Abstract
A 74-year-old female presented to the preoperative assessment clinic (PAC) prior to elective, right shoulder, arthroplasty. Her past medical history was significant for hypertension, osteoarthritis, and mild chronic obstructive pulmonary disease. She denied any cardiovascular symptoms with the exception of increasing exertional shortness of breath while walking her dog up the hill to her house. She attributed this change in exercise tolerance to “getting older and out of shape.” The patient quit smoking 25 years ago and regularly takes her antihypertensive regimen of hydrochlorothiazide and lisinopril. In the PAC her vital signs were the following; blood pressure 158/64 mmHg, pulse 78 beats/min, and room air oxygen saturation 100%. Auscultation of her chest revealed clear lung sounds and a regular cardiac rate and rhythm with a mid-grade (3 to 4/6) systolic ejection murmur radiating to her carotid arteries. A 12-lead electrocardiogram showed normal sinus rhythm and nonspecific S-T wave changes. A transthoracic echocardiogram was obtained which revealed a normal ejection fraction of 65%, impaired left ventricular relaxation, moderate concentric left ventricular hypertrophy, moderate left atrial enlargement, and severe aortic valve stenosis (AVA = 0.9 cm2; peak gradient 60 mmHg) with mild aortic regurgitation. The patient was referred to cardiology and subsequently underwent a coronary and right heart catheterization which showed nonobstructive coronary disease, a peak left ventricular pressure gradient of 75 mmHg and an end diastolic pressure of 22 mmHg.
The patient was scheduled for aortic valve replacement surgery with cardiopulmonary bypass (CPB). Intraoperative anesthetic and surgical care of the patient were uneventful; she was managed with an isoflurane and fentanyl-based anesthetic, and muscle relaxation was achieved with cisatracurium. A 23-mm stentless, bioprosthetic aortic valve was inserted and the patient was weaned from CPB without inotropes. Following closure of her sternum, transient episodes of hypotension (80/60 mmHg) occurred with concomitant echocardiographic evidence of left ventricular (LV) underfilling that responded to volume loading with colloid. The patient was hemodynamically stable upon transfer to the intensive care unit (ICU), sedated with dexmedetomidine, and on a low dose infusion of phenylephrine. During the first 6 hours in the ICU, the patient’s cardiac index dipped below 2.0 which corresponded to her relatively low cardiac filling pressure (LVEDP <18 mmHg) and labile blood pressure. Due to the minimal chest tube drainage and hemodynamic lability, a bedside TEE was performed which was negative for evidence of cardiac tamponade, but it did confirm a relative hypovolemia. The patient responded well to volume resuscitation and her hemodynamics stabilized at an LVEDP 24 mmHg. She was subsequently weaned from the ventilator on the morning of POD 1 and remained hemodynamically stable and cognitively intact before transfer to a floor bed on postoperative day two.
On POD 3 her family remarked that she was “not herself” and somewhat disoriented. Her oxygen requirements had increased over the preceding 24 hours, and her physical exam and chest x-ray were consistent with pulmonary edema. An electrocardiogram revealed atrial fibrillation. Her blood pressure was 95/60 mmHg. The patient was transferred to the ICU for closer observation, and for re-intubation after low oxygen saturation did not respond to face shield oxygen or Bi-PAP. Her cardiac rhythm was medically converted with amiodarone, and she was carefully diuresed with furosemide prior to extubation a day later. She progressed slowly and was discharged to a rehabilitation facility on POD 8 prior to returning to her home.
The above case represents a common scenario. Changes in the epidemiology of patients undergoing both cardiac and major noncardiac surgery (1–3), coupled with the growing number of older persons with heart failure (4,5), may make future perioperative care more difficult (6). Although our patient reached hospital discharge with no long-term sequelae, her postoperative course was prolonged and complicated. She exemplifies the limited physiologic reserve that characterizes many persons in her cohort. The question of how to recognize and manage this situation arises and is best addressed through an examination of the physiology of the early spectrum of cardiovascular disease, specifically that of aging and diastolic dysfunction. The valvular lesion that was discovered should not distract the reader from the physiologic derangements that were exposed after aortic valve repair; this patient’s hemodynamic lability, poor tolerance of volume shifts, cardiac arrhythmia, and eventual reintubation and ICU recidivism occurred despite normal systolic indices and a well-functioning aortic valve prosthesis. In addition, this patient could revisit the same set of perioperative issues when she returns for her shoulder arthroplasty.
This review will focus on the physiology and management of the patient with diastolic dysfunction from the standpoint of the cardiac anesthesiologist, echocardiographer, and general anesthesiologist. Diastolic dysfunction, the precursor of diastolic heart failure, has been termed the great masquerader (7). Because its clinical presentation may erroneously be ascribed to chronic obstructive pulmonary disease or to normal aging, diastolic heart disease may remain undiagnosed or ignored. Other than exercise intolerance (8,9), symptoms associated with isolated diastolic heart failure in the elderly include weakness, anorexia, fatigue, and mental confusion. One clue in identifying this disorder is the diastolic dysfunction phenotype; that is, the 65-year-old, postmenopausal, hypertensive female patient (10). Indeed, diastolic dysfunction represents a part of the physiologic spectrum that progresses from normal aging to advanced cardiovascular disease. Although the perioperative risk for the healthy, elderly patient with isolated diastolic dysfunction is not yet known (11,12), extrapolations from cardiac surgery and cardiology data suggest that it is associated with increased morbidity and mortality (13–17). Therefore, the perioperative physician is obliged to understand age-related changes in the heart and vasculature that impact diastolic function and to become knowledgeable of the diagnostic and prognostic echocardiographic measures of diastolic function so that perioperative management can be modified in a way that may improve outcome in the elderly.
Physiologic Changes of Aging and Diastolic Dysfunction
Several changes in cardiac structure and function occur with aging that contribute to diastolic dysfunction. On the structural level, there is a decrease in myocyte number, an increase in myocyte size, and an increase in the amount of connective tissue matrix (18,19). Myocyte number decreases because of cell necrosis and apoptosis. As myocytes are lost they are replaced with fibroblasts, and the remaining myocytes hypertrophy. As the fibroblasts produce collagen, interstitial fibrosis occurs and the heart becomes stiffer and less compliant. The stiffer and less compliant ventricle affects diastolic relaxation as well as systolic contraction. Chronically elevated afterload from stiff vasculature leads to left ventricular hypertrophy (LVH) and prolongation in systolic contraction time. Prolonged systolic contraction, in turn, impinges upon early diastole (20–23).
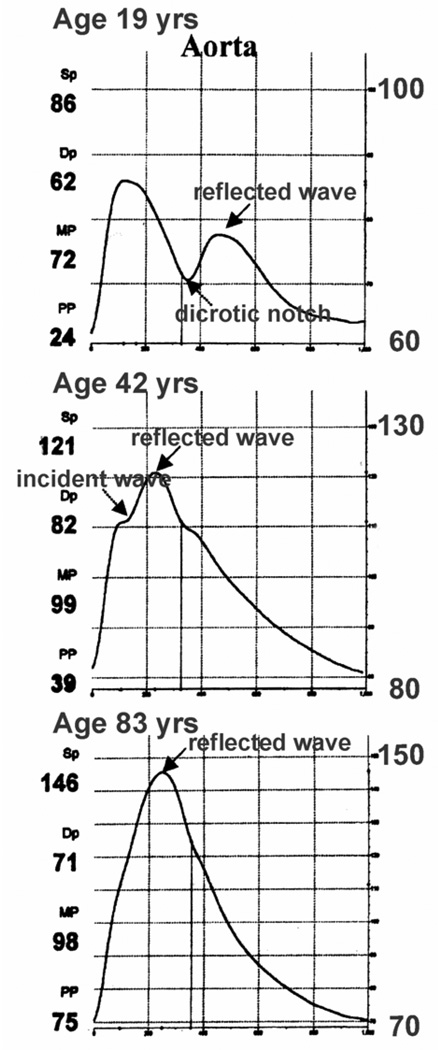
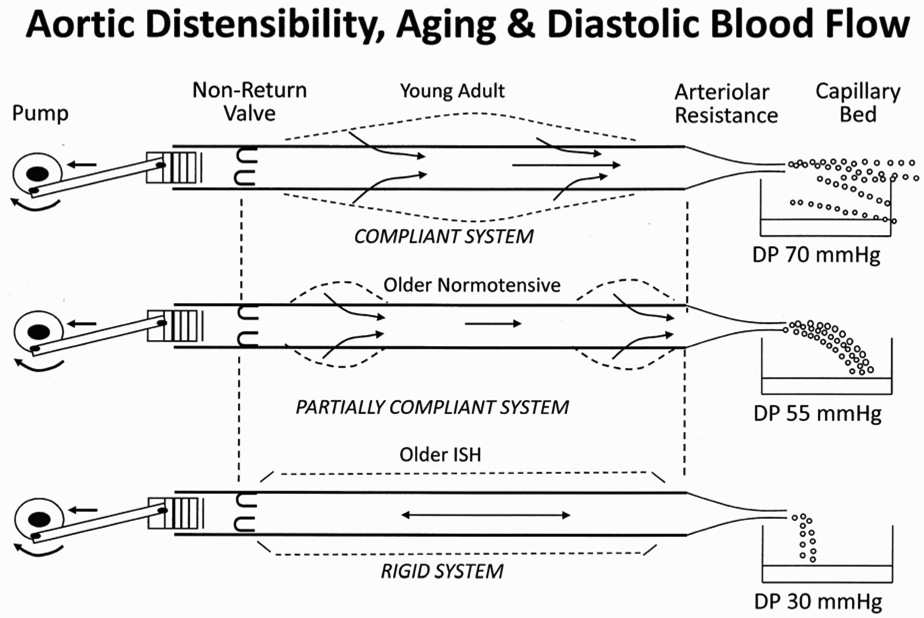
The two main consequences of age-related arterial stiffening are decreased aortic distensibility and increased pulse wave velocity (24,25). The aorta is responsible for cushioning the pulse energy generated by the heart and converting it into stored energy through the elastic recoil of the vessel. The loss of distensibility during systole results in a higher systolic pressure (Figure 1), and less stored energy to augment forward flow during diastole. This is manifested by a lower diastolic pressure (Figure 2). The resultant increased pulse pressure is an established risk factor for cardiovascular events (26–29).
