Abstract
The orexin / hypocretin system has recently been implicated in reward-processing and addiction. We examined the involvement of the orexin system in cue-induced reinstatement of extinguished cocaine-seeking by administering the orexin 1 receptor (OX1R) antagonist SB-334867, or the orexin 2 receptor (OX2R) antagonist 4-pyridylmethyl (S)-tert-leucyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (4PT), prior to reinstatement testing. Male Sprague-Dawley rats self-administered cocaine in 2-hour sessions for 10 days, followed by extinction training. Reinstatement of cocaine-seeking was elicited by presentation of tone + light cues previously paired with cocaine infusions. SB-334867 (10, 20, 30 mg/kg) dose-dependently decreased cue-induced reinstatement of cocaine-seeking without significantly affecting responding during late extinction. 4PT (10, 30 mg/kg) did not significantly alter cue-induced reinstatement. In separate experiments, the highest doses of SB-334867 and 4PT had no significant effect on established cocaine self-administration, and 4PT reduced spontaneous activity in a locomotor test to a greater extent than SB-334867. Finally, SB-334867 (30 mg/kg) had no effect on the acquisition of cocaine-paired cues during a Pavlovian cocaine-stimulus conditioning session in the operant chamber. Pretreatment with SB-334867 prior to the Pavlovian acquisition session had no effect on subsequent cue-induced reinstatement of cocaine-seeking elicited by those cues. However, pretreatment with SB-334867 prior to a second reinstatement session significantly attenuated the expression of cue-induced reinstatement. These results show that orexin transmission at OX1R, but not OX2R, is necessary for the reinstatement of cocaine-seeking elicited by drug-paired cues, and that orexin signaling is not critical for cocaine reinforcement or cocaine-stimulus conditioning.
Keywords: self-administration, addiction, reinstatement, relapse, hypothalamus
INTRODUCTION
Relapse prevention is a difficult aspect of addiction treatment, and pharmacotherapies for reducing cocaine relapse are very limited (O'Brien, 2005; Vocci et al., 2005; Karila et al., 2008). Drug-associated cues are powerful triggers for drug desire and relapse in cocaine addicts (Wallace, 1989; Childress et al., 1992; Sinha et al., 2003), and for reinstatement of an extinguished drug-seeking response in rodent models (Davis & Smith, 1976; Meil & See, 1996; for review, Shaham et al., 2003). Recently, we and others have examined whether the orexin system is involved in relapse to drug-seeking.
The orexins (or hypocretins) are two recently discovered hypothalamic neuropeptides that act at two G protein-coupled receptors, OX1R and OX2R (de Lecea et al., 1998; Sakurai et al., 1998). Orexins have been extensively implicated in maintenance of arousal states and narcolepsy with cataplexy (for review, Siegel, 2004; Nishino & Kanbayashi, 2005). Recent studies have shown that orexins are also involved in reward-seeking and neuroplasticity associated with drugs of abuse (Harris et al., 2005; Borgland et al., 2006; Narita et al., 2006; Harris et al., 2007). The OX1R antagonist SB-334867 reduced stress-induced reinstatement of cocaine-seeking, as well as yohimbine- and cue-induced reinstatement of ethanol-seeking (Boutrel et al., 2005; Lawrence et al., 2006; Richards et al., 2008). However, underlying neural circuitries are specific for the reinstatement modality and the drug being studied (Kalivas & McFarland, 2003; Shaham et al., 2003; Rogers et al., 2008), so these studies do not predict the potential involvement of orexin in cue-induced cocaine-seeking.
No studies to date have investigated the effects of OX2R antagonists on drug-seeking behavior. OX1R and OX2R differ in several ways. First, OX1R has 10-fold selectivity for orexin A, while OX2R is nonselective for orexin A and B (Sakurai et al., 1998). Second, OX1R is coupled exclusively to a Gq subclass of G proteins, whereas OX2R is coupled to both Gi/o and Gq proteins (Zhu et al., 2003). Third, the distributions of OX1R and OX2R differ substantially in most brain regions (Trivedi et al., 1998; Kilduff & de Lecea, 2001; Marcus et al., 2001). Finally, an OX2R mutation is associated with canine narcolepsy (Lin et al., 1999), linking this receptor with arousal and sleep functions. Thus, it is important to determine the relative contribution of orexin transmission at OX1 and OX2 receptors in addiction-related behaviors to find appropriate pharmacological targets.
We hypothesized that orexin signaling at OX1R is involved in cue-induced reinstatement of cocaine-seeking. We tested the effects of the OX1R antagonist SB-334867 (SB) (Porter et al., 2001), and the OX2R antagonist 4-pyridylmethyl (S)-tert-leucyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (4PT) (Hirose et al., 2003), on cue-induced reinstatement of cocaine-seeking following cocaine self-administration. We also determined whether orexin is necessary for the acquisition of cocaine-paired cues during self-administration, as it is involved in the acquisition of morphine-paired cues in a place preference paradigm (Narita et al., 2006; Harris et al., 2007). To further differentiate the effects of the two antagonists and to determine the specificity of behavioral effects, we tested antagonist effects on cocaine self-administration and spontaneous locomotion.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (initial weight 250-300 g; Charles River, Wilmington, MA) were single- or pair-housed under a reversed 12-hr light/dark cycle (lights off at 6 a.m.) and had ad libitum access to food and water. Animals were housed in a temperature- and humidity-controlled animal facility at MUSC (AAALAC-accredited; NCRR C06 grant RR015455). All experiments were approved by the Institutional Animal Care and Use Committee at MUSC and conducted according to specifications of the National Institutes of Health as outlined in the Guide for the Care and Use of Laboratory Animals.
Intravenous catheter surgery
Following acclimation to the animal facility, rats were implanted with chronic indwelling intravenous catheters while under ketamine/xylazine anesthesia (+ equithesin in some cases). A non-steroidal anti-inflammatory was administered as an analgesic prior to surgery. The catheters were constructed of silastic tubing (Dow Corning, Midland, MI) connected to a modified guide cannula (C313G-5UP-SPC12, Plastics One, Roanoke, VA), which was mounted on ProLite polypropylene monofilament mesh (Atrium, Hudson, NH) using Ortho-Jet acrylic (Lang Dental, Wheeling, IL); a small silicone bubble was placed 3.8 cm from the end of the silastic tubing. Briefly, the free end of silastic tubing was inserted into (and secured to) the right jugular vein, while the other end passed subcutaneously over the shoulder to the cannula which was mounted on the back and exited via a biopsy hole. Beginning 3 days after surgery, catheters were flushed once daily with 0.1 ml each of the antibiotic cefazolin (100 mg/ml) and heparin (100 U/ml). For each self-administration session, catheters were flushed with 0.1 ml saline to ensure patency prior to attachment to the cocaine infusion line (PE-50 tubing) and spring tether in the self-administration chamber, and flushed with 0.1 ml each of cefazolin and heparin following the session. Self-administration sessions began after one week of recovery from surgery.
