Abstract
The BET sub-family of bromodomain-containing genes is characterized by the presence of two bromodomains and a unique ET domain at their carboxyl termini. Here we show that the founding member of this sub-family, Brd2, is an essential gene by generating a mutant mouse line lacking Brd2 function. Homozygous Brd2 mutants are embryonic lethal, with most Brd2−/− embryos dying by embryonic day 11.5. Prior to death, the homozygous embryos were notably smaller and exhibited abnormalities in the neural tube where the gene is highly expressed. Brd2-deficient embryonic fibroblast cells were observed to proliferate more slowly than controls. Experiments to explore whether placental insufficiency could be a cause of the embryonic lethality showed that injecting diploid mutant embryonic stem cells into tetraploid wild-type blastocysts did not rescue the lethality; that is Brd2-deficient embryos could not be rescued by wild-type extra-embryonic tissues. Further, there were enhanced levels of cell death in Brd2-deficient embryos.
Keywords: Brd2, Bromodomain, Acetyl-lysine, Histone modification, Chromatin remodeling
INTRODUCTION
The bromodomain is an evolutionarily conserved 110 aa motif first identified by comparison of the Drosophila genes brahma and female sterile homeotic (fsh) and four other genes in yeast and human (Haynes et al., 1992; Tamkun et al., 1992). Approximately thirty bromodomain-containing genes exist in mouse and human and encode proteins involved in broad cellular processes, including replication, transcription, splicing, gene silencing, and chromatin remodeling (Florence and Faller, 2001; Jeanmougin et al., 1997; Winston and Allis, 1999; Yang, 2004; Zeng and Zhou, 2002). The bromodomain has been shown to interact with acetyl-lysine in histones and other proteins of diverse function [rev. in (Yang, 2004)], for example p53 (Barlev et al., 2001), HIV Tat (Mujtaba et al., 2004), c-Myb (Sano and Ishii, 2001), SF1 (Jacob et al., 2001), and MyoD (Polesskaya et al., 2001).
Brd2 is a member of the ‘BET’ subfamily of bromodomain-containing proteins, so designated because of the presence of two bromodomains along with an additional region of homology designated the ET domain (Florence and Faller, 2001). The original members of this unique sub-class include the Drosophila gene female sterile homeotic (fsh) (Haynes et al., 1989), the yeast S. cerevisiae gene bromodomain factor 1 (BDF1), and the human gene RING3 (Really Interesting New Gene 3) (BRD2) (Florence and Faller, 2001).
Drosophila Fsh was initially characterized as a maternal effect gene (Gans et al., 1975, 1980): maternal expression of fsh was required for normal embryonic development and maternal mutations could not be rescued by extra copies of the fsh gene from the sperm. Studies with conditional alleles of fsh found that, in addition to the maternal effect, zygotic expression of fsh is also required later in development, since mutant individuals die at various times from late embryogenesis through pupation, depending on the allele (Digan et al., 1986; Gans et al., 1975, 1980). Studies with conditional alleles of fsh at semi-permissive temperatures revealed that some individuals survived to adulthood while most died as segmented embryos or larvae. The majority of these progeny showed defects in segmentation, mostly partial or complete deletions of one or more thoracic anterior abdominal segments (Digan et al., 1986; Gans et al., 1975, 1980). Fsh was shown to interact synergistically with loci controlling patterning and the production of homeotic transformations during development, such as Ultrabithorax and trithorax.
Although there is only a single fsh gene in Drosophila, through alternative splicing it produces three transcripts: 5.9 kb, 7.6 kb, and 2.2 kb in length. The 5.9 kb transcript encodes an 1106 aa protein (FSH-S) and the 7.6 kb transcript encodes a 2038 aa protein. The two proteins share the same N-terminal region and both contain the double bromodomains and the ET domain. The 2.2 kb transcript is apparently a non-coding RNA, which overlaps the 3’ end of the 7.6 kb transcript and has the same direction of transcription (Haynes et al., 1989). Chang et al. (2007) reported that FSH-S acts directly on the ZESTE binding sites at the promoter of Ultrabithorax (Ubx) and activates Ubx expression. Recently, Florence and Faller (2008) found that a new allele of Fs(1)h, rancor (rnc), is a genetic interactor of another homeotic Hox gene, Deformed, and further, that Fs(1)h function is modulated by Ras signaling (Florence and Faller, 2008; Florence and McGinnis, 1998).
In mammals, the BET sub-family includes the genes (mouse designation/human designation) Brd2/BRD2, Brd3/BRD3, Brd4/BRD4, and Brdt/BRDT. Although the first mammalian BET gene, BRD2 (RING3), was identified over a decade ago (Beck et al., 1992), little is known about the roles it plays, particularly in vivo.
While Brd2 was shown to be expressed in a wide variety of tissues, it is highly expressed in tissues with epithelial compartments that undergo hormonally modulated remodeling, such as the mammary gland, uterus, and epididymis, and also ovary and testis (Rhee et al., 1998; Trousdale and Wolgemuth, 2004). The zebra fish homologue of mouse Brd2 and human BRD2 is highly expressed in the egg, early embryo, and developing nervous system (Dibenedetto et al., 2008). BRD2 has also been reported to be expressed in endothelial cells and the expression was elevated under VEGF stimulation (BelAiba et al., 2001). In the testis, Brd2 is most abundantly expressed in the germ cell lineage, while in the ovary, its expression was detected in both germ cells and somatic cells (Rhee et al., 1998; Trousdale and Wolgemuth, 2004). Interestingly, the sub-cellular distribution of Brd2 transcripts varied depending upon the stage of differentiation of the growing and meiotically maturing oocyte (Trousdale and Wolgemuth, 2004). Brd2 protein has been reported to be sequestered in the cytoplasm when cells are cultured in serum-free medium, but to translocate to the nucleus upon serum stimulation (Denis et al., 2000; Guo et al., 2000). Brd2 protein was also shown to be distributed differentially in the nucleus or cytoplasm depending upon the mode of proliferation in the developing neural tube and in dorsal root ganglia during embryogenesis in the mouse: Brd2 was in the nucleus during proliferation, but was predominantly cytoplasmic when cells are terminally differentiated (Crowley et al., 2004).
Nuclear BRD2 has been shown to interact with E2F and to transactivate promoters of genes encoding cell cycle regulatory proteins such as cyclin D1, cyclin A2, cyclin E, and dihydrofolate reductase (Denis et al., 2000; Guo et al., 2000; Sinha et al., 2005), and to selectively bind to acetylated lysine 12 on histone H4 (Kanno et al., 2004) in NIH/3T3 and HeLa cell lines. Brd2 has recently been demonstrated to have intrinsic histone chaperone activity and to be required for transcription of the cyclin D1 gene in vivo (LeRoy et al., 2008). It was further shown that Brd2 and Brd3 associate with hyperacetylated chromatin along the entire lengths of transcribed genes and that Brd2- and Brd3-associated chromatin is enriched in H4K5, H4K12, and H3K14 acetylation, confirming earlier experiments using FRET analysis (Kanno et al., 2004). Brd2 has been reported to form complexes with proteins such as E2F, Snf2β, Baf155, HDAC11, CAF1b, NAP1L3, some components of TAFIID, as well as histones (Denis et al., 2006). Brd2 has also been shown to interact with the major latency-associated nuclear antigen (LANA) of Kaposi’s sarcoma herpesvirus/human herpesvirus-8 (KSHV/HHV-8) and, similar to what was seen for Brd4 in the papillomavirus system (You et al., 2004), to tether the viral genome to mitotic chromosomes (An et al., 2005; Mattsson et al., 2002; Platt et al., 1999; Viejo-Borbolla et al., 2005). Finally, structural analysis of the two bromodomains of BRD2 as crystals and in solution has revealed the interesting finding that the first bromodomain of Brd2 can homodimerize (Huang et al., 2007; Nakamura et al., 2007; Umehara et al., 2007).
