Abstract
Aims
Our objective was to examine the association between body mass index (BMI) and survival according to the type of treatment in individuals with established coronary artery disease (CAD).
Methods and results
Patients with CAD were identified in the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) registry between January 2001 and March 2006. Analyses were conducted separately by treatment strategy [medical management only, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG)]. Patients were grouped according to six BMI categories. Multivariable-adjusted hazard ratios (HRs) for mortality were calculated using the Cox regression with the referent group for all analyses being normal BMI (18.5–24.9 kg/m2). The cohort included 31 021 patients with a median follow-up time of 46 months. In the medically managed only group, BMIs of 25.0–29.9 and 30.0–34.9 kg/m2 were associated with significantly lower mortality compared with normal BMI patients (adjusted HR 0.72; 95% CI 0.63–0.83 and adjusted HR 0.82; 95% CI 0.69.0–0.98, respectively). In the CABG group, BMI of 30.0–34.9 kg/m2 had the lowest risk of mortality (adjusted HR 0.75; 95% CI 0.61–0.94), whereas in the PCI group, BMI of 35.0–39.9 kg/m2 had the lowest risk of mortality (adjusted HR 0.65; 95% CI 0.47–0.90). Patients who were overweight or have mild or moderate obesity were also more likely to undergo revascularization procedures compared with those with normal BMI, despite having lower risk coronary anatomy.
Conclusion
A paradoxical association between BMI and survival exists in patients with established CAD irrespective of treatment strategy. Patients with obesity may be presenting earlier and receiving more aggressive treatment compared with those with normal BMI.
Keywords: Obesity, Coronary disease, Mortality, Epidemiology
Introduction
In Europe, the prevalence of obesity [body mass index (BMI) ≥30 kg/m2] in men ranges from 4.0 to 28.3% and in women from 6.2 to 36.5% with prevalence rates in Central, Eastern, and Southern Europe being higher than those in Western and Northern Europe.1 Obesity is considered an independent cardiovascular risk factor that is associated with poor clinical outcomes.2 In the general population, a higher BMI is associated with an increased risk of coronary artery disease (CAD),3 cardiovascular events,3 and new-onset heart failure (HF).4 This risk appears to be mediated through obesity-related co-morbidities such as diabetes mellitus, hypertension, and dyslipidaemia.2 Based largely on these observations, virtually all national and international guidelines recommend weight loss for overweight and obese patients for the primary prevention of cardiovascular disease.5,6 This has resulted in weight loss counselling being extended to obese patients with CAD7 and HF,8 despite a lack of supporting evidence of benefit in these patient populations. Although obesity is clearly a risk factor for developing CAD and HF, once CAD and HF are established, an inverse correlation of obesity with all-cause mortality,9 cardiovascular mortality,10 and need for repeat revascularization11 has been reported. There are, however, several limitations to previous studies. First, the relationship between obesity and mortality in patients with CAD and HF has been examined using analyses of retrospectively collected cohort data or post hoc analyses of randomized controlled trials originally designed to evaluate a specific drug or device,11–17 many of which have been limited by short follow-up times. Secondly, most previous studies were not powered to examine survival in severe or morbid obesity, i.e. BMI ≥ 40 kg/m2. Thirdly, prior studies have not adjusted for potential treatment- and time-specific differences in the risk of mortality between males and females with CAD, which could lead to biased measurements of the effect of BMI on survival.
Therefore, we sought to examine the association between BMI and survival in a broad population-based cohort of patients with CAD referred for catheterization and treated with medical management alone or revascularization.
Methods
We conducted a cohort study that included all adult patients undergoing coronary angiography in Alberta, Canada, between 1 January 2001 and 31 March 2006 using data from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) registry. The APPROACH registry is a population-based database that prospectively captures all cardiac catheterizations performed in the province of Alberta, Canada (population ∼3.3 million), since 1995. Details of the database and methods of data collection have been described previously.18 Briefly, the APPROACH database contains detailed clinical information, including the results from catheterization, and is merged quarterly with mortality data from the Vital Statistics Registry. The last merge date for these data was 31 October 2007. Data collected at catheterization included socio-demographic characteristics (sex, age, address, and postal code), clinical co-morbidities (renal insufficiency, hypertension, hyperlipidaemia, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, smoking status, pulmonary disease, liver/gastrointestinal disease, and malignancy), disease severity variables (congestive heart failure, prior myocardial infarction, prior thrombolytic therapy, Canadian Cardiovascular Society angina class, and results of non-invasive tests), and coronary angiography results (coronary anatomy, extent of coronary stenosis, and left ventricular ejection fraction). These data are collected in the catheterization laboratory from the patient's charts and inputted into the APPROACH registry. Individuals in the APPROACH registry are followed longitudinally after catheterization, thus allowing for assessment of subsequent procedures [i.e. percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)], as well as the outcomes of mortality and quality of life in patients who consent to follow-up. Body mass index is recorded at the time of the index catheterization by nursing staff. Height and weight are measured with a standard mechanical beam physician scale with the patient wearing a hospital gown. Unstable patients who arrive from the emergency department prior to catheterization have their weight and height established through self-report.
