Abstract
Introduction
Subarachnoidal hemorrhage (SAH) occurring after aneurismal rupture produces an inflammatory response in the cerebral circulation. Tumor necrosis factor (TNF)-α is a major cytokine in this process. Adhesion molecules provide information on inflammatory reactions taking place in the walls of blood vessels. Clinical evidence suggests a role of soluble intercellular adhesion molecule (sICAM)-1 in early hemorrhagic events. This study aimed to evaluate the implementation of early TNF-α and sICAM-1 serum measurement for the prognosis of patient outcome after intracranial aneurismal rupture.
Materials and Methods
The study consisted of 27 patients with a diagnosis of intracranial aneurysm. SAH was evaluated on admission according to the Fisher scale, patients-consciousness with the Glasgow Coma Scale, clinical grading with the Hunt and Hess scale, and clinical outcome with the Glasgow Outcome Scale (GOS). Blood samples were drawn within 72 h after arrival at the emergency room. Serum concentrations of TNF-α and sICAM-1 were assayed with the ELISA method.
Results
The initial serum TNF-α concentration in the aneurismal patients was low and did not correlate with radiological and clinical scores. The serum sICAM-1 level positively correlated with the severity of bleeding assessed by the Fisher scale and negatively with the patient’s scoring in the GOS.
Conclusions
This study demonstrated the absence of a systemic TNF-α-mediated inflammatory response at the onset of subarachnoid hemorrhage. Early measurement of serum sICAM-1 levels offers a potential prognostic value in the assessment of patients-outcome after brain aneurismal rupture.
Keywords: TNF-α, sICAM-1, brain, aneurismal rupture
Introduction
Intracranial aneurysms are often defined as weakened, abnormally dilated walls of the major arteries (Selman et al. 2000). The aneurysm is usually asymptomatic for a long time until rupture (Raps et al. 1993). A consequence of an aneurismal wall burst is blood leakage into the cerebral structures, which may lead to irreversible brain damage and death (Inagawa et al. 1995). The incidence of intracranial aneurismal in the general population is estimated to be about 4–6% (Rinkel et al. 1998). Inherited syndromes, traumatic or infectious diseases, and behavioral influences have been specified among the risk factors (De Braekeleer et al. 1996; Wardlaw and White 2000). Subjects of female gender, aged over 50, hypertensive, hypercholesterolemic, or with a current smoking history are prone to develop brain aneurysms (Wardlaw and White 2000). Coffee consumption may increase the incidence of aneurismal subarachnoidal hemorrhage (SAH) (Isaksen et al. 2002). The potential influence of alcohol consumption is under investigation (Wardlaw and White 2000).
SAH occurring after aneurismal rupture produces an inflammatory response in the cerebral circulation mediated by proinflammatory cytokines (Fassbender et al. 2001; Takizawa et al. 2001). Tumor necrosis factor (TNF)-α is one of the key immune activators of leukocytes counteracting the underlying pathological condition. Stimulated by cytokines, the inflammatory process may promote the development of cerebral vasospasm and ischemia, which negatively affect a patient’s outcome after SAH (Dumont et al. 2003; Hirashima et al. 1997).
Adhesion molecules provide information on inflammatory reactions which occur in the walls of blood vessels (Witkowska 2005). Soluble intercellular adhesion molecule (sICAM)-1 is a circulating form of ICAM-1, a molecule that is crucial for leukocyte adhesion to endothelial cells in the process of leukocyte migration through the vessel’s wall. Clinical evidence suggests that sICAM-1 may be engaged in the process of cerebral vasospasm (Mocco et al. 2002), and late elevated levels of serum sICAM-1 were predictors of poor outcome after aneurismal SAH (Mack et al. 2002).
Inflammatory cytokines and adhesion molecules may contribute to complications after intracranial hemorrhage, with dramatic consequences for the patient’s survival (Dhar and Diringer 2008; Dumont et al. 2003). Hence this study aimed to evaluate the relationships between early TNF-α and sICAM-1 serum levels and prognosis after aneurismal rupture.
Materials and Methods
The protocol of the study was approved by the local bioethics committee. The participants were 27 patients with a diagnosis of ruptured intracranial aneurysm admitted to the Department of Neurosurgery for life-saving surgery. Their detailed data are shown in Table 1. Seventeen healthy subjects of both genders, aged between 38–71 years (mean age: 55 years), took part in the examinations as the control group.
Table 1.