Cocaine self-administration, extinction, and reinstatement
Self-administration sessions were carried out in operant chambers housed in sound-attenuating cubicles and controlled via a MED-PC IV program (Med-Associates, St. Albans, VT). Rats learned to lever-press for intravenous cocaine (fixed ratio-1; 0.2 mg/50 μl infusion via motorized pump; 20-sec time-out after each infusion) in 2-hour daily sessions. Presses on an inactive lever had no programmed consequences. Except as noted, rats were given 10 self-administration sessions in which they earned ≥ 10 infusions. Rats then underwent daily extinction sessions, during which presses on either lever had no consequences (no drug or cues), until they met the criteria of two consecutive sessions with < 25 active lever presses (minimum of 7 sessions prior to the first reinstatement test; minimum of 2 sessions between subsequent reinstatement tests). During cue-induced reinstatement of cocaine-seeking, active lever presses once again resulted in delivery of tone + light cues, but no drug infusions.
Experiment 1: Cue-induced reinstatement
During self-administration sessions, cocaine infusions were paired with discrete tone + light cues (78 dB, 2900 Hz; white stimulus light above the active lever). The red house light (on the wall opposite the levers) was turned off during cocaine infusions and time-outs. Rats then underwent extinction training prior to reinstatement testing.
To test the effects of the OX1R antagonist SB-334867 (SB; 10, 20, or 30 mg/kg, i.p.) or the OX2R antagonist 4PT (10 or 30 mg/kg, i.p.) on cue-induced reinstatement of cocaine-seeking, animals were given up to 4 test sessions: 2 cue-induced reinstatement sessions with separate vehicle and antagonist pretreatment, and 2 late extinction sessions (no cues) with separate vehicle and antagonist pretreatment. In other words, all animals received both vehicle and antagonist pretreatment prior to separate sessions (2 extinction and 2 reinstatement) in a within-subjects design. The order of these sessions was counterbalanced within groups so that the 4 test sessions were presented in different orders to different animals. An animal received the same dose of antagonist for all tests (i.e., the same dose of antagonist was given prior to a cue-induced reinstatement session and extinction session, and vehicle was given prior to a cue-induced reinstatement session and extinction session).
Animals in SB experiments were given 10 days of cocaine self-administration followed by extinction, as described above. For cue-induced reinstatement testing, animals pretreated (30 min) with either 20 mg/kg (n = 9) or 30 mg/kg (n = 8) SB received all 4 test sessions described above. Animals pretreated with 10 mg/kg SB (n = 8) received only the 2 cue-induced reinstatement sessions (with separate vehicle and SB pretreatment) because preliminary studies indicated this dose to be ineffective, eliminating the need for control sessions during late extinction.
Animals in 4PT experiments were given 12 days of cocaine self-administration (due to 4PT pretreatment on the 10th day of self-administration for Experiment 3), followed by extinction. For cue-induced reinstatement testing, animals pretreated (15 min) with either 10 mg/kg (n = 9) or 30 mg/kg (n = 13) 4PT received all 4 test sessions.
Animals were excluded from all analyses (vehicle and antagonist sessions) if they did not receive some or all of the reinstatement tests due to insufficient extinction, or if they made less than 20 presses during the cue-induced reinstatement session with vehicle pretreatment (i.e., no reinstatement in response to cues). One animal in the 4PT group was excluded from all analyses due to high responding on the active lever during reinstatement (more than 2.5 standard deviations different from group mean).
Experiment 2: Locomotion
All animals used to test the effects of SB or 4PT on locomotor activity had previously been part of a cocaine self-administration experiment. At least 5 days after the last self-administration (or extinction/reinstatement) session, animals were tested for spontaneous locomotion in clear acrylic chambers (approx. 40 × 40 × 30 cm) equipped with Digiscan monitors (AccuScan Instruments, Inc., formerly Omnitech Electronics, Columbus, OH) containing a 16 ×16 photobeam array for the x/y axes (horizontal activity) and 16 photobeams for the z axis (vertical activity). Photobeam breaks were detected by a Digiscan analyzer and recorded by DigiPro software (Version 1.4). Animals were pretreated with antagonist (30 min for SB, 15 min for 4PT) prior to a 60-min test session, and data were collected in 5-min bins. Animals were tested twice for SB (0 and 30 mg/kg; n = 12) or 4PT (0 and 30 mg/kg; n = 12) in a counterbalanced fashion, with tests at least 2 days apart.
Experiment 3: Established self-administration
During self-administration sessions, cocaine infusions were paired with discrete tone + light cues (78 dB, 2900 Hz; white stimulus light above the active lever). The red house light (on the wall opposite the levers) was turned off during cocaine infusions and time-outs.
To test the effects of SB (30 mg/kg, i.p.; n = 12) or 4PT (30 mg/kg, i.p.; n = 10) on established cocaine self-administration, animals were pretreated with either SB or 4PT (30 min for SB, 15 min for 4PT) prior to the 10th self-administration session (animals had received 9 previous self-administration sessions in which they earned ≥ 10 infusions). Animals were subsequently given 2 additional self-administration sessions.
One animal in the 4PT group was excluded from all analyses due to extremely high responding on the active lever during self-administration (more than 3 standard deviations different from group mean).
Experiment 4: Acquisition and expression of Pavlovian-conditioned cues
To test the role of orexin in the acquisition of cocaine-paired cues during self-administration, we utilized a Pavlovian-conditioned cue paradigm (See, 2005). This paradigm affords the opportunity to study the acquisition of cocaine-cue associations in a single conditioning session. Briefly, animals are trained to self-administer cocaine in the absence of programmed stimuli, and then are given a single Pavlovian conditioning session during which they receive passive pairings of cocaine infusions with discrete stimulus cues (tone + light). Animals are then returned to daily cocaine self-administration in the absence of stimuli. Following extinction of responding in the absence of cocaine and cues, animals are given a reinstatement session where cues alone are presented in response to active lever presses, as described above.
More specifically, after 5 days of self-administration in the absence of cues (no discrete tone or light cues), animals were exposed to a single 2-hour Pavlovian conditioning session in the operant chamber, in which no levers were extended and the animals received passive infusions of cocaine paired with discrete tone + light cues. Time between infusions was fixed. The number of infusions was individually based on the average number of infusions during the 2 prior self-administration sessions for that animal. The animals then received 5 more days of self-administration in the absence of cues and extinction training. During reinstatement of cocaine-seeking elicited by the cocaine-associated cues, active lever presses resulted in delivery of tone + light cues, with no drug infusions.
To test the effects of SB (30 mg/kg, i.p.) on the acquisition of cocaine-associated cues, animals were pretreated (30 min) with either SB (n = 16) or vehicle (n = 11) prior to the Pavlovian conditioning session and then tested drug-free during a subsequent cue-induced reinstatement. To test the effects of SB on the expression of reinstatement induced by Pavlovian-conditioned cues, animals were pretreated (30 min) with either SB (n = 15) or vehicle (n = 12) prior to a second cue-induced reinstatement session (only a 1-hour session so that animals could be sacrificed for Fos analysis, not shown here). Animals were counterbalanced within this second reinstatement session so that some animals received the same pretreatment for both the acquisition and expression sessions, and some animals received different pretreatment for the two sessions.