We have previously developed and validated on a method for dealing with missing data in a prospective cardiac registry database.19 The method involves linking the prospectively derived cardiac registry data on a patient-by-patient basis to corresponding administrative data, followed by a process of mapping the specific clinical diagnoses present in both the registry data and the administrative data to create a single ‘final’ record of baseline risk factors present in a given patient. Patients with missing BMI values or BMI levels >70 or <11 kg/m2 were excluded as these values were deemed unlikely to be valid measurements. Long-term survival was assessed through linkage to records from the Alberta Bureau of Vital Statistics.18
Data analyses
Primary analysis
The primary outcome was all-cause mortality. Patients with one or more vessel CAD (Duke Coronary Index between 3 and 13)20 and without a previous catheterization were separated by initial treatment strategy (CABG, PCI, or medical management only). Coronary artery bypass grafting and PCI groups were chosen based on the first procedure following the index catheterization. All patients were grouped according to the World Health Organization (WHO) BMI classification system:21 underweight <18.5 kg/m2, normal 18.5–24.9 kg/m2, overweight 25.0–29.9 kg/m2, mildly obese 30.0–34.9 kg/m2, moderately obese 35.0–39.9 kg/m2, and severely obese ≥40 kg/m2. Univariate data are reported as mean ± SD for continuous variables or as percentage prevalence and count. Baseline characteristics of patients across the six BMI categories were examined by χ2 tests for linear trend for nominal variables and the Jonckheere–Terpstra test for trend for age and APPROACH Jeopardy score to account for the ordinal nature of the BMI categories. Unadjusted survival was examined using a Kaplan–Meier survival curve and the log-rank test. To evaluate the association between BMI and mortality, multivariable-adjusted hazard ratios (HRs) were calculated using the Cox proportional hazards regression models for each treatment strategy. All potential risk factors with a prevalence >1%, including the APPROACH Jeopardy score (representing percentage of myocardium at risk),22 were retained in each model regardless of statistical significance because our objective was to determine the adjusted HR for mortality in each BMI category while controlling for all measured clinical and co-morbid factors. The BMI category defined as ‘normal’ was considered the reference category and statistical significance was set at P < 0.05. All tests were two-sided. Interactions between BMI category and age, and BMI category and sex, were also tested using the likelihood ratio test. Since it has been suggested that sex-based differences in death rates after cardiac catheterization exist,23 the models were stratified by sex. We conducted an additional analysis to examine the dose–response relationship between BMI and all-cause mortality by modelling BMI as a continuous variable using fractional polynomial Cox regression,24 adjusted for treatment strategy.
Secondary analysis
As a secondary analysis, we examined the relationship between BMI and mortality in the patients who received a cardiac catheterization but were not diagnosed with CAD (<50% blockage in one vessel or normal coronaries). Baseline characteristics were compared across BMI categories in the same manner as above, as was unadjusted and adjusted survival.
Subgroup analyses
Pre-specified secondary analyses were undertaken in subgroups of patients who had HF as the indication for the cardiac catheterization and who had myocardial infarction (MI) as the indication for catheterization. Using the Cox regression, we adjusted for treatment strategy in addition to other baseline risk factors and co-morbidities. All analyses were performed using STATA version 9.
Results
A total of 46 663 patients underwent catheterization; 10 974 (23.5%) had normal coronaries, 460 (0.01%) were excluded for having undetermined or missing coronary anatomy, 4009 (8.6%) were excluded for having missing BMI values, and 199 (0.004%) were excluded due to implausible BMI levels (>70 or <11 kg/m2). An analysis of the excluded patients indicated that this group had more co-morbidities, higher emergent priority, and a significantly higher mortality at 30 days and 1 year (4.1 vs. 1.5% and 8.1 vs. 3.8%, respectively) compared with the remaining cohort, suggesting that these patients were sicker. Data were missing for priority for cardiac catheterization (1.3%) and ejection fraction (4.2%). Median length of follow-up for the study cohort was 46 months (inter-quartile range, 31–64 months).
There were 31 021 patients diagnosed with CAD. Patient characteristics by BMI category at the time of their cardiac catheterization are shown in Table 1. Of note, obese patients, particularly severely obese, were more likely to be younger, have hypercholesterolaemia, hypertension, pulmonary disease, and/or diabetes mellitus, but were less likely to smoke and less likely to have high-risk coronary anatomy (left main disease) and high percentage of myocardium at risk (APPROACH Jeopardy score). Medication data were available for a subset of 3583 patients. In all treatment groups, the numbers of patients receiving statins, ACE-inhibitors, nitrates, and beta-blockers were not significantly different across BMI categories (data not shown).
Table 1.
