Abstract
CEACAM1 (carcinoembryonic antigen-related cell adhesion molecule 1), a type I transmembrane glycoprotein involved in cell-cell adhesion, undergoes extensive alternative splicing, resulting in isoforms with 1–4 Ig-like extracellular domains (ECDs) with either long or short cytoplasmic tails. We have previously shown that CEACAM1-4L (4 ECDs with a long cytoplasmic domain) formed glands with lumena in humanized mammary mouse fat pads in NOD/SCID mice. In order to identify the key residues of CEACAM1-4L that play essential roles in lumen formation, we introduced phosphorylation mimic (e.g., Thr-457 or Ser-461 to Asp) or null mutations (Thr-457 or Ser-461 to Ala) into the cytoplasmic domain of CEACAM1-4L and tested them in both the in vivo mouse model and in vitro Matrigel model of mammary morphogenesis. MCF7 cells stably expressing CEACAM1-4L with the single mutation T457D or the double mutant T457D+S461D, but not the null mutants induced central lumen formation in 3D Matrigel and in humanized mammary fat pads. However, the single phosphorylation mimic mutation S461D, but not the null mutation blocked lumen formation in both models, suggesting that S461 has inhibitory function in glandular lumen formation. Compared to our results for the -4S isoform (Chen et al., J. Biol. Chem, 282: 5749–5760, 2008), the T457A null mutation blocks lumen formation for the -4L but not for the -4S isoform. This difference is likely due to the fact that phosphorylation of S459 (absent in the -4L isoform) positively compensates for loss of T457 in the -4S isoform, while S461 (absent in the -4S isoform) negatively regulates lumen formation in the -4L isoform. Thus, phosphorylation of these key residues may exert a fine control over the role of the -4L isoform (compared to the -4S isoform) in lumen formation.
Keywords: CEACAM1, phosphorylation, breast cancer, lumen formation
Introduction
CEACAM1 (carcinoembryonic antigen-related cell adhesion molecule 1) is a type I transmembrane glycoprotein involved in cell-cell adhesion [1, 2]. As a member of the CEA (carcinoembryonic antigen) gene family, CEACAM1 was originally identified in liver and bile as biliary glycoprotein (BGP) [3] and later shown to be expressed in epithelia of mammary glands, gastrointestinal tract, prostate, and in immune cells as well [4]. Through extensive alternative splicing, CEACAM1 has 11 different splice variants with most isoforms consisting of a transmembrane domain and either a long (L) or a short (S) cytoplasmic domain [5]. The long isoforms comprise 71 amino acids in the cytoplasmic tail, which has several serine (Ser), threonine (Thr) and tyrosine residues that may undergo posttranslational phosphorylation modifications that participate in signal transduction and protein–protein interactions with actin [6, 7], tropomyosin [7], calmodulin [8, 9], β3-integrin [10], and β-catenin [11]. In contrast, while the short isoforms only encode 12 cytoplasmic residues, this domain is still able to bind annexin II [12], actin, tropomyosin, and calmodulin [7]. Both long and short isoforms are co-expressed in most CEACAM1-expressing tissues, and the ratio between the two isoforms determines the signaling outcome [13, 14]. Importantly, two tyrosine residues within the long cytoplasmic domain form a functional immunoreceptor tyrosine-based inhibitory motif (ITIM) that appears to have inhibitory activity in different contexts [15–17].
CEACAM1 is believed to be a tumor suppressor for various types of cancer [4]. In normal breast tissue and benign lesions, CEACAM1 is consistently expressed at the apical sites of epithelial cells, however, the apical expression of CEACAM1 is lost upon development of malignant carcinomas, resulting in a predominant uniform membrane staining surrounding the atypical cells [18]. Furthermore, about 30% of breast cancer exhibit down-regulation of CEACAM1 [19], and transfection of the CEACAM1 gene into breast cancer cell lines reduces the tumorigenic phenotype in both cell culture and animal models [20].
Normal mammary glands are a collection of ductal and lobular structures [21]. During puberty in the female, the tissue of the mammary glands responds to the ovarian release of female sex hormones including estrogen and progesterone. These hormones stimulate the formation of additional ducts and milk secreting glands. There is also an increase in volume and elasticity of connective tissue, deposition of adipose tissue and increased vascularity in the mammary glands. In lactating mammary glands, the lobulo-alveolar structures are further developed due to hormonal stimulations [22]. A single layer of polarized epithelial cells surrounds central lumena where milk is secreted. These epithelial cells, in turn, are surrounded by myoepithelial cells that function in milk ejection, and a vascularized stroma that contains adipocytes and fibroblasts.
During breast cancer development, normal tissue architecture including glandular lumen formation is disrupted [23]. Previously, we have shown that CEACAM1 plays an essential role in reverting tumor cells to a normal lumen formation morphology in both the three-dimensional Matrigel model and the in vivo humanized mouse fat pad model of mammary morphogenesis [19, 24–26]. First, anti-CEACAM1 antibody and CEACAM1 anti-sense blocked lumen formation of the nonmalignant human breast cell line MCF10F that expresses CEACAM1 when grown in Matrigel [19]. Second, transfection of the CEACAM1-4S isoform into MCF7 mammary carcinoma cells that do not express CEACAM1 or form lumena when grown in Matrigel, reverted these cells to a normal morphogenic program [24, 26]. Third, when CEACAM1-4L transfected MCF7 cells were grown in a humanized mouse fat pad, they formed lumena with a single-layer of epithelium [25]. We have also shown that both CEACAM1-4S and CEACAM1-4L promoted lumen formation by mediating apoptosis of the central acinar cells that are not in contact with extracellular matrix [24, 25]. Although CEACAM1-4S transfected MCF7 cells did not form lumena in the humanized mouse mammary fat pad, the phosphorylation mimic mutations of Thr-457 (T457D) or Ser-459 (S459D) were able to induce lumen formation [25], suggesting that phosphorylation of Thr-457 or Ser-459 are essential for lumen formation. These results leave open the question of the role of CEACAM1-4L and its key residues in lumen formation, especially since both long and short isoforms are expressed in the human mammary gland [27] and they share amino acid residues through Thr-457, but differ after Gly-458 (Fig 1A).