Figure 1.
Age-related arterial stiffness and pressure waveform shapes. (Reprinted with permission from Dudley M, Groban L. Current Reviews in Clinical Anesthesia 2009;29:263-75.)
Figure 2.
Schematic of blood pressure as a result of ejection of blood (e.g., stroke volume) into a series of tubes whose diameters vary with pulsating pressure. In the young adult, the aorta cushions the cardiac pulsation by converting pressure energy into elastic energy through distension. Once the heart ceases ejection and the pressure falls, the walls of the aorta recoil and the elastic energy is reconverted into pressure energy. This reduces the magnitude of pressure change and allows for a steady flow beyond the arterioles. This accounts for the diastolic component of BP. With aging and hypertension, the aorta and other major conduit vessels become rigid leading to a loss of “cushioning” of the ejected energy. Accordingly, this loss of stored energy manifests in extremes in pressure--increased pulse pressure and very low diastolic pressure. (Adapted from Baird RN, Abbott WM. Lancet 1976;2:948-41.)
The pulse wave velocity is the speed at which the pressure wave, generated by the contracting heart, travels to the periphery and is responsible for a palpable pulse. As vessels become stiffer, the pulse wave becomes faster. The pressure wave is reflected from the periphery, mainly at arterial branch sites, similar to sound waves reflected as echoes. In the younger adult with compliant vessels, the reflected wave returns to the heart during diastole which augments aortic diastolic pressure and coronary perfusion. However, as the pulse wave velocity increases with stiffened vessels, the reflected wave returns during late systole which augments systolic pressure, increasing afterload and the pulse pressure width. This is analogous to a mistimed intra-aortic balloon pump (IABP). Inflation of an IABP prior to aortic valve closure leads to an increase in left ventricular end-diastolic volume (LVEDV), left ventricular end-diastolic pressure (LVEDP), left ventricular (LV) wall stress (afterload), and oxygen demand. Thus, the large vessel stiffening of advanced age can lead to greater myocardial stroke work, wall tension, and oxygen consumption in the older heart compared to the younger heart. Additionally, these arterial changes contribute to altered diastolic function; afterload directly affects LV relaxation, and is a stimulus for hypertrophy of the myocardium (30,31).
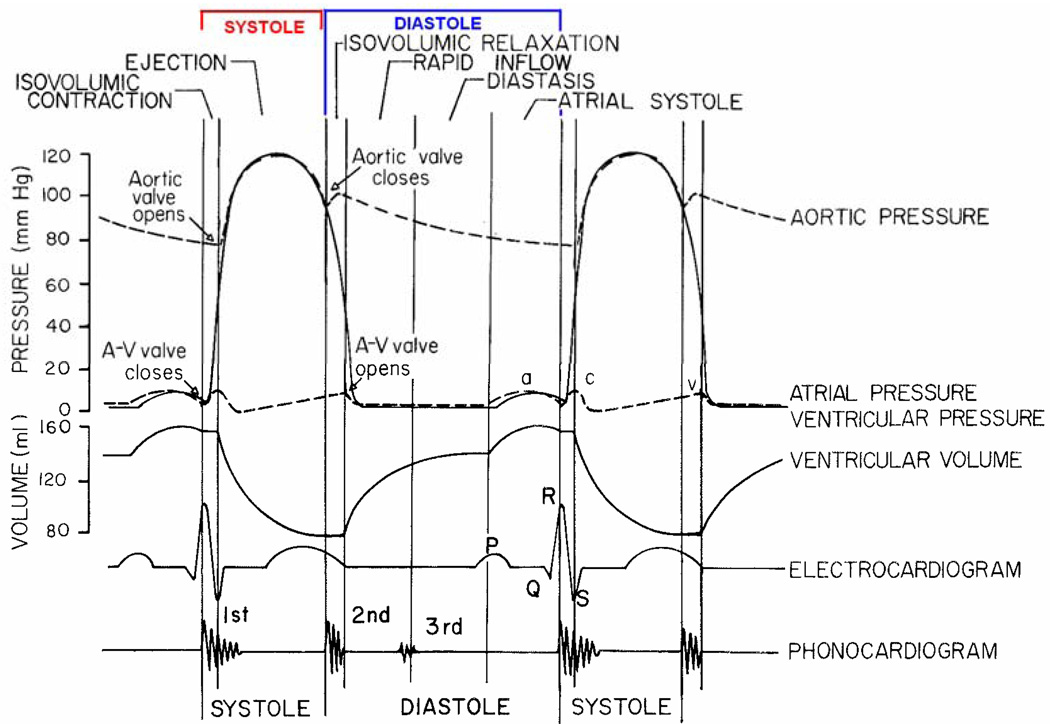
Although the majority of elderly patients presenting for surgery will have normal ejection fractions by echocardiogram, up to a one-third of these patients will have abnormal diastolic function (14). An understanding of the phases of diastole and associated physiologic determinants are important in order to understand how age-related changes in cardiac structure and function influence diastology (Table 1). At the mechanical level (Figure 3), diastole begins with aortic valve closure when the pressure within the left ventricle begins to fall, termed the isovolumic relaxation phase. The left ventricular pressure will continue to fall even after the opening of the mitral valve. In fact, left ventricular pressure falls below left atrial pressure as a result of elastic recoil, creating a “suction” effect. Rapid filling of the left ventricle occurs during this phase. Normally, left ventricular relaxation ends in the first third of rapid filling so that the majority of left ventricular filling is dependent on such properties as left ventricular compliance, ventricular interaction (e.g., synchronicity), and pericardial restraint. Finally, atrial systole contributes to the rest of left ventricular volume. In the young heart, approximately 80% of LV filling is complete by the end of the passive filling phase, with the remainder occurring during active atrial transport. In contrast, with advanced age, impairments in early diastolic relaxation and ventricular compliance alter filling dynamics such that atrial transport becomes the more important contributor to diastolic volume. This, so called, atrial “kick” is essential in order to maintain an adequate preload, particularly if the preceding three phases of diastole are adversely influenced by age-related changes in cardiac structure and function.
Table 1.
Principle Effects of Aging on the Cardiovascular System
| • Increased arterial stiffness |
| • Increased myocardial stiffness |
| • Impaired beta-adrenergic responsiveness |
| • Impaired endothelial function |
| • Reduced sinus node function |
| • Decreased baroreceptor responsiveness |
| • Net effect: marked reduction in cardiovascular reserve |
Figure 3.
Cardiac cycle with phases of diastole.