In vivo mutational analyses of Brd4 (Houzelstein et al., 2002) and Brdt (Shang et al., 2007) in the mouse model have revealed that these genes are essential for embryonic development and spermatogenesis, respectively. Although BRD2 is the prototype of BET genes in mammals, as it was the first one discovered, its in vivo function remains unknown. Using association analysis, Pal et al. (Pal et al., 2003) have identified BRD2 as EJM1, the locus on chromosome 6p21.3 linked to a form of common epilepsy, Juvenile Myoclonic Epilepsy (JME), suggesting that BRD2 may play a critical role in brain development and/or function.
In the present study, we generated a null mutation of the murine Brd2 gene using a gene-trap approach. Brd2 was shown to be an essential gene: Brd2-deficient embryos die during mid-gestation development. Further, the phenotype observed suggests a critical function for Brd2 in neural development and differentiation.
RESULTS
Generation of a null mutation of the Brd2 gene in mouse
We took advantage of a large-scale gene-trap strategy generating mutant mouse ES cell lines developed and commercialized by BayGenomics. These mutant cell lines have been deposited into a library that is available to the scientific research community at the following database (http://www.genetrap.org). A search of the library revealed that a Brd2 mutant ES cell line was available. For this mutant, the gene-trap vector pGT01 was inserted into the first intron (as the transcription start sites have not been precisely mapped and there are multiple mRNAs apparently transcribed from different promoters, the exon containing the first putative translation start codon ATG was defined as exon one) (Fig. 1). The gene-trap vector contained a splice acceptor of mouse En2 exon 2, a fusion of β-galactosidase and neomycin transferase (βgeo) selection cassette, and an SV40 polyadenylation signal, but no promoter. Only when the gene-trap vector is inserted into a gene after the endogenous promoter will the selection cassette be expressed. The splice acceptor interrupts normal splicing, abolishes the expression of the downstream part of the trapped gene, and causes the inserted vector sequence to be expressed (www.sanger.ac.uk/PostGenomics/genetrap). In our case, the insertion is in the first intron of Brd2, immediately after the translation start site. It was considered likely that the insertion would totally abolish expression of the endogenous gene, which was confirmed in the in situ hybridization data in Figure 2D. The stop codon of the selection cassette would result in a stop in translation of any mRNA that is produced.
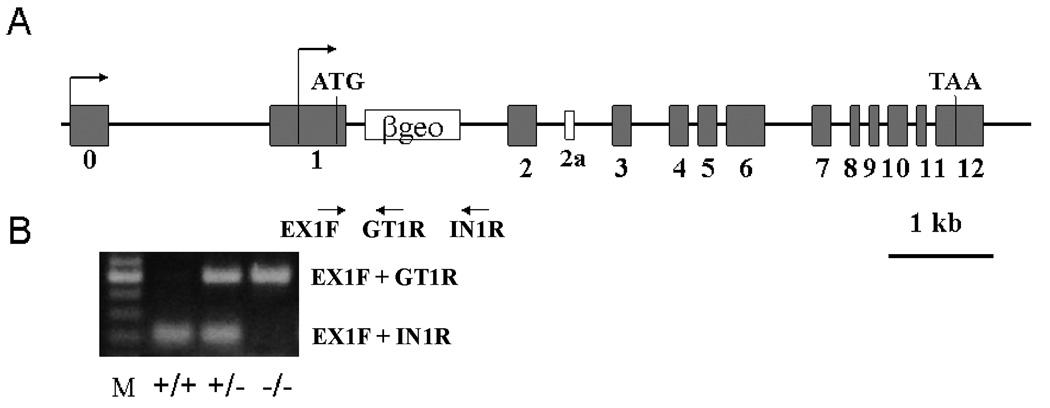
Figure 1.
Panel A: Schematic depiction of the inactivation of the Brd2 gene by a gene-trap approach. The gene trap vector was inserted into the first intron of the Brd2 gene, near the putative translation start codon ATG. The gene trap insertion is about 8 kb in size and is not depicted in proportion to the Brd2 gene. Arrows indicate the position of PCR primers used for verification of the insertion event and for genotyping progeny carrying the mutant or wild-type alleles. The primers are: EX1F (exon 1 forward), IN1R (intron 1 reverse), and GT1R (genetrap vector reverse). Panel B: Visualization of the PCR products used to genotype the progeny. The use of EX1F + GT1R gives the higher band (500 bp) representing the mutated allele; EX1F + IN1R produces the lower band (220 bp)--wildtype allele. Since the insertion is 8 kb, under our PCR condition (1 min extension time), this pair of primers would not yield a band corresponding to the mutant allele.
Figure 2.
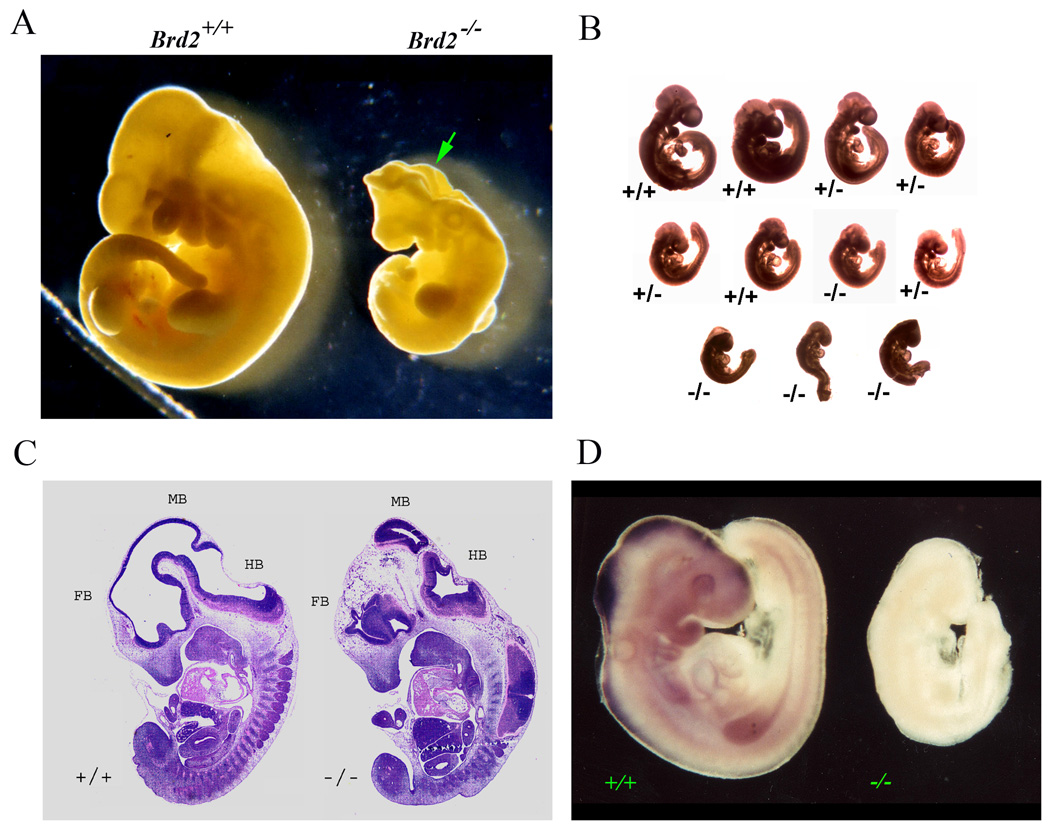
Panel A: Photographs of wild-type (Brd2+/+, left) and Brd2−/− (right) embryos at E9.5 of gestation. The mutant embryo is smaller and the developing neural tube is unfused (arrow). Panel B: A litter of 11 embryos at E9.5 of gestation were fixed in 4% paraformaldehyde and photographed together to portray the variations in phenotype among the embryos. The genotype and weight of the embryos are, from the top row and left to right: Top Row: +/+, 4.6 mg; +/+, 6.5 mg; +/−, 4.3 mg; +/−, 2.3 mg; Middle Row: +/−, 1.9 mg; +/+, 2.0 mg; −/−, 2.2 mg; +/−, 1.9 mg; and Bottom Row: −/−, 1.1 mg; −/−, 1.0 mg; −/−, 1.2 mg. The mean and SD for each genotype are: +/+, 5.1 ± 1.2 mg; +/−, 2.6 ± 1.3 mg; −/−, 1.4 ± 0.6 mg. Panel C: Sagittal sections of E11.5 embryos of wildtype and mutant embryos. Grossly normal appearing heart, lung, etc. are seen in the mutant, but the brain of the mutant is aberrant. FB, forebrain; MB, midbrain; HB, hindbrain. Panel D: Whole-mount in situ hybridization of wild-type and Brd2 mutant E9.5 embryos using digoxygenin-labeled Brd2 RNA probes (see Materials and Methods). Brd2 mRNA is detected in the wild-type developing neural system but is absent in the mutant.