Patient characteristics with coronary artery disease according to body mass index category
| Variable | Underweight (<18.5) | Normal (18.5–24.9) | Overweight (25.0–29.9) | Mild obesity (30.0–34.9) | Moderate obesity (35.0–39.9) | Severe obesity (≥40) | P-value* |
|---|---|---|---|---|---|---|---|
| n | 284 | 6718 | 12 904 | 7497 | 2492 | 1126 | — |
| Age (years) (mean ± SD) | 69 ± 12 | 67 ± 12 | 65 ± 11 | 63 ± 11 | 61 ± 11 | 60 ± 11 | <0.0001 |
| Sex (%F) | 49.3 (140) | 30.8 (2072) | 20.6 (2659) | 22.3 (1670) | 29.1 (726) | 34.9 (393) | <0.0001 |
| Cerebrovascular disease | 8.1 (23) | 9.9 (664) | 7.2 (933) | 6.9 (515) | 6.4 (159) | 7.2 (81) | <0.0001 |
| Chronic obstructive pulmonary disease | 28.2 (80) | 17.4 (1117) | 13.4(1731) | 14.0 (1052) | 15.7 (392) | 21.9 (247) | 0.008 |
| History of HF | 20.4 (58) | 15.7 (1052) | 12.1 (1565) | 12.0 (900) | 14.4 (358) | 16.5 (186) | 0.9 |
| Renal disease (serum creatinine >200 µmol/L) | 8.1 (23) | 5.4 (361) | 4.1 (529) | 3.3 (245) | 3.9 (97) | 5.6 (63) | 0.04 |
| Diabetes mellitus | 15.5 (44) | 18.7 (1258) | 22.8 (1940) | 30.2 (2266) | 38.4 (958) | 47.6 (536) | <0.0001 |
| Hypertension | 61.6 (175) | 59.6 (4005) | 63.7 (8216) | 70.3 (5272) | 75.5 (1881) | 79.1 (891) | <0.0001 |
| Hyperlipidaemia | 61.6 (175) | 72.5 (4872) | 78.2 (10,092) | 80.8 (6061) | 81.2 (2023) | 82.0 (923) | <0.0001 |
| Malignancy | 6.3 (18) | 5.4 (364) | 4.2 (545) | 3.7 (276) | 3.5 (88) | 3.0 (34) | <0.0001 |
| Peripheral vascular disease | 15.5 (44) | 10.1 (679) | 8.4 (1083) | 7.9 (591) | 8.4 (209) | 9.5 (107) | 0.2 |
| Liver/GI disease | 12.7 (36) | 8.0 (539) | 6.9 (884) | 6.8 (509) | 6.6 (164) | 8.3 (94) | 0.8 |
| Current smoker | 39.1 (111) | 32.5 (2181) | 28.1 (3623) | 29.2 (2191) | 28.5 (711) | 29.3 (330) | 0.06 |
| Quit smoking | 26.8 (76) | 30.8 (2066) | 39.0 (5034) | 41.7 (3126) | 43.1 (1075) | 40.0 (450) | <0.0001 |
| Prior MI | 62.0 (176) | 56.5 (3793) | 54.9 (7082) | 52.1 (3909) | 52.7 (1314) | 53.6 (603) | <0.0001 |
| Prior CABG | 2.8 (8) | 4.5 (304) | 5.2 (674) | 4.5 (337) | 4.3 (107) | 4.1 (46) | 0.07 |
| LVEF < 35% | 10.6 (30) | 7.7 (518) | 6.6 (850) | 5.6 (420) | 5.5 (136) | 4.9 (55) | <0.0001 |
| Emergent priority | 18.0 (51) | 14.1 (950) | 11.5 (1484) | 9.9 (744) | 9.3 (231) | 10.2 (115) | <0.0001 |
| MI indication for catheterization | 52.8 (150) | 46.9 (3151) | 43.3 (5589) | 40.7 (3049) | 40.4 (1007) | 40.5 (456) | <0.0001 |
| HF indication for catheterization | 3.2 (9) | 2.6 (176) | 1.7 (216) | 1.9 (144) | 2.2 (55) | 2.7 (30) | 0.9 |
| Left main disease | 8.5 (24) | 11.2 (753) | 10.6 (1374) | 9.7 (729) | 8.8 (220) | 8.0 (90) | <0.0001 |
| APPROACH Jeopardy score (% myocardium at risk) (mean ± SD) | 40 ± 30 | 49 ± 31 | 50 ± 30 | 48 ± 30 | 46 ± 29 | 46 ± 29 | 0.002 |
| Treatment | |||||||
| Medical management | 41.2 (117) | 28.5 (1915) | 24.2 (3126) | 22.7 (1704) | 25.0 (622) | 28.2 (317) | 0.1 |
| CABG | 11.3 (32) | 22.7 (1523) | 25.2 (3255) | 26.1 (1955) | 24.4 (608) | 21.7 (244) | 0.9 |
| PCI | 47.5 (135) | 48.8 (3280) | 50.6 (6523) | 51.2 (3838) | 50.6 (1262) | 50.2 (565) | 0.1 |
Values are expressed as % (count), unless otherwise noted.
HF, heart failure; GI, gastrointestinal; MI, myocardial infarction; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; APPROACH, Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease.
*P-values for χ2 test for linear trend or Jonckheere–Terpstra test for linear trend.
Survival
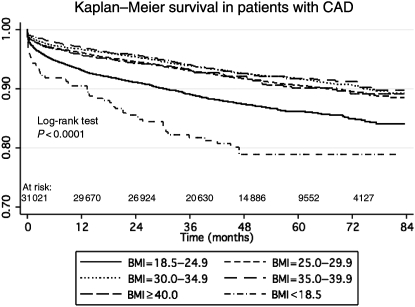
Crude survival by BMI category is presented in Figure 1. The log-rank test was significant for differences across BMI categories (P < 0.0001). Underweight patients were at the highest risk of death followed by patients with normal BMI.
Figure 1.
Kaplan–Meier survival by body mass index category.
