Abstract
Visualizing mitochondrial fusion in real time, we identified two classes of fusion events in mammalian cells. In addition to complete fusion, we observed transient fusion events, wherein two mitochondria came into close apposition, exchanged soluble inter-membrane space and matrix proteins, and re-separated, preserving the original morphology. Transient fusion exhibited rapid kinetics of the sequential and separable mergers of the outer and inner membranes, but allowed only partial exchange of integral membrane proteins. When the inner membrane fusion protein Opa1 level was lowered or was greatly elevated, transient fusions could occur, whereas complete fusions disappeared. Furthermore, transient fusions began from oblique or lateral interactions of mitochondria associated with separate microtubules, whereas complete fusions resulted from longitudinal merging of organelles travelling along a single microtubule. In contrast to complete fusion, transient fusions both required and promoted mitochondrial motility. Transient fusions were also necessary and sufficient to support mitochondrial metabolism. Thus, Opa1 expression and cytoskeletal anchorage govern a novel form of fusion that has a distinct function in mitochondrial maintenance.
Keywords: calcium, cytoskeleton, fusion, mitochondria, motility
Introduction
Cells expend considerable effort to continually merge and divide mitochondrial compartments that are essential for maintaining the metabolic function of these organelles as well as regulating their roles in cell signalling (Yaffe, 1999; Westermann, 2002; Chan, 2006; McBride et al, 2006; Tatsuta and Langer, 2008). Although it is unclear whether the causal link is to morphology, per se, or to the morphology-regulating proteins, most studies linked loss of fusion with mitochondrial dysfunction and greater susceptibility to apoptosis (Youle and Karbowski, 2005). The continual merging of mitochondria allows the recombination of mitochondrial DNA (Nakada et al, 2001; Ono et al, 2001), the damage of which is believed to be a key factor in age-related degeneration and some cancers (Taylor and Turnbull, 2005). Also, changes in the relative rates of fusion and fission alter the overall morphology of the mitochondria affecting the function of the organelles both as regulators of survival/apoptosis and in Ca2+ handling. It has been shown that fusion is blocked (Karbowski et al, 2004) and mitochondria become fragmented during apoptosis (Frank et al, 2001). However, enhanced fission alone does not induce apoptosis and has even been shown to protect against Ca2+-dependent apoptosis by preventing the propagation of harmful Ca2+ waves through the mitochondrial reticulum (Szabadkai et al, 2004). Furthermore, fission following selective fusion generates a subpopulation of non-fusing mitochondria with reduced ΔΨm and protein optic atrophy 1 (Opa1) to facilitate their removal by autophagy (Twig et al, 2008). Another function of mitochondrial fission may be the prevention of sustained excessive mitochondrial elongation that triggers cellular senescence (Lee et al, 2007). The stability of the overall mitochondrial morphology indicates that fusion is carefully balanced by fission in a wide range of conditions. However, the mechanism of the coupling between fusion and fission remains elusive.
The details of the mechanism to fuse four mitochondrial membranes to two have yet to be revealed; however, several dynamin-related GTPases, necessary for the fusion of mitochondria in mammals, have recently been described. Mitofusins 1 and 2 (Mfn1, Mfn2) are integral proteins of the outer mitochondrial membrane (OMM; Santel and Fuller, 2001) shown to act in trans in either homo- or heterotypic interactions to tether apposing mitochondria as the initial step of fusion (Koshiba et al, 2004). Mfn2 is also involved in other functions such as control of mitochondrial metabolism (Bach et al, 2003) and tethering of mitochondria to the ER (de Brito and Scorrano, 2008). Mutations in Mfn2 were recently reported to cause Charcot–Marie–Tooth neuropathy type 2A (Zuchner et al, 2004). The inner mitochondrial membrane (IMM) fusion protein Opa1, mutations of which cause dominant optic atrophy (Delettre et al, 2000), is required to tether and fuse IMMs (Meeusen et al, 2006) and to maintain the cristae (Frezza et al, 2006; Meeusen et al, 2006). This protein may also be active in fission (Chen et al, 2005), although its role in either process has to be further explored. Opa1 has genetically and molecularly distinct functions in mitochondrial fusion and cristae remodelling during apoptosis (Cipolat et al, 2006; Frezza et al, 2006; Olichon et al, 2007). Opa1 is cleaved by m-AAA paraplegin and AFG3L2 or i-AAA Ymel to control fusion (Duvezin-Caubet et al, 2006; Baricault et al, 2007; Griparic et al, 2007; Song et al, 2007; Guillery et al, 2008), or by PARL to control cristae remodelling in the apoptotic process (Cipolat et al, 2006).
Recently, fusion of mitochondria was shown to proceed through sequential events of OMM and IMM fusion (Meeusen et al, 2004; Malka et al, 2005). The advent of photoactivatable and photoswitching fluorescent proteins such as photoactivatable green fluorescent protein (Patterson and Lippincott-Schwartz, 2002), Kaede (Ando et al, 2002), and Kindling fluorescent protein (KFP; Chudakov et al, 2003), has opened a new opportunity to visualize the subcellular dynamics by selective labelling of a subset of organelles or proteins within living cells. A few studies have used these probes to monitor inter-mitochondrial interactions in certain paradigms (Arimura et al, 2004; Karbowski et al, 2004; Twig et al, 2006, 2008), but, to date, little is known of the modes of material exchange between mitochondria in vivo. Real-time imaging has also indicated that mitochondria are dynamically positioned to permit the fusion events. Mitochondrial fusion may occur between isolated mitochondria and in cells exposed to microfilament-disrupting agents (Mattenberger et al, 2003; Meeusen et al, 2004), but involvement of the cytoskeletal frame in shaping the mitochondria has also been suggested (De Vos et al, 2005; Bowes and Gupta, 2008). This study unravels two distinct modes of mitochondrial fusion–fission in mammalian cells, which result from discrete Opa1 dependence, cytoskeletal arrangements, and mitochondrial motor activities, and have different roles in mitochondrial metabolism and motility dynamics. The previously unrecognized transient fusions represent the mitochondrial version of the ‘kiss-and-run', and are competent by themselves to maintain metabolism and motility.
Results and discussion
Distinct forms of mitochondrial mergers: transient fusion and complete fusion
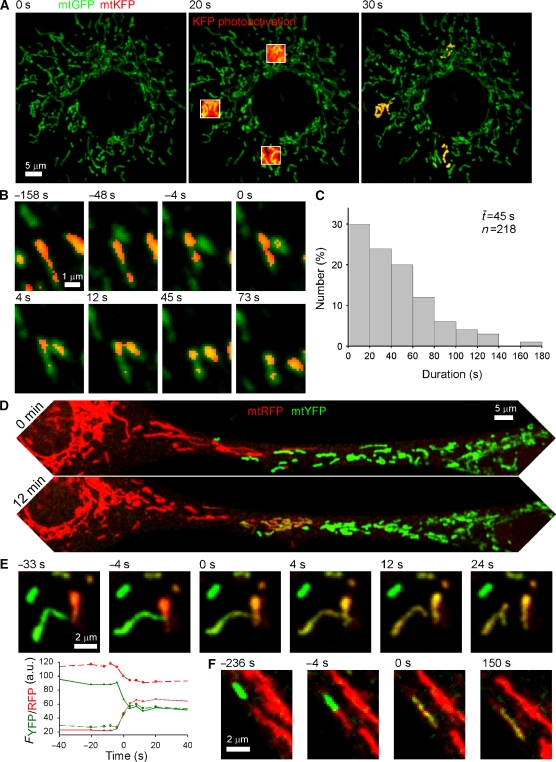
To visualize the exchange of matrix contents between individual mitochondria in real time, we have used the mitochondrial matrix-targeted green-photoactivated, red-fluorescent KFP (mtKFP) in combination with green or yellow fluorescent protein (mtGFP, mtYFP) or the cyan-photoactivated, green-fluorescence PAGFP (mtPAGFP) in combination with red fluorescence protein (mtRFP) in H9c2 cells. Using high resolution confocal imaging with region of interest scanning, we were able to illuminate 2–4 25 μm2 areas per cell and achieve an irreversible photoactivation within the mitochondria in those areas (mtKFP, Figure 1A and mtPAGFP, Supplementary Figure 1A). Monitoring cells for 7 min after photoactivation revealed interactions during which photoactivated KFP/PAGFP was transferred from one mitochondrion to another, often through unexpected, transient fusions. These events were remarkable both in the extremely short duration required for the opening and resealing of the four membranes between the matrices of conjugating mitochondria (as little as 4 s) and in the modes of interaction from which these events resulted. Whereas events that resulted in complete fusion tended to occur by longitudinal merging of oblong mitochondria (Figure 1F and Supplementary Figure 1C), transient fusions often began from oblique or lateral interactions (Figure 1B, Supplementary Figure 1B, and Supplementary movie), although some were also longitudinal. Transient fusions maintained the original shape of the participating mitochondria, indicating that separation of the membranes occurred close to the site of the merger, whereas complete fusions yielded a single organelle that later underwent fission at a different site. Thus, a difference between transient and complete fusion is that transient fusions could preserve the original topology, whereas complete fusions yielded a single organelle to make topological difference.