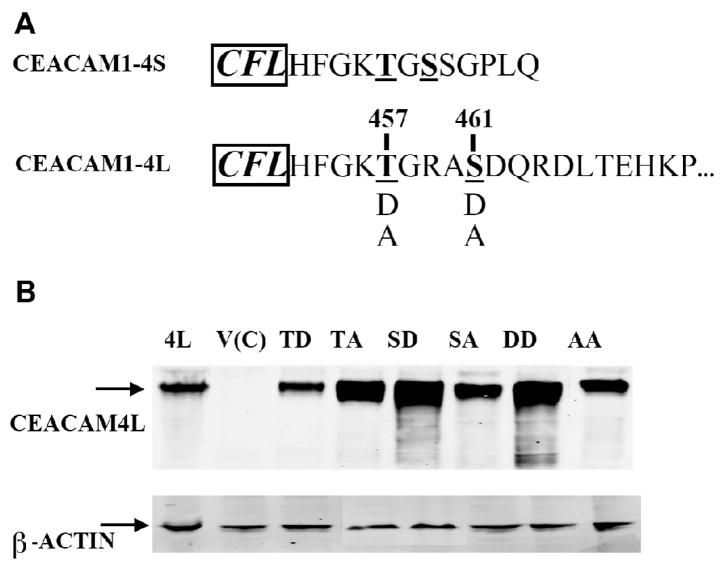
Figure 1. Amino acid sequences and mutational analysis of CEACAM1-4L cytoplasmic domain.
A) The partial amino acid sequences of the cytoplasmic domains of human CEACAM1-4S and CEACAM1-4L are shown for comparison. The three most N-terminal residues (shown to the left in bold and italic font) are assigned to the transmembrane region. The mutations made in this study are shown in single letter code: potential phosphorylation sites Thr-457 and Ser-461 were mutated to Asp (D) or Ala (A). B) Stably transfected MCF7 cell clones vector control (V(C)), CEACAM1-4L wild type (4L), T457D (TD), T457A (TA), S461D (SD), S461A (SA), T457D+S461D (DD), and T457A+S461A (AA) were analyzed by western blotting with CEACAM1 mAb T84.1 (β-actin was used as a loading control).
In order to address key residues involved in functions for lumen formation of CEACAM1-4L, we employed the in vivo mouse model developed by Kuperwasser and Weinberg, in which both the stromal and epithelial cells of the reconstructed mammary gland are of human origin [28]. Earlier studies have proposed that Thr-457 and Ser-461 in rat CEACAM1-4L could be phosphorylated [29], phosphorylation of Thr-457 in CEACAM1-4S is essential for lumen formation in vivo [25], and Thr-457 is shared by both CEACAM1-4L and CEACAM1-4S isoforms (Fig 1A). Therefore, we focused on two residues: Thr-457 and Ser-461 respectively. First, we show that the long cytoplasmic domain isoform CEACAM1-4L transfected MCF7 cells is sufficient to form glands with lumena in both the Matrigel and the in vivo model, but not as efficient as CEACAM1-4S in the Matrigel model. Second, mutational analysis of the long cytoplasmic domain isoform revealed that residues Thr-457 and Ser-461 are indeed key residues involved in lumen formation, but act in an opposite way. Cells with the T457A null mutant lost the capacity to form glandular lumena, while the phosphorylation mimic mutation T457D were as efficient as the wild type. In contrast, cells with the S461A null mutation formed lumena, while the phosphorylation mimic mutation S461D blocked glandular lumen formation, indicating that phosphorylation of Ser-461 has an inhibitory effect on lumen formation of CEACAM1-4L. Taken together, these results indicate that phosphorylation of these two residues may fine tune the lumen formation process, and that over-expression of CEACAM1-4L with phosphorylation of S461 may lead to diminished lumen formation, a situation mimicking lobular in situ carcinoma (Lobular Carcinoma in situ). These results strengthen our recent observation that breast cancers often over express the long isoform of CEACAM1 [30]. Thus, alteration of CEACAM1 isoform expression and/or its phosphorylation pattern may lead to an altered mammary gland morphology.
Materials and Methods
Cell Lines
The human mammary adenocarcinoma cell line MCF7 was obtained from ATCC (HTB-22™). Note: this cell line was found to be positive for mycoplasma on testing with a PCR kit (Boca Scientific, cat. No. 25233) and was cured of contamination with the MycoSolutions kit (Boca Scientific, Cat. No. MYC-BIOK100). MCF7 cells were cultured in MEM medium supplemented with 10% Fetal Bovine Serum, 1% Penicillin/Streptomycin/Am, 1% Sodium Pyruvate, 2% Sodium Bicarbonate, and 1% Non-essential Amino Acid. The primary human fibroblasts immortalized with human telomerase and stably expressing GFP (RMF/EG) were a gift from Dr. C. Kupperwasser (Tufts New England Medical Center, Boston, MA). RMF/EG cells were maintained in DMEM media supplemented with 10% Fetal Bovine Serum and 1% Penicillin/Streptomycin/Am.