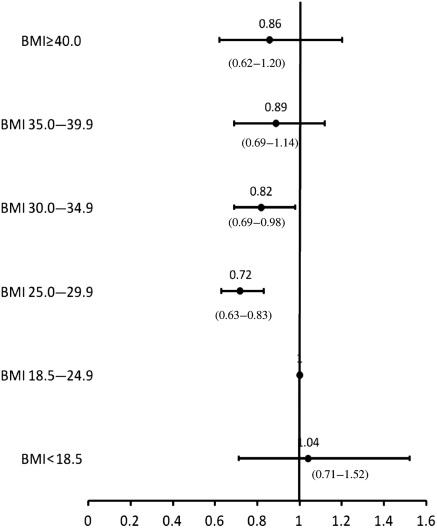
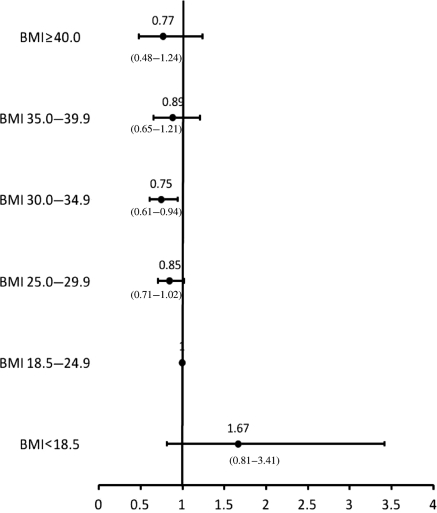
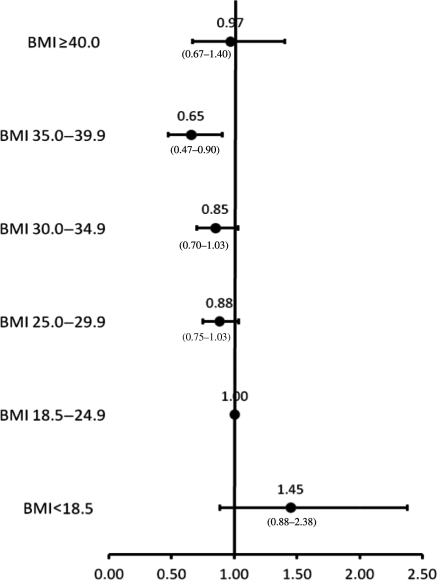
All variables presented in Table 1 of the patient characteristics were included in the regression models. Adjusted HRs for each BMI category within the pre-specified treatment strata are presented in Figures 2–4. There were no interactions between BMI category and age, and BMI category and sex. All covariates in the models met the proportionality assumption. In the medical management only, PCI, and CABG treatment groups, underweight patients were at higher adjusted risk of death compared with those with normal BMI; however, these associations were not significant. In the medically managed only group, overweight and mild obesity were associated with significantly lower mortality compared with normal BMI patients (adjusted HR 0.72; 95% CI 0.63–0.83 and 0.82; 95% CI 0.69–0.98, respectively). In the CABG group, those patients with mild obesity also had the lowest adjusted risk of death (adjusted HR 0.75; 95% CI 0.61–0.94). In the PCI group, moderate obesity was significantly associated with a reduced mortality risk (adjusted HR 0.65; 95% CI 0.47–0.90). There were no significant associations between severe obesity and mortality in any treatment group.
Figure 2.
Adjusted risk of mortality for patients treated with medical management only by body mass index category.
Figure 3.
Adjusted risk of mortality for patients treated with coronary artery bypass grafting by body mass index category.
Figure 4.
Adjusted risk of mortality for patients treated with percutaneous coronary intervention by body mass index category.
Body mass index as a continuous variable
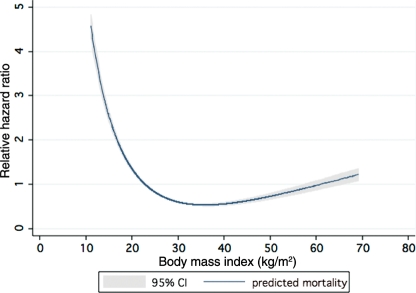
Examining BMI as a continuous variable (Figure 5) using polynomial Cox regression demonstrated aj-shaped association between BMI and adjusted all-cause mortality, where the mortality decreased with increasing BMI until ∼33 kg/m2, and then began to increase again at a BMI of ∼40 kg/m2.
Figure 5.
Association of body mass index as a continuous variable adjusted all-cause mortality using polynomial Cox regression.
Secondary analysis
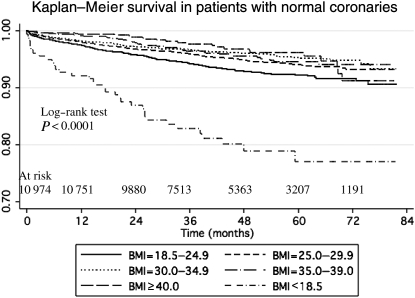
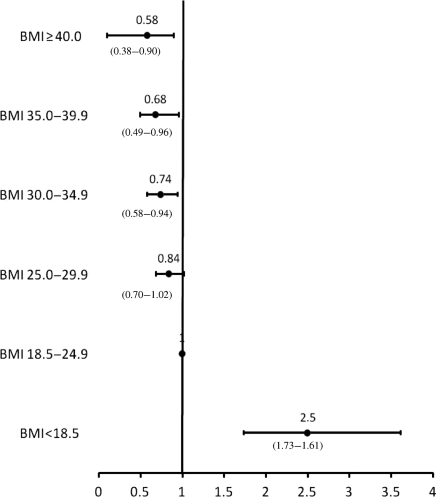
Patient characteristics of those with no CAD are presented in Table 2. Similar to the group with CAD, severely obese patients were younger and had more co-morbidities, but less likely to smoke compared with patients with normal BMI. Unadjusted survival is presented in Figure 6. The log-rank test was significant for differences across BMI categories (P < 0.0001). Underweight patients were at the highest risk of death followed by patients with normal BMI and severe obesity. Adjusted HRs are presented in Figure 7. Patients with mild, moderate, and even severe obesity had significantly reduced mortality compared with those with normal BMI (adjusted HR 0.74; 95% CI 0.58–0.94, adjusted HR 0.68; 95% CI 0.49–0.96, and adjusted HR 0.58; 95% CI 0.38–0.90, respectively).