Chimeric mice carrying the mutant Brd2 allele described above were generated and a total of ten chimeric mice were obtained, eight of which were male. All chimeric males gave agouti pups upon mating with C57Bl/6J females but only one transmitted the mutant allele yielding Brd2+/− heterozygous F1 offspring. The presence of the inserted gene-trap vector in the first intron of the Brd2 gene was confirmed by PCR with primers located in the first exon and the gene-trap vector (Fig. 1).
Brd2 is essential for normal embryonic development
The F1 heterozygous progeny were overtly normal, but subsequent heterozygous to heterozygous mating yielded no homozygous mutant offspring among three litters in the initial analysis and no homozygous live progeny have been observed in any of the over one hundred subsequent matings. This suggested that Brd2 was essential for embryonic development, but the timing of embryo demise was not known. Therefore, timed matings were set up between heterozygous animals, and fetuses were collected at selected days of gestational development, beginning at E7.5. Inspection of the embryos revealed that at E7.5, no obvious differences could be detected between wild-type and mutant embryos (data not shown). At E8.5, the mutant embryos were visibly smaller than the wild-type (data not shown), which was clearly evidenced at E9.5 (Fig. 2A). There were also defects in closure of the neural tube, in striking contrast to the degree of neural tube fusion in the normal embryos (Fig. 2A). As illustrated in Figure 2B, there was some heterogeneity in the degree of abnormal development among the mutant embryos at E9.5, but by E10.5 to E11.5, most mutant embryos began to degenerate. At E12.5, virtually all homozygous mutant embryos were resorbed.
We also observed size heterogeneity among heterozygous Brd2+/− embryos and even wildtype embryos, as shown in Fig 2B and detailed in the legend. The mean and standard deviation of the weights of each genotype were: +/+, 5.1 ± 1.2 mg; +/−, 2.6 ± 1.3 mg; and −/−, 1.4 ± 0.6 mg. In our experience, this kind of heterogeneity is not common in wildtype matings. The embryos appear otherwise normal and in the very rare Brd2−/− embryo that survived to E11.5 (Fig. 2C, histological section on right ), all the organ systems other than the brain seemed to be developing similarly with regard to patterning and relative size to those of the control (sectioned embryo on left) or heterozygous (data not shown).
Brd2 is highly expressed in the developing brain
We had previously shown that in histological sections of wild-type E11.5 mouse embryos hybridized in situ with radioactively-labeled antisense Brd2 RNA probe, Brd2 expression was detected in the embryonic neural tube (Crowley et al., 2004). To confirm that the mutant embryos indeed lacked expression of the Brd2 gene and to further explore the expression of Brd2 in the developing nervous system, we performed whole mount in situ hybridization with digoxygenin-labeled RNA probes on both wild-type and Brd2−/− embryos (Fig. 2D). These studies revealed that E9.5 wild-type embryos expressed Brd2 at high levels in the developing central neural system but homozygous null embryos showed no signal above background (Fig. 2D). At E9.5, expression was particularly obvious in the forebrain, midbrain, and hindbrain of the wild-type but not the mutant embryos.
Wildtype placenta did not rescue the lethal phenotype of Brd2−/− mutant embryos
While the defects in the closure of the neural tube in Brd2−/− embryos were striking, such defects in brain formation are not believed to be a direct cause of embryonic lethality in mid-gestation development (Papaioannou and Behringer, 2005). Rather, it has been suggested that placental insufficiency, defects in the cardiovascular system, and defective hematopoietic (red blood) cells are three likely causes of embryonic lethality occurring around E11.5, as was observed in the Brd2-deficient mice.
To begin to study the cause of lethality of the Brd2 mutant embryos, we first examined the possibility that the Brd2-deficient placenta was incapable of supporting embryonic development by performing a wildtype placenta-rescue experiment. Tetraploid cells can form functional extra-embryonic tissues, but have difficulty being incorporated into the embryo proper (Wang and Yuen, 1997). Conversely, diploid ES cells can form the embryo proper but not placenta. Thus, injection of either wild-type or mutant ES cells into tetraploid wild-type blastocysts can test whether the lethal phenotype is solely caused by placental defects. ES cells were derived from Brd2−/− mutant and wild-type blastocysts and injected into wild-type tetraploid blastocysts. The ES cell-injected blastocysts were transplanted into pseudopregnant foster mothers and embryos that developed from mutant ES cell injected blastocysts were examined at E12.5 (transplanted control blastocysts with wild-type ES cells were allowed to develop to term). We observed that virtually all of the Brd2−/− embryos died by E12.5 (25 out of 28, from 2 foster mothers), even in the presence of wildtype placenta. In contrast, the Brd2+/+ ES cells in the presence of the wildtype placenta resulted in live births (8 pups from 1 foster mother). This experiment excluded the possibility of placental insufficiency as the sole cause of the mid-gestation lethal phenotype of Brd2 knockout mice.
The proliferation capability of Brd2−/− MEFs was compromised and there was increased cell death in mutant embryos
As mentioned above, Brd2−/− mutant embryos were consistently significantly smaller than their wildtype and heterozygous littermates. It had been reported previously that inner cell mass cells that were null for the Brd4 member of the BET sub-family were defective in cell proliferation and that Brd4+/− mouse embryonic fibroblasts (MEFs) grew more slowly than wild-type cells in vitro (Houzelstein et al., 2002). We therefore initiated a series of preliminary experiments in which we derived MEFs from E9.5, E10.5, and E11.5 embryos of the three genotypes and measured their proliferation rate. Brd2−/− cells were very sensitive to the stress of in vitro culture: for the majority of the Brd2−/− embryos, the cells died after the first plating and did not grow to adequate numbers for sub-culturing. Cells of the few Brd2−/− embryos that did proliferate reasonably well were dispersed into 48-well plates and the cells were counted daily for 7 days.
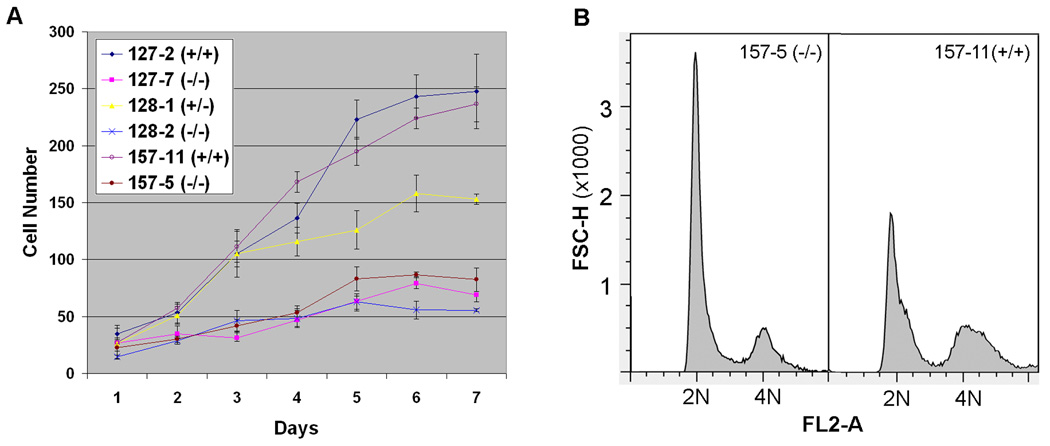
The initial results of this analysis are shown in Figure 3 and indicate that Brd2−/− MEFs grew more slowly than Brd2+/+ cells in vitro. The data also revealed that Brd2−/− cells stopped growing at a much lower density than Brd2+/+ cells. Furthermore, the heterozygous Brd2+/− MEFs proliferated at an intermediate rate, as compared to Brd2+/+ and Brd2−/− MEFs. To examine at which phase of the cell cycle the slowdown of the proliferation of the Brd2−/− MEFs occurred, we performed flow cytometric analysis of the cell cycle of the mutant and wildtype MEFs. Figure 3B shows that the mutant MEFs mainly delayed at the G1 phase of the cell cycle.