Figure 1.
Visualization of transient mitochondrial fusion by photoactivation or cell fusion. (A) Labelling of three subsets of mitochondria by photoactivation of KFP in an H9c2 cell expressing both mtKFP and mtGFP. (B) Time course of a typical mitochondrial transient fusion after photoactivation. The acceptor (green) mitochondrion moves into oblique apposition with the photoactivated donor (red). At 0 s the transfer of photoactivated KFP has begun. By 73 s the pair has re-separated at the apparent site of fusion and moved apart. (C) Duration of transient fusion. (D) A PEG-induced fusion between mtRFP- and mtYFP-expressing cells just before the intermixing of contents begins and 12 min later. (E) An individual transient fusion event from an mtRFP–mtYFP cell hybrid demonstrates the bidirectional exchange of contents. At 4 s both mitochondria have become yellow and re-separation occurs at 12 s. Bidirectionality is confirmed by the graphs showing a decrease in green and increase in red in the green donor (solid lines) and the inverse in the red donor (dashed lines). (F) A representative fusion from the PEG fusion assay shows the canonical end-to-end interaction of tubular mitochondria and also the bidirectional redistribution of matrix contents.
In the standard condition, using photoactivation of mtKFP we observed 162 instances of the exchange of mitochondrial matrix contents, 47 of which were transient and only 18 were complete fusions. The majority, 97 events, were classified as indeterminate, most often because the mitochondria involved remained distinct without separating before the end of imaging, and because the dynamic movement of the organelles made it impossible to determine the precise outcome. Also, the relatively weak fluorescence of the mtKFP made the classification of some events difficult. Using mtPAGFP, we observed 471 matrix mixing events, 223 of which were transient, 65 were complete fusion, and 183 events were indeterminate. Using the time of visual separation, the duration of association after the exchange of contents was determined for each transient fusion, yielding a range from less than 4 s to 5 min, with a mean post-exchange duration of association of 45 s for the 218 mtPAGFP transfer events (Figure 1C). Notably, the lifetime of transient fusion could be overestimated in the mtPAGFP assay since resealing of the membranes might have occurred earlier then the visual separation.
Because the photoactivation assay allows visualization of the transfer of matrix contents in only one direction, from a mitochondrion with photoactivated mtKFP to a naive one, it was unknown whether the transient fusion events represented a passive diffusion of contents through an unselective opening or an active unidirectional transfer of contents as in bacterial conjugation. To explore these options, we fused cells expressing different mitochondrial-targeted fluorescent proteins, mtYFP and mtRFP, using polyethylene glycol (PEG). Unlike previous reports that use the PEG fusion technique to monitor the extent of content mixing at discrete time points (Legros et al, 2002; Mattenberger et al, 2003), usually hours after the PEG treatment, we recorded 12-min time courses during the initial period, 45–135 min after cell fusion, to observe interactions between individual ‘virgin' RFP- and YFP-containing mitochondria (Figure 1D). In every observed interaction in which it was possible to make a determination whether transient or complete fusion, the transfer of contents was bidirectional with each mitochondrion ending with a mixture of RFP and YFP (Figure 1E and F). Thus, the mechanism of the transfer of soluble contents in both transient and complete fusion events is passive diffusion.
Sequential and separable mergers of the OMM and IMM during transient and complete fusion
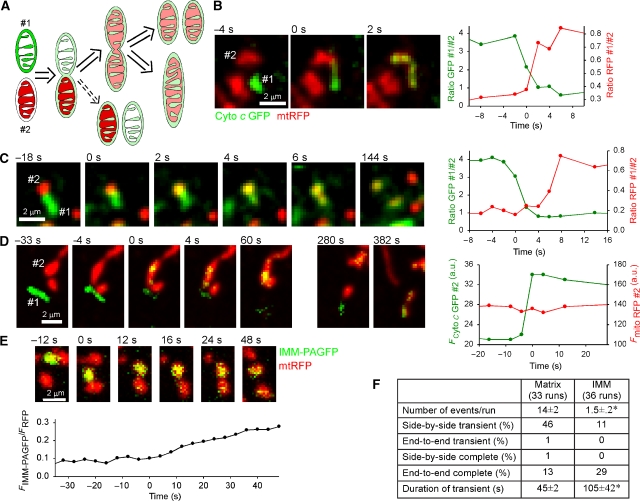
The transfer of matrix contents between mitochondria is only a partial description of mitochondrial transient fusion/complete fusion as it shows only the merging of the IMM. To study the OMM and IMM fusion steps separately, we set out to visualize the corresponding unifications of inter-membrane space (IMS) and matrix. We generated cell hybrids by PEG fusion from cells containing soluble IMS-targeted cytochrome c (cyto c) GFP (cyto c GFP; Heiskanen et al, 1999) and matrix-space mtRFP (Figure 2). In these experiments, complete as well as transient fusions between the cyto c GFP-(green) and mtRFP-(red)-containing mitochondria produced organelles that contained both GFP and RFP (yellow; Figure 2B and C). The time course for the distribution of both GFP and RFP between the merging organelles confirmed that the gap between IMS and matrix openings was usually quite short (Figure 2B, graph). However, in several examples (e.g., Figure 2C), cyto c GFP could be observed to diffuse into the RFP containing mitochondrion prior to the spreading of the mtRFP (∼6 s; Figure 2C, graph), showing the temporally distinct mergers of IMS and matrix. The mean delay between IMS and matrix merging was 2.4±0.5 s (n=27). In one case (out of 28), transient fusion of the IMS was observed in the absence of matrix exchange (Figure 2D). Transfer of a small amount of matrix marker could be difficult to visualize, but the lifetime of this merge was not particularly short and for every other events the mtRFP response was easily detectable in both the donor and the acceptor mitochondria. Thus, the lack of the mtRFP spreading suggests that the OMM and IMM fusions are not fundamentally coupled. This conclusion is also supported by a recent study that showed only OMM fusion in cells pretreated with valinomycin and uncoupler (Malka et al, 2005).
Figure 2.
Sequential and separable fusions of OMM and IMM observed during transient and complete fusion events. (A) Scheme showing the possible paths of transient/complete fusion observed by PEG fusion of cells expressing IMS-targeted cyto c GFP with mtRFP-expressing cells. Fusion of the OMM results in diffusion of the cyto c GFP into the ‘red' mitochondrion usually followed by IMM fusion and diffusion of the mtRFP into the ‘green' mitochondrion. In rare cases, the OMM fusion is reversed without IMM fusion having occurred (dashed arrow). (B) Close temporal coupling of OMM and IMM mergers observed with cyto c GFP and mtRFP as described above. The separation of events is not visible in the images, although the graph reveals an ∼1-s lag between exchange of IMS and matrix contents. (C) Clearly separated OMM and IMM fusions during transient fusion. Mitochondrion #2 becomes yellow from intake of cyto c GFP ∼6 s before mtRFP enters #1. (D) OMM fusion can be completely uncoupled from IMM fusion: an example of the rare OMM-only transient fusion. Mitochondrion #1 donates cyto c GFP to #2 and re-separates (60 s) without having merged matrices. Minutes later #1 undergoes a matrix fusion with another mitochondrion (382 s). (E) Slow and partial mixing of integral IMM proteins during transient fusion after photoactivation in H9c2 cell expressing both IMM-PAGFP and mtRFP. (F) Comparison of the transfer of soluble matrix and integral IMM proteins during transient and complete fusions in cells expressing mtPAGFP/mtRFP or IMM-PAGFP/mtRFP (mean±s.e.m.; *P⩽0.001).
Never, in either transient and complete fusion, was there exchange of mtRFP without simultaneous diffusion of cyto c GFP, indicating that transient fusion involves the same double-membrane-merging steps as complete fusion, and not a direct connection of matrices via a pore-like structure as seemed possible given the short duration of transient fusion. From this result and the passive nature of the exchange of soluble contents in both transient and complete fusion, it seems likely that there is a single mechanism underlying both types of event, which in some cases can be rapidly reversed and in others proceeds to complete fusion. A scheme depicting the sequential mergers of the OMMs and IMMs during both transient and complete fusions is shown in Figure 2A.