Drugs
Cocaine HCl (NIDA, Research Triangle Park, NC) was dissolved in 0.9% saline. SB-334867 (1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride; purchased from Tocris, Ellisville, MO, or generously donated by Eli Lilly, Indianapolis, IN) was suspended in 2% DMSO and 10% 2-hydroxypropyl-β-cyclodextrin (Sigma) in sterile water; 0, 10, 20, or 30 mg/kg was given in a volume of 4 ml/kg (i.p.) 30 min prior to testing. 4PT (4-pyridylmethyl (S)-tert-leucyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline; generously donated by Eli Lilly) was suspended in either: a) 5% Solutol HS 15 (BASF, Ludwigshafen, Germany) and 10% 2-hydroxypropyl-β-cyclodextrin in sterile water, or b) 2% DMSO and 10% 2-hydroxypropyl-β-cyclodextrin in sterile water; 0, 10, or 30 mg/kg was given in a volume of 4 ml/kg (i.p.) 15 min prior to testing. SB has 50-fold selectivity for OX1R over OX2R and 100-fold selectivity over approximately 50 other molecular targets (Porter et al., 2001; Smart et al., 2001). 4PT has 250-fold selectivity for OX2R over OX1R, as well as over 50 other receptors and targets (Hirose et al., 2003).
Data analyses
One-way or mixed-model ANOVAs were utilized for most analyses, with test session or time as a repeated-measure when appropriate. Post-hoc analyses were computed with the Tukey-Kramer test. To evaluate the effects of SB or 4PT on cue-induced reinstatement, the 4 test sessions for the groups given 20 or 30 mg/kg SB, or 10 or 30 mg/kg 4PT (vehicle and antagonist pretreatment prior to 2 separate cue-induced reinstatement sessions, and vehicle and antagonist pretreatment prior to 2 separate extinction sessions), were analyzed with one-way repeated-measures ANOVAs within each group. The 2 tests for the group given 10 mg/kg SB (vehicle and SB pretreatment prior to 2 separate cue-induced reinstatement sessions) were analyzed with a paired t-test. To evaluate the effects of SB or 4PT on locomotion, the 60-min test sessions were divided into 5-min bins and analyzed with mixed-model ANOVAs (2-way ANOVA with session time as a repeated-measure); when overall ANOVAs were significant, individual paired t-tests were used to evaluate treatment differences within each 5-min bin. To evaluate the effects of SB or 4PT on self-administration, the antagonist-pretreated self-administration session was compared to the preceding and following sessions using one-way repeated-measures ANOVAs. For Pavlovian acquisition and expression, t-tests were used to evaluate differences between vehicle and SB pretreatment for reinstatement.
RESULTS
Experiment 1: Effects of SB or 4PT on cue-induced reinstatement of cocaine-seeking
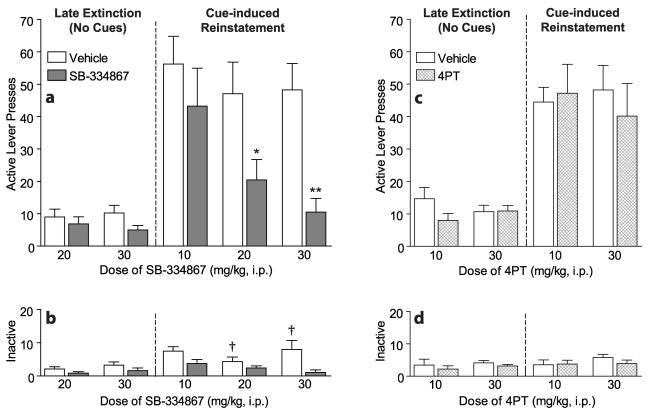
Figure 1 shows the mean (± SEM) numbers of lever presses during cue-induced reinstatement when animals were pretreated with SB (Fig. 1a, b) or 4PT (Fig. 1c, d). Active lever (Fig. 1a, c) and inactive lever (Fig. 1b, d) responding is shown for late extinction sessions and cue-induced reinstatement sessions following vehicle or antagonist pretreatment.
Figure 1.
Attenuation of cue-induced reinstatement of cocaine-seeking by the OX1R antagonist SB-334867, but not the OX2R antagonist 4PT. In a within-subjects design, rats were pretreated with a single dose of antagonist (10, 20, or 30 mg/kg, i.p.) or vehicle prior to late extinction sessions (no cues or cocaine) and cue-induced reinstatement sessions (tone + light cues) in a counterbalanced fashion. Mean (± SEM) numbers of presses on the active and inactive levers in 2-hour sessions in the self-administration chamber are shown. a, Active lever responding during cue-induced reinstatement sessions was significantly attenuated by SB at 20 mg/kg (n = 9) and 30 mg/kg (n = 8), but not 10 mg/kg (n = 8), with no significant effect on late extinction session responding (*p < 0.01, **p < 0.001). b, Inactive lever responding was significantly reduced during late extinction and cue-induced reinstatement sessions by 20 and 30 mg/kg SB, as compared to cue-induced reinstatement with vehicle pretreatment (†p < 0.05). c, Active lever responding during late extinction and cue-induced reinstatement sessions was not significantly affected by 4PT at 10 mg/kg (n = 9) or 30 mg/kg (n = 13). d, Inactive lever responding was also not significantly affected by 4PT.
OX1R antagonist - SB-334867 (SB)
SB effects on active lever responding (Fig. 1a, 2)
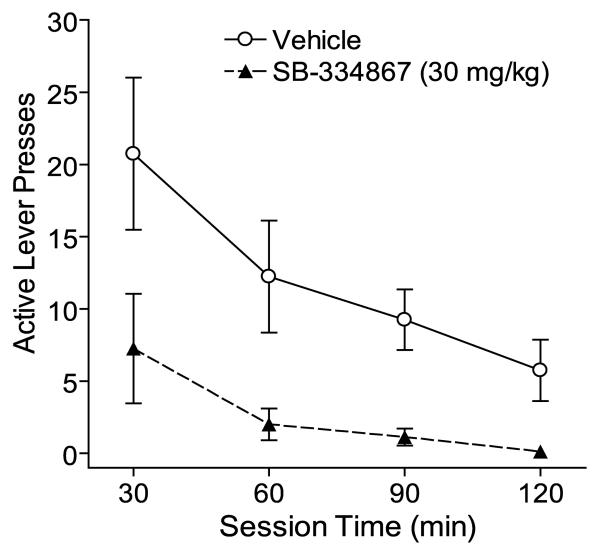
Figure 2.
Attenuation of cue-induced reinstatement of cocaine-seeking by the OX1R antagonist SB-334867 across the 2-hour session. Mean (± SEM) numbers of active lever presses for each 30-min time bin during cue-induced reinstatement sessions in animals pretreated with 30 mg/kg SB (i.p.) and vehicle (n = 8). Active lever responding across the 30-min time bins was significantly attenuated by SB (p = 0.01).