Figure 3.
Panel A: Growth parameters of mouse embryonic fibroblast (MEFs) derived from Brd2+/+, Brd2+/−, and Brd2−/− embryos of E9.5 (157-5 and 157-11), E10.5 (128-1 and 128-2), and E11.5 (127-2 and 127-7). The cells were plated at 104 cells/well at day 0 and the number of cells/well was monitored at daily intervals. At each time point, 3 wells of cells were counted and the number of cells is shown as average of the three samples. The culture media was changed at day 4. The Brd2−/− cells grew very poorly as compared to the wild-type cells, and the heterozygous Brd2+/− MEFs grew at an intermediate rate as compared to the Brd2+/+ and Brd2−/−cells. Panel B: Flow cytometry analysis of cell cycle of MEFs of E9.5 Brd2−/− (157-5) and Brd2+/+ (157-11) embryos, showing an elevated G1 peak (2N) in the mutant cells, indicating that the cell cycle of Brd2−/− MEFs is delayed at the G1 phase.
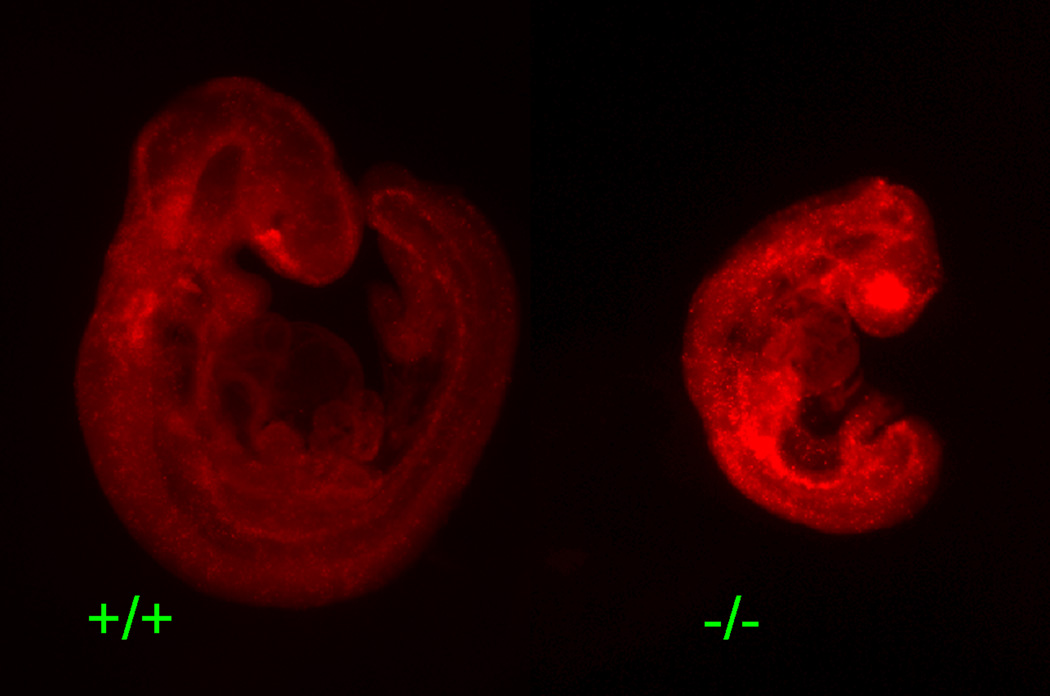
We next examined the embryos directly for evidence of differences in the proportion of cells undergoing cell death, as smaller embryos and abnormal differentiation can also result from enhanced rates of cell death. We therefore performed whole mount Lysotracker staining, which stains for lysosomal activity. Lysosomal activity increases at late stages of apoptosis in tissues when macrophages and neighboring cells phagocytose apoptotic bodies and is thus an indicator of cell death (Dunty et al., 2002). As shown in Figure 4, Brd2−/− embryos at E9.5 of gestation exhibited elevated levels of cell death.
Figure 4.
Lysotracker staining to detect apoptotic cells in E9.5 embryos. Embryos were dissected and processed for Lysotracker staining in the whole embryos (see materials and Methods). Photomicrographs of the entire embryos of Brd2+/+ versus Brd2−/− are shown. There are enhanced levels of positive lysotracker staining in the Brd2-deficient embryo.
DISCUSSION
Among the four mammalian BET family members, Brd2 and Brd3 show greater similarity to one another than to Brdt or Brd4, while Brdt and Brd4 appear more closely related, with regard to the size of the protein produced or the level of overall homology. Brdt is testis-specific and is essential for spermatogenesis (Shang et al., 2007; Shang et al., 2004), while Brd4 is essential for embryonic development (Houzelstein et al., 2002). In the present study we found that Brd2 is also required for embryonic development in the mouse but with a strikingly different phenotype than that observed for Brd4, which resulted in embryonic lethality prior to implantation. In contrast, Brd2-deficient embryos survived until mid-gestation. E9.5 Brd2−/− embryos were noticeably smaller than Brd2+/+ or Brd2+/− littermates and some showed unfused neural tubes. Brd2−/− mutant embryos began to die by E9.5 and were rarely detected after E11.5. Brd2−/− MEFs proliferated more slowly in vitro, which is consistent with the observation of smaller mutant embryos in vivo. Interestingly, Brd2+/− MEFs also proliferated more slowly than wildtype cells in vitro, while Brd2+/− mice are apparently normal. This is similar to Brd4+/− mice: Brd4+/− MEFs grow more slowly than wildtype cells, but Brd4+/− mice were grossly normal (Houzelstein et al., 2002).
Studies in the rat model found that Brd2 mRNA levels were elevated in cells undergoing apoptosis, both in cultured PC12 cells and in developing neurons in vivo (Wang et al., 1997). However, in our Brd2 knockout mouse model, w observed that in the Brd2−/− mutant embryos the level of cell death increased. This suggested that Brd2 not only has an important role in cell proliferation, but is also involved in cell death, either inducing apoptosis or as a response for preventing death in cells facing stress. Because the level of cell death increased in the absence of Brd2 function, we speculate that it may normally function to prevent cell death.
The human BRD2 gene (originally designated RING3) was identified during a chromosome walk in the region of the class II major histocompatibility complex (MHC) on chromosome 6 (Beck et al., 1992). Although RING3/BRD2 is localized in the vicinity of the MHC genes and its expression is elevated in lymphocytes from patients with leukemia and in leukemic cell lines, there has been no direct evidence reported suggesting a role in immune function (Denis and Green, 1996; Thorpe et al., 1996). However, a role for BRD2 in disease is suggested by the observation that overexpression of BRD2 in lymphoid cells in mouse resulted in splenic B-cell lymphoma and B-cell leukemia (Greenwald et al., 2004; Lenburg et al., 2007).