Patients’ data
| Sex (male/female) | 14/13 |
| Age (years) — mean (range) | 56 (33–77) |
| Deaths/survivals after aneurysm rupture | 5/22 |
| Body mass index — mean (range) | 28 (21–42) |
| Cigarette smokers/nonsmokers/data not available | 8/9/10 |
| Alcohol drinkers (spirits ≥3 times/week)/occasional drinkers (spirits <3 times/week) or abstainers/data not available | 0/17/9 |
| Coffee drinkers (coffee consumption ≥3 times/week)/occasional coffee drinkers (<3 times/week) or nondrinkers/data not available | 9/8/10 |
SAH was evaluated on admission using computed tomography and graded according to the Fisher scale (Fisher et al. 1980). The patients’ consciousness was graded with the Glasgow Coma Scale (GCS) (Teasdale and Jennett 1974). Clinical grading was performed with the Hunt and Hess scale (Hunt and Hess 1968). Grading with the Fisher scale, GCS, and Hunt and Hess scale was available for 24 patients (the other 3 patients died before clinical assessment). Clinical outcome of the patients was assessed according to the Glasgow Outcome Scale (GOS) (Jennett and Bond 1975).
Venous blood samples were drawn within 72 h after arrival at the emergency room. Blood was collected into Vacutainer System test tubes containing a clotting activator (Becton Dickinson, France). The samples were allowed to clot for 30 min. After 10-minute centrifugation at an relative centrifugal force of 1000 g, the serum was pipetted into Eppendorf test tubes and kept frozen at −20 C. The serum samples were thawed and assayed for TNF-α and sICAM-1 concentrations with commercially available sandwich ELISA (Bender MedSystems Diagnostics GmbH, Vienna, Austria) according to the manufacturer’s directions.
Statistical calculations were based on parametric tests. Group comparisons were performed using Student’s t-test. Correlation of radiological and clinical grading with the serum TNF-α and sICAM-1 concentrations was made with Pearson’s test. A probability level of 5% was considered statistically significant. Statistica 6.1 software (StatSoft, Inc.) was implemented for data analysis.
Results
The patients’ clinical presentation and radiological assessment by computed tomography are shown in Table 2. On admission, 67% of the subjects showed signs of cerebral bleeding of different degrees (grades 2–4 on the Fisher scale). The patients’ grading according to the GCS was between 11 and 15. Seventy-four percent of the patients presented with grades 0–1 on the Hunt and Hess scale. The patients’ rating on the GOS was between 3 and 5 for 81% of the subjects. The death rate was about 19% (5 patients died during the hospitalization).
Table 2.
Patients’ radiological and clinical grading
| Grade | Fisher scale n (%) | GCS n (%) | Hunt and Hess scale n (%) | GOS n (%) |
|---|---|---|---|---|
| 0 | – | – | 6 (22.2) | – |
| 1 | 6 (22.2) | 0 | 14 (51.9) | 5 (18.5) |
| 2 | 8 (29.6) | 0 | 0 | 0 |
| 3 | 5 (18.5) | 0 | 2 (7.4) | 2 (7.4) |
| 4 | 5 (18.5) | 0 | 2 (7.4) | 7 (25.9) |
| 5 | – | 0 | 0 | 13 (48.1) |
| 6 | – | 0 | – | – |
| 7 | – | 0 | – | – |
| 8 | – | 0 | – | – |
| 9 | – | 0 | – | – |
| 10 | – | 0 | – | – |
| 11 | – | 1 (3.7) | – | – |
| 12 | – | 0 | – | – |
| 13 | – | 4 (14.8) | – | – |
| 14 | – | 1 (3.7) | – | – |
| 15 | – | 18 (66.7) | – | – |
n – number of patients.
Comparisons of the parameters tested in the patients with intracranial aneurysms vs. controls are shown in Table 3. No differences in the mean serum TNF-α and sICAM-1 concentrations of the aneurismal subjects and controls were observed.
Table 3.
Serum TNF-α and sICAM-1 concentrations (mean±SD) in patients with intracranial aneurysms vs. controls
| Intracranial aneurysms (n=27) | Controls (n=17) | p-value | |
|---|---|---|---|
| TNF-α (pg/ml) | 12.42±9.70 | 11.29±8.80 | NS |
| sICAM-1 (ng/ml) | 255±90 | 225±50 | NS |
NS — not significant.
The serum TNF-α concentration did not correlate with the radiological and clinical scores (Table 4). The concentration of sICAM-1 positively correlated with the Fisher grade (r=0.439) and negatively with the GOS (r= −0.423).