For the 30 mg/kg SB group (n = 8), there was a significant effect of test session (F(3,4) = 16.90; p < 0.0001), and post-hoc analyses revealed that active lever responding during the cue-induced reinstatement session with vehicle pretreatment was significantly different from the other 3 sessions (i.e., cue-induced reinstatement with SB pretreatment, and extinction sessions with either vehicle or SB pretreatment, p < 0.001). For the 20 mg/kg group (n = 9), there was a significant effect of test session (F(3,5) =14.57; p < 0.001), and post-hoc analyses revealed that active lever responding during the cue-induced reinstatement session with vehicle pretreatment was significantly different from the other 3 sessions (i.e., cue-induced reinstatement with SB pretreatment, p < 0.01; extinction sessions with either vehicle or SB pretreatment, p < 0.001). For the 10 mg/kg SB group (n = 8), active lever responding during cue-induced reinstatement sessions with either vehicle or SB pretreatment was not significantly different (T(7) =1.11; p = 0.30). When the SB groups were compared across doses (between-group analyses), there were no significant differences between SB groups for cue-induced reinstatement sessions with vehicle pretreatment, but a significant difference between SB groups for cue-induced reinstatement sessions with SB pretreatment (F(2,22) = 4.32; p < 0.05). Post-hoc analyses revealed a significant difference between the 10 and 30 mg/kg SB groups only (p < 0.05). These data show that 20 or 30 mg/kg, but not 10 mg/kg, SB attenuated active lever responding during cue-induced reinstatement to extinction levels.
Figure 2 shows the mean (± SEM) numbers of active lever presses for each 30-min bin during cue-induced reinstatement sessions in animals pretreated with 30 mg/kg SB and vehicle (n = 8). When SB-pretreated reinstatement was compared to vehicle-pretreated reinstatement in the same animals, there was a significant effect of pretreatment (F(1,14) = 8.66; p = 0.01), but no significant effect of time and no significant interaction.
SB effects on inactive lever responding (Fig. 1b)
For the 30 mg/kg SB group, there was a significant effect of test session (F(3,4) = 4.65; p < 0.05), and post-hoc analyses revealed that inactive lever responding during cue-induced reinstatement with vehicle pretreatment was significantly different from cue-induced reinstatement and extinction with SB pretreatment (p < 0.05). For the 20 mg/kg SB group, there was a significant effect of test session (F(3,5) = 3.25; p < 0.05), and post-hoc analyses revealed that inactive lever responding during cue-induced reinstatement with vehicle pretreatment was significantly different from extinction with SB pretreatment (p < 0.05). For the 10 mg/kg SB group, inactive lever responding during the cue-induced reinstatement sessions with either vehicle or SB pretreatment was not significantly different (T(7) = 1.90; p= 0.10). When the SB groups were compared across doses, there were no significant differences between SB groups for cue-induced reinstatement sessions with either vehicle or SB pretreatment. These data indicate a minimal but significant effect of 20 or 30 mg/kg SB on inactive lever responding.
Baseline responding
Self-administration data were analyzed to assess differences in responding between SB groups before SB administration. No significant differences were observed between groups for cocaine intake or active lever responding during the last two self-administration sessions, for active lever responding during the first 7 days of extinction, or for active lever responding during cue-induced reinstatement following vehicle pretreatment. On the last two self-administration days, the across-group (n = 25 total) mean (± SEM) for cocaine infusions was 34.4 (± 2.1) and 32.6 (± 1.8) per session (18.8 (±1.2) and 17.5 (±0.9) mg/kg), and active lever presses was 47.2(± 4.4) and 42.0 (± 2.9) per session. During extinction, there was a significant main effect for extinction session (F(6,22) =30.14; p < 0.001), indicating that animals showed a significant decrease in active lever responding across extinction sessions. The across-group mean (± SEM) for active lever presses on days 1 and 7 of extinction was 79.0 (± 9.8) and 13.5 (± 1.8). Active lever responding during cue-induced reinstatement following vehicle pretreatment as compared to the prior extinction session was not significantly different between SB groups, but there was a significant main effect for cue-induced reinstatement (F(1,22) = 88.83; p < 0.001), and post-hoc analyses showed significant cue-induced reinstatement of active lever responding for all groups (p < 0.001). There were no significant differences for inactive lever responding between SB groups during cue-induced reinstatement in this analysis.
OX2R antagonist - 4PT
Lack of effect of 4PT on active lever responding (Fig. 1c)
For the 30 mg/kg 4PT group (n = 13), there was a significant effect of test session (F(3,9) = 10.50; p < 0.0001), and post-hoc analyses revealed that active lever responding during the cue-induced reinstatement session with vehicle pretreatment was significantly different from the late extinction sessions (vehicle or 4PT pretreatment; p < 0.001). Similarly, responding during the cue-induced reinstatement session with 4PT pretreatment was significantly different from extinction sessions (vehicle or 4PT pretreatment; p < 0.01). For the 10 mg/kg 4PT group (n = 9), there was a significant effect of test session (F(3,5) = 16.22; p < 0.0001), and post-hoc analyses revealed that active lever responding during the cue-induced reinstatement session with vehicle pretreatment was significantly different from the late extinction sessions (vehicle or 4PT pretreatment; p < 0.01). Also, active lever responding during the cue-induced reinstatement session with 4PT pretreatment was significantly different from extinction sessions (vehicle or 4PT pretreatment; p < 0.001). Within the 10 and 30 mg/kg 4PT groups, cue-induced reinstatement sessions with vehicle or 4PT pretreatment were not significantly different from each other. When the 4PT groups were compared across doses (between-group analyses), there were no significant differences for cue-induced reinstatement sessions with vehicle pretreatment or sessions with 4PT pretreatment. These data show that 10 or 30 mg/kg 4PT had no significant effects on active lever responding during cue-induced reinstatement.
Lack of effect of 4PT on inactive lever responding (Fig. 1d)
For 10 or 30 mg/kg 4PT groups, there were no significant effects of test session for inactive lever responding during cue-induced reinstatement or extinction sessions.
Baseline responding
Self-administration data were analyzed to assess differences in baseline responding between 4PT groups before 4PT administration. No significant differences were observed between groups for cocaine intake or active lever responding during the last two self-administration sessions, for active lever responding during the first 7 days of extinction, or for active lever responding during cue-induced reinstatement (following vehicle pretreatment). On the last two days of self-administration, the across-group (n = 22 total) mean (± SEM) for cocaine infusions was 44.3 (± 2.4) and 41.6 (± 2.1) per session (23.4 (±1.3) and 21.8 (± 1.1) mg/kg), and active lever presses was 66.7 (± 9.5) and 85.3 (± 26.0) per session. During extinction, there was a significant main effect of extinction session (F(6,20) = 17.64; p < 0.0001), indicating that animals showed a significant decrease in active lever responding across extinction sessions. The across-group mean (± SEM) for active lever presses on days 1 and 7 of extinction was 58.0 (± 7.6) and 17.0 (± 2.5). Active lever responding during cue-induced reinstatement (following vehicle pretreatment) as compared to the prior extinction session was not significantly different between 4PT groups, but there was a significant main effect for cue-induced reinstatement (F(1,20) = 49.21; p < 0.0001), and post-hoc analyses showed significant cue-induced reinstatement of active lever responding for all groups (p < 0.005). No significant differences were observed for the inactive lever during cue-induced reinstatement in this analysis.