Transfection and Selection of Stable Cell Lines
The cloning of CEACAM1-4L into the pHβ-actin expression vector was previously described [24]. The phosphorylation-mimic and null mutants of the cytoplasmic domain of CEACAM1-4L, Thr-457 to Ala (T457A), Ser-461 to Ala (S461A), both Thr-457 and Ser-461 to Ala (T457A+S461A), Thr-457 to Asp (T457D), Ser-461 to Asp (S461D), and both Thr-457 and Ser-461 to Asp (T457D+S461D) were generated on the pHβ–CEACAM1-4L vector using the QuickChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). CEACAM1-4L mutant plasmids were transfected into MCF7 cells with Lipofectamine 2000 (Invitrogene, CA). Forty-eight hours later transfected MCF7 cells were selected in 1.0 mg/ml Geneticin (G418) and stable transfected cells were maintained in MEM medium containing 0.5 mg/ml of Geneticin (G418).
Western blotting
Proteins (15μg) from 1% NP40 cell lysates were separated by SDS-gel electrophoresis, transferred to nitrocellulose membranes and probed with mAb T84.1 (1 μg/ml) [31] at room temperature (RT) for 1 hour, followed by infrared-labeled secondary antibody (LI-COR Biosciences, Lincoln, Nebraska) at RT for 1 hour. β-Actin was detected with 1:5,000 diluted mouse anti-β-actin primary antibody (Abcam, Cambridge, UK). Signals were detected with the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, Nebraska).
Immunofluorescence
CEACAM1 expression was detected on cells plated on 12-well plate using anti-CEACAM1 monoclonal antibody 5F4 (kind gift from Dr. Richard Blumberg, Boston, MA). 5F4 (1 μg/ml) was added to culture media for 40 min at 37 °C followed by goat anti-mouse Alexa 488 (Molecular Probes, Carlsbad, CA) adding to culture media for 30 min at 37 °C. Staining was visualized on an inverted Olympus IX81 microscope.
Matrigel Culture and Staining
The three-dimensional Matrigel Sandwich assay has been previously described [26]. Briefly, 12-well plates were coated with 150μl Matrigel and incubated at 37 °C for 30 min until the Matrigel solidified. Cells (1×105) in 500 μl of mammary epithelial basal medium (MEBM) (Clonetics, CA) plus pituitary gland extract (Cambrex, East Rutherford, NJ) were added to each well. After 3 hr of incubation, the floating cells were removed and the bound cells were overlaid with 150μl Matrigel. The MEBM culture medium covering the top layer of Matrigel was changed every other day. After 10 days, the Matrigel cultures were fixed with 2% paraformaldehyde at RT for 20 min and permeablized with PSG (0.01% saponinin phosphate-buffered saline) containing 0.1% Nonidet P-40 for 20 min at RT. Cells were stained for F-actin with Texas Red-conjugated phalloidin (Molecular Probes, Carlsbad, CA). MCF7 cells grown in a Matrigel sandwich assay were scored for lumen formation in about a hundred acini. Statistical analysis was performed using Fisher’s exact test.
Animals and Surgery
The procedures were described previously [25]. Briefly, NOD/SCID mice were anesthetized and the fourth mammary glands were removed. Two weeks later, 2.0 × 105 unirradiated RMF/EG fibroblasts and 2.0 × 105 irradiated (4 Gy) fibroblasts suspended in 50μl PBS were injected into each cleared fat pad 24 hr after irradiation. Two weeks later, MCF7 or CEACAM1 transfected MCF7 cells (2.0 × 105) with 2.0 × 105 RMF/EG fibroblasts were injected into the humanized fat pad sites. MCF7 or CEACAM1 transfected MCF7 cells were mixed with fibroblasts and resuspended in 100μl of a 3:1 rat Collagen I (Upstate Biotechnology, Lake Placid, NY): Phenol-red free Matrigel (Becton Dickinson, Bedford, MA) mixture and 50μl were injected into each site. Eight weeks later, mice were sacrificed. All animal studies were approved by the Research Animal Care Committee of the City of Hope (RACC# 05024).
Histology and Immunohistochemistry
Fresh xenograft tissue was obtained from humanized mammary fat pads and fixed in 10% neutral buffered formalin. Five-micron sections were deparaffinized, rehydrated through graded alcohols, and subjected to antigen retrieval [32]. Immunohistochemistry was performed using antibodies against CEACAM1 (5F4, kind gift from Dr. R. S. Blumberg), and M30 (Roche, Nutley, NJ). Immune complexes were visualized by the ABC peroxidase method, and sections were counterstained with hematoxylin.
Results
Generating CEACAM1-4L mutants in transfected MCF7 cell lines
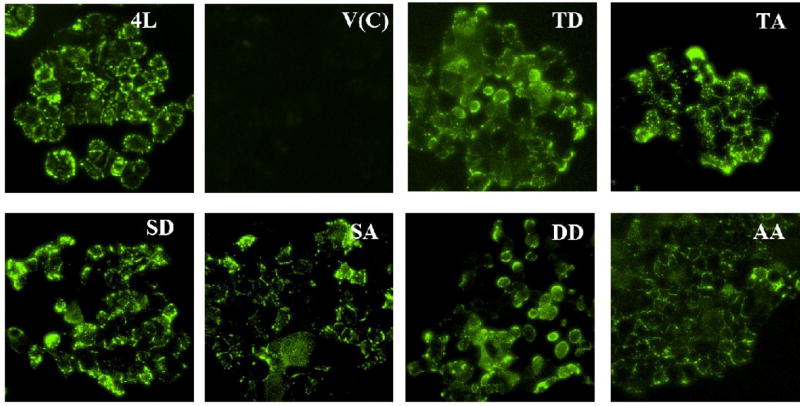
Previous studies have shown that CEACAM1-4L transfected MCF7 cells were fully capable to form glandular lumen in an in vivo model of mammary morphogenesis, while CEACAM1-4S transfected MCF7 cells were not able to form lumen in this model [25]. In this in vivo model, human mammary epithelial cells are grown in humanized mammary fat pads in NOD/SCID mice [28]. Yokoyama et al. have also demonstrated that phosphorylation mimic mutations at either or both Thr-457 and Ser-459 of CEACAM1-4S protein can induce glandular lumen formation in this in vivo mouse model, indicating that posttranslational changes such as phosphorylation play essential roles in lumen formation [25]. In fact, the short isoform and the long isoforms share the same the sequence through Thr-457, but differ after Gly-458 (Figure 1A). Previous work by Obrink and coworkers has proposed that residues Thr-457 and Ser-461 in rat CEACAM1-4L could be phosphorylated [29]. Thus, the corresponding residues Thr-457 and Ser-461 of human CEACAM1-4L may be candidates for phosphorylation and play a role in lumen formation. In order to understand if these two residues of CEACAM1-4L are involved in lumen formation, we generated four single and two double mutants: T457D, T457A, S461D, S461A, T457D+S461D and T457A+S461A (Figure 1A). The acidic D (Asp) mutations were made to mimic phosphorylated forms of T457 and S461, while the A (Ala) mutations were considered null mutants. MCF7 cells stably expressing each CEACAM1-4L mutant as well as wild type and vector controls were established. Each mutant cell line (but to a lesser extent in the T457D mutant) as well as the wild type had high expression of CEACAM1 as shown by western blot analysis, while there were no expression of CEACAM1 in the vector control cell line (Figure 1B). On the other hand, the expression of β-actin was similar among these different cells (Figure 1B). To confirm the localization of CEACAM1-4L in stably transfected MCF7 cells, immunofluorescence with monoclonal antibody against CEACAM1 was performed. Strong surface expression was observed on the wild type and six mutant cell lines, but not on the vector control cells (Figure 2).
