Abstract
Randall, Charles C. (University of Mississippi School of Medicine, Jackson), Lanelle G. Gafford, Robert W. Darlington, and James M. Hyde. Composition of fowlpox virus and inclusion matrix. J. Bacteriol. 87:939–944. 1964.—Inclusion bodies of fowlpox virus infection are especially favorable starting material for the isolation of virus and inclusion matrix. Electron micrographs of viral particles and matrix indicated a high degree of purification. Density-gradient centrifugation of virus in cesium chloride and potassium tartrate was unsatisfactory because of inactivation, and clumping or disintegration. Chemical analyses of virus and matrix revealed significant amounts of lipid, protein, and deoxyribonucleic acid, but no ribonucleic acid or carbohydrate. Approximately 47% of the weight of the virus and 83% of the matrix were extractable in chloroform-methanol. The lipid partitions of the petroleum ether extracts were similar, except that the phospholipid content of the matrix was 2.2 times that of the virus. Viral particles were sensitive to diethyl ether and chloroform.
Full text
PDF
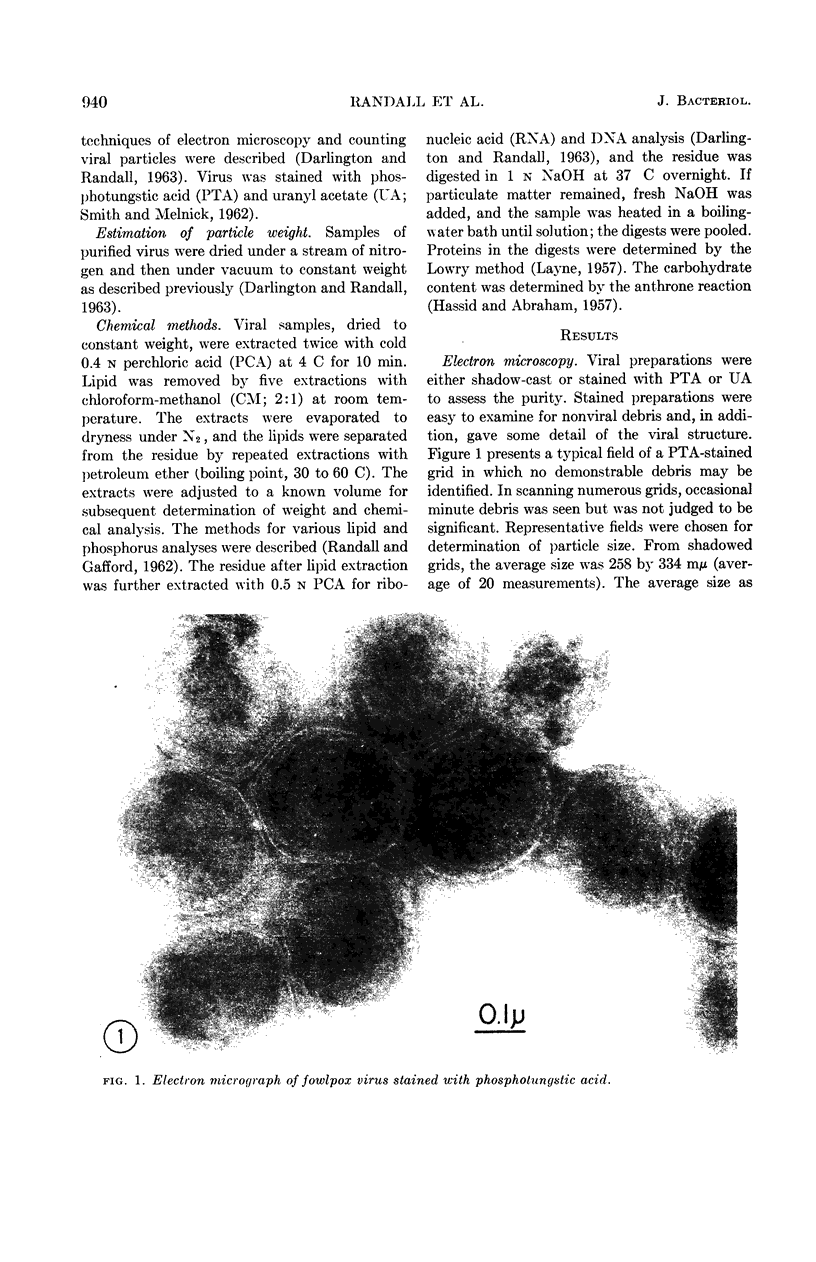
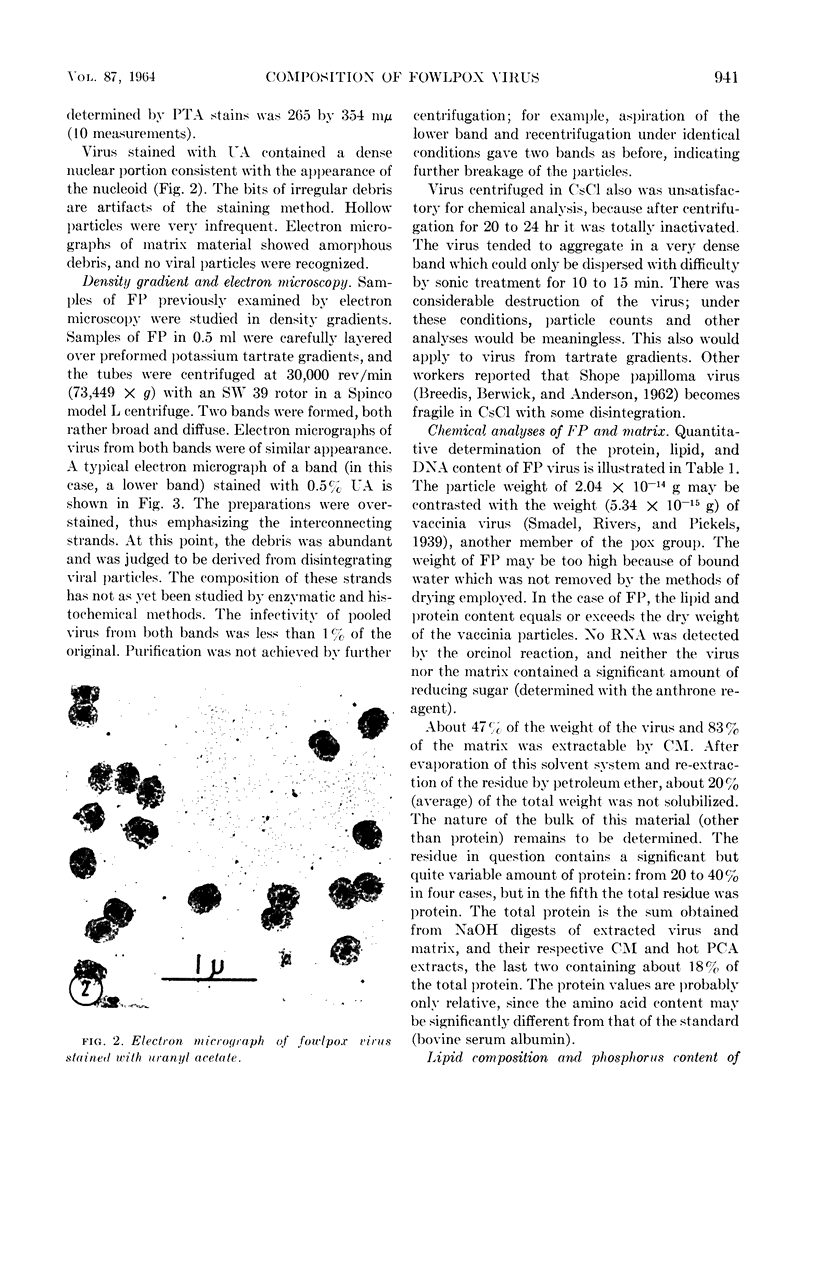


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., BURKE D. C. The nucleic acid contents of viruses. J Gen Microbiol. 1962 Feb;27:181–194. doi: 10.1099/00221287-27-2-181. [DOI] [PubMed] [Google Scholar]