Table 2.
Patient characteristics normal coronaries according to body mass index category
| Variable | Underweight (<18.5) | Normal (18.5–24.9) | Overweight (25.0–29.9) | Mild obesity (30.0–34.9) | Moderate obesity (35.0–39.9) | Severe obesity (≥40) | P-value* |
|---|---|---|---|---|---|---|---|
| n | 178 | 2802 | 3889 | 2478 | 973 | 654 | — |
| Age (years) (mean ± SD) | 60 ± 14 | 59 ± 14 | 59 ± 13 | 58 ± 12 | 57 ± 12 | 55 ± 11 | <0.0001 |
| Sex (%F) | 36.0 (64) | 45.3 (1269) | 59.5 (23.13) | 56.7 (1406) | 50.8 (494) | 43.3 (283) | 0.4 |
| Cerebrovascular disease | 9.0 (16) | 6.0 (167) | 4.2 (163) | 5.4 (133) | 4.3 (42) | 5.0 (33) | 0.9 |
| Chronic obstructive pulmonary disease | 37.1 (66) | 20.6 (577) | 18.3 (713) | 19.3 (479) | 21.2 (206) | 33.8 (221) | <0.0001 |
| History of HF | 27.0 (48) | 19.3 (541) | 15.9 (615) | 16.0 (397) | 17.6 (171) | 24.5 (160) | 0.02 |
| Renal disease (serum creatinine >200 µmol/L) | 5.6 (10) | 4.5 (127) | 2.6 (103) | 2.9 (71) | 2.9 (28) | 4.4 (29) | 0.5 |
| Diabetes mellitus | 11.8 (21) | 9.4 (264) | 11.9 (462) | 17.5 (434) | 25.5 (248) | 37.3 (244) | <0.0001 |
| Hypertension | 45.5 (81) | 44.8 (1256) | 51.8 (2016) | 61.0 (1512) | 67.3 (655) | 72.8 (476) | <0.0001 |
| Hyperlipidaemia | 40.4 (72) | 51.1 (1432) | 60.5 (2353) | 64.3 (1594) | 65.0 (632) | 61.6 (403) | <0.0001 |
| Malignancy | 3.9 (7) | 4.9 (132) | 5.0 (193) | 4.0 (98) | 3.5 (34) | 2.1 (14) | 0.001 |
| Peripheral vascular disease | 7.3 (13) | 6.1 (170) | 5.4 (209) | 4.9 (122) | 5.5 (54) | 6.6 (43) | 1.0 |
| Liver/GI disease | 8.4 (15) | 9.1 (256) | 8.4 (325) | 7.7 (192) | 9.7 (94) | 10.1 (66) | 0.8 |
| Current smoker | 30.3 (54) | 24.5 (686) | 19.9 (775) | 18.4 (457) | 19.5 (190) | 19.6 (128) | 0.005 |
| Quit smoking | 23.6 (42) | 29.0 (812) | 34.4 (1338) | 37.7 (934) | 39.6 (385) | 35.6 (233) | <0.0001 |
| Prior MI | 23.0 (41) | 16.4 (459) | 14.5 (565) | 15.7 (388) | 13.3 (129) | 13.5 (88) | 0.4 |
| Prior CABG | 0 | 0.6 (16) | 0.3 (12) | 0.4 (10) | 0.2 (2) | 0.8 (5) | 0.6 |
| LVEF < 35% | 5.6 (10) | 6.0 (167) | 5.6 (216) | 5.6 (138) | 6.1 (59) | 4.7 (31) | 0.5 |
| Emergent priority | 2.8 (5) | 3.0 (83) | 2.7 (105) | 1.7 (42) | 2.4 (23) | 2.0 (13) | 0.03 |
| MI indication for catheterization | 23.0 (41) | 15.0 (420) | 12.2 (476) | 10.9 (269) | 8.2 (80) | 8.7 (57) | <0.0001 |
| HF indication for catheterization | 5.6 (10) | 4.3 (120) | 3.7 (144) | 4.4 (109) | 5.43 (51) | 7.6 (50) | <0.0001 |
| Treatment | |||||||
| Medical management only | 84.3 (150) | 88.2 (2302) | 83.5 (3246) | 86.3 (2138) | 87.5 (851) | 88.2 (577) | <0.0001 |
| Percutaneous coronary intervention | 15.2 (27) | 17.5 (490) | 15.8 (615) | 13.2 (326) | 11.8 (115) | 11.2 (73) | <0.0001 |
| Coronary artery bypass grafting | 0.6 (1) | 0.4 (10) | 0.7 (28) | 0.6 (14) | 0.7 (7) | 0.6 (4) | 0.4 |
Values are expressed as % (count), unless otherwise noted.
HF, heart failure; GI, gastrointestinal; MI, myocardial infarction; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; APPROACH, Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease.
*P-values for χ2 test for trend or Jonckheere–Terpstra test for trend.
Figure 6.
Kaplan–Meier survival by body mass index category in patients with normal coronaries.
Figure 7.
Adjusted hazard ratios of mortality for patients with normal coronaries by body mass index category.