Figure 2. Surface expressions of CEACAM1-4L on stably transfected MCF7 cells.
Stable MCF7 cell clones transfected with vector control (V(C)), CEACAM1-4L wild type (4L), T457D (TD), T457A (TA), S461D (SD), S461A (SA), T457D+S461D (DD), and T457A+S461A (AA) were grown in 12-well plates and were stained with CEACAM1 mAb 5F4 followed by Alexa-488 conjugated rabbit anti-murine IgG staining. Cells were analysed by inverted fluorescence microscope. (Magnification 100×)
Comparison of CEACAM1-4L mutants transfected MCF7 cells in the three-dimensional Matrigel model
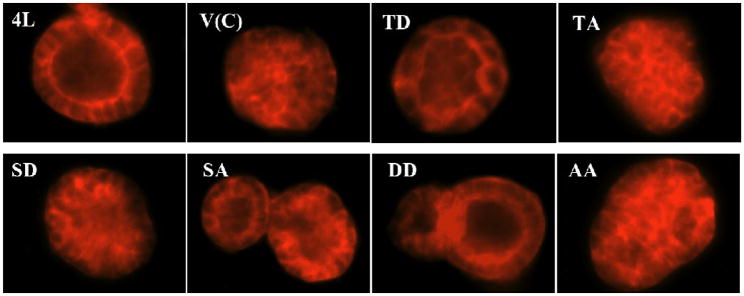
To investigate the effects of different mutants of CEACAM1-4L on lumen formation, we first cultured these stably transfected MCF7 cells in the three-dimensional Matrigel model of mammary morphogenesis. MCF7 cells stably expressing wild type CEACAM1–4L were grown in a sandwich Matrigel assay for 10 days (see “Materials and Methods”), and about 44% formed lumena with a defined lumenal ring of actin as seen by staining with phalloidin for F-actin (Figure 3, Table 1). In contrast, the majority (over 90%) of vector control transfected MCF7 cells formed acini without lumena when grown in the sandwich Matrigel assay (Figure 3, Table 1), which is significantly different from wild type CEACAM1-4L (p< 0.0001). Notably, this result is in contrast to the short isoform CEACAM1-4S that forms over 90% lumena in Matrigel compared to vector transfected control cells [26]. When MCF7 cells stably expressing the T457D mutant, S461A mutant or T457D+S461D double mutants were analyzed, the percentage of lumen formation was similar to that found for wild type CEACAM1-4L (Table 1, Figure 3) and markedly higher than vector control (p< 0.0001). However, MCF7 cells expressing the T457A mutant, S461D mutant, or T457A+S461A double mutants had similar percentages of lumen formation compared to vector control cells (Table 1, Figure 3). Overall, the T457D mutant, S461A mutant or T457D+S461D double mutants-transfected MCF7 cells formed about 4-fold more acini with lumena compared to vector control-transfected cells (Table 1) (p<0.0001).
Figure 3. Lumen formation of different stably transfected MCF7 cells grown in three-dimensional Matrigel model of mammary morphogenesis.
Stable MCF7 cell clones transfected with vector control (V(C)), CEACAM1-4L wild type (4L), T457D (TD), T457A (TA), S461D (SD), S461A (SA), T457D+S461D (DD), and T457A+S461A (AA) were grown in Matrigel Sandwich for 10 days and were stained for F-actin with Texas Red-conjugated phalloidin. Cells were analysed by inverted fluorescence microscope. (Magnification 200×)
Table 1.
Lumen formation of CEACAM1-4L mutants in Matrigel and in humanized mammary fat pads1
| Cell Line/Mutant | Percent Lumen Formation in Matrigel | Lumen Formation in Humanized Mammary Fat Pads |
|---|---|---|
| Vector control | 9% | No |
| Wild type | 44%*** | Yes |
| T457D | 44%*** | Yes |
| T457A | 7% | No |
| S461D | 6% | No |
| S461A | 47%*** | Yes |
| T457D+S461D | 35%*** | Yes |
| T457A+S461A | 14% | No |
MCF7 cells transfected with vector, wild type, or various mutants of CEACAM1 were grown in Matrigel for 10 days and scored for percent lumen formation. A hundred acini were counted for each experiment and the p value calculated versus vector control using Fisher’s exact test (
, p<0.0001). The ability of the transfectants to form lumena in humanized mammary fat pads could not be qunatitated, but is listed as positive or negative based on histochemistry.