Florence and Faller (2008) recently reported that the function of the Drosophila Brd2 homolog Fs(1)h is modulated by Ras signaling; specifically, that fs(1)h is required for the regulation of the Ras targets tailless (tll) and huckebein (hkb) during early embryogenesis. Ras and BRD2 (RING3) were also shown to synergize in transactivating promoters of cell cycle regulatory genes (Denis et al., 2000). Our observation of decreased cell proliferation in Brd2 mutant cultured MEF cells is consistent with these findings. Further, cell cycle analysis of Brd2−/− MEFs revealed that the cell cycle of the mutant cells delayed at G1 phase. These data collectively support the notion that Brd2 activates cell cycle genes like cyclin D1, cyclin E1, and cyclin A2 (Denis et al., 2000; Sinha et al., 2005), which are involved in G1 entry, and that Brd2 is involved in Ras signaling (Florence and Faller, 2008), which intermediates signals from growth factor to cell division. In Drosophila, Fsh interacts with the homeobox genes ultrabithorax, trithorax, ash-1, and deformed to control segmentation. In our Brd2 mutant embryos, the global reduction of size and rapid degeneration obviated detailed analysis of the morphological ramifications on patterning of loss of Brd2 function; however, in the occasional embryos that survived to later stages, organogenesis in general appeared normal except for neural tube defects. It will be of interest in future studies to determine whether Hox gene expression is altered in Brd2-deficient embryos.
Human BRD2 has been shown to be the EJM1 gene, which is related to the expression of the syndrome of Juvenile Myoclonic Epilepsy (JME), an adolescent-onset epilepsy that is thought to be entirely genetic in origin. EJM1 was mapped to the region by linkage analysis using HLA and microsatellite markers (Durner et al., 1991; Greenberg et al., 1988; Greenberg et al., 2000; Sander et al., 1997; Weissbecker et al., 1991). The identity of EJM1 as BRD2 was determined by association analysis using Single Nucleotide Polymorphisms (SNPs) (Pal et al., 2003) and confirmed in two other studies (Cavalleri et al., 2007; Lorenz et al., 2006). Additional recent evidence from other investigators has suggested that BRD2 is an underlying cause not only of JME but also in sensitivity to photic stimulation (in which epilepsy-related electroencephalographic patterns manifest when lights are flashed at a subject) (Lorenz et al., 2006; Tauer et al., 2005). Although both the linkage and association studies have been replicated, to date no mutation has been found in the coding region of the BRD2 genes of JME patients (Pal et al., 2003). However, some polymorphisms were observed in the non-coding region (UTRs and introns) and in the promoter, polymorphisms that appear to associate with JME (Greenberg et al., 2000; Pal et al., 2003).
JME is one of the most common types of adolescent-onset epilepsy, accounting for about 10% of all epilepsies (Annegers, 1994). The mechanisms underlying the development of JME are not understood, but we favor the hypothesis that developmental abnormalities are, at least in part, involved. That is, aberrant development and differentiation during the formation of specific regions of the brain could enhance the subsequent susceptibility to the induction of seizures. In this light, it is of interest that in the present study we showed that Brd2 expression was highest in the brain during mid-gestation embryonic development and that a recent report also showed that the homologue in zebra fish is also highly expressed in the developing nervous system (Dibenedetto et al., 2008). Although the reduced size of the Brd2−/− mouse embryos was global in nature, in that all of the organs appeared to be also smaller, the most severe phenotype was observed in the brain and neural tissue. These findings reinforced the idea that BRD2 plays a critical role in brain development. Furthermore, the idea that dysregulation of Brd2 expression might lead to structural brain abnormalities during development that predispose to epilepsy in adolescence was first proposed by Pal et al (2003), and was supported by MRI results from Woermann et al (1999), and volumetric analysis by Tae et al (2006).
We also observed size heterogeneity among heterozygous Brd2+/− embryos and even wildtype embryos. Since Brd2 is abundantly expressed in the oocyte (Trousdale and Wolgemuth 2004), we speculate that a maternal effect could be involved. That is, in the heterozygous by heterozygous matings, even the wild type embryos are the product of a heterozygous Brd2+/− egg, in which Brd2 levels would likely be reduced by one half. The possibility of a maternal effect is intriguing in light of the observation that, in humans, JME may be maternally inherited. Linkage evidence (Greenberg et al., 2000) and segregation evidence (Pal et al., 2006) support maternal inheritance of JME, that is, JME-affected offspring inherit the disease-related BRD2 allele only from the mother. This observation is consistent with the finding that Drosophila fsh is a maternal effect gene (Gans et al., 1975, 1980).
In conclusion, we have shown in the present study the first in vivo functional analysis for Brd2, a member of the novel BET family of bromodomain-containing genes; demonstrating that it is indispensable for embryonic development. It was further demonstrated that Brd2 is highly expressed in the central neural system and that the lack of Brd2 expression causes deleterious deficits in the developing brain. In parallel with the finding that human BRD2 is associated with JME, these observations suggest that further research into BRD2’s in vivo function during brain development will provide insight into the etiology of seizure susceptibility and neuronal differentiation.
EXPERIMENTAL PROCEDURES
Generation of Brd2-null mice
We identified an embryonic stem (ES) cell line, RRE050, which contained an insertional mutation of the Brd2 gene in the database of mutant ES cell lines at Baygenomics (http://www.genetrap.org). The mutant cell line was requested and blastocyst injection was performed at the transgenic facility at the University of California Davis in order to generate chimeric mice. Ten chimeric mice were obtained and transferred to the animal facility at Columbia University Medical Center. Male chimeras were mated to C57Black/6J females to produce heterozygous offspring. Agouti pups and subsequent progeny were genotyped by PCR with primers that spanned the junction of the Brd2 gene and the inserted gene-trap vector. The primers were: EX1F, (exon 1 forward, in exon 1), 5’-AAAACGTGACTCCCCACAAG-3’; IN1R, (intron 1 reverse, in intron 1), 5’-GCTGCTCAGACGCAGTACCT-3’; and GT1R, (genetrap vector reverse, in gene-trap vector), 5’-TAGAATCGGAAGAGGCTGGA-3’.
Collection of embryos
Heterozygous Brd2+/− male and female mice were mated and the day of the appearance of vaginal plug was designated as embryonic day 0.5 (E0.5). Embryos were collected at specified time points and fixed in specific fixatives as noted below, depending upon the subsequent analysis to be performed. The genotype of the embryos was determined from DNA isolated from yolk sac.
Whole mount in situ hybridization
E8.5, E9.5 and E10.5 embryos were collected and fixed for 2–5 hours (depending on the different stages of the embryos) in freshly prepared 4% paraformaldehyde (PFA) in 0.1M sodium phosphate buffer, pH7.4, 0.15M NaCl (PBS) and then processed for whole-mount in situ hybridization according to protocols from Hogan et al. (Hogan et al., 1994). Digoxigenin-labeled anti-sense and sense RNA probes were synthesized with T7 and T3 RNA polymerases, respectively, using the Roche Diagnostics RNA labeling kit (www.roche-applied-science.com), according to the manufacturer’s instructions. Brd2 cDNA corresponding to nt 855 to 3180 (according to NM_010238) in pBluescript SK vector were linearized with BamHI and XhoI, respectively, and used as templates.
Briefly, fixed embryos were stored in 100% methanol at −20°C before in situ hybridization. The embryos were rehydrated in PBST (1x PBS, 0.1% Tween 20), washed in RIPA buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1mM EDTA, 50 mM Tris-Cl, pH7.6), post-fixed with 4% PFA, and then washed with PBST. They were pre-hybridized in 50% formamide, 5 X SSC, 20 mM sodium phosphate buffer, pH7.4, 0.1% Tween 20, 100 ug/ml yeast tRNA, 100 ug/ml ssDNA at 70° C for 3 hr and hybridized in the same solution plus probe overnight in a chamber humidified with 50% formamide in water at 70° C. The embryos were then washed 3 times and treated with blocking solution (10% sheep serum plus 3% BSA in PBS) for 2 hr, and then with 1:2000 dilution of anti-digoxygenin alkaline phosphatase-tagged antibody (Roche Diagnostic) and incubated overnight at 4° C. The samples were then washed in TBST (150 mM NaCl, 25 mM Tris-Cl, pH 7.6, 0.1% Tween 20) at 4° C overnight with gentle rocking, followed by washing 3 times in NTMT (100 mM NaCl, 100 mM Tris-HCl, pH 9.5, 50 mM MgCl2, 0.1% Tween-20). The washing solution was removed and the reaction mix (125 ug/ml BCIP and 259 ug/ml NBT in NTMT) was added and allowed to develop overnight at 4°C. Following staining, the embryos were post-fixed in 4% PFA and washed in PBS. The intact embryos were viewed and photographed under a Wild Heerbrugg photomicroscope with dark field.