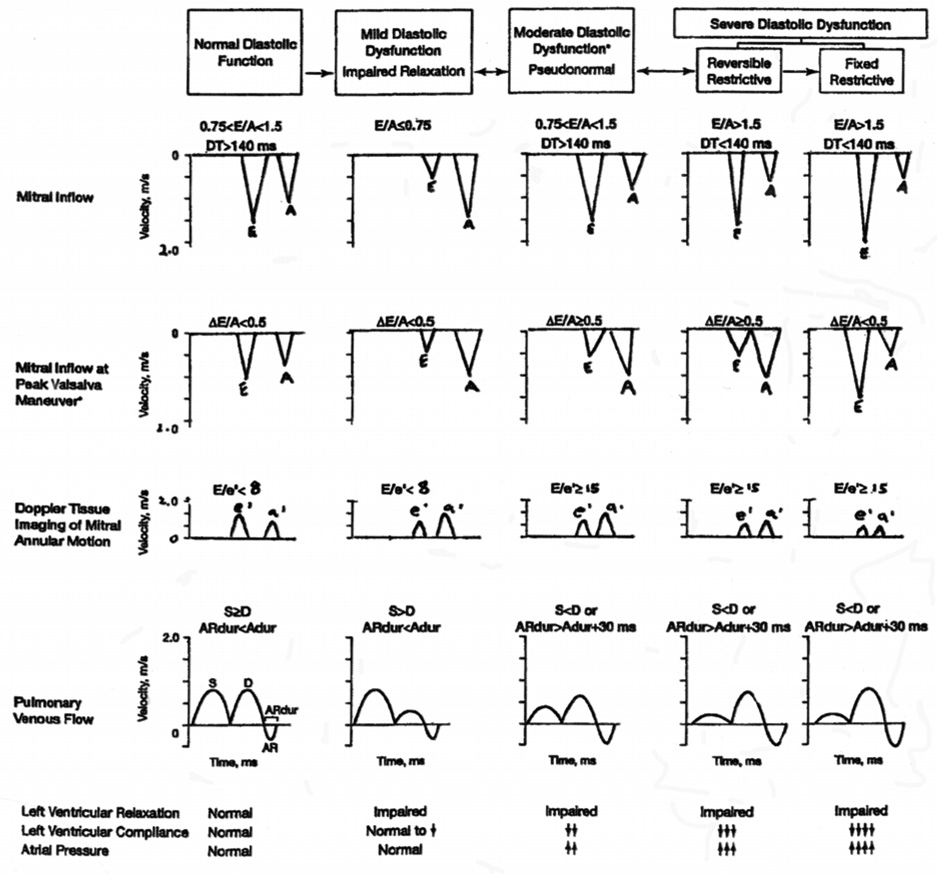
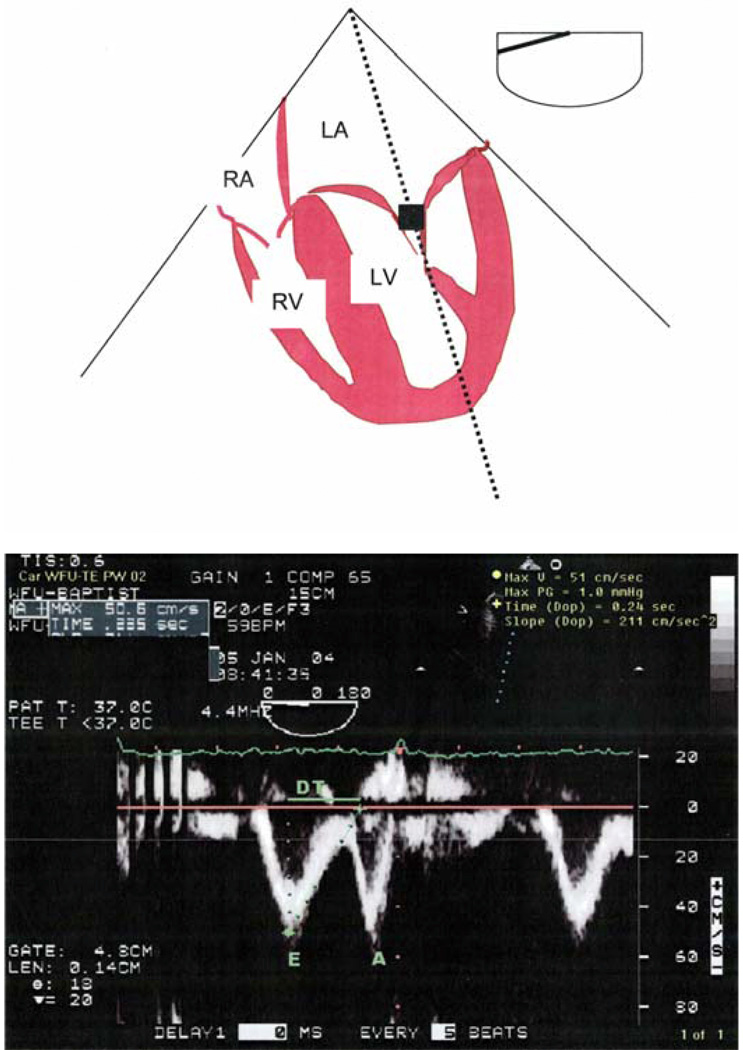
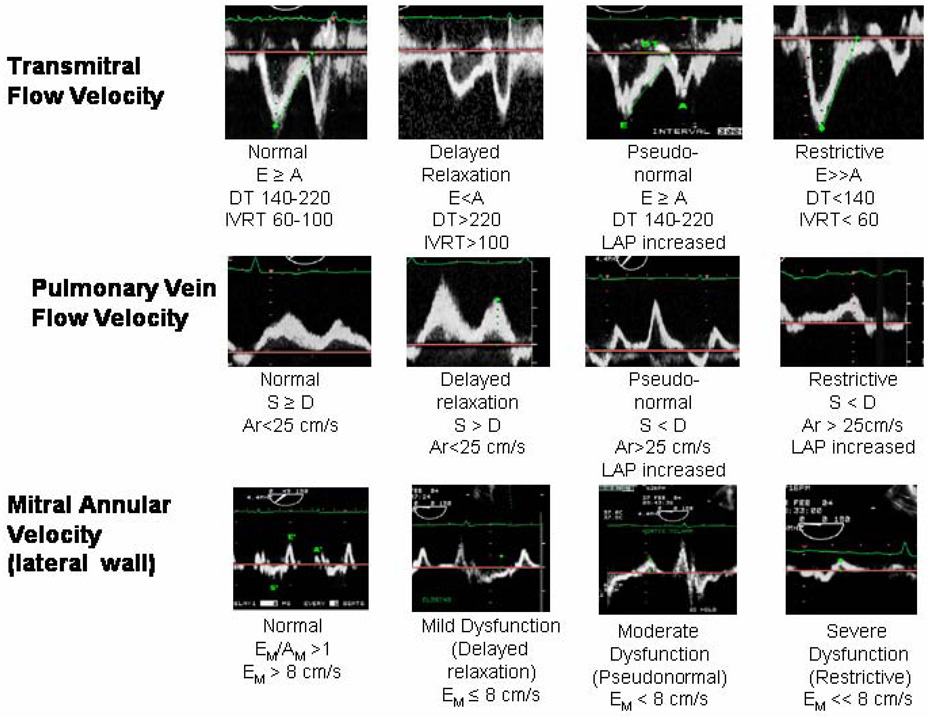
The diagnosis of diastolic dysfunction can be made from cardiac catheterization and Doppler LV diastolic filling patterns. Catheterization data show increases in ventricular diastolic pressure (>16mmHg) with preserved systolic function and normal ventricular volumes. Most Doppler LV diastolic filling patterns can be categorized into one of four distinct categories (Figure 4). The normal pattern is seen in healthy young and middle-aged persons. In sinus rhythm, there are two peaks in the Doppler diastolic filling profile that occur in response to the pressure gradient between the left atrium (LA) and left ventricle; early in diastole following mitral valve opening when LV pressure falls below LA pressure, and late in diastole when atrial contraction increases LA pressure above LV pressure. The LV filling pattern in healthy young subjects is characterized by predominant rapid filling early in diastole with modest additional filling during atrial contraction. The filling pattern can be quantified by measuring the peak early diastolic flow velocity (E) and the peak flow velocity during atrial contraction (A), and expressing this as E/A ratio (Figure 5). Normally, the E/A ratio in young subjects is greater than one.
Figure 4.
Doppler criteria for classification of diastolic function. (Reprinted with permission from Chest 2005;128:3652–3663.) (Figure 4)
Figure 5.
Top: Schematic of the midesophageal four-chamber view with pulsed wave Doppler (PWD) imaging sample volume at the level of the tips of the open mitral valve leaflets.