Comparison of CEACAM1-4L mutants transfected MCF7 cells in the humanized mouse mammary fat pad model
Next, to evaluate CEACAM1-4L and mutants’ function in vivo, we adapted the humanized chimeric murine mammary fat pad model, an orthotopic xenograft model in which both the stromal and epithelial cells of the reconstructed mammary gland are of human origin [28]. In this model, cleared mammary fat pads of NOD/SCID mice are first inoculated with immortalized but nontumorigenic human mammary fibroblasts, followed by trypsinized cell suspension of MCF7 cells transfected with vector, CEACAM1-4L or mutants two weeks later. After 8 weeks of growth, nodules 5–10 mm in diameter were harvested from the humanized mouse fat pads and subjected to analysis by immunohistochemistry. H&E staining for xenografts from either the vector control (Figure 4A) or CEACAM1-4L (Figure 4D) transfected MCF7 cells showed that the CEACAM1-4L transfected MCF7 cells were capable of forming glandular structures with a single layer of epithelium surrounding a central lumen populated with apoptotic cells (characterized by condensed nuclei), while the vector control cells formed solid tumors (Figure 4A). This is consistent with our previous observations [25]. Immunostaining of serial sections with an anti-CEACAM1 antibody revealed no staining for the vector controls (Figure 4B), and intense lumenal staining for the CEACAM1-4L transfected cells (Figure 4E). In addition, staining with antibody M30 that recognizes cleaved cytokeratin 18 and is specific for apoptotic cells [33, 34], revealed intense staining in the lumena of CEACAM1-4L transfected cells (Figure 4F), consistent with our previous data that demonstrated lumen formation is a result of apoptosis of the central luminal cells [24].
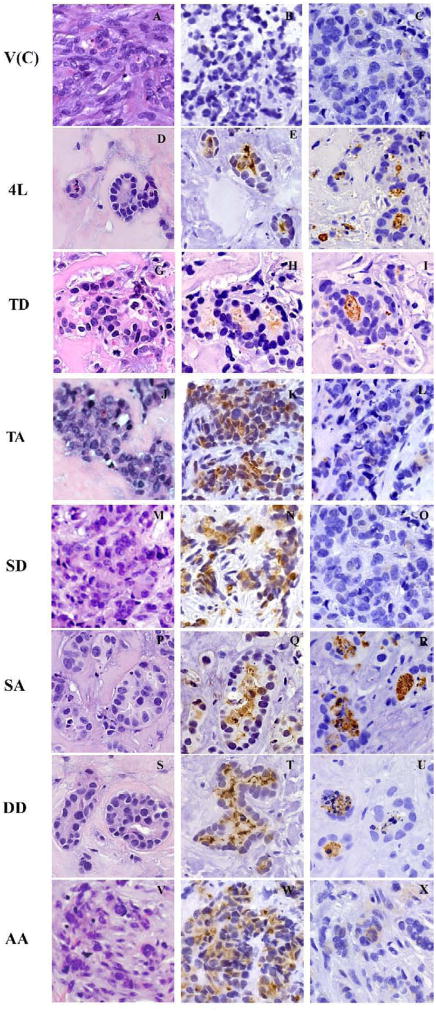
Figure 4. Lumen formation of CEACAM1-4L and mutants transfected MCF7 cells grown in humanized mammary fat pads in NOD/SCID mice exhibiting apoptosis of the central cells.
Paraffin sections of xenograft outgrowths of CEACAM1-4L and mutants in MCF7 cells grown in humanized mammary fat pads were stained with hematoxylin and eosin (H&E). Stable MCF7 cell clones transfected with vector control (A), CEACAM1-4L wild type (D), T457D (G), T457A (J), S461D (M), S461A (P), T457D+S461D (S), and T457A+S461A (V) are shown. Immunostaining was performed with anti-CEACAM1 antibody 5F4 for stable MCF7 cell clones transfected with vector control (B), CEACAM1-4L wild type (E), T457D (H), T457A (K), S461D (N), S461A (Q), T457D+S461D (T), and T457A+S461A (W). Immunostaining with M30 antibody was performed for stable MCF7 cell clones transfected with vector control (C), CEACAM1-4L wild type (F), T457D (I), T457A (L), S461D (O), S461A (R), T457D+S461D (U), and T457A+S461A (X). (Magnification 400×)
Similarly, single CEACAM1-4L mutants T457D, T457A, S461D, S461A and double mutants T457D+S461D, T457A+S461A in transfected MCF7 cells were inoculated in humanized fat pads of female NOD/SCID mice. The phosphorylation mimic T457D mutant formed simple epithelium (Figure 4G), with a single layer of epithelial cells surrounding a central lumen that was moderately positive for CEACAM1 (Figure 4H) in the central apoptotic cells and strongly positive for cleaved CK18 (Figure 4I). To the contrary, the null mutant T457A in transfected MCF7 cells exhibited diminished lumen formation forming solid tumors (Figure 4J–L). These results demonstrate that phosphorylation at Thr-457 of CEACAM1-4L is required in glandular formation with simple glandular epithelium in the in vivo model, which is consistent with our findings in Matrigel culture (Figure 3).
The opposite result was found for mutations at S-461. The null mutation (S461A) formed acini with central lumena surrounded by a single layer of epithelial cells in vivo (Figure 4P–R). The lumena were strongly stained for CEACAM1 (Figure 4Q) and for cleaved CK18 (Figure 4R). On the other hand, the phosphorylation mimic mutant S461D in transfected MCF7 cells exhibited diminished lumen formation in vivo (Figure 4M–O). In the tumors formed by S461D transfected MCF7 cells, CEACAM1 staining was strong in tumor cells (Figure 4N), while there were no staining of cleaved CK18 (Figure 4O). This result is consistent with our findings in Matrigel culture (Figure 3). Thus, the null mutation of Ser-461 (S461A) was able to form lumen both in vitro and in vivo, while the phosphorylation mimic mutation at Ser-461 (S461D) blocked lumen formation in both cases, suggesting that phosphorylation at Ser-461 of CEACAM1-4L has an inhibitory role in lumen formation.