To visualize the transfer of integral IMM proteins between individual mitochondria in real time, we have used an IMM protein marker (ABCB10-fused PAGFP, IMM-PAGFP; Twig et al, 2006). IMM-PAGFP fluorescence increase showed a slow kinetic in the acceptor mitochondrion during both transient fusion (Figure 2E) and complete fusion (not shown). In Figure 2E, IMM-PAGFP fluorescence transfer was still in progress when the two mitochondria separated at 36 s. This was sharp contrast of the rapid mixing of the soluble matrix proteins, which required 6 s or less (Figure 2B–D and Supplementary Figure 1B). The events of IMM-PAGFP transfer were also observed less frequently than the mtKFP or mtRFP exchange events (1.5/run versus 14/run; Figure 2F). This could be in part due to the relatively weak expression of IMM-PAGFP, but also reflected a less efficient integral membrane protein transfer during brief transient fusions. In total, transient fusion accounted for 46% of the matrix mixing events, but only 11% of the integral IMM protein mixing. On the other hand, complete fusion represented a relatively large fraction of the IMM mixing events (soluble matrix 13% and integral IMM 29%). Furthermore, transient fusions showing mtIMM-PAGFP exchange were significantly longer than the ones showing mtPAGFP exchange (IMM 105±42 s versus matrix 45±2 s, P⩽0.003; Figure 2E and F). Thus, exchange of the integral IMM proteins is a slow process that is limited during transient fusion events.
Dependence of transient and complete fusion on ΔΨm
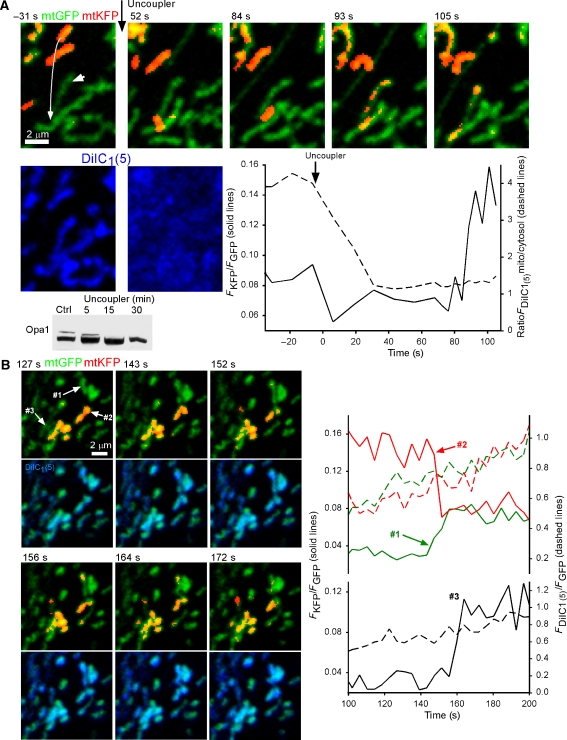
IMM potential (ΔΨm) is essential for mitochondrial content mixing in the PEG fusion assay (Legros et al, 2002; Mattenberger et al, 2003) and in the in vitro assay (Meeusen et al, 2004). We developed an assay using the far-red fluorescent ΔΨm-sensor probe, 1,1′,3,3,3′,3′-hexamethylindodicarbocyanine iodide (DiIC1(5)) to monitor ΔΨm simultaneously with fusion by KFP photoactivation (Figure 3A). To avoid the creation of harmful products during the illumination required for photoactivation, 50 nM DiIC1(5) was added immediately after photoactivation and was observed as it accumulated in a ΔΨm-dependent manner to the mitochondria. The time course of the DiIC1(5) accumulation was similar both within and out of the area of the photoactivation, indicating that the metabolic function was maintained in the mitochondria containing the photoactivated KFP (not shown). FCCP (5 μM), an uncoupler, was added at the end of each ΔΨm measurement to confirm the ΔΨm-dependent compartmentalization of DiIC1(5). Unexpectedly, after complete release of the dye from the mitochondria (Figure 3A, lower row, 52 s) the exchange of photoactivated mtKFP could still be observed (Figure 3A, upper row, 93 s; white arrows marking the interacting mitochondria and their pre-fusion movement (left), −31 s). This would indicate that ΔΨm per se is not required for transient fusion/complete fusion. To confirm this result, we performed the KFP photoactivation assay (without addition of DiIC1(5)) on cells treated with uncoupler or antimycin A (5 μM) and oligomycin, which was proven to eliminate ΔΨm within <5 min by the release of TMRE, and also by loss of mitochondrial Ca2+ uptake (Szalai et al, 2000). Cells treated with ΔΨm-dissipating drugs showed gradual loss of fusion-competency, but one which was not closely coupled with loss of potential as would be expected if ΔΨm were an absolute requirement for fusion. Short-term drug treatment (1–15 min, uncoupler; 5–20 min, antimycin A and oligomycin) yielded 21 events in 17 of the 7 min mtKFP recordings (average 1.2 events/record), whereas long-term treatment (1–2 h with either) had just six events in 15 runs (0.4 events/record), compared with 80 events in 33 control runs (2.4 events/record). The loss of fusion after long-term treatment with the metabolic inhibitors confirms previous observations (Legros et al, 2002; Mattenberger et al, 2003; Meeusen et al, 2004). Collectively, these data indicate that loss of ΔΨm, or at least presence of membrane-depolarizing drugs, results in a significant impairment of mitochondrial fusion, but this, in fact, is secondary to the loss of ΔΨm per se.
Figure 3.
IMM ΔΨm and transient and complete mitochondrial fusion. To avoid light-induced damage, the far-red fluorescent ΔΨm-sensor dye DiIC1(5) (50 nM) was added to cells immediately after photoactivation and was monitored as it accumulated in the mitochondria. (A) Mitochondrial fusion after dissipation of the ΔΨm. After 7-min monitoring in the presence of DiIC1(5), uncoupler (5 μM FCCP, 5 μg/ml oligomycin) was added resulting in a loss of ΔΨm and consequent release of the probe to the cytosol. The graph shows that redistribution of the dye was complete ∼30 s after addition of uncoupler (dotted line); still, ∼60 s later, an exchange of matrix contents between two mitochondria occurred (solid line). These mitochondria are marked by white arrows in the upper left image. Immunoblots show time-dependent loss of the long form of Opa1 in cells treated with uncoupler. (B) ΔΨm is not lost during transient or complete fusion. Two representative events are shown in a cell after photoactivation and addition of DiIC1(5). In neither case is there a disturbance in the ΔΨm during the exchange of contents as indicated by the steady increase in DiIC1(5) fluorescence shown in blue in the lower row of images and the dashed lines in the graphs.
The recent discovery that dissipation of ΔΨm facilitates the processing of Opa1 to a form that is not competent to support fusion by itself (Duvezin-Caubet et al, 2006; Ishihara et al, 2006; Song et al, 2007) offers a physico-chemical coupling mechanism to underlie the ΔΨm dependence of the mitochondrial fusion. Indeed, the decrease in fusion was paralleled by an FCCP-induced gradual loss of the long-form Opa1 (Figure 3A, immunoblot). Based on the time course, Opa1 cleavage may mediate the FCCP-induced fusion inhibition. The probability of transient fusion was 37% (18 out of 48) in control cells, whereas 35% (8 out of 23) in FCCP-pretreated (15 min) cells, indicating that transient fusion does not show distinct dependence on the ΔΨm.
In no case was a disturbance in the continual uptake of DiIC1(5) observed in either mitochondrion during a transient or complete fusion (n=28; Figure 3B). Furthermore, using TMRE measurements in mtPAGFP-expressing cells, no significant change in TMRE fluorescence was observed in either mitochondrion during transient or complete fusion (n=26). While we cannot eliminate the possibility of a short-lived or small depolarization (or hyperpolarization) of the IMM because of the temporal resolution of the imaging (0.25 s−1) or sensitivity of the potentiometric probe, it appears that both transient and complete fusion proceeds without major alteration to the mitochondrion's ΔΨm-generating capacity.
Distinct dependence of transient and complete fusion on Opa1
The mechanism of both transient and complete fusion almost certainly involves Mfn proteins, as the presence of at least one Mfn isoform on each mitochondrion has been shown to be essential for the mixing of contents in the PEG fusion assay (Chen et al, 2003). A key role for the IMM fusion protein, Opa1 (Olichon et al, 2002; Cipolat et al, 2004), in both transient and complete fusion is supported by the sequential fusion of the OMM and IMM in these processes (see above). However, it remained a question whether transient and complete fusions show similar dependence on Opa1.