Bottom: Transmitral blood flow velocity profile obtained with PWD imaging at the midesophageal four-chamber view. (Reprinted with permission from Chest 2005;128:3652–3663.) (Figure 2A and B)
The first pattern of altered LV filling is termed “delayed relaxation” (Figure 4). In this pattern there is reduced peak rate and amount of early filling, and the relative importance of atrial filling is enhanced, resulting in a reversed E/A ratio of less than one (e.g., E<A). This decreased rate of early filling is owing to a decreased early diastolic LA to LV pressure gradient, caused by a slowed rate of LV relaxation. While a delayed relaxation pattern can be seen in patients with LV hypertrophy, atrial hypertension and coronary artery disease, it is the one normally seen in healthy older persons who are free of cardiovascular disease.
The other two patterns (Figure 4) of altered LV filling are always abnormal, including in the elderly. The first has been termed pseudo-normalization, as the E/A ratio is greater than 1 (as seen in young normals). This pattern results from an increase in LA pressure that compensates for the slowed rate of LV relaxation and restores early diastolic LV pressure gradient to the baseline level seen in younger persons. The left atrium “pushes” to fill the LV, whereas in the young patient, the left ventricle fills by creating a “suction” effect. Elevated left atrial pressure results in left atrial enlargement due to pressure and volume overload. It has been suggested that left atrial enlargement is associated with age (32,33); however, there is evidence that increased left atrial size is not a normal result of aging (34,35) and is more likely a compensatory response to impaired left ventricular relaxation. Left atrial volume increases with progressively worsening diastolic function (36–38), and is a risk factor for complications including atrial fibrillation and embolic stroke. In order to differentiate normal from pseudo-normal, the patient’s preload can be reduced using nitroglycerin or with the introduction of a Valsalva maneuver, potentially uncovering an E<A pattern and impaired relaxation. Another way to circumvent the preload dependency of transmitral Doppler is the use of myocardial (or annular) velocities by tissue Doppler (TDI) as discussed later.
In the final altered LV filling pattern, called “restrictive”, early filling is increased abnormally, even above that seen in young normals. Moreover, as a result of diminished atrial filling, due to reduced atrial contractility, the E/A ratio is often greater than two. This pattern is seen in patients with severe diastolic dysfunction, pulmonary congestion, and end-stage dilated cardiomyopathy. Similar to pseudonormalization, reversible and irreversible restrictive disease can be distinguished from each other by Valsalva maneuver. In reversible, restrictive disease the mitral inflow pattern becomes abnormal with the A > E whereas in irreversible, restrictive disease the E wave remains greater than the A wave owing to the very stiff ventricle and very high filling pressures. Taken together, each abnormal filling pattern results from a variable combination of delayed early relaxation, increased LA pressure, and increased LV chamber stiffness. Indeed, these patterns represent a continuum from normal to severe diastolic dysfunction, with progressively increasing LV chamber stiffness.
It is important to distinguish the difference between diastolic dysfunction and diastolic heart failure (Table 2). Diastolic dysfunction is a physiologic or preclinical state in which abnormal relaxation or increased LV stiffness is compensated for by increasing LA pressure so that LV preload remains adequate. These patients may be considered American College of Cardiology/American Heart Association (ACC/AHA) stage A or stage B since they are asymptomatic (39). Progression to diastolic heart failure, ACC/AHA stage C or D, is characterized by signs and symptoms of HF with normal ejection fraction (>50%), the absence of valvular disease, and echocardiographic evidence of diastolic dysfunction. Diastolic heart failure is a true heart failure syndrome, as neurohormonal activation is triggered in a similar manner to that which occurs in systolic heart failure (40,41).
Table 2.
Risk Factors for Diastolic Heart Failure
| • Age >70, hypertensive female |
| • Systolic hypertension, increased pulse pressure (>60 mmHg) |
| • Diabetes, chronic renal insufficiency |
| • Echo: Normal EF, delayed relaxation, left LAE >50 mm, LVH |
| • ECG: previous MI, LVH, AF |
| • Recent weight gain (fluid overload) |
| • Exercise intolerance |
| • BNP >120 (BNP of 200 pg/ml may not be clinically significant in older, post-menopausal women) |
The pathophysiology of diastolic heart failure is characterized by a low cardiac output state resulting from a stiff, thickened ventricle with a small cavity. Relaxation is slow in early diastole and offers greater resistance to filling in late diastole, so that diastolic pressures are elevated. Elevated left atrial pressure is transmitted backward through the valveless pulmonary veins to the pulmonary capillary bed. Under normal resting conditions, the patient may be asymptomatic. However, periods of activity or stress which increase heart rate, stroke volume, end diastolic volume or blood pressure result in pulmonary overload, manifesting as shortness of breath, fatigue, and most commonly, exertional dyspnea (8). Accordingly, because patients with diastolic dysfunction are often asymptomatic at rest, it is important to inquire about exercise tolerance (9). Indeed, the presentation of heart failure in older patients may be insidious or sudden with the onset of severe shortness of breath usually attributable to pulmonary edema. However, patients may complain only of fatigue or lack of energy, which may be attributable to physical deconditioning. Even though signs/symptoms and clinical examination can provide useful information, such as atrial fibrillation, displaced apex, jugular venous distension, accurately diagnosing older patients with suspected heart failure can be difficult. While its beyond the scope of this review, investigations for an older patient with suspected heart failure should include a combination of simple blood tests such as serum electrolytes, 12-lead electrocardiogram, chest radiology, B-type natriuretic peptide, and echocardiography.
The Cardiac Surgery Patient with Diastolic Dysfunction
It is well established that complications following cardiac surgery are encountered in patients of advanced age. Other risk stratification characteristics that are typically encountered include prolonged CPB time, female sex, and diminished systolic function (42,43); however, there may exist a group of patients who are still at elevated risk for a more complicated hospital course who do not necessarily display these characteristics. Specifically, the echocardiographic identification of diastolic dysfunction and the presence of elevated diastolic filling pressures can yield meaningful information that can help identify these patients and guide perioperative management.
As discussed previously, the LV inflow Doppler is the most commonly used measurement in the echocardiographic examination of diastolic function because transmitral flow patterns and associated deceleration times represent increasing degrees of LV diastolic impairment. Because these measurements, along with the pulmonary venous waveform patterns, change rapidly with preload variations, heart rate and rhythm disturbances (44–46), tissue Doppler imaging is considered to be a more sensitive tool in the assessment of diastolic function (Figure 6). Tissue Doppler imaging (TDI) is a modality that measures myocardial velocity, in contrast to traditional Doppler, which measures blood flow velocity and may not represent actual myocardial properties (47). Mitral annular motion has been shown in experimental animal work and in humans to relate well with invasive indices of relaxation (48–51). The measurements e′, representing the early diastolic active relaxation phase, and a′, the late diastolic atrial contraction phase, can be used to identify and quantify diastolic dysfunction (Figure 6 and Figure 7).
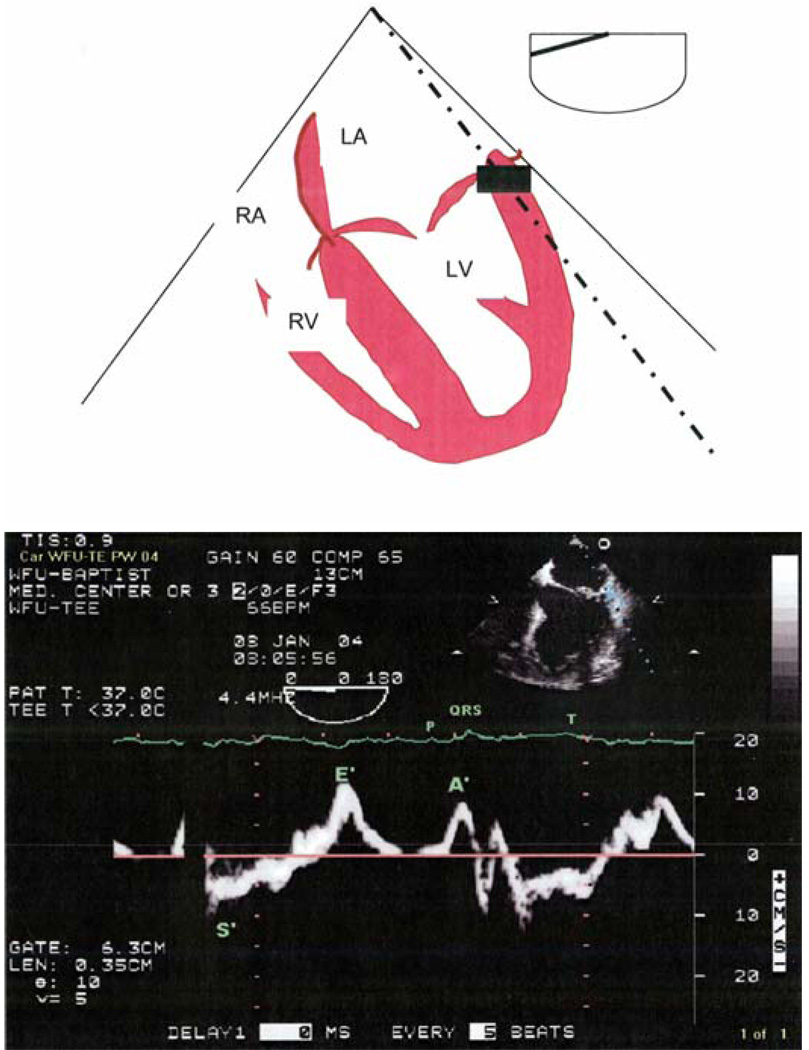
Figure 6.