We also tested the double mutants, T457D+S461D and T457A+S461A. Double mutants T457D+S461D in transfected MCF7 cells formed central lumena with single layer of epithelial cells (Figure 4S), that were strongly positive for CEACAM1 (Figure 4T) in the central apoptotic cells which were also strongly positive for cleaved CK18 (Figure 4U); while the double null mutants T457A+S461A in transfected MCF7 cells failed to form lumen in vivo (Figure 4V–X), with no positive staining for cleaved CK18 (Figure 4X). These results suggest that phosphorylation at Thr-457 of CEACAM1-4L is required and essential for glandular formation with simple glandular epithelium in the in vivo model. Furthermore, we conclude that if Thr-457 is already “phosphorylated”, as in the double mutant T457D+S461D, then the phosphorylation of Ser-461, which otherwise is inhibitory, is unable to inhibit lumen formation. Vice versa, when Thr-457 cannot be phosphorylated, as in the double mutant T457A+S461A, the null mutation of Ser-461 (removal of its inhibitory activity) by itself is unable to promote lumen formation.
Discussion
The normal mammary gland is comprised of lobulo-alveolar structures with central lumena into which milk is secreted. During breast cancer development, breast carcinomas start to lose their ability to differentiate into a normal structure, thus presenting an abnormal histological morphology, including less lumen formation and development of stratified epithelium [35]. Although only 30% of breast cancer exhibit down-regulated CEACAM1 expression [18, 19], it appears that this decrease of CEACAM1 in mammary gland is linked to diminished lumen formation, as introducing CEACAM1 into breast carcinoma cells restored normal morphology both in vitro and in vivo [24–26]. Furthermore, stratified epithelium development may also result from less phosphorylation of key residues in the short cytoplasmic domain isoforms, as phosphorylation mimic mutations of these residues (Thr-457 and Ser-459) of CEACAM1-4S could induce lumen formation in the humanized mouse fat pad [25], while the wild type failed to form lumen in this in vivo model. In contrast, expression of the wild type long cytoplasmic domain isoforms could provoke lumen formation in vivo and in vitro by itself [25] (and see Results). Obviously, the difference between short and long cytoplasmic domain isoforms resides in the cytoplasmic tail, and importantly, both isoforms share the first five amino acids including the potential phosphorylation site Thr-457. Indeed, phosphorylation of Thr-457 is also essential for lumen formation of CEACAM1-4L, because a null mutation of this amino acid (T457A) blocks normal glandular lumen formation, while a phosphorylation mimic mutation (T457D) is still able to form lumena. Moreover, CEACAM1-4L appears to have additional regulatory residues in lumen formation functions. A phosphorylation mimic mutation at Ser-461 (S461D) blocked glandular lumen formation of CEACAM1-4L both in vitro and in vivo, while non-phosphorylated Ser-461 (S461A) was able to form lumena, suggesting phosphorylation of Ser-461 has inhibitory functions for lumen formation of CEACAM1-4L. This difference is likely due to the fact that phosphorylation of S459 (absent in the -4L isoform) positively compensates for loss of T457 in the -4S isoform, while S461 (absent in the -4S isoform) negatively regulates lumen formation in the -4L isoform.
Thus there is a fundamental difference in regulation of lumen formation between CEACAM1-4S and CEACAM1-4L. This may explain why wild type CEACAM1-4L is only able to make only 44% lumena in Matrigel, or less than half the lumen formation of CEACAM1-4S [26]. Nonetheless, wild type CEACAM1-4L is more efficient at lumen formation in humanized fat pads than wild type CEACAM1-4S, perhaps because phosphorylation of CEACAM1-4S’s T457 residue is catalyzed by a different kinase or is inhibited by an unknown mechanism that is operative in vivo, but not in vitro.
It should be noted that in an earlier publication [24], we found that MCF7 cells transfected with the -4L isoform underwent cell death by necrosis in Matrigel. Subsequent studies in our lab demonstrated that this phenomenon was due to contamination of the parent MCF7 cell line with a low level of mycoplasma (detected only by PCR or EM) and that even fresh cells from ATCC were similarly contaminated. Once the MCF7 cells were cured of contamination and retransfected with the -4L isoform, the cells no longer necrosed in Matrigel. However, it is interesting that this phenomenon was not observed with the -4S isoform, suggesting some subtle difference between the behavior of the two isoforms in Matrigel.
A major question is which kinases are responsible for the phosphorylation of Thr-457 or Ser-461 in CEACAM1-4L? Edlund et al. have shown that Serine and Threonine residues in rat CEACAM1-4S can be phosphorylated by PKC isozymes [29], however, human CEACAM1 sequences differ sufficiently from the murine, making the human sequence an unlikely candidate for phosphorylation by PKC isozymes. When synthetic peptides containing the cytoplasmic domains of either the short or long cytoplasmic domains of human CEACAM1 are subjected to in vitro phosphorylation with various PKC isozymes, little or no phosphorylation is observed (unpublished data). Therefore, we have begun to look into other kinases that are potential candidates, but so far without much luck. Although we haven’t identified the kinase(s) responsible for the phosphorylation of Thr-457 or Ser-461 in CEACAM1-4L, we speculate that these two residues are phosphorylated by two different kinases, because the phosphorylation of these two residues appears to have distinct effects on the lumen inducing capacity of CEACAM1-4L. Thus, the ultimate control of lumen formation by CEACAM1-4L may depend on at least two kinases, activated by different environmental cues such as extracellular matrix and cell-cell adhesion, as well as others.