Experiment 2: Effects of SB or 4PT on locomotion
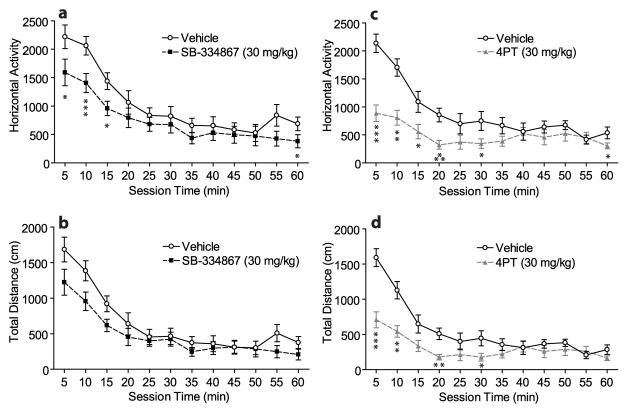
Figure 3 shows the horizontal activity (number of photobeam breaks; Fig. 3a, c) and total distance traveled (cm; Fig. 3b, d) during a 60-min locomotion test following pretreatment with either SB (0 or 30 mg/kg; n = 12) or 4PT (0 or 30 mg/kg; n = 12). In all analyses, there was a significant effect of session time during the 60-min test (p < 0.0001).
Figure 3.
Reduction in spontaneous locomotor activity by the OX1R antagonist SB-334867 and OX2R antagonist 4PT. In a within-subjects design, rats were pretreated with either SB, 4PT (30 mg/kg, i.p. each) or vehicle prior to two separate locomotor tests (60 min). Mean (± SEM) horizontal activity (number of photobeam breaks in the x/y axes) and total distance traveled (cm) is shown for each 5-min time bin. SB (n = 12) significantly affected overall horizontal activity (p < 0.05) (a), but not overall total distance (b). 4PT (n = 12) significantly affected overall horizontal activity (p < 0.0005) (c), and overall total distance (p < 0.0005) (d). Individual paired t-tests within each 5-min bin revealed specific time points for significant differences (*p < 0.05, **p < 0.005, ***p < 0.0005).
OX1R antagonist – SB-334867 (SB)
There was a significant effect of SB treatment for horizontal activity (F(1,22) = 5.16; p < 0.05) but not for total distance. There were no significant interactions between treatment and time for either locomotor measure. Individual paired t-tests for each 5-min bin of horizontal activity revealed that 30 mg/kg SB was significantly different from vehicle only during the first 3 time bins (p < 0.05, 0.0001, 0.01, respectively) and the last time bin (p < 0.05).
OX2R antagonist - 4PT
There was a significant effect of 4PT treatment for horizontal activity (F(1,22) = 18.24; p < 0.0005) and total distance (F(1,22) = 16.55; p = 0.0005), and significant interactions between treatment and session time for horizontal activity (F(11,242) = 5.72; p < 0.0001) and total distance (F(11,242) = 6.45; p < 0.0001). Individual paired t-tests for each 5-min bin of horizontal activity revealed that 30 mg/kg 4PT was significantly different from vehicle during the first 4 time bins (p < 0.0001, 0.001, 0.05, 0.0005, respectively), sixth bin (p < 0.05), and last bin (p < 0.05). Similar analyses of total distance revealed that 30 mg/kg 4PT was significantly different from vehicle during the first 2 time bins (p < 0.0001, 0.005), fourth bin (p < 0.001), and sixth bin (p < 0.05).
When SB and 4PT groups were compared, there were significant differences between the groups during antagonist-pretreated sessions for both horizontal activity (F(1,22) = 5.59; p < 0.05) and total distance (F(1,22) = 6.72; p <0.05), and significant interactions between group and time for horizontal activity (F(11,242) = 2.80; p < 0.005) and total distance (F(11,242) = 2.68; p < 0.005). There were no significant differences between SB and 4PT groups during vehicle-pretreated sessions for either horizontal activity or total distance (F(1,22) < 1.3; p > 0.25), and no significant interactions between group and time. These analyses indicate that 4PT caused significantly greater reductions in locomotor activity than SB.
Experiment 3: No effect of SB or 4PT on cocaine self-administration
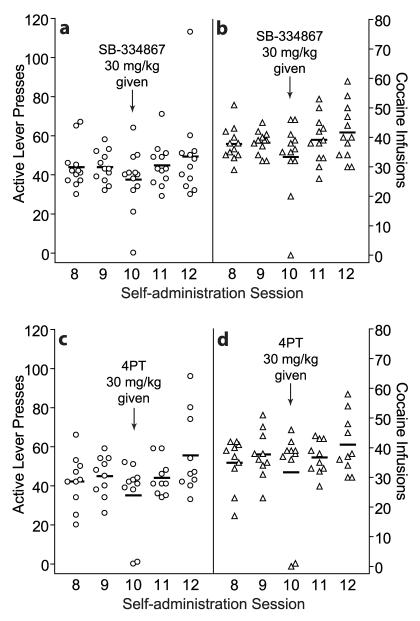
Figure 4 shows the number of active lever presses (Fig. 4a, c) and cocaine infusions (Fig. 4b, d) for individual animals on sessions 8 through 12 of self-administration (means for each session are represented by horizontal line segments). Animals were pretreated with either SB (30 mg/kg; n = 12) or 4PT (30 mg/kg; n = 10) prior to session 10 of self-administration.
Figure 4.
No effects of the OX1R antagonist SB-334867 or the OX2R antagonist 4PT on cocaine self-administration. Rats were pretreated with either SB or 4PT (30 mg/kg, i.p.) prior to their 10th cocaine self-administration session (0.2 mg/infusion; FR-1; 20-sec time-out; 2-hour sessions). Number of active lever presses (left y-axis) and cocaine infusions (right y-axis) for individual animals across self-administration sessions 8 through 12 are shown. Means for each session are represented by horizontal line segments. Analyses of sessions 9 through 11 revealed that SB (n = 12) caused no significant effect on active lever responding (a) or number of cocaine infusions (b) across the sessions. 4PT (n = 10) also caused no significant effect on active lever responding (c) or number of cocaine infusions (d) across the sessions.