Table 4.
Serum TNF-α and sICAM-1 concentrations in relation to radiological and clinical scores
| Grade | Fisher scale n=24 | GCS n=24 | Hunt and Hess scale n=24 | GOS n=27 |
|---|---|---|---|---|
| TNF-α | r =−0.055 | r =0.143 | r =−0.042 | r =0.077 |
| p=0.799 | p=0.504 | p=0.846 | p=0.722 | |
| sICAM-1 | r =0.439 | r =−0.191 | r =0.376 | r=−0.423 |
| p=0.032 | p=0.372 | p=0.071 | p=0.039 |
n — number of patients.
Bold letters represent statistical significance.
Serum sICAM-1 concentration in relation to the Fisher and the GOS grading is shown in Fig. 1. A gradually increasing sICAM-1 concentration in relation to the Fisher score was observed. The patients who scored 4 had significantly elevated serum sICAM-1 levels compared with the controls. The sICAM-1 concentration negatively correlated with the scores obtained from the GOS. The patients who were graded 4 and 5 had low serum sICAM-1 levels, comparable to those of the controls. The patients who scored 1 or 3 had significantly higher sICAM-1 levels than the controls and the patients who scored 5.
Fig. 1.
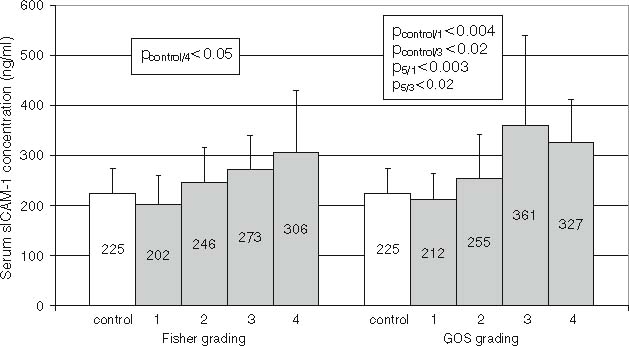
Serum sICAM-1 concentration (means±SD) according to the Fisher scale and the Glasgow Outcome Scale (GOS).
Discussion
SAH is a life-threatening consequence of an intracranial aneurysm’s rupture, which may be lethal for nearly 50% of patients (Inagawa et al. 1995). Disruption of a cerebral artery causes blood extravasations which may induce cytokine-stimulated immune reactions contributing to the development of cerebral vasospasm and ischemia. Systemic inflammation develops in a few days after hemorrhage. TNF-α is a major cytokine in the initiation of inflammatory reactions. Significantly increased TNF-α levels in the cerebrospinal fluid were observed 4–10 days after SAH (Mathiesen et al. 1997). In the present study, early TNF-α levels were studied. Blood samples were taken from the patients within 72 h after admittance to the hospital. The data analysis showed similar TNF-α levels in the aneurysmal patients and controls. No correlation of TNF-α levels with the radiological and clinical scores was observed. In many pathological conditions, TNF-α stimulates an inflammatory response. The low TNF-α levels observed in the sera of the aneurismal patients within three days of hospitalization suggest the absence of systemic inflammation during the early hemorrhagic events.
Leukocyte penetration through the blood-brain barrier into the subarachnoidal space is facilitated by adhesion molecules on the surface of endothelial cells. sICAM-1, a released form of the membrane molecule, is present in cerebrospinal fluid and blood. Several studies demonstrated an increase in serum sICAM-1 after aneurismal rupture (Frijns et al. 2006; Mack et al. 2002; Nissen et al. 2001). Interesting observations concerning patients’ outcome after SAH were made by Mack et al. (Mack et al. 2002). They observed that sICAM-1 levels were elevated in early hemorrhagic events and that late high sICAM-1 levels were predictors of critical outcome. Several other studies did not find significant associations between patient outcome and serum sICAM-1 levels measured at the onset of SAH (Frijns et al. 2006; Nissen et al. 2001). Contrary to these findings, the results obtained in the present study show associations of early serum sICAM-1 levels with patient outcome after SAH. The serum sICAM-1 concentration correlated with the scores obtained in the Fisher and the GOS. The former results demonstrate a clear connection between serum sICAM-1 concentration and the intensity of bleeding. At the same time, high sICAM-1 had a predictive value of poor outcome. The patients who were graded low in the GOS (1 or 3 points) had significantly elevated sICAM-1 titers compared with the healthy controls and the patients with the best scores.