Assessment of proliferation and cell cycle analysis in mouse embryonic fibroblasts
Mouse embryonic fibroblasts (MEFs) were isolated and cultured following a protocol described in Hogan et al. (Hogan et al., 1994). Briefly, E9.5, E10.5, and E11,5 embryos were minced after removing the head, incubated in 0.25% trypsin for 10 min at 37°C, and cultured in 6-well plates individually in Dulbecco’s Modification of Eagle’s Medium (DMEM) (MediaTech, www.cellgro.com) plus 10% fetal bovine serum (FBS). After expansion and genotyping, cells were plated into 48-well plates at a density of 104 cells per well in 200ul. For analysis of proliferation, the MEFs were harvested at selected time points by incubation in 0.25% trypsin for 20–30 min at 37° C and counted using a hemocytometer. For cell cycle analysis, MEFs were harvested after 2 days in culture, fixed with 70% cold ethanol and stored at −20°C. Cells were stained with prodidium iodide (100 ug/ml PI, 20 ug/ml RNase A, 0.3% Triton-X 100, 1 mg/ml sodium citrate) and analyzed with a BD FACSCalibur Cell Analyzer.
Assessment of cell death in the developing neural tube
E9.5 and E10.5 embryos were dissected and incubated for 30 min in 5uM Lysotracker Red (Invitrogen, L7528) in Hank’s balanced salt solution at 37 ° C, 5% CO2 in air. They were then washed 3 times 10 min each with PBS with 0.2% BSA with rocking, at room temperature. The embryos were then fixed overnight at 4° C, in the dark, in 4% paraformaldehyde in PBS and cleared with BABB (1:2 benzyl alcohol: benzyl benzoate) for better observation. Staining in the embryos was photographed with Nikon fluorescence microscope using 568 nm light source.
Placental rescue of Brd2-deficient embryos
Brd2+/− female mice at 6–8 weeks of age were super-ovulated with 5 IU PMSG (Pregnant mare’s serum gonadotrophin, Sigma-Aldrich, St. Louis, Missouri) and 5 IU hCG (human chorionic gonadotrophin, Sigma-Aldrich) at intervals of 48 hr. The females were mated individually to Brd2+/− males, and checked for the presence of a vaginal plug the following morning. Plugged females were sacrificed by cervical dislocation at 3.5 days after hCG injection for the collection of embryos at the morula or blastocyst stage. Embryos were subsequently cultured in KSOM+ AA medium (Specialty Media, Phillipsburg, NJ) in vitro at 37° C under 5% CO2 in air to the blastocyst stage. Zonae pellucidae of the blastocysts were removed with brief exposure to Tyrode’s saline acidified to pH 2.5. These denuded embryos were plated individually into a well covered with feeder cells in 96-well plates, and cultured with ES Derivation Medium (ESDM) (Bryja et al., 2006) at 37° C in 5% CO2 in humidified air for 4–5 days. Cell clumps originating from the blastocysts were trypsinized in 20µl of 0.025% Trypsin, 0.75mM EDTA (Specialty Media, Cat# SM-2004-C) for 5 min, and 200µl of ESDM was added to each well to stop the reaction. Cells were dispersed by pipetting up and down at least 20 times with a 200µl pipetteman, and the medium with cell suspension was transferred to a well in a 96-well plate with freshly seeded feeder cells. Cell colonies could be observed 2–3 days after the first trypsinization. Colony expansion of the ES cells proceeded from 96-well to 48-well to 6-well plates with feeder cells in ESDM, and then to gelatinized 25-cm2 flasks for routine culture in ES culture medium, with 15% fetal calf serum (FCS) and 1,000 IU/ml LIF. The established ES cell lines were cryopreserved or used for genotyping.
Embryos of B6D2F1 mice from The Jackson Laboratory (Bar Harbor, Maine) at the 2-cell stage were subjected to electrofusion for induction of tetraploidy (Nagy et al., 1993). Briefly, embryos were washed in 0.3M d-mannitol (Sigma-Aldrich) and 0.3% BSA (Sigma-Aldrich) for 20 seconds and transferred to a fusion chamber attached to an ECM2001 Electrocell Manipulator (BTX Inc., San Diego, CA). Two-cell embryos were aligned by alternating current (A.C.) and double direct current (D.C.); pulses of 1,000V/cm for 30µs were applied. Following application of the electric field, embryos were transferred to KSOM + AA media and cultured in the incubator. Blastomere fusion was checked at 2 hr after the application of the electric field; those fused embryos were moved to new KSOM+AA micro-drops covered with mineral oil, and the culture was continued under 5% CO2 at 37° C.
Genotyped ES cells (Brd2−/− or Brd2+/+) were injected into tetraploid blastocysts. ES cells were trypsinized, resuspended in normal ES medium without LIF (Bryja et al., 2006), and kept on ice. A flat tip microinjection pipette was used for ES cell injection. ES cells were picked up in the end of the injection pipette and 15–20 ES cells were injected into each blastocyst. The injection pipette was used to facilitate clumping of the ES cells close to the inner cell mass of the blastocyst. The injected blastocysts were kept in KSOM+AA until embryo transfer. Ten injected blastocysts were transferred into each uterine horn of 2.5-days-postcoitum pseudopregnant ICR females. Embryos from Brd2−/− ES cells were dissected at E12.5 and the wild type Brd2+/+ embryos were allowed to develop to term.
ACKNOWLEDGEMENTS
This work was supported in part by a grant from the NIH, NS27941 and DK31775 (DAG).
We thank Dr. Deb Pal for helpful discussion, Dr. Naiche Adler for advice on the Lysotracker methodologies, Ms Irene Tsui for performing the MEF cell culture, and Ms Shelby Sutton for technical assistance.