OX1R antagonist - SB-334867 (SB)
Analyses comparing session 10 (with SB pretreatment) with sessions 9 and 11 (no SB pretreatment) revealed no significant differences in responding on the active (Fig. 3a; F(2,9) = 2.30; p > 0.1) or inactive lever (F(2,9) = 2.20; p > 0.1; not shown) across the self-administration sessions, as well as no significant differences for cocaine infusions (Fig. 3b; F(2,9) = 2.90; p > 0.05) across the sessions.
OX2R antagonist - 4PT
Analyses comparing session 10 (with 4PT pretreatment) with sessions 9 and 11 (no 4PT pretreatment) revealed no significant differences in active lever responding (Fig. 3c; F(2,7) = 1.80; p > 0.1) or cocaine infusions (Fig. 3d; F(2,7) = 3.06; p > 0.05). There was a significant effect of session on inactive lever responding (F(2,7) = 3.97; p < 0.05; not shown); post-hoc analyses revealed a significant difference between sessions 10 and 11 (p < 0.05).
Experiment 4: Effects of SB on acquisition and expression of Pavlovian-conditioned cues
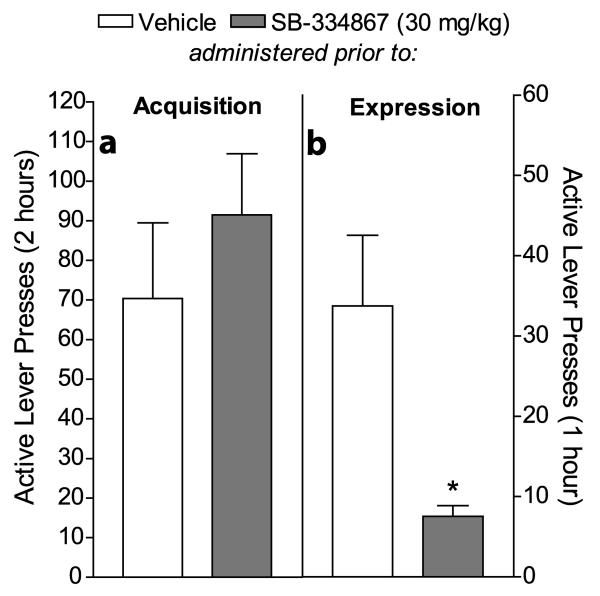
Figure 5 shows the mean (± SEM) numbers of active lever presses during cue-induced reinstatement when animals were pretreated with SB or vehicle prior to the acquisition of the Pavlovian-conditioned cues used for reinstatement (Fig. 5a) or the expression of reinstatement elicited by those cues (Fig. 5b).
Figure 5.
Attenuation of the expression, but not acquisition, of Pavlovian-conditioned cocaine-associated cues by the OX1R antagonist SB-334867. After 5 days of cocaine self-administration in the absence of cues, rats were exposed to a single 2-hour Pavlovian conditioning session in the operant chamber, in which passive cocaine infusions were paired with discrete tone + light cues. Animals then received 5 more days of self-administration in the absence of cues and extinction training. Animals were pretreated with either SB (30 mg/kg, i.p.) or vehicle prior to the Pavlovian conditioning session and then tested for reinstatement elicited by the Pavlovian cues. Rats were then pretreated with SB or vehicle prior to a 2nd reinstatement session. Mean (± SEM) number of presses on the active lever in the self-administration chamber is shown. a, Active lever responding during a 2-hour cue-induced reinstatement session was not significantly different between animals pretreated with SB (n = 16) or vehicle (n = 11) prior to the Pavlovian conditioning session (acquisition of cues). b, Active lever responding during a 1-hour cue-induced reinstatement session was significantly different between animals pretreated with SB (n = 15) or vehicle (n = 12) prior to the reinstatement session (expression of cues) (*p < 0.005).
SB effects on active lever responding
Pretreatment with 30 mg/kg SB (n = 16) prior to the acquisition of Pavlovian-conditioned cues had no significant effect on active lever responding during the expression of cue-induced reinstatement, as compared to pretreatment with vehicle in separate animals (n = 11) (unpaired t-test; T(1,25) = 0.86; p = 0.40), indicating that SB did not affect the acquisition of cocaine-stimulus conditioning. In contrast, pretreatment with 30 mg/kg SB (n = 15) prior to the expression of cue-induced reinstatement significantly reduced active lever responding during that reinstatement session, as compared to pretreatment with vehicle in separate animals (n = 12) (T(1,25) = 3.28; p < 0.005).
SB effects on inactive lever responding
Pretreatment with 30 mg/kg SB had no significant effects on inactive lever responding during cue-induced reinstatement sessions.
Baseline responding
Self-administration data were analyzed to assess differences in baseline responding between test groups (e.g., vehicle vs. SB groups for acquisition, vehicle vs. SB groups for expression). No significant differences were observed between groups for the number of cocaine infusions received during the Pavlovian conditioning sessions, for cocaine intake or active lever responding during the last two self-administration sessions, or for active lever responding during the first 7 days of extinction. The across-group (n = 27 total) mean (± SEM) for the number of infusions during the Pavlovian session was 27 (± 1.8). On the last two self-administration days, the across-group mean (± SEM) for cocaine infusions was 32.8 (± 2.4) and 34.6 (± 3.3) per session, and active lever presses was 46.1 (± 5.4) and 51.4 (± 10.3) per session. During extinction, there was a significant main effect for extinction session (F(6,23) = 12.94; p < 0.001), and the across-group mean (± SEM) for active lever presses on days 1 and 7 of extinction was 54.8 (±5.0) and 16.7 (±4.7).
DISCUSSION
The present studies indicate that orexin signaling at OX1R, but not OX2R, is critical for cue-induced reinstatement of extinguished cocaine-seeking in the self-administration paradigm. The OX1R antagonist SB dose-dependently attenuated cue-induced reinstatement of cocaine-seeking, whereas the OX2R antagonist 4PT did not attenuate reinstatement of extinguished responding (Fig. 1). This indicates an important functional difference between orexin signaling at OX1R and OX2R. Locomotor studies showed that 4PT caused more dramatic reductions in locomotor activity than SB (Fig. 3), which confirms that 4PT was administered at a behaviorally effective dose, and suggests that reduced locomotor activity does not account for the observed attenuation of cue-induced reinstatement following SB administration. Neither antagonist significantly attenuated established cocaine-taking behavior during self-administration (Fig. 4), indicating that orexin signaling is not critical for cocaine reinforcement. This was further supported by the finding that SB had no effect on the acquisition of cocaine-stimulus conditioning in the Pavlovian conditioned-cue paradigm (Fig.5), which indicates that signaling at OX1R is not necessary for cocaine reinforcement or learning cocaine-stimulus associations.