This study demonstrates the absence of a systemic inflammatory TNF-α-mediated response at the onset of subarachnoid hemorrhage. Early measurement of serum sICAM-1 levels offers a prognostic value in the assessment of patient outcome after brain aneurismal rupture.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- SAH
subarachnoidal hemorrhage
- sICAM-1
soluble intercellular adhesion molecule-1
- TNF-α
tumor necrosis factor α
- GCS
Glasgow Coma Scale
- GOS
Glasgow Outcome Scale
References
- De Braekeleer M, Perusse L, Cantin L et al (1996) A study of inbreeding and kinship in intracranial aneurysms in the Saguenay Lac-Saint-Jean region (Quebec, Canada). Ann Hum Genet 60: 99-104 [DOI] [PubMed]
- Dhar R, Diringer MN (2008) The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care 8: 404-412 [DOI] [PMC free article] [PubMed]
- Dumont AS, Dumont RJ, Chow MM et al (2003) Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery 53: 123-133 [DOI] [PubMed]
- Fassbender K, Hodapp B, Rossol S et al (2001) Inflammatory cytokines in subarachnoid hemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry 70: 534-537 [DOI] [PMC free article] [PubMed]
- Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6: 1-9 [DOI] [PubMed]
- Frijns CJ, Fijnheer R, Algra A et al (2006) Early circulating levels of endothelial cell activation markers in aneurismal sub- arachnoid haemorrhage: associations with cerebral ischaemic events and outcome. J Neurol Neurosurg Psychiatry 77: 77-83 [DOI] [PMC free article] [PubMed]
- Hirashima Y, Nakamura S, Endo S et al (1997) Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with sub- arachnoid hemorrhage. Neurochem Res 22: 1249-1255 [DOI] [PubMed]
- Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28: 14-50 [DOI] [PubMed]
- Inagawa T, Tokuda Y, Ohbayashi N et al (1995) Study of aneurismal subarachnoid hemorrhage in Izumo City, Japan. Stroke 26: 761-766 [DOI] [PubMed]
- Isaksen J, Egge A, Waterloo K et al (2002) Risk factors for aneurysmal subarachnoid haemorrhage: the Tromsø study. J Neurol Neurosurg Psychiatry 73: 185-187 [DOI] [PMC free article] [PubMed]
- Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1: 480-484 [DOI] [PubMed]
- Mack WJ, Mocco J, Hoh DJ et al (2002) Outcome prediction with serum intercellular adhesion molecule-1 levels after aneurismal subarachnoid hemorrhage. J Neurosurg 96: 71-75 [DOI] [PubMed]
- Mathiesen T, Edner G, Ulfarsson E et al (1997) Cerebrospinal fluid interleukin-1 receptor antagonist and TNF-alpha following subarachnoid hemorrhage. J Neurosurg 87: 215-220 [DOI] [PubMed]
- Mocco J, Mack WJ, Kim GH et al (2002) Ride in serum soluble intercellular adhesion molecule-1 levels with vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 97: 537-541 [DOI] [PubMed]
- Nissen JJ, Mantle D, Gregson B et al (2001) Serum concentration of adhesion molecules in patients with delayed ischaemic neurological deficit after aneurismal subarachnoid haemorrhage: the immunoglobulin and selectin superfamilies. J Neurol Neurosurg Psychiatry 71: 329-333 [DOI] [PMC free article] [PubMed]
- Raps EC, Rogers JD, Galetta SL et al (1993) The clinical spectrum of unruptured intracranial aneurysms. Arch Neurol 50: 265-268 [DOI] [PubMed]
- Rinkel GJ, Djibuti M, Algra A et al (1998) Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 29: 251-256 [DOI] [PubMed]
- Selman WR, Tarr RW, Ratcheson RA (2000) Intracranial aneurysms and subarachnoid hemorrhage. In: Bradley WG et al (eds): Neurology in clinical practice. Butterworth- Heinemann, Boston, pp 1189-1190
- Takizawa T, Tada T, Kitazawa K et al (2001) Inflammatory cytokine cascade released by leukocytes in cerebrospinal fluid after subarachnoid hemorrhage. Neurol Res 23: 724-730 [DOI] [PubMed]
- Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2: 81-84 [DOI] [PubMed]
- Wardlaw JM, White PM (2000) The detection and management of unruptured intracranial aneurysms. Brain 123: 205-221 [DOI] [PubMed]
- Witkowska AM (2005) Soluble ICAM-1: a marker of vascular inflammation and lifestyle. Cytokine 31: 127-134 [DOI] [PubMed]