- BANG F. B., LEVY E., GEY G. O. Some observations on host-cell-virus relationships in fowl pox. I. Growth in tissue culture. II. The inclusion produced by the virus on the chick chorio-allantoic membrane. J Immunol. 1951 Mar;66(3):329–345. [PubMed] [Google Scholar]
- BREEDIS C., BERWICK L., ANDERSON T. F. Fractionation of Shope papilloma virus in cesium chloride density gradients. Virology. 1962 May;17:84–94. doi: 10.1016/0042-6822(62)90084-3. [DOI] [PubMed] [Google Scholar]
- DARLINGTON R. W., RANDALL C. C. The nucleic acid content of equine abortion virus. Virology. 1963 Mar;19:322–327. doi: 10.1016/0042-6822(63)90071-0. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C., GAFFORD L. G. Histochemical andbiochemical studies of isolated viral inclusions. Am J Pathol. 1962 Jan;40:51–62. [PMC free article] [PubMed] [Google Scholar]
- SZYBALSKI W., ERIKSON R. L., GENTRY G. A., GAFFORD L. G., RANDALL C. C. Unusual properties of fowlpox virus DNA. Virology. 1963 Apr;19:586–589. doi: 10.1016/0042-6822(63)90056-4. [DOI] [PubMed] [Google Scholar]
- TODD W. M., RANDALL C. C., CONIGLIO J. G. Quantitative changes in lipid composition of tissues infected with fowlpox virus. Proc Soc Exp Biol Med. 1958 May;98(1):65–67. doi: 10.3181/00379727-98-23942. [DOI] [PubMed] [Google Scholar]