Top: Schematic of the midesophageal four-chamber view with pulsed Doppler imaging sample volume located at the lateral mitral annular wall for tissue Doppler imaging (TDI) assessment of diastolic function.
Bottom: Lateral mitral annular tissue Doppler waveforms for the assessment of left ventricular diastolic function. (Reprinted with permission from Chest 2005;128:3652–3663.) (Figures 7A and B)
Figure 7.
Transmitral Doppler imaging, pulmonary view Doppler imaging, and tissue Doppler imaging (TDI) profiles corresponding to normal, delayed relaxation, pseudonormal, and restrictive filling patterns. (Reprinted with permission from Chest 2005;128:3652–3663.) (Figure 5)
In the normal heart, e′ may be influenced by alterations in preload (52); however, in the presence of diastolic dysfunction, e′ decreases and becomes preload independent (53). This allows for the severity of diastolic dysfunction to be quantified by a decreasing e′ value. Age influences the e′ and a′ values; an e′ <10 cm/s in those less than 50 years of age, and e′ <8 in those over 50 should be considered abnormal (52). The e′ to a′ ratio verifies abnormal diastolic function (e′/a′ > 1 is considered normal), as an e′/a′ ratio of less than one during Valsalva confirms the presence of diastolic dysfunction (54). Age also influences the e′/a′ ratio, and after the age of 50 e′/a′ <1 is frequently encountered and should be correlated with other echocardiographic measurements.
A robust quantification of elevated left ventricular filling pressures in diastolic dysfunction is the ratio of trans-mitral E wave velocity to mitral annular velocity (E/e′) (55–58). This ratio normalizes early transmitral left ventricular filling to mitral annular motion and is used to estimate mean left atrial pressure (with values >15 representing elevated filling pressures, and <8 reflecting normal filling pressures) (17,50,55). Moreover, accuracy of this measurement has been shown to be relatively independent of LV systolic function, rhythm abnormalities (such as tachycardia and atrial fibrillation), LV hypertrophy, and functional mitral regurgitation (17,50,59–62).
Although e′ relates to global indices of LV relaxation, it must be realized that it is a regional index, as errors can occur in patients with regional wall motion abnormalities at the Doppler sampling site. A limitation to E/e′, a′, and e′ is that myocardial motion at the lateral annulus is higher than the septal annulus, as the septum is tethered to the right ventricle and other structures in the middle of the heart (55,63). For this reason, and because of its accessibility with transesophageal echocardiography, the lateral mitral annular velocity may be easier to use in the intraoperative transesophageal setting. Though relatively independent of ejection fraction (EF), the reliability of E/e′ in predicting pulmonary capillary wedge pressure in decompensated advanced systolic heart failure has been called into question (64). The previously mentioned age-related changes and influence of preload in the normal heart when using TDI measures must also be kept in mind. Despite these limitations, tissue Doppler imaging is a powerful tool for identifying whether mitral valve inflow velocity patterns represent pseudonormalization and elevated filling pressures.
Doppler echocardiography of diastolic function has shown utility as a diagnostic tool in predicting outcome in patients undergoing cardiac surgery. A recent prospective report of 191 CABG patients found greatly increased mortality (12% vs 0%) following cardiac surgery in patients with significant diastolic dysfunction; risk scores based on systolic function and patient characteristics were less accurate in predicting complications in this patient group than were markers of diastolic dysfunction (65). Bernard, et al, identified diastolic dysfunction as an independent predictor of difficult separation from CPB (66). Liu et al. (13) identified that pseudonormal or restrictive transmitral flow patterns were predictive of cardiac events following CABG, while LVEF and the presence of left main coronary artery disease were not independent predictors of poor outcome.
The importance of diastolic dysfunction in cardiac surgery patients is supported by the mechanism of progression of myocardial dysfunction in ischemic heart disease. Diastolic dysfunction has been identified as the earliest potential marker of myocardial ischemia (67–69), and thereby may represent an early range of the spectrum of myocardial dysfunction that occurs prior to gross systolic impairment, nonetheless representing a diseased myocardium. An incremental relationship between severity of diastolic dysfunction and outcomes has been demonstrated by Whalley et al., in which non-surgical congestive heart failure patients with restrictive diastolic filling patterns had more complications than those with pseudonormal filling patterns or abnormal relaxation (70). Furthermore, non-surgical patients with both preserved and depressed EF admitted for acute myocardial ischemia (AMI) could be stratified for risk using E/e′ to identify patients with diastolic dysfunction with elevated filling pressures (17). In that study group, AMI patients were shown to have a higher incidence of heart failure and poor outcomes with restrictive and pseudonormal LV filling patterns. Elevated filling pressures in that study group were identified by an E/e′ >15 mmHg, consistent with several previous reports (17,50,55).
This evidence suggests that elevated LV diastolic filling pressures may be the factor most important in poor outcomes, rather than simply the existence of delayed relaxation (71). Elevated filling pressures have also been found to be a predictor of mortality in cardiac surgery patients independent of systolic function (16). In that group, those patients identified to have LV filling pressures above 22 mmHg were found to have twice the mortality of patients with filling pressures less than 14 mmHg.
There is some indication that elevated LV filling pressures may predict a prolonged and more complicated ICU and/or hospital stay following cardiac surgery. In a retrospective study of 205 cardiac surgery patients, a 12% increase in hospital length of stay was observed in those patients who had tissue-Doppler based evidence of elevated filling pressures as defined by E/e′ >17 (72). Ejection fraction and patient comorbidities were equal between groups. Also, in a study of ICU re-admissions following cardiac surgery, ICU recidivism has been shown to be more likely in those with diastolic dysfunction. This analysis examined 41 ICU re-admissions and their likelihood of requiring re-intubation. With similar EF, age, baseline BP, HR, and renal function, those who required re-intubation were observed to have worse diastolic function, increased E/e′, and increased left atrial size on the preoperative echocardiogram (17).
Diastolic dysfunction and elevated filling pressures should alert the clinician that the cardiac surgery patient may be more challenging than appreciated, even if systolic function is normal. The increased sensitivity of the cardiovascular system to acute changes in loading conditions, and thus the need for strict management of volume status, is of critical importance. The speed with which intravenous fluids are administered may be more significant, with patients of poor diastolic function less able to tolerate rapid volume shifts. Myocardial protection strategies are, as always, of paramount importance, but may need to be reexamined on a patient-by-patient basis in the presence of diastolic dysfunction to ensure an optimal strategy. Myocardial calcium regulation is abnormal in diastolic dysfunction, and may affect the choice to utilize an inotrope, or to administer specific agents. Lusitropic agents such as milrinone may be of particular benefit in weaning off of cardiopulmonary bypass. Although there is no directed strategy for acutely improving diastolic function, these are a few strategies that have been utilized in the management of these patients.
The newer, TDI based diastolic variables e′, a′, and E/e′ are simple to incorporate into the echocardiographic examination, and can give valuable information with respect to postoperative complications following cardiac surgery. These measures are easy to obtain, and can identify patients without traditional predictors of complications following cardiac surgery who still may be at high risk.