In addition, the long cytoplasmic domain of CEACAM1-4L has about 70 amino acids; thus its down-stream sequences and their interaction partners may also influence its activity. For instance, this long cytoplasmic domain has been shown to interact with actin [6, 7], tropomyosin [7], calmodulin [8, 9], β3-integrin [10], and β-catenin [11]. Its function in lumen formation appears to involve cytoskeletal reorganization and inhibition of mitogenesis associated with the differentiated phenotype. Thus, the cytoplasmic domain of CEACAM1-4L may recruit various components of a signaling complex through activation of the cytoskeleton at specific locations between interacting cells, and its phosphorylation status may be a key event in triggering these interactions. Noteworthy, not all CEACAM1-4L transfected MCF7 cells (about 44%), when cultured in three-dimensional Matrigel, formed lumena. This suggests that lumen formation is a finely controlled process, requiring not only a proper microenvironment of extracellular matrix but also the correct ratio of CEACAM1 isoforms. In addition, two tyrosine residues within the long cytoplasmic domain form a functional immunoreceptor tyrosine-based inhibitory motif (ITIM) that appears to have the inhibitory activity in different contexts [15–17]. We have not yet explored the potential role of these residues, nor the more recently discovered β-catenin binding motif [11] in the control of lumen formation.
Notably, phosphorylation of Thr-457 and Ser-461 has opposite effects on glandular lumen formation of CEACAM1-4L, i.e., phosphorylation of Thr-457 promotes lumen formation, whereas which of Ser-461 inhibits lumen formation. These intriguing results may suggest there is a delicate balance between phosphorylation of Thr-457 and Ser-461 residues of CEACAM1-4L in a mammary gland that has a preponderance of the long isoform. During breast cancer development, tumor cells might disrupt this balance via activity changes in key kinases, resulting in decreased phosphorylation of Thr-457 or increased phosphorylation of Ser-461. Either change would lead to aberrant acinous formation, thus leading to the kind of hyperplasia seen in LCIS (Lobular Carcinoma in situ) and perhaps in DCIS (Ductal Carcinoma in situ). This hypothesis needs to be further evaluated.
In conclusion, wild type CEACAM1-4L formed glandular epithelia with central lumena in both three-dimensional Matrigel and in humanized mouse mammary fat pads. Phosphorylation mimic mutation at Thr-457 (T457D) was also able to induce glandular lumen formation, while null mutation at Thr-457 (T457A) in CEACAM1-4L inhibited lumen formation. Thus, phosphorylation of Thr-457 is essential for the lumen formation function of CEACAM1-4L, which is similar to CEACAM1-4S isoforms. Furthermore, phosphorylation mimic mutation of Ser-461 (S461D) blocked lumen formation of CEACAM1-4L, suggesting phosphorylation of Ser-461 has inhibitory effect for lumen formation. These findings suggest that both Thr-457 and Ser-461 are key residues involved in lumen formation of CEACAM1-4L.
Acknowledgments
We would like to thank the Microscopy and Pathology cores at City of Hope for helping us process the images and perform immunohistochemistry. We are very grateful to Dr. Blumberg for providing antibodies and Dr. Kuperwasser for providing immortalized human fibroblasts. This work was supported by NIH grant CA84202.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–46. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 2.Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–71. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svenberg T, Hammarstrom S, Zeromski J. Immunofluorescence studies on the occurrence and localization of the CEA-related biliary glycoprotein I (BGP I) in normal human gastrointestinal tissues. Clin Exp Immunol. 1979;36:436–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Obrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol. 1997;9:616–26. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett TR, Drake L, Pickle W., 2nd Human biliary glycoprotein gene: characterization of a family of novel alternatively spliced RNAs and their expressed proteins. Mol Cell Biol. 1993;13:1273–82. doi: 10.1128/mcb.13.2.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadekova S, Lamarche-Vane N, Li X, Beauchemin N. The CEACAM1-L glycoprotein associates with the actin cytoskeleton and localizes to cell-cell contact through activation of Rho-like GTPases. Mol Biol Cell. 2000;11:65–77. doi: 10.1091/mbc.11.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schumann D, Chen CJ, Kaplan B, Shively JE. Carcinoembryonic antigen cell adhesion molecule 1 directly associates with cytoskeleton proteins actin and tropomyosin. J Biol Chem. 2001;276:47421–33. doi: 10.1074/jbc.M109110200. [DOI] [PubMed] [Google Scholar]
- 8.Edlund M, Obrink B. Evidence for calmodulin binding to the cytoplasmic domains of two C-CAM isoforms. FEBS Lett. 1993;327:90–4. doi: 10.1016/0014-5793(93)81046-3. [DOI] [PubMed] [Google Scholar]
- 9.Blikstad I, Wikstrom T, Aurivillius M, Obrink B. C-CAM (Cell-CAM 105) is a calmodulin binding protein. FEBS Lett. 1992;302:26–30. doi: 10.1016/0014-5793(92)80276-m. [DOI] [PubMed] [Google Scholar]
- 10.Brummer J, Ebrahimnejad A, Flayeh R, Schumacher U, Loning T, Bamberger AM, Wagener C. cis Interaction of the cell adhesion molecule CEACAM1 with integrin beta(3) Am J Pathol. 2001;159:537–46. doi: 10.1016/s0002-9440(10)61725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin L, Li Y, Chen CJ, Sherman MA, Le K, Shively JE. Direct interaction of tumor suppressor CEACAM1 with beta-catenin: identification of key residues in the long cytoplasmic domain. Exp Biol Med (Maywood) 2008 doi: 10.3181/0712-RM-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirshner J, Schumann D, Shively JE. CEACAM1, a cell-cell adhesion molecule, directly associates with annexin II in a three-dimensional model of mammary morphogenesis. J Biol Chem. 2003;278:50338–45. doi: 10.1074/jbc.M309115200. [DOI] [PubMed] [Google Scholar]