REFERENCES
- An FQ, Compitello N, Horwitz E, Sramkoski M, Knudsen ES, Renne R. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16 INK4A-induced cell cycle arrest. J Biol Chem. 2005;280:3862–3874. doi: 10.1074/jbc.M407435200. [DOI] [PubMed] [Google Scholar]
- Annegers JF. Epidemiology and genetics of epilepsy. Neurologic Clinics. 1994;12:15–29. [PubMed] [Google Scholar]
- Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, Berger SL. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell. 2001;8:1243–1254. doi: 10.1016/s1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- Beck S, Hanson I, Kelly A, Pappin DJ, Trowsdale J. A homologue of the Drosophila female sterile homeotic (fsh) gene in the class II region of the human MHC. DNA Seq. 1992;2:203–210. doi: 10.3109/10425179209020804. [DOI] [PubMed] [Google Scholar]
- BelAiba RS, Baril P, Chebloune Y, Tabone E, Boukerche H. Identification and cloning of an 85-kDa protein homologous to RING3 that is upregulated in proliferating endothelial cells. Eur J Biochem. 2001;268:4398–4407. doi: 10.1046/j.1432-1327.2001.02355.x. [DOI] [PubMed] [Google Scholar]
- Bryja V, Bonilla S, Cajanek L, Parish CL, Schwartz CM, Luo Y, Rao MS, Arenas E. An efficient method for the derivation of mouse embryonic stem cells. Stem Cells. 2006;24:844–849. doi: 10.1634/stemcells.2005-0444. Epub 2005 Dec 2009. [DOI] [PubMed] [Google Scholar]
- Cavalleri GL, Walley NM, Soranzo N, Mulley J, Doherty CP, Kapoor A, Depondt C, Lynch JM, Scheffer IE, Heils A, Gehrmann A, Kinirons P, Gandhi S, Satishchandra P, Wood NW, Anand A, Sander T, Berkovic SF, Delanty N, Goldstein DB, Sisodiya SM. A multicenter study of BRD2 as a risk factor for juvenile myoclonic epilepsy. Epilepsia. 2007;48:706–712. doi: 10.1111/j.1528-1167.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- Chang YL, King B, Lin SC, Kennison JA, Huang DH. A double-bromodomain protein, FSH-S, activates the homeotic gene ultrabithorax through a critical promoter-proximal region. Mol Cell Biol. 2007;27:5486–5498. doi: 10.1128/MCB.00692-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T, Brunori M, Rhee K, Wang X, Wolgemuth DJ. Change in nuclear-cytoplasmic localization of a double-bromodomain protein during proliferation and differentiation of mouse spinal cord and dorsal root ganglia. Brain Res Dev Brain Res. 2004;149:93–101. doi: 10.1016/j.devbrainres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Denis GV, Green MR. A novel, mitogen-activated nuclear kinase is related to a Drosophila developmental regulator. Genes Dev. 1996;10:261–271. doi: 10.1101/gad.10.3.261. [DOI] [PubMed] [Google Scholar]
- Denis GV, McComb ME, Faller DV, Sinha A, Romesser PB, Costello CE. Identification of Transcription Complexes that Contain the Double Bromodomain Protein Brd2 and Chromatin Remodeling Machines. J Proteome Res. 2006;5:502–511. doi: 10.1021/pr050430u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis GV, Vaziri C, Guo N, Faller DV. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Differ. 2000;11:417–424. [PMC free article] [PubMed] [Google Scholar]
- Dibenedetto AJ, Guinto JB, Ebert TD, Bee KJ, Schmidt MM, Jackman TR. Zebrafish brd2a and brd2b are paralogous members of the bromodomain-ET (BET) family of transcriptional coregulators and show structural and expression divergence. BMC Dev Biol. 2008;8:39. doi: 10.1186/1471-213X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digan ME, Haynes SR, Mozer BA, Dawid IB, Forquignon F, Gans M. Genetic and molecular analysis of fs(1)h, a maternal effect homeotic gene in Drosophila. Dev Biol. 1986;114:161–169. doi: 10.1016/0012-1606(86)90392-1. [DOI] [PubMed] [Google Scholar]
- Dunty WC, Jr, Zucker RM, Sulik KK. Hindbrain and cranial nerve dysmorphogenesis result from acute maternal ethanol administration. Dev Neurosci. 2002;24:328–342. doi: 10.1159/000066748. [DOI] [PubMed] [Google Scholar]
- Durner M, Sander T, Greenberg DA, Johnson K, Beck-Mannagetta G, Janz D. Localization of idiopathic generalized epilepsy on chromosome 6p in families of juvenile myoclonic epilepsy patients. Neurology. 1991;41:1651–1655. doi: 10.1212/wnl.41.10.1651. [DOI] [PubMed] [Google Scholar]
- Florence B, McGinnis W. A genetic screen of the Drosophila X chromosome for mutations that modify Deformed function. Genetics. 1998;150:1497–1511. doi: 10.1093/genetics/150.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence B, Faller DV. You bet-cha: a novel family of transcriptional regulators. Front Biosci. 2001;6:D1008–D1018. doi: 10.2741/florence. [DOI] [PubMed] [Google Scholar]
- Florence B, Faller DV. Drosophila female sterile (1) homeotic is a multifunctional transcriptional regulator that is modulated by Ras signaling. Dev Dyn. 2008;237:554–564. doi: 10.1002/dvdy.21432. [DOI] [PubMed] [Google Scholar]
- Gans M, Audit C, Masson M. Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster. Genetics. 1975;81:683–704. doi: 10.1093/genetics/81.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans M, Forquignon F, Masson M. The role of dosage of the region 7D1-7D5-6 of the X chromosome in the production of homeotic transformations in Drosophila melanogaster. Genetics. 1980;96:887–902. doi: 10.1093/genetics/96.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Delgado-Escueta AV, Widelitz H, Sparkes RS, Treiman L, Maldonado HM, Park MS, Terasaki PI. Juvenile myoclonic epilepsy (JME) may be linked to the BF and HLA loci on human chromosome 6. Am J Med Genet. 1988;31:185–192. doi: 10.1002/ajmg.1320310125. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Durner M, Keddache M, Shinnar S, Resor SR, Moshe SL, Rosenbaum D, Cohen J, Harden C, Kang H, Wallace S, Luciano D, Ballaban-Gil K, Tomasini L, Zhou G, Klotz I, Dicker E. Reproducibility and complications in gene searches: linkage on chromosome 6, heterogeneity, association and maternal inheritance in juvenile myoclonic epilepsy. American Journal of Human Genetics. 2000;66:508–516. doi: 10.1086/302763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Tumang JR, Sinha A, Currier N, Cardiff RD, Rothstein TL, Faller DV, Denis GV. E mu-BRD2 transgenic mice develop B-cell lymphoma and leukemia. Blood. 2004;103:1475–1484. doi: 10.1182/blood-2003-06-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Faller DV, Denis GV. Activation-induced nuclear translocation of RING3. J Cell Sci. 2000;113:3085–3091. doi: 10.1242/jcs.113.17.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes SR, Mozer BA, Bhatia-Dey N, Dawid IB. The Drosophila fsh locus, a maternal effect homeotic gene, encodes apparent membrane proteins. Dev Biol. 1989;134:246–257. doi: 10.1016/0012-1606(89)90094-8. [DOI] [PubMed] [Google Scholar]
- Haynes SR, Dollard C, Winston F, Beck S, Trowsdale J, Dawid IB. The bromodomain: a conserved sequence found in human, Drosophila and yeast proteins. Nucleic Acids Res. 2603;20 doi: 10.1093/nar/20.10.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. A laboratory manual. Plainview: Cold Spring Harbor Laboratory Press; 1994. Manipulating the Mouse Embryo; p. 497. [Google Scholar]
- Houzelstein D, Bullock SL, Lynch DE, Grigorieva EF, Wilson VA, Beddington RS. Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol Cell Biol. 2002;22:3794–3802. doi: 10.1128/MCB.22.11.3794-3802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Shen W, Wang X, Wu J, Wu J, Shi Y. Solution structure of the second bromodomain of Brd2 and its specific interaction with acetylated histone tails. BMC Struct Biol. 2007;7:57. doi: 10.1186/1472-6807-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob AL, Lund J, Martinez P, Hedin L. Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. J Biol Chem. 2001;276:37659–37664. doi: 10.1074/jbc.M104427200. [DOI] [PubMed] [Google Scholar]
- Jeanmougin F, Wurtz JM, Le Douarin B, Chambon P, Losson R. The bromodomain revisited. Trends Biochem Sci. 1997;22:151–153. doi: 10.1016/s0968-0004(97)01042-6. [DOI] [PubMed] [Google Scholar]