Perioperative Implications and Anesthetic Management of Diastolic Dysfunction for the General Surgical Patient
Given the cardiovascular changes that occur with diastolic dysfunction and in the elderly (Table 3), the perioperative management of these patients can be challenging. A thorough preoperative assessment is in order to risk stratify these patients. Particularly in the elderly, it is important to inquire about functional capacity as individuals unable to climb a flight of stairs (4 METS), walk indoors around the house, or do light house work (1 MET), are at an increased risk for complications. The functional capacity evaluation may further alert the anesthesiologist to signs of clinically significant diastolic dysfunction. Since a heart failure history, independent of coronary artery disease, is associated with increased morbidity and mortality after noncardiac surgery (73), risk factors for heart failure should be sought in the preoperative evaluation. Although not specific to the elderly per se, the reader should refer to the latest ACC/AHA published guidelines for a complete discussion of perioperative care and evaluation of cardiac patients undergoing non-cardiac surgery (74). In brief, patients with asymptomatic heart disease can safely undergo elective noncardiac surgery without first requiring angioplasty or coronary bypass grafting to lower the risk for surgery. Noninvasive and invasive preoperative cardiac testing should not necessarily be performed unless results will affect patient management. Patients with severe or symptomatic cardiovascular disease and/or active cardiac conditions should undergo evaluation by a cardiologist and treatment before noncardiac surgery. Statins should not be discontinued before surgery. If a cardiac intervention is required before elective noncardiac surgery, then the patient should have angioplasty with the use of a bare-metal stent followed by 4 to 6 weeks of antiplatelet therapy plus aspirin.
Table 3.
Age Related Cardiovascular Changes and Implications
| Age-related Change | Mechanism | Consequences | Anesthetic Implications |
|---|---|---|---|
| Myocardial hypertrophy | Apoptotic cells are not replaced and there is compensatory hypertrophy of existing cells; reflected waves during late systole create strain on myocardium leading to hypertrophy | Increased ventricular stiffness, prolonged contraction and delayed relaxation | Failure to maintain preload leads to an exaggerated decrease in CO; excessive volume more easily increases filling pressures to congestive failure levels; dependence on sinus rhythm and low-normal HR |
| Myocardial stiffening | Increased interstitial fibrosis, amyloid deposition | Ventricular filling dependent on atrial pressure | |
| Reduced LV relaxation | Impaired calcium homeostasis; reduced beta receptor responsiveness, early reflected wave | Diastolic dysfunction | |
| Reduced beta receptor responsiveness | Diminished coupling of beta receptor to intracellular adenylate cyclase activity, decreased density of beta receptors | Increased circulating catecholamines; limited increase in HR and contractility in response to endogenous and exogenous catecholamines; impaired baroreflex control of BP | Hypotension from anesthetic blunting of sympathetic tone, altered reactivity to vasoactive drugs; increased dependence on Frank-Starling mechanism to maintain CO; labile BP, more hypotension |
| Conduction system abnormalities | Apoptosis, fibrosis, fatty infiltration, and calcification of pacemaker and His-bundle cells | Conduction block, sick sinus syndrome, atrial fibrillation, decreased contribution of atrial contraction to diastolic volume | Severe bradycardia with potent opioids, decreased CO from decrease in end-diastolic volume |
| Stiff arteries | Loss of elastin, increased collagen, glycosylation cross linking of collagen | Systolic hypertension Arrival of reflected pressure wave during end-ejection leads to myocardial hypertrophy and impaired diastolic relaxation | Labile BP; diastolic dysfunction, sensitive to volume status |
| Stiff veins | Loss of elastin, increased collagen, glycosylation cross linking of collagen | Decreased buffering of changes in blood volume impairs ability to maintain atrial pressure | Changes in blood volume cause exaggerated changes in cardiac filling |
During anesthesia, the cardiovascular changes discussed in the preceding sections predispose the elderly patient to greater hemodynamic instability and greater sensitivity to volume status (6,19,22,75). Several mechanisms can explain the hemodynamic instability. First, the elderly have a higher resting sympathetic tone and have altered beta receptor sensitivity. Removal of the baseline sympathetic tone with the induction of general or neuraxial anesthesia often results in hypotension. Secondly, older patients have a greater sensitivity to volume status. They often arrive on the day of surgery with a depleted intravascular volume because of more frequent use of diuretics, a decreased thirst response to hypovolemia, and age-related changes in renal function. As they are intensely dependent on preload to fill the left ventricle, the reduction in preload induced by anesthesia may result in profound hypotension. Thirdly, the direct effects of intravenous and volatile anesthetics impair cardiac inotropy and lusitropy, and produce both arterial and venous vasodilatation.
Anesthetic management of the elderly patient must be planned on a case-by-case basis. Instead of a specific type of anesthetic for the older patient, we offer suggestions on a set of principles that address the problems often encountered with the elderly patient. Monitoring volume status is critical to management of the older patient. For patients with known heart failure, coronary artery disease, and/or moderate diastolic dysfunction (e.g., delayed relaxation with indications of elevated filling pressures) the decision to place an intraarterial cannula for invasive blood pressure measurement and frequent blood sampling is based on the same considerations applied to the younger patient. Certainly, age-related alterations and coexisting disease may persuade the experienced anesthesiologist to institute such monitoring. However, because no clear evidence exists to specifically recommend this practice before or after induction of anesthesia, the timing of direct arterial pressure monitoring is best based on experience and local practice. For major surgery or vascular surgery, it is imperative that normovolemia be maintained. In such cases, consider use of central venous catheter, pulmonary artery catheter, or transesophageal echocardiography for intraoperative monitoring. Because evidence regarding the efficacy of central venous pressure, pulmonary artery pressure, or transesophageal echocardiographic monitoring as a means to evaluate intravascular volume in the elderly has not been specifically addressed in the perioperative setting, it is not possible to recommend any of these for routine monitoring at this time. Moreover, given the inability of several noninvasive devices, such as the esophageal Doppler or arterial pulse contour, to measure pressures in the central circulation, their utility in patients in whom a concern over the development of pulmonary edema exists, remains limited (76). Indeed, future studies are warranted to determine their potential benefit in the elderly surgical patient when combined with an intraoperative goal-directed fluid strategy.
Induction of anesthesia should be accomplished in a smooth and controlled manner. The elderly require a reduced dose of any given induction agent to produce unconsciousness. The induction dose of most agents is decreased by 30–50% in the elderly. In addition, induction may be prolonged due to a slow circulation time. Therefore, consider titrating induction agents and waiting for an effect before administering additional doses. It is also important to prevent hypoxemia and hypercarbia, as these patients are prone to pulmonary hypertension. Adequate mask ventilation should be initiated as early as possible. Control of the patient’s blood pressure is also essential. It is reasonable to maintain the systolic BP within 10% of the baseline. At the same time, diastolic BP must be maintained, as a low diastolic BP can lead to myocardial ischemia. An attempt should be made to keep the pulse pressure less than the diastolic blood pressure; an increased pulse pressure is indicative of increased aortic impedance which, as described earlier, will increase wall stress, alter ventricular contraction time, and impair early diastolic filling.
Simultaneous infusions of low dose nitroglycerin and titrated phenylephrine can help to alleviate these physiologic alterations. Administered alone, however, these agents may worsen cardiac function in the elderly. For example, phenylephrine “stiffens” the vasculature and increases the return of the reflective wave (manifested by an increase in the pulse pressure), potentially impinging on systole and increasing myocardial work. Nitroglycerin alone decreases vascular tone and preload; ultimately reducing cardiac output. However, in contrast to phenylephrine, nitroglycerin decreases the amplitude of the reflected wave and when used under normovolemic conditions, it reduces the pulse pressure (77). Thus, the benefits of using combination low dose infusions of phenylephrine and nitroglycerine in the elderly are 1) the preservation of vascular distensibility, 2) avoidance of reductions in preload and coronary perfusion pressure and 3) maintenance of stroke volume with minimal cardiac work. In addition, heart rate should be maintained in the low to normal range (60–70 bpm). At this rate, there is adequate time in diastole to fill the noncompliant ventricle. In general, these principles can be remembered by using the “Rule of 70s.” For patients age >70, maintain DBP >70, PP <70, and HR = 70.
In the early postoperative period, patients with known diastolic heart disease should be watched over closely. As illustrated by this case scenario, elderly patients with diastolic dysfunction can acutely decompensate after initially appearing stable. Hypoxemia and/or atrial fibrillation are among the most common complications these patients may encounter in the postoperative anesthesia care unit as a consequence of volume overload. Importantly, when vascular sympathetic tone is restored upon emergence from general anesthesia or resolution of neuraxial blockade, the noncompliant heart may not be able to tolerate the increased shift in central blood volume thus resulting in pulmonary edema and/or atrial fibrillation. Indeed, maintaining the low dose infusion of nitroglycerin (e.g., 25 mcg/min), as discussed previously, may mitigate this from occurring due to its advantageous actions on the pulmonary vasculature. Nonetheless, the assessment of the postoperative patient with suspected heart failure should include an electrocardiogram for signs of ischemia, left ventricular hypertrophy, atrial fibrillation, left bundle branch block. If the ECG is abnormal, a further objective assessment of the patient is required. In most cases, this would involve an echocardiogram. Echocardiography is the ideal investigation as information can be obtained about cardiac valves as well as ventricular function. Particularly in older patients, obstructive valvular disease can be detected and other factors influencing the left ventricular preload, including diastolic dysfunction. If echocardiography is not readily available, a chest radiograph may be obtained to provide information about the presence or absence of cardiomegaly and the presence of pulmonary fluid. Also, before treatment commences, additional blood tests such as arterial blood gas, serum electrolytes, and CBC should be performed in the older patient with confirmed heart failure. While treatment options include a carefully chosen dose of intravenous diuretic therapy, a beta blocker or calcium channel blocker for heart rate control, and a venodilator such as nitroglycerin (if tolerated), treatment is best when delivered as part of a multidisciplinary team.