- 13.Singer BB, Scheffrahn I, Obrink B. The tumor growth-inhibiting cell adhesion molecule CEACAM1 (C-CAM) is differently expressed in proliferating and quiescent epithelial cells and regulates cell proliferation. Cancer Res. 2000;60:1236–44. [PubMed] [Google Scholar]
- 14.Turbide C, Kunath T, Daniels E, Beauchemin N. Optimal ratios of biliary glycoprotein isoforms required for inhibition of colonic tumor cell growth. Cancer Res. 1997;57:2781–8. [PubMed] [Google Scholar]
- 15.Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, Morales V, Rudd CE, Utku N, Blumberg RS. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol. 2004;172:3535–43. doi: 10.4049/jimmunol.172.6.3535. [DOI] [PubMed] [Google Scholar]
- 16.Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, Chen D, Fuss IJ, Utku N, Lewicki DN, Becker C, Gallagher TM, Holmes KV, Blumberg RS. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471–82. doi: 10.1084/jem.20030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izzi L, Turbide C, Houde C, Kunath T, Beauchemin N. cis-Determinants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene. 1999;18:5563–72. doi: 10.1038/sj.onc.1202935. [DOI] [PubMed] [Google Scholar]
- 18.Riethdorf L, Lisboa BW, Henkel U, Naumann M, Wagener C, Loning T. Differential expression of CD66a (BGP), a cell adhesion molecule of the carcinoembryonic antigen family, in benign, premalignant, and malignant lesions of the human mammary gland. J Histochem Cytochem. 1997;45:957–63. doi: 10.1177/002215549704500705. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Hardy JD, Sun Y, Shively JE. Essential role of biliary glycoprotein (CD66a) in morphogenesis of the human mammary epithelial cell line MCF10F. J Cell Sci. 1999;112 (Pt 23):4193–205. doi: 10.1242/jcs.112.23.4193. [DOI] [PubMed] [Google Scholar]
- 20.Luo W, Wood CG, Earley K, Hung MC, Lin SH. Suppression of tumorigenicity of breast cancer cells by an epithelial cell adhesion molecule (C-CAM1): the adhesion and growth suppression are mediated by different domains. Oncogene. 1997;14:1697–704. doi: 10.1038/sj.onc.1200999. [DOI] [PubMed] [Google Scholar]
- 21.Strange R, Metcalfe T, Thackray L, Dang M. Apoptosis in normal and neoplastic mammary gland development. Microsc Res Tech. 2001;52:171–81. doi: 10.1002/1097-0029(20010115)52:2<171::AID-JEMT1003>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.McManaman JL, Neville MC. Mammary physiology and milk secretion. Adv Drug Deliv Rev. 2003;55:629–41. doi: 10.1016/s0169-409x(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 23.Guinebretiere JM, Menet E, Tardivon A, Cherel P, Vanel D. Normal and pathological breast, the histological basis. Eur J Radiol. 2005;54:6–14. doi: 10.1016/j.ejrad.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Kirshner J, Chen CJ, Liu P, Huang J, Shively JE. CEACAM1-4S, a cell-cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci U S A. 2003;100:521–6. doi: 10.1073/pnas.232711199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoyama S, Chen CJ, Nguyen T, Shively JE. Role of CEACAM1 isoforms in an in vivo model of mammary morphogenesis: mutational analysis of the cytoplasmic domain of CEACAM1-4S reveals key residues involved in lumen formation. Oncogene. 2007;26:7637–46. doi: 10.1038/sj.onc.1210577. [DOI] [PubMed] [Google Scholar]
- 26.Chen CJ, Kirshner J, Sherman MA, Hu W, Nguyen T, Shively JE. Mutation analysis of the short cytoplasmic domain of the cell-cell adhesion molecule CEACAM1 identifies residues that orchestrate actin binding and lumen formation. J Biol Chem. 2007;282:5749–60. doi: 10.1074/jbc.M610903200. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Simpson JF, Glackin C, Riethorf L, Wagener C, Shively JE. Expression of biliary glycoprotein (CD66a) in normal and malignant breast epithelial cells. Anticancer Res. 1998;18:3203–12. [PubMed] [Google Scholar]
- 28.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edlund M, Wikstrom K, Toomik R, Ek P, Obrink B. Characterization of protein kinase C-mediated phosphorylation of the short cytoplasmic domain isoform of C-CAM. FEBS Lett. 1998;425:166–70. doi: 10.1016/s0014-5793(98)00222-1. [DOI] [PubMed] [Google Scholar]
- 30.Gaur S, Shively JE, Yen Y, Gaur RK. Altered splicing of CEACAM1 in breast cancer: identification of regulatory sequences that control splicing of CEACAM1 into long or short cytoplasmic domain isoforms. Mol Cancer. 2008;7:46. doi: 10.1186/1476-4598-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagener C, Clark BR, Rickard KJ, Shively JE. Monoclonal antibodies for carcinoembryonic antigen and related antigens as a model system: determination of affinities and specificities of monoclonal antibodies by using biotin-labeled antibodies and avidin as precipitating agent in a solution phase immunoassay. J Immunol. 1983;130:2302–7. [PubMed] [Google Scholar]
- 32.Shi SR, Cote RJ, Taylor CR. Antigen retrieval techniques: current perspectives. J Histochem Cytochem. 2001;49:931–7. doi: 10.1177/002215540104900801. [DOI] [PubMed] [Google Scholar]
- 33.Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaekers FC, Bjorklund B, Nap M, Jornvall H, Schutte B. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–72. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 34.Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–94. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005;205:248–54. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]