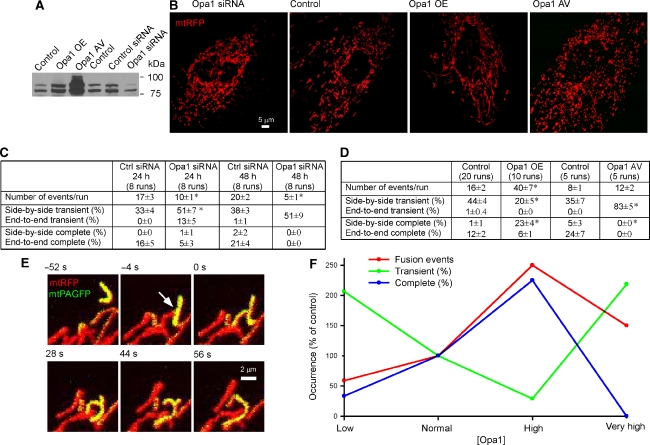
We used genetic approaches to establish four levels of Opa1 expression: low (siRNA), normal (control), high (overexpression using plasmid DNA), and very high (adenoviral expression (AV); Figure 4A). Opa1 siRNA induced mitochondrial fragmentation and swelling (48 h). Opa1 overexpression by plasmid DNA resulted in elongated mitochondria, whereas Opa1 AV caused mitochondrial fragmentation (Figure 4B). These Opa1-dependent changes in mitochondrial morphology were consistent with recent observations by several groups (Opa1 silencing-induced fragmentation (Olichon et al, 2003; Griparic et al, 2004; Lee et al, 2004; Chen et al, 2005); Opa1 overexpression-induced mitochondrial elongation (Cipolat et al, 2004); and Opa1 overexpression-induced mitochondrial fragmentation (Chen et al, 2005; Twig et al, 2008)). Twenty-four and 48 h of Opa1 silencing caused a progressive decrease in the total fusion events (60 and 25% as compared with their respective control). Complete fusion was suppressed even as percent of all fusion events at 24 h and was completely eliminated at 48 h of Opa1 silencing (Figure 4C). At the same time, transient fusion was maintained at 24 h (Figure 4C). Interestingly, at 24 h Opa1 silencing many transient fusion events resulted from longitudinal interactions (Figure 4C) that were normally reserved for complete fusion (Figure 1).
Figure 4.
Opa1 amount controls both fusion quantity and quality. (A) Effects of Opa1 siRNA, overexpression (OE) or AV on expression levels of Opa1. (B) Effects of Opa1 siRNA, OE, or AV on mitochondrial morphology. (C) Effects of Opa1 siRNA on mitochondrial fusion quantity and quality. Means±s.e.m.; P⩽0.03. (D) Effects of Opa1 OE or AV mitochondrial fusion quantity and quality. Means±s.e.m.; *P⩽0.003. (E) The time course of typical side-by-side complete fusion after photoactivation in Opa1-overexpressing cells. The donor (yellow) mitochondria moves into oblique opposition with the accepter. At 0 s the transfer of photoactived mtPAGFP has begun. Two mitochondria completely fuse to one at 56 s. (F) Summary of Opa1 amount effects on fusion quantity and quality.
Moderate overexpression of Opa1 greatly increased the total number of fusion events (2.5-fold; Figure 4D). Complete fusions became frequent in particular, whereas transient fusion represented a smaller fraction of all fusion events and were more prolonged (Figure 4D and Supplementary Table 1). In this condition, many complete fusion events resulted from lateral/oblique interactions (side-by-side or end-to-side; Figure 4D), which was rare in the control. The image sequence in Figure 4E shows an end-to-side complete fusion event in an Opa1-overexpressing cell. The donor met the acceptor at −4 s, showed matrix exchange at 0 s, and gradually progressed from T shape to a simple longitudinal shape in the next 56 s. In Opa1 AV cells, despite the fragmented mitochondrial morphology the number of fusion events was increased 1.5-fold (Figure 4D). However, no complete fusion occurred and transient fusion became frequent (83%).
Figure 4F summarizes the relationship between Opa1 amount and fusion quantity and quality. Total fusion events decreased when Opa1 amount was low, showed robust increase at moderate overexpression, and declined to a small increase at high amount. The percent of complete fusion also peaked at moderate Opa1 overexpression, and decreased and disappeared when Opa1 was either lowered or largely elevated. However, the fraction of transient fusion showed a complementary pattern, increased at low or very high Opa1 level, and decreased at moderate amount. The total number of transient fusion showed the greatest increase at high level of Opa1. Thus, transient and complete fusion exhibit distinct dependence on Opa1 amount. In mammalian cells, Opa1 has eight mRNA splice variants and subsequent proteolytic processing (Delettre et al, 2001). Recent studies showed that long and short forms of Opa1 have little activity alone and need to complement each other to support fusion activity (Song et al, 2007). Furthermore, the pro-fusion effect of Opa1 is also dependent on other fusion- or fission-promoting factors. Indeed, Opa1 overexpression in mouse embryonic fibroblasts induced mitochondrial fragmentation (Chen et al, 2005), whereas elongation occurred in cells where the mitochondria were naturally fragmented (Olichon et al, 2002, Cipolat et al, 2004). Thus, the quantitative relationship between different Opa1 isoforms and other components of the fusion machinery is different for transient and complete fusion.
Transient fusion involve Drp1-dependent fusion–fission dynamics
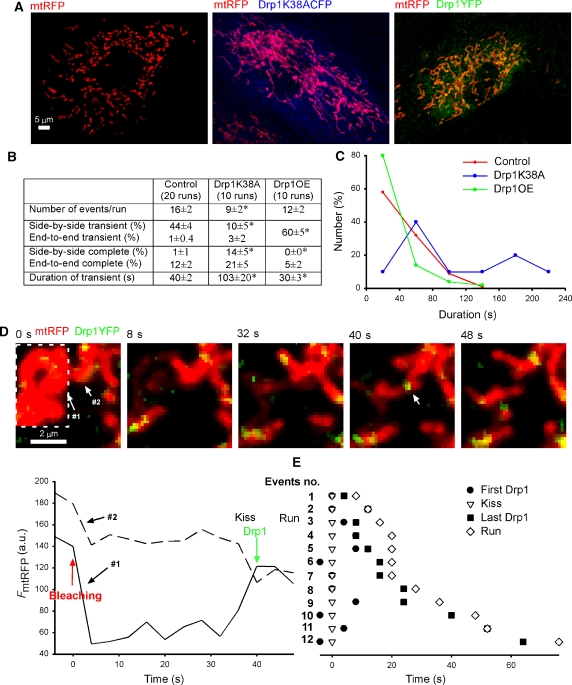
Is the separation phase of transient fusion reverse fusion or fission? A possible role for reverse fusion emerged because transient fusion preserved the mitochondrial topology and showed a rapid time course. Reverse fusion would be mediated by fusion proteins, whereas fission is dependent on Drp1 (Pitts et al, 1999; Santel and Fuller, 2001), which utilizes interactions with Fis1 (James et al, 2003). To test the dependence of transient fusion on Drp1, the fusion quantity and quality was investigated in cells expressing a dominant-negative Drp1 construct (Drp1K38ACFP) and mtRFP or overexpressed Drp1YFP and mtRFP. In cells expressing Drp1K38ACFP, the CFP fluorescence showed broad cytoplasmic distribution and mtRFP fluorescence showed elongated mitochondria (Figure 5A, middle). Formation of Drp1K38ACFP cytoplasmic clusters (Pitts et al, 1999; James et al, 2003) was seldom observed in H9c2 cells. In Drp1YFP-overexpressing cells, YFP fluorescence was mainly in the cytoplasm, but bright YFP spots, which were often globular, were also observed on mitochondria (Figure 5A, right). Fusion was decreased in Drp1K38A-expressing cells and was not significantly altered in Drp1-overexpressing cells (Figure 5B). Less fusion events could occur in Drp1K38A-expressing cells because most mitochondria formed an interconnected network and few interactions took place between discrete mitochondria. Similar mitochondrial morphology in DrpK38A-expressing cells were observed (Pitts et al, 1999; James et al, 2003). In Drp1K38A-expressing cells, the fraction of transient fusion decreased from 45 to 13%, and complete fusion frequently resulted from lateral/oblique interactions (side-by-side or end-to-side) (increased from 1 to 14%). These results suggest that in Drp1K38A-expressing cells many transient fusions turned to complete fusion because of the lack of Drp1-dependent fission. On the other hand, in Drp1-overexpressing cells the percent of transient fusions increased from 45 to 60%. Notably, many of the fusion events in Drp1-overexpressing cells occurred among globular mitochondria and therefore it was not feasible to decide about the orientation of the interacting organelles. Furthermore, the transient fusion in Drp1K38A-expressing cells lasted longer (103±20 s), whereas that in Drp1-overexpressing cells was shorter (30±3 s) as compared with the control (40±2 s; Figure 5B). Histograms of the durations of transient fusions in these three conditions illustrated that in Drp1K38A-expressing cells longer events became more frequent, whereas in Drp1-overexpressing cells 80% of the events were within 40 s. Thus. the separation phase of transient fusions depends on the availability of Drp1.
Figure 5.
Drp1 is required for separation during transient fusion. (A) Effect of Drp1 domain negative or Drp1YFP overexpression on mitochondrial morphology. H9c2 cells were transfected with mtRFP, mtRFP/Drp1K38ACFP, and mtRFP/Drp1YFP. (B) Effect of Drp1 domain negative or Drp1YFP overexpression (OE) on mitochondrial fusion quantity and quality. Statistically significant differences from the control are marked by an asterisk (P⩽0.04). (C) Effect of Drp1 domain negative or Drp1YFP overexpression on durations of transient fusion. (D) The time course of Drp1YFP fluorescence involved in mitochondrial transient fusion. At 0 s the photobleaching of mtRFP fluorescence is finished. (E) Transient fusion and Drp1YFP fluorescence time chart for individual mitochondria.