Conclusion
As the number of persons aged 65 and older continues to grow, the anesthesiologist will more frequently encounter this demographic. Cardiovascular changes that occur in this patient population present difficult anesthetic challenges and place these patients at a high risk of perioperative morbidity and mortality. The anesthesiologist should be knowledgeable about these age-related cardiovascular changes, the pathophysiology underlying them, and the appropriate perioperative management. Whether presenting for cardiac or general surgery, the anesthesiologist must identify patients with altered physiology due to aging or to diastolic dysfunction and be prepared to modify the care plan accordingly. With a directed preoperative assessment that focuses on certain aspects of the cardiovascular system, and the assistance of powerful echocardiographic tools such as tissue Doppler, this can be achieved.
Acknowledgments
Supported in part by grants to L. Groban from the Hartford Foundation Project, American Geriatrics Society, Anesthesia Initiative on Aging Education: Geriatrics for Specialists Initiative and Paul Beeson Award, National Institutes of Aging K08 AG-026764-04.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engoren M, Arslanian-Engoren C, Steckel D, et al. Cost, outcome, and functional status in octogenarians and septuagenarians after cardiac surgery. Chest. 2002;122:1309–1315. doi: 10.1378/chest.122.4.1309. [DOI] [PubMed] [Google Scholar]
- 2.Fruitman DS, MacDougall CE, Ross DB. Cardiac surgery in octogenarians: can elderly patients benefit? Quality of life after cardiac surgery. Ann Thorac Surg. 1999;68:2129–2135. doi: 10.1016/s0003-4975(99)00818-8. [DOI] [PubMed] [Google Scholar]
- 3.Inpatient Procedures. Fast Stats A–Z, National Center for Health Statistics, US Department for Health and Human Services, Centers for Disease Control. 2009 March; Available at: http://www.cdc.gov/nchs/fastats/insurg.htm. Accessed March 30, 2009.
- 4.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 6.Priebe HJ. The aged cardiovascular risk patient. Br J Anaesth. 2000;85:763–778. doi: 10.1093/bja/85.5.763. [DOI] [PubMed] [Google Scholar]
- 7.White SE. Anesthesiology: perioperative medicine or “when the anesthetic is a diuretic”. J Clin Anesth. 2004;16:130–137. doi: 10.1016/j.jclinane.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Kitzman DW, Groban L. Exercise intolerance. Heart Fail Clin. 2008;4:99–115. doi: 10.1016/j.hfc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little WC, Kitzman DW, Cheng CP. Diastolic dysfunction as a cause of exercise intolerance. Heart Fail Rev. 2000;5:301–306. doi: 10.1023/a:1026503028065. [DOI] [PubMed] [Google Scholar]
- 10.Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez AF, Whellan DJ, Stroud S, et al. Outcomes in heart failure patients after major noncardiac surgery. J Am Coll Cardiol. 2004;44:1446–1453. doi: 10.1016/j.jacc.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 12.Hammill BG, Curtis LH, Bennett-Guerrero E, et al. Impact of heart failure on patients undergoing major noncardiac surgery. Anesthesiology. 2008;108:559–567. doi: 10.1097/ALN.0b013e31816725ef. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Tanaka N, Murata K, et al. Prognostic value of pseudonormal and restrictive filling patterns on left ventricular remodeling and cardiac events after coronary artery bypass grafting. Am J Cardiol. 2003;91:550–554. doi: 10.1016/s0002-9149(02)03304-0. [DOI] [PubMed] [Google Scholar]
- 14.Phillip B, Pastor D, Bellows W, et al. The prevalence of preoperative diastolic filling abnormalities in geriatric surgical patients. Anesth Analg. 2003;97:1214–1221. doi: 10.1213/01.ANE.0000083527.45070.F2. [DOI] [PubMed] [Google Scholar]
- 15.Vaskelyte J, Stoskute N, Kinduris S, et al. Coronary artery bypass grafting in patients with severe left ventricular dysfunction: predictive significance of left ventricular diastolic filling pattern. Eur J Echocardiogr. 2001;2:62–67. doi: 10.1053/euje.2000.0051. [DOI] [PubMed] [Google Scholar]
- 16.Salem R, Denault AY, Couture P, et al. Left ventricular end-diastolic pressure is a predictor of mortality in cardiac surgery independently of left ventricular ejection fraction. Br J Anaesth. 2006;97:292–297. doi: 10.1093/bja/ael140. [DOI] [PubMed] [Google Scholar]
- 17.Møller JE, Pellikka PA, Hillis GS, et al. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction. Circulation. 2006;114:438–444. doi: 10.1161/CIRCULATIONAHA.105.601005. [DOI] [PubMed] [Google Scholar]
- 18.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 19.Groban L. Diastolic dysfunction in the older heart. J Cardiothorac Vasc Anesth. 2005;19:228–236. doi: 10.1053/j.jvca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Gillebert TC, Leite-Moreira AF, De Hert SG. Load dependent diastolic dysfunction in heart failure. Heart Fail Rev. 2000;5:345–355. doi: 10.1023/a:1026563313952. [DOI] [PubMed] [Google Scholar]
- 21.Leite-Moreira AF, Correia-Pinto J, Gillebert TC. Afterload induced changes in myocardial relaxation: a mechanism for diastolic dysfunction. Cardiovasc Res. 1999;43:344–353. doi: 10.1016/s0008-6363(99)00099-1. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, Nakayama M, Nevo E, et al. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–1227. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi M, Hay I, Fetics B, et al. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 24.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 26.Meaume S, Benetos A, Henry OF, et al. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 27.Chae CU, Pfeffer MA, Glynn RJ, et al. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 28.Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 29.Domanski M, Norman J, Wolz M, et al. Cardiovascular risk assessment using pulse pressure in the first national health and nutrition examination survey (NHANES I) Hypertension. 2001;38:793–797. doi: 10.1161/hy1001.092966. [DOI] [PubMed] [Google Scholar]
- 30.Abhayaratna WP, Barnes ME, O'Rourke MF, et al. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients > or =65 years of age. Am J Cardiol. 2006;98:1387–1392. doi: 10.1016/j.amjcard.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 31.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 32.Nikitin NP, Witte KK, Thackray SD, et al. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr. 2003;4:36–42. doi: 10.1053/euje.2002.0611. [DOI] [PubMed] [Google Scholar]
- 33.Triposkiadis F, Tentolouris K, Androulakis A, et al. Left atrial mechanical function in the healthy elderly: new insights from a combined assessment of changes in atrial volume and transmitral flow velocity. J Am Soc Echocardiogr. 1995;8:801–809. doi: 10.1016/s0894-7317(05)80004-5. [DOI] [PubMed] [Google Scholar]
- 34.Pearlman JD, Triulzi MO, King ME, et al. Left atrial dimensions in growth and development: normal limits for two-dimensional echocardiography. J Am Coll Cardiol. 1990;16:1168–1174. doi: 10.1016/0735-1097(90)90549-5. [DOI] [PubMed] [Google Scholar]
- 35.Thomas L, Levett K, Boyd A, Leung DY, et al. Compensatory changes in atrial volumes with normal aging: is atrial enlargement inevitable? J Am Coll Cardiol. 2002;40:1630–1635. doi: 10.1016/s0735-1097(02)02371-9. [DOI] [PubMed] [Google Scholar]
- 36.Leung DY, Boyd A, Ng AA, et al. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J. 2008;156:1056–1064. doi: 10.1016/j.ahj.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Tsang TS, Barnes ME, Gersh BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 38.Osranek M, Seward JB, Buschenreithner B, et al. Diastolic function assessment in clinical practice: the value of 2-dimensional echocardiography. Am Heart J. 2007;154:130–136. doi: 10.1016/j.ahj.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 39.Hunt SA. American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Schunkert H, Jackson B, Tang SS, et al. Distribution and functional significance of cardiac angiotensin converting enzyme in hypertrophied rat hearts. Circulation. 1993;87:1328–1339. doi: 10.1161/01.cir.87.4.1328. [DOI] [PubMed] [Google Scholar]