Subgroup analyses
In the HF subgroup, patients with moderate obesity had the lowest mortality hazard compared with those with normal BMI (adjusted HR 0.48; 95% CI 0.22–1.03). Compared with patients with a normal BMI, underweight HF patients had a significantly higher risk of mortality (adjusted HR 2.99; 95% CI 1.25–7.81). In the MI subgroup, overweight, mild obesity, and moderate obesity were all associated with a significantly reduced risk of mortality (adjusted HR 0.82; 95% CI 0.72–0.93, adjusted HR 0.82; 95% CI 0.70–0.97, and adjusted HR 0.72; 95% CI 0.54–0.94, respectively) compared with patients with normal BMI. Severe obesity was not associated with mortality in either of these subgroups.
Post hoc analysis
Given that patients with obesity had lower risk coronary anatomy compared with those with normal BMI, we sought to determine whether obese patients were less likely to receive revascularization procedures. Logistic regression was used to determine the odds ratios for receiving PCI or CABG for each of the BMI categories, with the normal BMI group as the reference category. The same covariates used in the previous Cox models (except for type of treatment) were used in the logistic regression models.
Patients who were underweight were less likely to be revascularized, whereas patients who were overweight, had either mild or moderate, but not severe, obesity were significantly more likely to be treated with CABG or PCI compared with CAD patients with a normal BMI (Table 3).
Table 3.
Odds ratios for patients undergoing revascularization by body mass index category
| BMI category | Odds ratio for undergoing PCI/CABG revascularization | 95% CI |
|---|---|---|
| Underweight (<18.5) | 0.67 | 0.5–0.89 |
| Normal (18.5–24.9) | 1.0 (reference) | — |
| Overweight (25.0–29.9) | 1.21 | 1.13–1.31 |
| Mild obesity (30.0–34.9) | 1.31 | 1.20–1.43 |
| Moderate obesity (35.0–39.9) | 1.12 | 1.05–1.33 |
| Severe obesity (≥40) | 0.97 | 0.83–1.14 |
Discussion
In this large population-based study of all consecutive patients with a confirmed diagnosis of CAD seen in a defined geographic locale over 5 years and followed for a median of 46 months, we found that mildly and/or moderately obese patients with established CAD had reduced mortality compared with normal BMI patients, whether treated with medical management only, PCI, or CABG. Although our findings may appear counterintuitive given the epidemiological link between obesity and cardiovascular disease in the general population,2 they are very much in line with previous reports of an inverse relationship between excess weight and all-cause and cardiovascular mortalities in individuals with established coronary disease. This observation has been referred to as the ‘obesity paradox’ or ‘reverse epidemiology’.25 Our large Canadian-based study adds to the increasing knowledge and awareness in this evolving field of survival paradoxes in chronic disease states.
It has been previously suggested that the obesity paradox post-CABG or post-PCI may reflect the possibility that only the low-risk obese patients are being selected for revascularization;26 however, our data suggest that mild to moderately obese patients who are not revascularized within 1 year of catheterization and instead are treated with medical management alone and those without a diagnosis of CAD also demonstrate lower mortality rates. The reduction in mortality rates was also consistent among the HF and MI subgroups and persisted after adjustment for known prognostic factors. The lowest adjusted mortality was noted in patients with BMI between 30.0 and 39.9 kg/m2, whereas a progressive increase in mortality was evident below this range, and a plateau or slight increase was observed above this range in patients with CAD. These findings are similar to recent meta-analyses conducted in patients post-PCI,9 post-CABG,9 post-MI,27 and in patients with HF.10
Our finding of the obesity paradox in patients who have received a cardiac catheterization but without coronary disease is unexpected. It is possible that patients who received a cardiac catheterization but did not show diseased coronaries had other cardiac risk factors that would classify them as having ‘pre-clinical’ disease, and that having a higher BMI offers protection against mortality even in this population. However, another possibility is that this is indicative of a referral and treatment bias in CAD. Being obese is a visible risk factor that may predispose physicians to refer them for a cardiac catheterization earlier than those of normal BMI. We also showed that patients with mild or moderate (but not severe) obesity are more likely to be revascularized with either CABG or PCI in patients with CAD, despite having lower risk coronary anatomy. This is similar to Yancy et al.28 who also showed that individuals with a BMI of 25.0–35.0 had the highest rates of coronary procedure utilization, whereas patients with a BMI of ≥40 kg/m2 had the lowest odds of receiving cardiac catheterization PCI or CABG.
There have been several proposed explanations for the obesity paradox. Similar to previous studies,29 our data show that compared with normal weight patients, obese patients are younger and have less severe disease at the time of cardiac catheterization. It has been suggested that obese patients who present earlier have more recognizable and aggressively treated co-morbidities. In addition, as patients who are obese tend to have higher systolic blood pressure, this may permit more aggressive upward titration of disease-modifying medications such as ACE-inhibitors and beta-blockers. However, data regarding the association between guideline-recommended treatment and obesity are conflicting30,31 and our data did not suggest that patients with obesity are being more aggressively treated with statins, ACE-inhibitors, beta-blockers, and nitrates, nor that BMI is associated with more invasive treatment strategy. The discrepancies in reported associations between obesity and medical and invasive treatment likely reflect differences in centre-specific practice patterns. Other potential explanations include obesity as protective against protein-energy malnutrition post-revascularization32 and altered neuroendocrine profiles (N-terminal BNP) of obese patients that may play a role in modulating heart disease progression.33
Our study has a number of strengths. We report on a large, well-categorized, consecutively enrolled, population-based cohort of patients. We were able to assess the effect of BMI on mortality in those with and without CAD according to treatment strategy in all patients undergoing cardiac catheterization from multiple centres. The large number of patients provided adequate power to evaluate survival in those with severe obesity, and our results from the stratified analyses are unbiased estimates of the effect of BMI on mortality in both males and females. Our study provides long-term follow-up data (median 46 months, maximum 84 months). In addition, the data are linked to up-to-date records from the Alberta Bureau of Vital Statistics and thus we had no right censoring in our survival analyses.
