Abstract
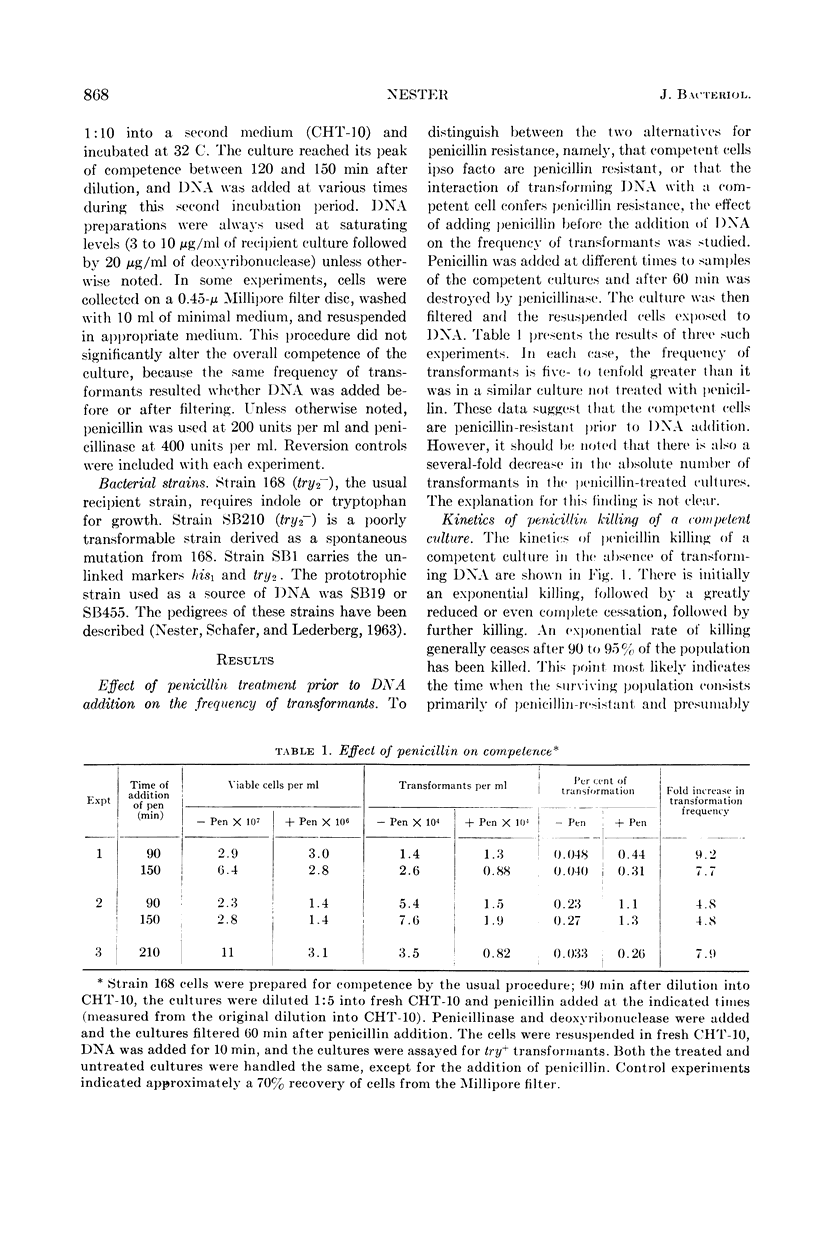
Nester, E. W. (University of Washington, Seattle). Penicillin resistance of competent cells in deoxyribonucleic acid transformation of Bacillus subtilis. J. Bacteriol. 87:867–875. 1964.—Transformants are resistant to penicillin killing for several hours after deoxyribonucleic acid (DNA) addition. The present study indicates that this resistance is a consequence of such cells still remaining competent and is not the result of any interaction of donor DNA with the recipient cell. The following data support this conclusion: (i) the frequency of transformation can be increased five- to tenfold if penicillin acts on a competent culture prior to DNA addition; (ii) the percentage of competent cells in such a penicillin-treated culture calculated on the basis of a random coincidence of DNA molecules entering the same cell increases some 25-fold over that of a penicillin-nontreated population; (iii) the kinetics of penicillin killing of a recipient culture are identical whether or not transforming DNA has been added; (iv) the extent of killing by penicillin is related to the level of competence of the recipient culture; and (v) the kinetics of appearance and disappearance of competence in a population as well as in individual cells indicate that a cell may remain competent for 3 to 4 hr.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Induction of streptomycin resistance in sensitive Hemophilus influenzae by extracts containing desoxyribonucleic acid from resistant Hemophilus influenzae. J Exp Med. 1953 Jan;97(1):17–31. doi: 10.1084/jem.97.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTENBERN R. A. Reversion of L forms and spheroplasts of Proteus mirabilis. J Bacteriol. 1963 Feb;85:269–272. doi: 10.1128/jb.85.2.269-272.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S. Deoxyribonucleic acid incorporation by transformed bacteria. Biochim Biophys Acta. 1957 Oct;26(1):83–85. doi: 10.1016/0006-3002(57)90056-2. [DOI] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Initiation of bacterial transformation. Nature. 1957 Jun 29;179(4574):1322–1325. doi: 10.1038/1791322a0. [DOI] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMAN L. S., TOLMACH L. J. Genetic transformation. I. Cellular incorporation of DNA accompanying transformation in Pneumococcus. Biochim Biophys Acta. 1957 Oct;26(1):68–82. doi: 10.1016/0006-3002(57)90055-0. [DOI] [PubMed] [Google Scholar]
- MICHAEL J. G., MASSELL B. F., PERKINS R. E. EFFECT OF SUBLETHAL CONCENTRATIONS OF PENICILLIN ON THE VIRULENCE AND ANTIGENIC COMPOSITION OF GROUP A STREPTOCOCCI. J Bacteriol. 1963 Jun;85:1280–1287. doi: 10.1128/jb.85.6.1280-1287.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAVA G. C., GALIS A., BEISER S. M. Bacterial transformation: an antigen specific for 'competent' pneumococci. Nature. 1963 Mar 2;197:903–904. doi: 10.1038/197903b0. [DOI] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., STOCKER B. A. BIOSYNTHETIC LATENCY IN EARLY STAGES OF DEOXYRIBONUCLEIC ACIDTRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1963 Oct;86:785–796. doi: 10.1128/jb.86.4.785-796.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUY J. H. Transformability of Haemophilus influenzae. J Gen Microbiol. 1962 Nov;29:537–549. doi: 10.1099/00221287-29-3-537. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. Physiological and genetic factors affecting transformation of Bacillus subtilis. J Bacteriol. 1961 May;81:823–829. doi: 10.1128/jb.81.5.823-829.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]