These results are consistent with previous findings that the orexin system is involved in stimulus-induced responding for other drugs. SB blocked olfactory discriminative cue-induced reinstatement of ethanol-seeking in a self-administration paradigm, and reduced expression of preference for a morphine-paired environment in a conditioned place preference paradigm (Harris et al., 2005; Lawrence et al., 2006). The current data also correspond with findings that orexin neurons are activated by stimuli associated with rewards such as morphine, cocaine, ethanol, and food (Harris et al., 2005; Harris & Aston-Jones, 2006; Dayas et al., 2008). On the other hand, Hamlin et al. (2008) found that non-orexin (but not orexin) lateral hypothalamic neurons were Fos activated by re-exposure to a self-administration context during renewal-associated reinstatement of cocaine-seeking. This is in contrast to the present data showing involvement of orexin signaling at OX1R in cue-induced reinstatement of cocaine-seeking, and may indicate that orexin is differently involved in cocaine-seeking elicited by discrete cues vs. context, or that Fos may not accurately reflect the role of orexin in stimulus-induced cocaine-seeking.
Taken together with the results of Boutrel et al. (2005), these findings indicate that orexin signaling at OX1R is necessary for cocaine-seeking elicited by either previously drug-paired cues or stress. However, the current studies also show that orexin transmission at OX1R is not necessary for established cocaine self-administration on a fixed ratio schedule, whereas previous studies have shown a role for orexin in self-administration of ethanol, nicotine, and high-fat food, but not self-administration of sucrose (Lawrence et al., 2006; Hollander et al., 2008; Nair et al., 2008; Richards et al., 2008). Similarly, other studies have shown that orexin is necessary for the acquisition of morphine place preference (Narita et al., 2006; Harris et al., 2007), while the current findings show that SB does not affect the acquisition of Pavlovian-conditioned stimuli associated with cocaine. These findings may reflect important differences in the involvement of orexin in the reinforcing properties of different types of rewards (e.g., food, cocaine, opiates, ethanol) (Aston-Jones et al., 2009).
Orexin function in arousal
In the current studies, there were no overt signs of motor impairment or other obvious behavioral side effects following 30 mg/kg SB injections. Animals generally appeared active and non-sedated. On the other hand, motor impairments and slight sedation were apparent in several animals following injections of 30 mg/kg 4PT. 30 mg/kg SB significantly reduced horizontal activity during a spontaneous locomotor activity test; however, 4PT caused significantly greater reductions in measures of horizontal activity as well as total distance traveled, which suggests that the effect of SB on cue-induced reinstatement cannot be fully explained by locomotor reductions, and that signaling at OX1R vs. OX2R may be associated with different functions of the orexin system (e.g., conditioned motivation vs. general arousal). Other groups have reported slight reductions in spontaneous locomotor activity following 30 mg/kg, but not 20 mg/kg, administration of the OX1R antagonists SB-334867 or SB-408124 (Rodgers et al., 2001; Richards et al., 2008; Dugovic et al., 2009), but there were no significant changes to sleep-wake states following administration of 30 mg/kg of SB-334867 or SB-408124 alone (Smith et al., 2003; Dugovic et al., 2009). In contrast, the OX2R antagonists JNJ-10397049 or EMPA reduced spontaneous locomotion (Dugovic et al., 2009; Malherbe et al., 2009), and JNJ-10397049 also caused significant decreases in the latency to nREM sleep and increases in sleep time (Dugovic et al., 2009). These studies indicate that orexin signaling at OX2Rs, and not OX1Rs, is involved in sleep-wake architecture and arousal.
Data presented here also indicate that attenuation of cue-induced reinstatement by SB is unlikely to be due to a generalized locomotor effect. First, SB had no significant effect on active or inactive lever responding during a late extinction session (Fig. 1a, b). Although significant effects on inactive lever responding were observed during cue-induced reinstatement with SB pretreatment, inactive lever responding may reflect changes in drug-seeking under reinstatement conditions (e.g., a decrease in drug-seeking may be reflected in decreased responding on the inactive lever as well as the active lever) and is not necessarily an indicator of impaired motor activity. Second, 30 mg/kg SB had no significant effect on active lever responding or cocaine intake during a self-administration session (Fig. 4a, b), and 20 mg/kg SB has previously been shown to have no effect on operant self-administration of 5% sucrose (Richards et al., 2008), showing that SB does not impair lever-pressing behavior in general. Reductions in cocaine self-administration following SB or 4PT pretreatment in a small number of rats may have been due to adverse reactions to the antagonist injection itself in those animals (4 ml/kg suspension), and were not comparable to the magnitude of the effect observed during cue-induced reinstatement. Third, 30 mg/kg SB reduced spontaneous horizontal activity in a locomotor test predominantly in the first 30 minutes only (Fig. 3a, b), whereas SB reduced reinstatement behavior across the full 2-hour session (Fig. 2). 30 mg/kg SB reaches peak brain levels at 30 minutes and has a terminal elimination half-life of approximately 4 hours (Ishii et al., 2005), which indicates that SB should be fully active for the entire duration of these tests. Reductions predominantly in initial locomotor activity might be explained by anxiolytic effects of either SB or 4PT in a novel environment (Chang et al., 2007; Samson et al., 2007). Lastly, 30 mg/kg 4PT caused greater reductions in spontaneous locomotion than SB (both horizontal activity and total distance traveled; Fig. 3c, d), but did not attenuate cue-induced reinstatement (Fig. 1c). Thus, self-administration behavior can proceed unabated despite substantially decreased locomotor activity. This last result also indicates that 30 mg/kg 4PT is a behaviorally active dose. Further evidence that 4PT and SB should be active at similar doses comes from in vitro studies which show that similar concentrations of SB and 4PT inhibited orexin-mediated responses at OX1R and OX2R, respectively (Smart et al., 2001; Hirose et al., 2003). Additionally, 4PT has been shown to have in vivo efficacy as an orexin antagonist (Chang et al., 2007).
We hypothesize that signaling at the OX2R, as opposed to the OX1R, is primarily related to the arousal-associated functions of orexin, as others have also discussed (Marcus et al., 2001; Willie et al., 2003; Akanmu & Honda, 2005). Dysfunction in the orexin system has been closely associated with narcolepsy that co-occurs with cataplexy in humans and animals (Chemelli et al., 1999; Lin et al., 1999; Nishino et al., 2000; Beuckmann et al., 2004), and orexin null or orexin-deficient mice show slight decreases in locomotor activity (Mochizuki et al., 2004; Zhang et al., 2007). This hypothesis of OX2R function is based in part on findings by Lin et al. (1999) that familial canine narcolepsy is caused by a mutation in the OX2R gene which renders the receptor nonfunctional, as well as findings by Willie et al. (2003) that OX2R and orexin null mice had similar abnormalities in the transition between wake and non-REM states, whereas OX1R knockout mice did not display overt behavioral abnormalities and had only mild fragmentation of sleep states. However, OX2R null mice exhibited only mild cataplexy attacks of REM sleep, indicating that disruption at both OX1 and OX2 receptors may be necessary to achieve the full narcolepsy with cataplexy syndrome (Willie et al., 2003). Recent studies using specific antagonists for the OX1R and OX2R also support a role for signaling at OX2R in the sleep-wake functions of the orexin system (as discussed above).