- 41.Flesch M, Schiffer F, Zolk O, et al. Angiotensin receptor antagonism and angiotensin converting enzyme inhibition improve diastolic dysfunction and Ca(2+)-ATPase expression in the sarcoplasmic reticulum in hypertensive cardiomyopathy. J Hypertens. 1997;15:1001–1009. doi: 10.1097/00004872-199715090-00011. [DOI] [PubMed] [Google Scholar]
- 42.Royster RL, Butterworth JF, IV, Prough DS, et al. Preoperative and intraoperative predictors of inotropic support and long-term outcome in patients having coronary artery bypass grafting. Anesth Analg. 1991;72:729–736. doi: 10.1213/00000539-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Rao V, Ivanov J, Weisel RD, et al. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg. 1996;112:38–51. doi: 10.1016/s0022-5223(96)70176-9. [DOI] [PubMed] [Google Scholar]
- 44.Garcia MJ, Smedira NG, Greenberg NL, et al. Color M-mode Doppler flow propagation velocity is a preload insensitive index of left ventricular relaxation: animal and human validation. J Am Coll Cardiol. 2000;35:201–208. doi: 10.1016/s0735-1097(99)00503-3. [DOI] [PubMed] [Google Scholar]
- 45.Hurrell DG, Nishimura RA, Ilstrup DM, et al. Utility of preload alteration in assessment of left ventricular filling pressure by Doppler echocardiography: a simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardiol. 1997;30:459–467. doi: 10.1016/s0735-1097(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 46.Møller JE, Poulsen SH, Søndergaard E, et al. Preload dependence of color M-mode Doppler flow propagation velocity in controls and in patients with left ventricular dysfunction. J Am Soc Echocardiogr. 2000;13:902–909. doi: 10.1067/mje.2000.106572. [DOI] [PubMed] [Google Scholar]
- 47.Maurer MS, Spevack D, Burkhoff D, et al. Diastolic dysfunction: can it be diagnosed by Doppler echocardiography? J Am Coll Cardiol. 2004;44:1543–1549. doi: 10.1016/j.jacc.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 48.Oki T, Tabata T, Yamada H, et al. Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol. 1997;79:921–928. doi: 10.1016/s0002-9149(97)00015-5. [DOI] [PubMed] [Google Scholar]
- 49.Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 50.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 51.Nagueh SF, Sun H, Kopelen HA, et al. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol. 2001;37:278–285. doi: 10.1016/s0735-1097(00)01056-1. [DOI] [PubMed] [Google Scholar]
- 52.Skubas N. Intraoperative Doppler tissue imaging is a valuable addition to cardiac anesthesiologists' armamentarium: a core review. Anesth Analg. 2009;108:48–66. doi: 10.1213/ane.0b013e31818a6c4c. [DOI] [PubMed] [Google Scholar]
- 53.Firstenberg MS, Greenberg NL, Main ML, et al. Determinants of diastolic myocardial tissue Doppler velocities: influences of relaxation and preload. J Appl Physiol. 2001;90:299–307. doi: 10.1152/jappl.2001.90.1.299. [DOI] [PubMed] [Google Scholar]
- 54.Dumesnil JG, Paulin C, Pibarot P, et al. Mitral annulus velocities by Doppler tissue imaging: practical implications with regard to preload alterations, sample position, and normal values. J Am Soc Echocardiogr. 2002;15:1226–1231. doi: 10.1067/mje.2002.123396. [DOI] [PubMed] [Google Scholar]
- 55.Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 56.Groban L, Dolinski SY. Transesophageal echocardiographic evaluation of diastolic function. Chest. 2005;128:3652–3663. doi: 10.1378/chest.128.5.3652. [DOI] [PubMed] [Google Scholar]
- 57.Dokainish H, Zoghbi WA, Lakkis NM, et al. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation. 2004;109:2432–2439. doi: 10.1161/01.CIR.0000127882.58426.7A. [DOI] [PubMed] [Google Scholar]
- 58.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 59.Nagueh SF, Kopelen HA, Quiñones MA. Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation. 1996;94:2138–2145. doi: 10.1161/01.cir.94.9.2138. [DOI] [PubMed] [Google Scholar]
- 60.Nagueh SF, Mikati I, Kopelen HA, et al. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation. 1998;98:1644–1650. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 61.Bruch C, Stypmann J, Gradaus R, et al. Usefulness of tissue Doppler imaging for estimation of filling pressures in patients with primary or secondary pure mitral regurgitation. Am J Cardiol. 2004;93:324–328. doi: 10.1016/j.amjcard.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 62.Nagueh SF, Lakkis NM, Middleton KJ, et al. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254–261. doi: 10.1161/01.cir.99.2.254. [DOI] [PubMed] [Google Scholar]
- 63.Hadano Y, Murata K, Tanaka N, et al. Ratio of early transmitral velocity to lateral mitral annular early diastolic velocity has the best correlation with wedge pressure following cardiac surgery. Circ J. 2007;71:1274–1278. doi: 10.1253/circj.71.1274. [DOI] [PubMed] [Google Scholar]
- 64.Mullens W, Borowski AG, Curtin RJ, et al. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009;119:62–70. doi: 10.1161/CIRCULATIONAHA.108.779223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merello L, Riesle E, Alburquerque J, et al. Risk scores do not predict high mortality after coronary artery bypass surgery in the presence of diastolic dysfunction. Ann Thorac Surg. 2008;85:1247–1255. doi: 10.1016/j.athoracsur.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 66.Bernard F, Denault A, Babin D, et al. Diastolic dysfunction is predictive of difficult weaning from cardiopulmonary bypass. Anesth Analg. 2001;92:291–298. doi: 10.1097/00000539-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Higashita R, Sugawara M, Kondoh Y, et al. Changes in diastolic regional stiffness of the left ventricle before and after coronary artery bypass grafting. Heart Vessels. 1996;11:145–151. doi: 10.1007/BF01745172. [DOI] [PubMed] [Google Scholar]
- 68.Castello R, Pearson AC, Kern MJ, et al. Diastolic function in patients undergoing coronary angioplasty: influence of degree of revascularization. J Am Coll Cardiol. 1990;15:1564–1569. doi: 10.1016/0735-1097(90)92827-o. [DOI] [PubMed] [Google Scholar]
- 69.Kunichika H, Katayama K, Sakai H, et al. The effect of left ventricular chamber compliance on early diastolic filling during coronary reperfusion. Jpn Circ J. 1995;59:762–771. doi: 10.1253/jcj.59.762. [DOI] [PubMed] [Google Scholar]
- 70.Whalley GA, Doughty RN, Gamble GD, et al. Pseudonormal mitral filling pattern predicts hospital re-admission in patients with congestive heart failure. J Am Coll Cardiol. 2002;39:1787–1795. doi: 10.1016/s0735-1097(02)01868-5. [DOI] [PubMed] [Google Scholar]
- 71.Møller JE, Søndergaard E, Poulsen SH, et al. Pseudonormal and restrictive filling patterns predict left ventricular dilation and cardiac death after a first myocardial infarction: a serial color M-mode Doppler echocardiographic study. J Am Coll Cardiol. 2000;36:1841–1846. doi: 10.1016/s0735-1097(00)00965-7. [DOI] [PubMed] [Google Scholar]
- 72.Sanders D, Houle T, Kon N, Zvara D, Groban L. Diastolic dysfunction predicts adverse outcome after cardiac surgery. Anesthesiology. 2008;109 Suppl:A1592. [Google Scholar]
- 73.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 74.American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society for Vascular Surgery, Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Anesth Analg. 2008;106:685–712. doi: 10.1213/01/ane.0000309024.28586.70.
- 75.Rooke GA. Cardiovascular aging and anesthetic implications. J Cardiothorac Vasc Anesth. 2003;17:512–523. doi: 10.1016/s1053-0770(03)00161-7. [DOI] [PubMed] [Google Scholar]
- 76.Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108:887–897. doi: 10.1213/ane.0b013e31818ffd99. [DOI] [PubMed] [Google Scholar]
- 77.Pauca AL, Kon ND, O'Rourke MF. Benefit of glyceryl trinitrate on arterial stiffness is directly due to effects on peripheral arteries. Heart. 2005;91:1428–1432. doi: 10.1136/hrt.2004.057356. [DOI] [PMC free article] [PubMed] [Google Scholar]