Previous studies have shown Drp1YFP fluorescence on the mitochondria before fission (Smirnova et al, 2001), and this observation was also confirmed in the present study (Supplementary Figure 2A). To visualize the interaction of Drp1 with the mitochondria during transient fusion, Drp1YFP was used, whereas the matrix exchange was monitored by Fluorescence Recovery after Photobleaching (FRAP) of mtRFP (Figure 5D). The graphs show that the transfer of matrix content began at 36 s. A Drp1YFP fluorescence spot appeared at the site of fusion at 40 s and then separation of the organelles happened at 48 s. Figure 5E summarizes the timeline for 12 events of transient fusion synchronized by the time of fusion (0 s). A bright Drp1YFP spot was always recorded close to the beginning of matrix exchange (−4 to 8 s) and a spot was also present in the final 12 s before separation took place. These data further support that the separation during transient fusion requires Drp1. Although, the Drp1YFP spot was not visible in every image, it was always apparent in the same area, consistent with the possibility that Drp1 could remain associated with the Mfn complex during transient fusion. A Drp1YFP spot also appeared at the site of complete fusion, but subsequently it disappeared and was observed at a different site when fission took place (Supplementary Figure 2). Collectively, our results demonstrate that the process of transient fusion consists of fusion and Drp1-dependent fission. Drp1 appears when fusion occurs and soon mediates fission during transient fusion. One potential recruitment site is formed by activated Mfn2 that has been shown to interact with Bax (Neuspiel et al, 2005; Karbowski et al, 2006). Recording of fluorescence resonance energy transfer between CFP-Mfn2 and Drp1YFP we could visualize interaction between Mfn2 and Drp1 at the fusion sites (data not shown). This initial recruitment of Drp1 may be sufficient to support fission if other conditions also favor to the separation. One of these factors is likely to be the arrangement of Opa1 proteins in the IMM. Another potential factor is the extent of tension that promotes the dynamin-mediated fission of the endocytotic vesicles (Roux et al, 2006).
Differential contribution of mitochondrial movements to transient and complete fusion
Since fusion usually occurred among mitochondria that displayed dynamic movements (movie), and the spatial relationship between the interacting mitochondria was different for transient and complete fusions (transient: seven longitudinal interactions and 216 lateral out of 223 and complete: 60 longitudinal and five lateral out of 65), a prediction was made that distinct cytoskeletal anchorage of the mitochondria and the forces produced by the motor proteins are relevant for the different forms of mitochondrial mergers.
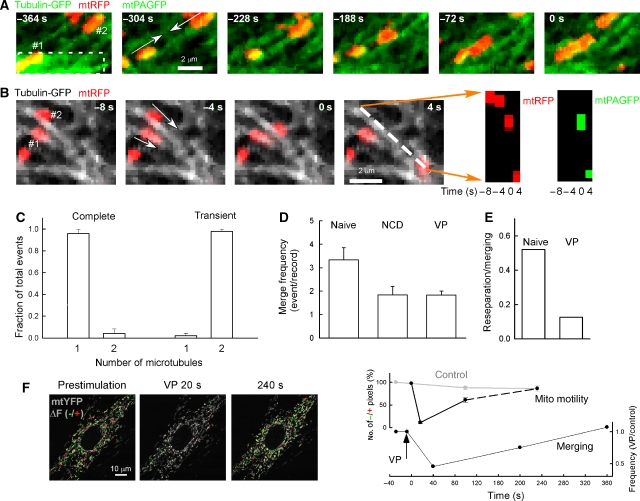
In H9c2 cells, almost every mitochondrion was aligned with microtubules (Supplementary Figure 3A, left) that provide the primary tracks for long-distance mitochondrial movements (Yi et al, 2004), and a smaller fraction appeared in association with the microfilaments (Supplementary Figure 3A, right). We have used mtPAGFP/mtRFP in combination with tubulin-GFP to visualize both mitochondrial movement along the microtubules and fusion in real time. We applied photoactivation in narrow areas (width ∼1 μm) perpendicular to the direction of microtubular tracks so photobleaching of tubulin-GFP fluorescence was minimized and fusion events could be observed during mitochondrial movements (Supplementary Figure 3B). Figure 6A shows an end-to-end interaction between a yellow mitochondrion, containing the photoactivated mtPAGFP (lower left) and a red one (upper right) moving towards each other along a single microtubule. After these organelles met, they both became orange, indicating exchange of their matrix content. Figure 6B shows a transient fusion event between two mitochondria (shown in red) moving along two separate microtubules (shown in grayscale). The donor mitochondrion (#1) stayed at its microtubule. The acceptor mitochondrion (#2) met the donor when it moved along another microtubule. To illustrate the transfer of the photoactivated mtPAGFP, the pre-photoactivation green fluorescence was subtracted and a linescan-type image was created by selecting a line parallel to the direction of the movement (white dashed line in the last image of, Figure 6B), obtaining the corresponding fluorescent signal from every image in the time series and stacking the successive lines vertically. This presentation shows the appearance of the mtPAGFP fluorescence in the acceptor after it met the donor (0 s; Figure 6B, right). In total, 30 out of 31 transient fusions events originated from side-by-side interactions between mitochondria that were linked to two separate microtubules, whereas 15 out of 16 complete fusion events came from end-to-end interactions between mitochondria that move towards each other along a single microtubule (Figure 6C). These data provided evidence that mitochondrial movements differently contributed to transient and complete fusion.
Figure 6.
Dependence of transient and complete fusion on mitochondrial movements and anchorage to the cytoskeleton (A) Longitudinal (end-to-end) interaction between two mitochondria along a common microtubular track resulted in complete fusion. Tubulin-GFP and mtRFP fluorescence is shown in green and red, respectively. mtPAGFP fluorescence gained after photoactivation is also shown in green. The white dash marks the area of photoactivation, the white arrows indicate the vector for the force produced by the motor proteins, and the numbers in white indicate the mtPAGFP donor (#1) and acceptor (#2) mitochondria, respectively. (B) Oblique (side-by-side) interaction between two mitochondria travelling along separate microtubular tracks. Here, tubulin-GFP fluorescence is shown in grayscale and mtRFP fluorescence is shown in red, whereas mtPAGFP is shown in green only in the linescan images. The mtPAGFP donor is marked by #1 and the acceptor is labeled #2. The direction of movement is marked by white arrows. A line scan-type image was created for both mtRFP (red) and mtPAGFP (green, after subtraction of the pre-photoactivation image), respectively, by selecting a line parallel to the direction of movement of the acceptor, obtaining the corresponding fluorescent signal from every 2D images in the time series and stacking the successive lines horizontally. mtRFP is visible in the acceptor mitochondrion at every time point, but mtPAGFP appears only after the interaction with the donor took place at 0 s. (C) The number of microtubular tracks supporting the movement of the pair of mitochondria undergoing complete or transient fusions. (means±s.e.m.). (D) Merging of mitochondria is suppressed in NCD-pretreated and VP-stimulated cells. The KFP fusion assay was conducted in naive, NCD-pretreated (10 μM for 30 min) cells and in cells stimulated with 100 nM VP after photoactivation. Occurrence of fusion events/measurement is plotted (naive, n=43; NCD, n=14; VP, n=15). (E) Rapid re-separation of merged mitochondria is inhibited by VP. The fraction of merging mitochondria that showed re-separation is shown for both naive (n=47) and VP-stimulated (n=9) cells. (F) Comparison of the kinetics of the inhibition of mitochondrial motility and suppression of the merging of the mitochondria induced by VP (100 nM). Confocal images show both mtYFP fluorescence (grayscale) and at each time point, the sites of mitochondrial movement calculated by subtraction of sequential images (red for positive changes and green for negative changes). To quantitate mitochondrial motility, the pixels that change more than a threshold value were calculated and normalized as the percentage loss from the average prior to stimulation (control) (Yi et al, 2004, means±s.e.m., upper graph). The time of the merging of mitochondria was also recorded and the frequency of events in the VP-treated cells (n=23) was normalized to the control (n=158, lower graph).
Next, we sought for mitochondrial movement inhibitors to test the relevance of mitochondrial motility for transient and complete fusion events. Pretreatment with nocodazol (NCD, for 20 min), a microtubule disrupting agent, caused disappearance of the microtubules and suppression of the mitochondrial movements, but ΔΨm generation and Ca2+ handling by the mitochondria was sustained (Yi et al, 2004). In the NCD-treated cells, less mtKFP transfer events occurred than in the control (Figure 6D), suggesting that the mitochondrial anchorage to the microtubules or the movements is important for fusion. Among the remaining fusion events, the frequency of complete fusion increased from 14 to 31% in NCD-treated cells (n=15). Furthermore, rapid re-separation seldom occurred in the NCD-treated cells (7%), indicating preferential inhibition of transient fusion/support of complete fusion.