Orexin function in drug-seeking
The current data show that orexin transmission at OX1R is necessary for cue-elicited drug-seeking, but not for cocaine reinforcement. Further, orexin is necessary for reinstatement driven by discrete cocaine-associated cues regardless of whether the cues are present throughout all self-administration sessions or during a single Pavlovian conditioned session with non-contingent cocaine. Orexin signaling at OX1R may be necessary for the reinforcing properties of conditioned stimuli, or the conditioned motivation (craving) triggered by drug-associated stimuli. In response to the renewed availability of conditioned stimuli, animals typically show increased stimulus-driven behavior (i.e., reinstatement of extinguished responding), despite drug unavailability. Blockade of OX1R via SB specifically reduced the responding elicited by the conditioned stimuli. However, SB did not significantly affect cocaine-taking behavior, indicating that orexin signaling is not necessary for the unconditioned reinforcing properties of cocaine. Animals given 20-30 mg/kg SB prior to a cue-induced reinstatement session showed active lever responding similar to late extinction sessions, and not a complete blockade of responding; therefore, it appears that once animals were unrewarded by their lever pressing (i.e., no cocaine), they attenuated drug-seeking behavior just as they would during a late extinction session when no cues were available (Fig. 2).
The mechanism of action for orexin during reinstatement of drug-seeking is unknown. Orexin may be acting in the ventral tegmental area (VTA) during cue-induced reinstatement of cocaine-seeking. Orexin has projections to and actions in VTA (Peyron et al., 1998; Fadel & Deutch, 2002; Korotkova et al., 2003; Vittoz et al., 2008), and intra-VTA injections of SB attenuated behavioral responses associated with cocaine and morphine (Harris et al., 2005; Borgland et al., 2006; Narita et al., 2006; Harris et al., 2007). Cocaine-seeking was reinstated by intra-VTA injections of orexin A (non-selective agonist for OX1R and OX2R), but not orexin B (selective agonist for OX2R), supporting the hypothesis that signaling at OX1R in particular is involved in drug-seeking (Wang et al., 2009). Importantly, SB does not affect basal dopamine function in VTA, which is an important consideration therapeutically (Borgland et al., 2006; Rasmussen et al., 2007). Given the divergent projections of the orexin system, however, several addiction-associated brain regions may be involved in SB effects, such as nucleus accumbens, amygdala, and prefrontal cortex, all of which have been previously implicated in cue-induced reinstatement of cocaine-seeking (Feltenstein & See, 2008). Recent self-administration studies suggest that orexin might play a role in drug-seeking via interactions with norepinephrine and corticotropin-releasing factor systems in the brain (Boutrel et al., 2005; Richards et al., 2008). Topographic differences in orexin neurons in lateral and medial hypothalamus may underlie these different possible roles for orexin in reward-cue associations and stress, as we recently hypothesized (Harris & Aston-Jones, 2006). Experiments are currently underway to test these hypotheses.
Conclusions
The current results indicate that orexin signaling at OX1R, but not OX2R, is necessary for reinstatement of cocaine-seeking elicited by discrete drug-paired stimuli in the self-administration paradigm, and that orexin signaling is not necessary for the reinforcing properties of cocaine or the maintenance of established drug-taking. Together with previous studies, these findings support a critical role for orexin in cue- and stress-induced reinstatement of cocaine- and ethanol-seeking (Boutrel et al., 2005; Lawrence et al., 2006; Richards et al., 2008), and provide evidence that the orexin system may be an important therapeutic target for future addiction treatments. Signaling at OX1R may be a particularly important target for combating relapse elicited by drug-associated stimuli.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants F31-DA019733, R01-DA017289, R37-DA06214, and P50-DA015369. We thank Paul Knackstedt, Shannon Ghee, Anthony Carnell, George Khouri, and Phong Do for their excellent technical assistance and Eli Lilly (Indianapolis, IN) for providing the orexin receptor antagonists.
ABBREVIATIONS
- OX1R
orexin 1 receptor
- OX2R
orexin 2 receptor
- SB
SB-334867
- SEM
standard error of mean
- VTA
ventral tegmental area
- 4PT
4-pyridylmethyl (S)-tert-leucyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline
REFERENCES
- Akanmu MA, Honda K. Selective stimulation of orexin receptor type 2 promotes wakefulness in freely behaving rats. Brain Res. 2005;1048:138–145. doi: 10.1016/j.brainres.2005.04.064. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56:112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, Yanagisawa M. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci. 2004;24:4469–4477. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Saito T, Ohiwa N, Tateoka M, Deocaris CC, Fujikawa T, Soya H. Inhibitory effects of an orexin-2 receptor antagonist on orexin A- and stress-induced ACTH responses in conscious rats. Neurosci Res. 2007;57:462–466. doi: 10.1016/j.neures.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, Rohsenow DJ, Robbins SJ, O'Brien CP. Classically conditioned factors in drug dependence. In: Lowinson JW, Luiz P, Millman RB, Langard G, editors. Substance Abuse: A Comprehensive Textbook. Williams and Wilkins; Baltimore, MD: 1992. pp. 56–69. [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlov J Biol Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, Bonaventure P, Yun S, Li X, Lord B, Dvorak CA, Carruthers NI, Lovenberg TW. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M, Egashira S, Goto Y, Hashihayata T, Ohtake N, Iwaasa H, Hata M, Fukami T, Kanatani A, Yamada K. N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline: the first orexin-2 receptor selective non-peptidic antagonist. Bioorg Med Chem Lett. 2003;13:4497–4499. doi: 10.1016/j.bmcl.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Jeffrey P, Summerfield S, Rodgers RJ. Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res. 2005;157:331–341. doi: 10.1016/j.bbr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lepine JP. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, de Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin-concentrating hormone receptors: networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Borroni E, Gobbi L, Knust H, Nettekoven M, Pinard E, Roche O, Rogers-Evans M, Wettstein J, Moreau JL. Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX receptor. Br J Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Kanbayashi T. Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/orexin system. Sleep Med Rev. 2005;9:269–310. doi: 10.1016/j.smrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, Jewitt F, Jeffrey P, Austin N. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Hsu MA, Yang Y. The orexin-1 receptor antagonist SB-334867 blocks the effects of antipsychotics on the activity of A9 and A10 dopamine neurons: implications for antipsychotic therapy. Neuropsychopharmacology. 2007;32:786–792. doi: 10.1038/sj.npp.1301239. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am J Physiol Regul Integr Comp Physiol. 2007;292:R382–387. doi: 10.1152/ajpregu.00496.2006. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MI, Piper DC, Duxon MS, Upton N. Evidence implicating a role for orexin-1 receptor modulation of paradoxical sleep in the rat. Neurosci Lett. 2003;341:256–258. doi: 10.1016/s0304-3940(03)00066-1. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Wallace BC. Psychological and environmental determinants of relapse in crack cocaine smokers. J Subst Abuse Treat. 1989;6:95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol. 2007;581:649–663. doi: 10.1113/jphysiol.2007.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, Sakurai T, Goto K. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92:259–266. doi: 10.1254/jphs.92.259. [DOI] [PubMed] [Google Scholar]