- Kanno T, Kanno Y, Siegel RM, Jang MK, Lenardo MJ, Ozato K. Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol Cell. 2004;13:33–43. doi: 10.1016/s1097-2765(03)00482-9. [DOI] [PubMed] [Google Scholar]
- Lenburg ME, Sinha A, Faller DV, Denis GV. Tumor-specific and proliferation-specific gene expression typifies murine transgenic B cell lymphomagenesis. J Biol Chem. 2007;282:4803–4811. doi: 10.1074/jbc.M605870200. Epub 2006 Dec 4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell. 2008;30:51–60. doi: 10.1016/j.molcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz S, Taylor KP, Gehrmann A, Becker T, Muhle H, Gresch M, Tauer U, Sander T, Stephani U. Association of BRD2 polymorphisms with photoparoxysmal response. Neurosci Lett. 2006;400:135–139. doi: 10.1016/j.neulet.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Mattsson K, Kiss C, Platt GM, Simpson GR, Kashuba E, Klein G, Schulz TF, Szekely L. Latent nuclear antigen of Kaposi's sarcoma herpesvirus/human herpesvirus-8 induces and relocates RING3 to nuclear heterochromatin regions. J Gen Virol. 2002;83:179–188. doi: 10.1099/0022-1317-83-1-179. [DOI] [PubMed] [Google Scholar]
- Mujtaba S, He Y, Zeng L, Yan S, Plotnikova O, Sachchidanand, Sanchez R, Zeleznik-Le NJ, Ronai Z, Zhou MM. Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol Cell. 2004;13:251–263. doi: 10.1016/s1097-2765(03)00528-8. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC, Nagy PD, Bujarski JJ. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells targeting the site of RNA-RNA recombination in brome mosaic virus with antisense sequences. Proc Natl Acad Sci U S A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Umehara T, Nakano K, Jang MK, Shirouzu M, Morita S, Uda-Tochio H, Hamana H, Terada T, Adachi N, Matsumoto T, Tanaka A, Horikoshi M, Ozato K, Padmanabhan B, Yokoyama S. Crystal structure of the human BRD2 bromodomain: insights into dimerization and recognition of acetylated histone H4. J Biol Chem. 2007;282:4193–4201. doi: 10.1074/jbc.M605971200. [DOI] [PubMed] [Google Scholar]
- Pal DK, Durner M, Klotz I, Dicker E, Shinnar S, Resor S, Cohen J, Harden C, Moshe SL, Ballaban-Gill K, Bromfield EB, Greenberg DA. Complex inheritance and parent-of-origin effect in juvenile myoclonic epilepsy. Brain Dev. 2006;28:92–98. doi: 10.1016/j.braindev.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal DK, Evgrafov OV, Tabares P, Zhang F, Durner M, Greenberg DA. BRD2 (RING3) is a probable major susceptibility gene for common juvenile myoclonic epilepsy. Am J Hum Genet. 2003;73:261–270. doi: 10.1086/377006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou VE, Behringer R. Mouse phenotypes : a handbook of mutation analysis. Cold Spring Harbor. N.Y: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- Platt GM, Simpson GR, Mittnacht S, Schulz TF. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J Virol. 1999;73:9789–9795. doi: 10.1128/jvi.73.12.9789-9795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya A, Naguibneva I, Duquet A, Bengal E, Robin P, Harel-Bellan A. Interaction between acetylated MyoD and the bromodomain of CBP and/or p300. Mol Cell Biol. 2001;21:5312–5320. doi: 10.1128/MCB.21.16.5312-5320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee K, Brunori M, Besset V, Trousdale R, Wolgemuth DJ. Expression and potential role of Fsrg1, a murine bromodomain-containing homologue of the Drosophila gene female sterile homeotic. J Cell Sci. 1998;111:3541–3550. doi: 10.1242/jcs.111.23.3541. [DOI] [PubMed] [Google Scholar]
- Sander T, Bockenkamp B, Hildmann T, Blasczyk R, Kretz R, Wienker TF, Volz A, Schmitz B, Beck-Mannagetta G, Riess O, Epplen JT, Janz D, Ziegler A. Refined mapping of the epilepsy susceptibility locus EJM1 on chromosome 6. Neurology. 1997;49:842–847. doi: 10.1212/wnl.49.3.842. [DOI] [PubMed] [Google Scholar]
- Sano Y, Ishii S. Increased affinity of c-Myb for CREB-binding protein (CBP) after CBP-induced acetylation. J Biol Chem. 2001;276:3674–3682. doi: 10.1074/jbc.M006896200. [DOI] [PubMed] [Google Scholar]
- Shang E, Nickerson HD, Wen D, Wang X, Wolgemuth DJ. The first bromodomain of Brdt, a testis-specific member of the BET sub-family of double-bromodomain-containing proteins, is essential for male germ cell differentiation. Development. 2007;134:3507–3515. doi: 10.1242/dev.004481. Epub 2007 Aug 3529. [DOI] [PubMed] [Google Scholar]
- Shang E, Salazar G, Crowley TE, Wang X, Lopez RA, Wang X, Wolgemuth DJ. Identification of unique, differentiation stage-specific patterns of expression of the bromodomain-containing genes Brd2, Brd3, Brd4, and Brdt in the mouse testis. Gene Expr Patterns. 2004;4:513–519. doi: 10.1016/j.modgep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Sinha A, Faller DV, Denis GV. Bromodomain analysis of Brd2-dependent transcriptional activation of cyclin A. Biochem J. 2005;387:257–269. doi: 10.1042/BJ20041793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae WS, Hong SB, Joo EY, Han SJ, Cho JW, Seo DW, Lee JM, Kim IY, Byun HS, Kim SI. Structural brain abnormalities in juvenile myoclonic epilepsy patients. Korean J Radiol. 2006;7:162–172. doi: 10.3348/kjr.2006.7.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, Kaufman TC, Kennison JA. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992;68:561–572. doi: 10.1016/0092-8674(92)90191-e. [DOI] [PubMed] [Google Scholar]
- Tauer U, Lorenz S, Lenzen KP, Heils A, Muhle H, Gresch M, Neubauer BA, Waltz S, Rudolf G, Mattheisen M, Strauch K, Nurnberg P, Schmitz B, Stephani U, Sander T. Genetic dissection of photosensitivity and its relation to idiopathic generalized epilepsy. Ann Neurol. 2005;57:866–873. doi: 10.1002/ana.20500. [DOI] [PubMed] [Google Scholar]
- Thorpe KL, Abdulla S, Kaufman J, Trowsdale J, Beck S. Phylogeny and structure of the RING3 gene. Immunogenetics. 1996;44:391–396. doi: 10.1007/BF02602785. [DOI] [PubMed] [Google Scholar]
- Trousdale RK, Wolgemuth DJ. Bromodomain containing 2 (Brd2) is expressed in distinct patterns during ovarian folliculogenesis independent of FSH or GDF9 action. Mol Reprod Dev. 2004;68:261–268. doi: 10.1002/mrd.20059. [DOI] [PubMed] [Google Scholar]
- Umehara T, Wakamori M, Tanaka A, Padmanabhan B, Yokoyama S. Purification, crystallization and preliminary X-ray diffraction of the C-terminal bromodomain from human BRD2. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63:613–615. doi: 10.1107/S1744309107028473. Epub 2007 Jun 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viejo-Borbolla A, Ottinger M, Bruning E, Burger A, Konig R, Kati E, Sheldon JA, Schulz TF. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi's Sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J Virol. 2005;79:13618–13629. doi: 10.1128/JVI.79.21.13618-13629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, Yuen PW. Development and therapeutic potential of calpain inhibitors. Adv Pharmacol. 1997;37:117–152. doi: 10.1016/s1054-3589(08)60949-7. [DOI] [PubMed] [Google Scholar]
- Wang S, Dibenedetto AJ, Pittman RN. Genes induced in programmed cell death of neuronal PC12 cells and developing sympathetic neurons in vivo. Dev Biol. 1997;188:322–336. doi: 10.1006/dbio.1997.8655. [DOI] [PubMed] [Google Scholar]
- Weissbecker KA, Durner M, Janz D, Scaramelli A, Sparkes RS, Spence MA. Confirmation of linkage between juvenile myoclonic epilepsy locus and the HLA region of chromosome 6. Am J Med Genet. 1991;38:32–36. doi: 10.1002/ajmg.1320380109. [DOI] [PubMed] [Google Scholar]
- Winston F, Allis CD. The bromodomain: a chromatin-targeting module? Nat Struct Biol. 1999;6:601–604. doi: 10.1038/10640. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122:2101–2108. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- Yang XJ. Lysine acetylation and the bromodomain: a new partnership for signaling. Bioessays. 2004;26:1076–1087. doi: 10.1002/bies.20104. [DOI] [PubMed] [Google Scholar]
- Zeng L, Zhou MM. Bromodomain: an acetyl-lysine binding domain. FEBS Lett. 2002;513:124–128. doi: 10.1016/s0014-5793(01)03309-9. [DOI] [PubMed] [Google Scholar]