Mitochondrial motility is also suppressed during stimulation of the cells with vasopressin (VP), a Ca2+-mobilizing hormone, but the microtubular tracks remain intact (Yi et al, 2004). In VP-stimulated cells, the mtKFP transfer among mitochondria was also attenuated (Figure 6D). Furthermore, VP-induced suppression of the mitochondrial motility was transient and resulted in transient inhibition of the mtKFP transfer events, which closely followed the time course for the change in motility (Figure 6F). These effects of VP indicate that the mitochondrial movements, presumably the force produced by the mitochondrial motors, are directly relevant for fusion. Although co-sedimentation of the organelles is sufficient to permit mitochondrial fusion in vitro (Meeusen et al, 2004), cytoskeletal transport controls the distance among individual mitochondria in the cell. Analysis of the re-separation of the organelles that retained fusion activity in the VP-treated cells revealed that the rapid reversal of the fusion was practically abolished (Figure 6E). Since the VP-insensitive mtKFP transfer was mostly long-lasting fusion and the frequency of these events was similar to the frequency of complete fusion in non-stimulated cells, the complete fusion activity was maintained in the period of depressed mitochondrial motility. These results suggest that the inhibition of the mitochondrial motility causes selective inhibition of the transient fusion activity. Notably, prolonged calcium signals have been shown to cause mitochondrial Drp1 localization and fragmentation (Cereghetti et al, 2008), but during the above described VP treatment no obvious mitochondrial fragmentation occurred.
Complete fusions commonly occur as end-to-end interactions, whereas transient fusions are produced in side-by-side orientation. End-to-end interactions are likely to take place between mitochondria that move towards each other along a single microtubule (Figure 6A). Therefore, the entire force produced by the active motors supports the connection between the merging organelles. Differently, side-by-side interactions are formed between mitochondria that are linked to two separate microtubules (Figure 6B), and in this case the force vectors always have a component that favors the separation of the organelles. Recent evidence shows that tension promotes the dynamin-mediated fission of the endocytotic vesicles (Roux et al, 2006). Similarly, mitochondrial membrane re-separation mediated by Drp1, a dynamin-family GTPase is likely to be supported by tension. Collectively, these observations suggest that the mitochondrial transport machinery and the fusion/fission proteins come together to establish a ‘kiss-and-run' mechanism for the mitochondria.
Function of transient fusion in mitochondrial motility and bioenergetics
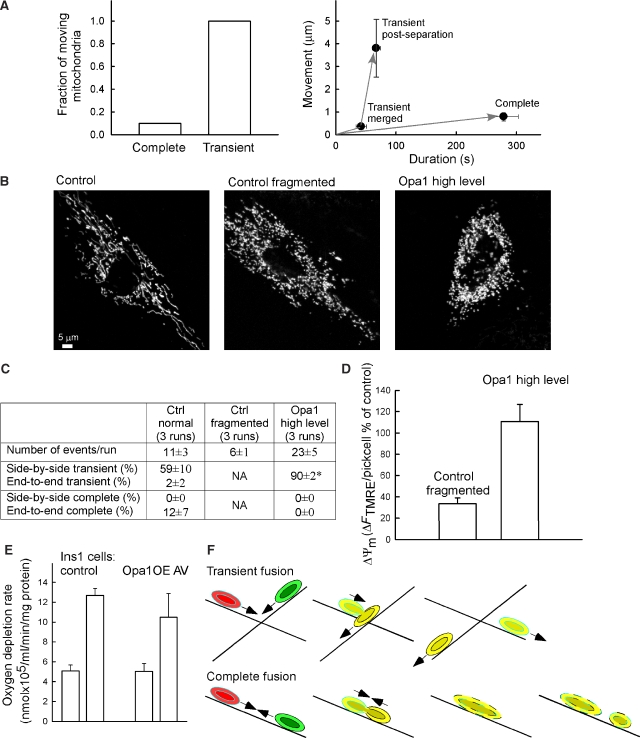
Dynamic positioning of individual mitochondria is fundamental for cellular metabolism, specialization, and ion transport (Li et al, 2004; Yi et al, 2004). Thus, a spectrum of cell functions might depend on the transportability of individual mitochondria. The bioenergetics also depends on the presence of the fusion proteins (Chen et al, 2005). Thus, the effect of transient fusions on the transport and metabolic performance of the individual mitochondria was analyzed. Both complete and transient fusion mitochondria showed similar movement activity in the pre-merger period (1.3±0.2 and 0.9±0.2 μm/20 s, n=10) and a decrease when the merger took place (0.4±0.1 and 0.4±0.1 μm/20 s; P<0.05, n=10) (Figure 7A, left). However, the decay in motility was prolonged only for complete fusions, whereas transient fusions restored (even increased) movement activity immediately after the re-separation of the mitochondria (Figure 7A, right). Thus, transient fusion allows mitochondria to minimize the time spent in a reduced motility state.
Figure 7.
Mitochondrial movements and metabolism depend on transient fusion. (A) Differential control of mitochondrial motility during transient and complete fusion. Left: Fraction of merging mitochondria that showed >1.5 μm/5-min displacement after complete and transient fusion is shown (n=10 and 11, respectively). Right: Maximal displacement is plotted against the elapsed time for complete fusion (0–279 s, n=10), for the merging (0–42 s, n=11) and post re-separation (47–67 s, n=6) phases of transient fusion. The grey arrows indicate the temporal sequence of the events. (B) Mitochondrial morphology in a control cell, a control cell with fragmented mitochondria, and an Opa1-overexpressing (high level) cell. (C) Comparing the fusion quantity and quality between control cell, control cell with fragmented mitochondria, and Opa1-overexpressing (high level) cells. (D) TMRE loading of control cells with fragmented mitochondria, and Opa1-overexpressing (high level) cells. The TMRE loading of mitochondria was normalized to the response in the non-transfected control cells. Means±s.e.m.; *P⩽0.01. (E) Oxygen depletion rate of Opa1OE AV and control Ins1 cells in the absence and presence of an uncoupler. (F) Mitochondrial movement and fusion dynamics. Mitochondrial composition and position relative to the microtubules (gray) is depicted during the time course of the transient (upper) and complete fusion (lower). The IMS/matrix space contents are marked in green and red and the membrane contents are shown in black and sky for the interacting mitochondria, respectively. Direction of the movement is marked by arrows.
The contribution of transient fusions to the maintenance of the metabolic activity of the individual mitochondria was first tested by the ATP synthase inhibitor oligomycin. In healthy mitochondria that produce ATP at the expense of their ΔΨm, oligomycin causes slight hyperpolarization. By contrast, in mitochondria that use cytoplasmic ATP to maintain the ΔΨm through a reverse ATP synthase activity, oligomycin causes depolarization (Ward et al, 2000). In naive cells, oligomycin caused a barely detectable mitochondrial depolarization, indicating that most mitochondria were not ATP consumers. Because of the rapid reversal of the inhibition (Supplementary Figure 4A), VP could not be used for sustained suppression of the mitochondrial dynamics. To interfere with the transient fusions, the cells were treated with NCD for 3 h. Gross cellular and mitochondrial morphology and TMRE accumulation were preserved in the NCD-treated cells (images in Supplementary Figure 4A). However, the mitochondria became very sensitive to the oligomycin-induced depolarization (Supplementary Figure 4A), indicating that a large fraction of the mitochondria lost the capacity to produce ATP during the prolonged treatment with NCD. Notably, 15-min pretreatment with NCD did not promote oligomycin-induced depolarization, indicating that it was not caused by an acute toxic effect of the drug (not shown). As an alternative approach to immobilize the mitochondria and suppress transient fusion, we used a synthetic inter-organellar linker (Csordas et al, 2006) that fastened mitochondria to the plasma membrane. In cells expressing the synthetic plasma membrane-mitochondrial linker, the ΔΨm was lower whereas no decrease was found in cells expressing only the mitochondrial part of the linker (OMM-RFP; Supplementary Figure 4B).
An attractive strategy for isolation of transient fusion from complete fusion came from the observation that cells expressing massive amounts of Opa1 showed only transient fusion (Figure 4). Figure 7C shows difference of fusion quantity and quality between control cells containing normal mitochondria, control cells of fragmented mitochondria, and Opa1-overexpressing (high level) cells. The number of total fusion events in control cells with fragmented mitochondria was decreased by 50%, whereas in the Opa1-overexpressing cells it was similar to the control. Strikingly, the ΔΨm was decreased in the fragmented mitochondria of the control cells, but was sustained in the fragmented mitochondria of the Opa1-overexpressing cells (Figure 7B and D). Finally, to evaluate the competency of transient fusion in supporting respiration, we used control and Opa1 AV-infected, insulin-secreting Ins1 cells and measured O2 consumption (Figure 7E). Opa1 AV Ins1 cells display fragmented mitochondria and high level of transient fusion (Twig et al, 2008). Measurements of O2 consumption showed that respiration was maintained in the cells that displayed only transient fusion events. Collectively, the results obtained using four different approaches indicate that transient fusion is competent to maintain mitochondrial metabolism and bioenergetics. Transient fusion would function as a ‘pit stop' for the mitochondria, providing a mechanism for quickly replenishing some soluble factors by IMS and matrix exchange and for rapid recovery of the movement activity.
