. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 16.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 17.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 18.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–397. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 19.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Hla KM, Skatrud JB, Finn L, et al. The effect of correction of sleep-disordered breathing on BP in untreated hypertension. Chest. 2002;122:1125–1132. doi: 10.1378/chest.122.4.1125. [DOI] [PubMed] [Google Scholar]
- 21.Adams KF, Jr., Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 23.Spaak J, Egri ZJ, Kubo T, et al. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension. 2005;46:1327–1332. doi: 10.1161/01.HYP.0000193497.45200.66. [DOI] [PubMed] [Google Scholar]
- 24.Kaye DM, Mansfield D, Aggarwal A, et al. Acute effects of continuous positive airway pressure on cardiac sympathetic tone in congestive heart failure. Circulation. 2001;103:2336–2338. doi: 10.1161/01.cir.103.19.2336. [DOI] [PubMed] [Google Scholar]
- 25.Tkacova R, Rankin F, Fitzgerald FS, et al. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98:2269–2275. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 26.Naughton MT, Rahman MA, Hara K, et al. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91:1725–1731. doi: 10.1161/01.cir.91.6.1725. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 28.Franklin KA. Sleep apnoea screening in heart failure? Not until benefit is proven! Eur Respir J. 2007;29:1073–1074. doi: 10.1183/09031936.00030507. [DOI] [PubMed] [Google Scholar]
- 29.Verbeke G, Molenberghs G. New York, NY: Springer; 2000. Linear mixed models for longitudinal data. [Google Scholar]
- 30.Jarjoura D. Crossing controls to treatment in repeated-measures trials. Control Clin Trials. 2003;24:306–323. doi: 10.1016/s0197-2456(02)00341-0. [DOI] [PubMed] [Google Scholar]
- 31.Crager MR. Analysis of covariance in parallel-group clinical trials with pretreatment baselines. Biometrics. 1987;43:895–901. [PubMed] [Google Scholar]
- 32.Yoshinaga K, Burwash IG, Leech JA, et al. The effects of continuous positive airway pressure on myocardial energetics in patients with heart failure and obstructive sleep apnea. J Am Coll Cardiol. 2007;49:450–458. doi: 10.1016/j.jacc.2006.08.059. [DOI] [PubMed] [Google Scholar]
- 33.Baratz DM, Westbrook PR, Shah PK, et al. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest. 1992;102:1397–1401. doi: 10.1378/chest.102.5.1397. [DOI] [PubMed] [Google Scholar]
- 34.Gray A, Goodacre S, Newby DE, et al. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 35.Bendjelid K, Schutz N, Suter PM, et al. Does continuous positive airway pressure by face mask improve patients with acute cardiogenic pulmonary edema due to left ventricular diastolic dysfunction? Chest. 2005;127:1053–1058. doi: 10.1378/chest.127.3.1053. [DOI] [PubMed] [Google Scholar]
- 36.Chadda K, Annane D, Hart N, et al. Cardiac and respiratory effects of continuous positive airway pressure and noninvasive ventilation in acute cardiac pulmonary edema. Crit Care Med. 2002;30:2457–2461. doi: 10.1097/00003246-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Khayat R, Jarjoura D, Patt B, et al. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009 doi: 10.1016/j.cardfail.2009.05.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintana-Gallego E, Villa-Gil M, Carmona-Bernal C, et al. Home respiratory polygraphy for diagnosis of sleep-disordered breathing in heart failure. Eur Respir J. 2004;24:443–448. doi: 10.1183/09031936.04.00140603. [DOI] [PubMed] [Google Scholar]