A limitation of our study is that the observational nature provides associative, not causal, evidence and therefore the possibility of selection bias and residual confounding cannot entirely be ruled out. However, the observational nature of this study also provides real-world data on the largest cohort studied to date. Secondly, although BMI is the most commonly used epidemiological measure of obesity, it is imperfect and does not directly distinguish between adipose and lean tissue or central and peripheral adiposity. Thirdly, we were unable to control for the role of non-purposeful weight loss prior to study entry. Our risk-adjusted analysis, however, did include age, current smoking status, and history of malignancy, which are factors that could lead to non-purposeful weight loss. Fourthly, since this was a record linkage study, we did not have data on cause-specific mortality. Finally, 8.6% of the patients were excluded for missing or implausible BMI values. This excluded group indeed could have differing results than those included in the analysis; however, given the size of the cohort analysed, it is unlikely that the results would have changed substantially.
In conclusion, we found that a paradoxical association exists between higher BMI and survival in patients with established CAD irrespective of medical, interventional, or surgical treatment strategy. This paradoxical observation is also seen in patients who were referred for cardiac catheterization but who have normal coronary arteries. Despite having lower risk coronary anatomy in obese patients with CAD, patients who are overweight, have either mild or moderate (but not severe) obesity, are more likely to receive revascularization procedures compared with those with normal BMI. Further studies are needed to explore the possibility of a treatment/referral bias as an explanation for the obesity paradox in the CAD population.
Funding
A.O. is supported by the Canadian Cardiovascular Outcomes Research Team (CCORT) and the Heart and Stroke Foundation of Canada; F.A.M. is supported by a salary award from the Alberta Heritage Foundation for Medical Research (AHFMR); K.K.-Z. is supported by research grants from the National Institute of Diabetes, Digestive, and Kidney Disease of the National Institute of Health (R01 DK078106 and R21 DK078012), an American Heart Association grant in aids (0655776Y) and a philanthropic grant from Mr Harold Simmons; C.M.N. is an AHFMR Population Health Investigator, a Canadian Institute of Health Research (CIHR) New Investigator and a co-investigator of GENESIS (Gender and Gender Determinants of Circulatory Health) and CCORT (Canadian Cardiovascular Outcomes Research Team); G.C.F. is supported by the Ahmanson Foundation (Los Angeles, CA, USA) and holds the Eliot Corday Chair in Cardiovascular Medicine and Science. APPROACH was initially funded with a grant from the W. Garfield Weston Foundation. The ongoing operation of this project has been made possible by contributions from the Provincial Wide Services Committee of Alberta Health and Wellness and the following industry sponsors: Merck Frosst Canada Inc., Roche Canada, Eli Lilly Canada Inc., Bristol–Myers Squibb, Philips Medical Systems Canada, Searle Pharmaceuticals, Guidant Corporation, Boston Scientific Ltd, and Cordis. We appreciate support from the Calgary Health Region, Capital Health Authority, Libin Cardiovascular Institute, and Mazankowski Heart Institute.
Conflict of interest: none declared.
Acknowledgements
We gratefully acknowledge Drs Yonfei Wang and Peter Faris for their statistical consultation, as well as the cardiac personnel for their diligence in data collection and entry: Foothills Medical Center, Royal Alexandra Hospital, and the University of Alberta Hospital.
References
- 1.Berghofer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8:200. doi: 10.1186/1471-2458-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calle E, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans J, Levy D, Wilson P, Benjamin E, Larson M, Kannel W, Vasan R. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Cats VM, Orth-Gomer K, Perk J, Pyorala K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D. European guidelines on cardiovascular disease prevention in clinical practice: third joint task force of European and other societies on cardiovascular disease prevention in clinical practice. Eur J Cardiovasc Prev Rehabil. 2003;10:S1–S10. doi: 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- 6.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 7.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 8.Malcom J, Arnold O, Howlett JG, Ducharme A, Ezekowitz JA, Gardner MJ, Giannetti N, Haddad H, Heckman GA, Isaac D, Jong P, Liu P, Mann E, McKelvie RS, Moe GW, Svendsen AM, Tsuyuki RT, O'Halloran K, Ross HJ, Sequeira EJ, White M. Canadian Cardiovascular Society Consensus Conference guidelines on heart failure—2008 update: best practices for the transition of care of heart failure patients, and the recognition, investigation and treatment of cardiomyopathies. Can J Cardiol. 2008;24:21–40. doi: 10.1016/s0828-282x(08)70545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oreopoulos A, Padwal R, Norris C, Mullen M, Kalantar-Zadeh K. Effect of obesity on short- and long-term mortality post coronary revascularization: a meta-analysis. Obesity. 2008;16:442–450. doi: 10.1038/oby.2007.36. [DOI] [PubMed] [Google Scholar]