Conclusions
Mitochondrial transient fusion represents a novel mechanism by which organelles can undergo exchange of contents necessary for proper functioning while maintaining a desired morphology. Even the short-term transient fusion events ensure equilibration of the soluble IMS and matrix factors. These include small solutes, mRNA, and proteins. However, transient fusions would support less effectively the redistribution of integrant membrane proteins that display slow lateral transport in the mitochondrial membranes or mtDNA that has been shown to diffuse more slowly through the reticulum than matrix proteins in the PEG fusion assay (Legros et al, 2004) or in the DNA complementation (Ono et al, 2001). The more prolonged complete fusion events may allow redistribution of both soluble and membrane components. Thus, a basic difference between transient fusion and complete fusion lies in the extent of the mixing of the mitochondrial components. The restricted mixing of integral membrane components during transient fusion may be of relevance for preservation of the original morphology of the merging organelles.
Mitochondrial transient fusion is mediated by the same fusion proteins that mediate complete fusion (Mfns and Opa1). It is possible that OMM fusion, kiss IMM fusion, and complete IMM fusion occur successively when the conditions are favorable for complete fusion events. However, transient fusion has different Opa1 requirement than complete fusion. When Opa1 level is too low or too high to support complete fusion, it can still effectively support transient fusion. It is predicted that the spatial arrangements and interactions of Opa1 are instrumental in controlling the reaction steps from kiss to complete IMM fusion. The separation phase of transient fusion depends on Drp1 such as fission, indicating that it is not simply reversal of fusion. A novel observation in this study is that Drp1 binds to the fusion site and initiates fission promptly if the fusion is not complete.
The interaction between mitochondrial motility and fusion/fission dynamics uncovered in this work is illustrated in Figure 7F. Mitochondria travelling along separate microtubules engage oblique or lateral interactions that result in exchange of the IMS and matrix content, but tend to rapidly reverse due to the force generated by the motor proteins and mediated by the anchorage to the microtubules (upper row). The tension may promote recruitment or scission ring formation of Drp1 (Ingerman et al, 2005) as it has been described for dynamin (Roux et al, 2006). Re-separation of the membranes restores the original shape of the mitochondria and enables prompt recovery of the movement activity. By contrast, mitochondria that move towards each other along a single microtubule undergo longitudinal interaction, leading to fusion that is continually reinforced by the output of the motors (lower row). The stable interaction permits the exchange of all mitochondrial components and attenuates organellar motility. Fission of the mosaic organelle may occur at a distinct site, forming two new individual mitochondria that finally regain their movement activity. Fission of the completely fused organelles may also be aided by the force produced by the mitochondria-associated motors, but the observation that the mitochondria show a small degree of fragmentation in NCD-treated cells (not shown) indicates that fission may occur in the absence of any movements along the microtubules. Also, the microfilaments provide the main tracks for mitochondrial movements in many cell types and are likely to assume the role in fusion played by the microtubules in the present model (De Vos et al, 2005). Overall, the interplay between movements and fusion/fission produces a ‘kiss-and-run' pattern of the mitochondrial dynamics. In addition to the lack of complete intermixing of the membrane components and the rapid reversal of the interaction, the dependence on dynamin-family GTPases is also shared between the kiss-and-run mechanism of neurosecretion and mitochondrial transient fusion (Taraska et al, 2003). However, kiss-and-run is discriminated from full-collapse exocytosis by the lack of clathrin coat (Rizzoli and Jahn, 2007), whereas mitochondrial kiss-and-run and complete fusion seem to involve common fusion proteins, though depending differently on Opa1 amount. Another difference in the regulation of these processes is that exocytosis is triggered by [Ca2+] rise, whereas clamping [Ca2+] at a low level (⩽20 nM; Yi et al, 2004) did not prevent mitochondrial fusion (n=3). In fact, Ca2+ may decrease the chance for mitochondrial transient fusions by suppressing mitochondrial movements. Importantly, the involvement of the mitochondrial movements presents a novel site for the control of the fusion dynamics by cell signalling mechanisms.
Each mitochondrial unit is competent to generate ΔΨm, but maintenance of their metabolic activity relies on the fusion dynamics, because presumably the individual organelles have limited reserves and cannot restock their constituents on their own. Transient fusion provides an efficient recharging mechanism by allowing rapid equilibration of the soluble IMS and matrix proteins and probably, smaller molecules including mRNA between the organelles. Since a short-term decrease in the ΔΨm does not seem to preclude transient or complete fusion, these processes may provide a means to rescue the function of organelles that ran short in some components. However, there is limited time for repair and if this time is exceeded the damaged organelles are likely to be recycled. In this model, transient fusions represent an important element of the mitochondrial quality control. Although maintenance of the autonomous mitochondria by transient fusions consumes cellular energy, the individual organelles offer some advantages, including a greater mobility that seems to be important for a range of cell functions. Thus, transient fusions enhance the functional stability and plasticity of mitochondria, providing a means to optimize the use of the mitochondrial pool in a variety of cellular activities.
Materials and methods
Cells
H9c2 cells were cultured in DMEM as described (Szalai et al, 2000). INS-1 832/13 cells were cultured in RPMI media as described (Twig et al, 2008).
Transient expression
Transfection was performed using either Lipofectamine 2000 (Invitrogen) with 2 μg/ml of each vector or by electroporation (2–3 × 106 cells and 4–20 μg of each plasmid DNA per 250 μl). All mtFP vectors use the targeting sequence of cyto c oxidase subunit VIII to achieve mitochondrial matrix localization. IMM-PAGFP is the mitochondrial targeting sequence (105AA) of ABCB10 fused with PAGFP (Twig et al, 2006). ABCB10 is an IMM erythroid transporter involved in heme biosynthesis. The transfected cells were further incubated in the culture for 24–72 h before the imaging experiments. For high level of Opa1 overexpression, 3 μl Opa1 AV was used in 0.5 ml medium for 5–6 h as described previously (Twig et al, 2008). The infected cells were further incubated in the culture for 48 h before the imaging experiments.
siRNA preparation and transfection
An AA(N19)UU sequence (5′-AAGUCAUCAGUCUGAGCCAGGUU-3′) at position c.1811–1833 of the rat Opa1 open reading frame was selected that is shared by all Opa1 isoforms (Delettre et al, 2001). The sequence of the region was targeted by an Opa1-siRNA (Dharmacon). Transfections with Opa1-siRNA or a scrambled control were performed using an siRNA transfection reagent (GeneSilencer).
Cell fusion by PEG
After transfection in suspension by electroporation, cells were mixed in equal parts of mtRFP and either mtYFP or cyto c GFP and plated at high density on glass coverslips so as to be ∼70% confluent once attached to the glass. PEG fusion was performed 18–36 h post-transfection. After >30-min pretreatment with 50 μM cycloheximide, cells were washed with PBS and fused using 800 μl 50% PEG 2000 in growth medium without FBS for 45–60 s. After extensive washing with medium containing FBS, cells were returned to growth conditions for 45–120 min before imaging both in the presence of 50 μM cycloheximide.
Live-cell microscopic imaging
Most experiments were performed using a Bio-Rad Radiance system fitted to an Olympus IX70 microscope using a × 40 objective (Uapo340, NA 1.35) recording 512 × 512 pixel image pairs or triplicates at 0.25 s−1. An He–Cd laser (442 nm) was used for photoactivation of PAGFP and for excitation of CFP. A dual-line Kr/Ar ion laser source was used for imaging of GFP and YFP at 488-nm excitation, and at 568 nm for TMRE, RFP, and KFP (including photoactivation of KFP). DiIC1(5) was excited by a diode laser at 637 nm. Imaging measurements were performed in an extracellular medium containing 0.25% BSA at 35°C (Yi et al, 2004). The simultaneous use of 488-nm excitation for GFP or YFP monitoring was effective at preventing ongoing photoactivation by continuously quenching the reversible portion of photoactivation if the 568-nm intensity for monitoring of KFP was kept at 9% of maximum.
Imaging at higher temporal resolutions in the PEG fusion assay (0.5 s−1 or 1 s−1) was accomplished using a Nipkow spinning-disk confocal system (Solamere TG) also fitted to an Olympus IX70 using a × 60 objective (PlanApo NA 1.45). Images were acquired by a Stanford Photonics XR/Mega-10 intensified CCD camera with two-binning for 512 × 512 resolution using QED software (Media Cybernetics). Excitation was supplied by a 2 W multi-line argon-ion laser (Spectra Physics) using the 488- and 568-nm lines as with the Radiance system.