- 10.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow G, McAlister F. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Gruberg L, Mercado N, Milo S, Boersma E, Disco C, van Es GA, Lemos PA, Ben Tzvi M, Wijns W, Unger F, Beyar R, Serruys PW Arterial Revascularization Therapies Study I. Impact of body mass index on the outcome of patients with multivessel disease randomized to either coronary artery bypass grafting or stenting in the ARTS trial: the obesity paradox II? Am J Cardiol. 2005;95:439–444. doi: 10.1016/j.amjcard.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Bozkurt B, Deswal A. Obesity as a prognostic factor in chronic symptomatic heart failure. Am Heart J. 2005;150:1233–1239. doi: 10.1016/j.ahj.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Kenchaiah S, Pocock S, Wang D, Finn P, Zornoff L, Skali H, Pfeffer M, Yusuf S, Swedberg D, Michelson E, Granger C, McMurray F, Solomon S. Body mass index and prognosis in patients with chronic heart failure. Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Program. Circulation. 2007;116:627–636. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 14.Cicoira M, Maggioni A, Latini R, Barlera S, Carretta D, Janosi A, Soler J, Anand I, Cohn J. Body mass index, prognosis and mode of death in chronic heart failure: results from the Valsartan Heart Failure Trial. Eur J Heart Fail. 2007;9:397–402. doi: 10.1016/j.ejheart.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson F, Kragelund CB, Torp-Pedersen C, Seibaek M, Burchardt H, Akkan D, Thune JJ, Kober L. Effect of obesity and being overweight on long-term mortality in congestive heart failure: influence of left ventricular systolic function. Eur Heart J. 2005;26:58–64. doi: 10.1093/eurheartj/ehi022. [DOI] [PubMed] [Google Scholar]
- 16.Gurm HS, Whitlow PL, Kip KE Investigators B. The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization. Insights from the bypass angioplasty revascularization investigation (BARI) J Am Coll Cardiol. 2002;39:834–840. doi: 10.1016/s0735-1097(02)01687-x. [DOI] [PubMed] [Google Scholar]
- 17.Butler J, Howser R, Portner P, Pierson RN., III Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg. 2005;79:66–73. doi: 10.1016/j.athoracsur.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Ghali WA, Knudtson ML. Overview of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease. On behalf of the APPROACH investigators. Can J Cardiol. 2000;16:1225–1230. [PubMed] [Google Scholar]
- 19.Norris CM, Ghali WA, Knudtson ML, Naylor CD, Saunders LD. Dealing with missing data in observational health care outcome analyses. J Clin Epidemiol. 2000;53:377–383. doi: 10.1016/s0895-4356(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 20.Smith LR, Harrel FE, Jr, Rankin JS, Califf JM, Pryor DB, Muhlbaier LH, Lee KL, Mark D, Jones DH, Oldham HN, Glower DD, Reves JG, Sabiston DC., Jr Determinants of early versus late cardiac death in patients undergoing coronary artery bypass graft surgery. Circulation. 1991;84(Suppl. III):III-245–III-253. [PubMed] [Google Scholar]
- 21.Calle E, Rodriguez C, Walker-Thurmond K, Thun M. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 22.McAlister FA, Oreopoulos A, Norris CM, Graham MM, Tsuyuki RT, Knudtson M, Ghali WA. Exploring the treatment-risk paradox in coronary disease. Arch Intern Med. 2007;167:1019–1025. doi: 10.1001/archinte.167.10.1019. [DOI] [PubMed] [Google Scholar]
- 23.King KM, Ghali WA, Faris PD, Curtis MJ, Galbraith PD, Graham MM, Knudtson ML. Sex differences in outcomes after cardiac catheterization: effect modification by treatment strategy and time. JAMA. 2004;291:1220–1225. doi: 10.1001/jama.291.10.1220. [DOI] [PubMed] [Google Scholar]
- 24.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, Morley JE. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433–442. doi: 10.1097/MCO.0b013e3281a30594. [DOI] [PubMed] [Google Scholar]
- 26.Yancy W, Olsen M, Curtis L, Schulman K, Cuffe M, Oddone E. Variations in coronary procedure utilization depending on body mass index. Arch Intern Med. 2005;165:1381–1387. doi: 10.1001/archinte.165.12.1381. [DOI] [PubMed] [Google Scholar]
- 27.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 28.Yancy WS, Jr, Olsen MK, Curtis LH, Schulman KA, Cuffe MS, Oddone EZ. Variations in coronary procedure utilization depending on body mass index. Arch Intern Med. 2005;165:1381–1387. doi: 10.1001/archinte.165.12.1381. [DOI] [PubMed] [Google Scholar]
- 29.Rubinshtein R, Halon D, Jaffe R, Shahla J, Lewis B. Relation between obesity and severity of coronary artery disease in patients undergoing coronary angiography. Am J Cardiol. 2000;97:1277–1280. doi: 10.1016/j.amjcard.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg BA, Cannon CP, Hernandez AF, Pan W, Peterson ED, Fonarow GC. Medical therapies and invasive treatments for coronary artery disease by body mass: the ‘obesity paradox’ in the Get With The Guidelines database. Am J Cardiol. 2007;100:1331–1335. doi: 10.1016/j.amjcard.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Shubair MM, Prabhakaran P, Pavlova V, Velianou JL, Sharma AM, Natarajan MK. The relationship of body mass index to outcomes after percutaneous coronary intervention. J Interv Cardiol. 2006;19:388–395. doi: 10.1111/j.1540-8183.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- 32.Abel R, Fischer FE, Buckly MJ, Barnett GO, Austen G. Malnutrition in cardiac surgery patients. Arch Surg. 1976;111:45–50. doi: 10.1001/archsurg.1976.01360190047008. [DOI] [PubMed] [Google Scholar]
- 33.Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, Canham RM, Chung AK, Leonard D, Wians FH, Jr, de Lemos JA. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]