To assess ΔΨm, fluorescence imaging was conducted with TMRE-loaded cells (dequenching mode, 500 nM for 25 min; Supplementary Figure 3B and in quenching mode, 25 nM for 15 min; Figure 7 and Supplementary Figure 4) using a CCD camera (Szalai et al, 2000).
All images were analyzed using Spectralyzer imaging software. Organellar movements were quantified in the mtGFP or mtYFP image sequences using a difference image protocol as described before (Yi et al, 2004).
O2 consumption
Oxygen consumption in INS1 cells was measured by an XF24 bioenergetic assay (Seahorse Bioscience, Billerica, MA, USA) as described earlier (Twig et al, 2008).
Western blot analysis
Equal amounts of cell lysates were run on 10% polyacrylamide gels and transferred to nitrocellulose filters. Filters were blocked overnight followed by incubation with anti-Opa1 at dilution 1:1000 (Lee et al, 2004) or 1:500 (BD). After incubation with the primary antibody, bound antibodies were visualized using horseradish peroxidase-coupled secondary antibody with the Dura Signal chemiluminescence-developing kit (Pierce).
Statistics
The data are shown as mean±s.e.m. (n⩾3). Significance of differences from the relevant controls was calculated by Student's t test.
Supplementary Material
Supplementary Movie
Supplementary Material
Review Process File
Acknowledgments
We thank Drs Tamas Balla, Atan Gross, Carmen Mannella, Heidi McBride, Peter Varnai, and Richard Youle for reagents and helpful discussions. This work was supported by a grant from NIH to GH (AA017773).
Footnotes
The authors declare that they have no conflict of interest.
References
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A (2002) An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci USA 99: 12651–12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura S, Yamamoto J, Aida GP, Nakazono M, Tsutsumi N (2004) Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc Natl Acad Sci USA 101: 7805–7808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A (2003) Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278: 17190–17197 [DOI] [PubMed] [Google Scholar]
- Baricault L, Segui B, Guegand L, Olichon A, Valette A, Larminat F, Lenaers G (2007) OPA1 cleavage depends on decreased mitochondrial ATP level and bivalent metals. Exp Cell Res 313: 3800–3808 [DOI] [PubMed] [Google Scholar]
- Bowes T, Gupta RS (2008) Novel mitochondrial extensions provide evidence for a link between microtubule-directed movement and mitochondrial fission. Biochem Biophys Res Commun 376: 40–45 [DOI] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105: 15803–15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125: 1241–1252 [DOI] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192 [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudakov DM, Belousov VV, Zaraisky AG, Novoselov VV, Staroverov DB, Zorov DB, Lukyanov S, Lukyanov KA (2003) Kindling fluorescent proteins for precise in vivo photolabeling. Nat Biotechnol 21: 191–194 [DOI] [PubMed] [Google Scholar]
- Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101: 15927–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D'Adamio L, Derks C, Dejaegere T, Pellegrini L, D'Hooge R, Scorrano L, De Strooper B (2006) Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126: 163–175 [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174: 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP (2005) Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol 15: 678–683 [DOI] [PubMed] [Google Scholar]
- Delettre C, Griffoin JM, Kaplan J, Dollfus H, Lorenz B, Faivre L, Lenaers G, Belenguer P, Hamel CP (2001) Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet 109: 584–591 [DOI] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26: 207–210 [DOI] [PubMed] [Google Scholar]
- Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS (2006) Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem 281: 37972–37979 [DOI] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525 [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189 [DOI] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM (2007) Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol 178: 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griparic L, van der Wel NN, Orozco IJ, Peters PJ, van der Bliek AM (2004) Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem 279: 18792–18798 [DOI] [PubMed] [Google Scholar]
- Guillery O, Malka F, Landes T, Guillou E, Blackstone C, Lombes A, Belenguer P, Arnoult D, Rojo M (2008) Metalloprotease-mediated OPA1 processing is modulated by the mitochondrial membrane potential. Biol Cell 100: 315–325 [DOI] [PubMed] [Google Scholar]
- Heiskanen KM, Bhat MB, Wang HW, Ma J, Nieminen AL (1999) Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J Biol Chem 274: 5654–5658 [DOI] [PubMed] [Google Scholar]
- Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J (2005) Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol 170: 1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Fujita Y, Oka T, Mihara K (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J 25: 2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC (2003) hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem 278: 36373–36379 [DOI] [PubMed] [Google Scholar]
- Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ (2004) Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol 164: 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ (2006) Role of Bax and Bak in mitochondrial morphogenesis. Nature 443: 658–662 [DOI] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305: 858–862 [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H (2007) Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem 282: 22977–22983 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F, Lombes A, Frachon P, Rojo M (2002) Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell 13: 4343–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F, Malka F, Frachon P, Lombes A, Rojo M (2004) Organization and dynamics of human mitochondrial DNA. J Cell Sci 117: 2653–2662 [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119: 873–887 [DOI] [PubMed] [Google Scholar]
- Malka F, Guillery O, Cifuentes-Diaz C, Guillou E, Belenguer P, Lombes A, Rojo M (2005) Separate fusion of outer and inner mitochondrial membranes. EMBO Rep 6: 853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattenberger Y, James DI, Martinou JC (2003) Fusion of mitochondria in mammalian cells is dependent on the mitochondrial inner membrane potential and independent of microtubules or actin. FEBS Lett 538: 53–59 [DOI] [PubMed] [Google Scholar]
- McBride HM, Neuspiel M, Wasiak S (2006) Mitochondria: more than just a powerhouse. Curr Biol 16: R551–R560 [DOI] [PubMed] [Google Scholar]
- Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J (2006) Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell 127: 383–395 [DOI] [PubMed] [Google Scholar]
- Meeusen S, McCaffery JM, Nunnari J (2004) Mitochondrial fusion intermediates revealed in vitro. Science 305: 1747–1752 [DOI] [PubMed] [Google Scholar]
- Nakada K, Inoue K, Ono T, Isobe K, Ogura A, Goto YI, Nonaka I, Hayashi JI (2001) Inter-mitochondrial complementation: mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat Med 7: 934–940 [DOI] [PubMed] [Google Scholar]
- Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H (2005) Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem 280: 25060–25070 [DOI] [PubMed] [Google Scholar]
- Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G (2003) Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 278: 7743–7746 [DOI] [PubMed] [Google Scholar]
- Olichon A, Elachouri G, Baricault L, Delettre C, Belenguer P, Lenaers G (2007) OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death Differ 14: 682–692 [DOI] [PubMed] [Google Scholar]
- Olichon A, Emorine LJ, Descoins E, Pelloquin L, Brichese L, Gas N, Guillou E, Delettre C, Valette A, Hamel CP, Ducommun B, Lenaers G, Belenguer P (2002) The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett 523: 171–176 [DOI] [PubMed] [Google Scholar]
- Ono T, Isobe K, Nakada K, Hayashi JI (2001) Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet 28: 272–275 [DOI] [PubMed] [Google Scholar]
- Patterson GH, Lippincott-Schwartz J (2002) A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297: 1873–1877 [DOI] [PubMed] [Google Scholar]
- Pitts KR, Yoon Y, Krueger EW, McNiven MA (1999) The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell 10: 4403–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Jahn R (2007) Kiss-and-run, collapse and ‘readily retrievable' vesicles. Traffic 8: 1137–1144 [DOI] [PubMed] [Google Scholar]
- Roux A, Uyhazi K, Frost A, De Camilli P (2006) GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 441: 528–531 [DOI] [PubMed] [Google Scholar]
- Santel A, Fuller MT (2001) Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114: 867–874 [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C, Chan DC (2007) OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol 178: 749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R (2004) Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell 16: 59–68 [DOI] [PubMed] [Google Scholar]
- Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G (2000) Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem 275: 15305–15313 [DOI] [PubMed] [Google Scholar]
- Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W (2003) Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci USA 100: 2070–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T, Langer T (2008) Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 27: 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Turnbull DM (2005) Mitochondrial DNA mutations in human disease. Nat Rev Genet 6: 389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Graf SA, Wikstrom JD, Mohamed H, Haigh SE, Elorza A, Deutsch M, Zurgil N, Reynolds N, Shirihai OS (2006) Tagging and tracking individual networks within a complex mitochondrial web with photoactivatable GFP. Am J Physiol Cell Physiol 291: C176–C184 [DOI] [PubMed] [Google Scholar]
- Ward MW, Rego AC, Frenguelli BG, Nicholls DG (2000) Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci 20: 7208–7219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B (2002) Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep 3: 527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MP (1999) The machinery of mitochondrial inheritance and behavior. Science 283: 1493–1497 [DOI] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnoczky G (2004) Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol 167: 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Karbowski M (2005) Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6: 657–663 [DOI] [PubMed] [Google Scholar]
- Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V et al. (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot–Marie–Tooth neuropathy type 2A. Nat Genet 36: 449–451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie
Supplementary Material
Review Process File