Abstract
Background
Potassium homeostasis is essential for normal myocardial function, and low serum potassium may cause fatal arrhythmias. However, the association of low potassium and long-term mortality and morbidity in heart failure (HF) is largely unknown.
Methods and results
We studied 6845 HF patients in the Digitalis Investigation Group trial with serum potassium levels ≤5.5 mEq/L. Of these, 1189 had low potassium (<4 mEq/L). Propensity scores for low potassium were calculated for each patient and were used to match 1187 low-potassium patients with 1187 normal-potassium (4–5.5 mEq/L) patients. Effects of low potassium on outcomes were assessed using matched Cox regression analyses. All-cause mortality occurred in 379 (rate, 1103/10000 person-years) normal-potassium and 441 (rate, 1330/10000 person-years) low-potassium patients, respectively during 3437 and 3315 years of follow-up [hazard ratio (HR), 1.25; 95% confidence interval (CI), 1.07–1.46; P=0.006]. Cardiovascular mortality occurred in 297 (864/10000 person-years) normal-potassium and 356 (1074/10000 person-years) low-potassium patients (HR, 1.27, 95% CI, 1.06–1.51, P=0.009). Cardiovascular hospitalization occurred in 284 (rate, 2361/10000 person-years) normal-potassium and 301 (rate, 2704/10000 person-years) low-potassium patients (HR, 1.13; 95% CI, 0.99–1.29; P=0.082).
Conclusions
In a cohort of ambulatory chronic systolic and diastolic HF patients who were balanced in all measured baseline covariates, serum potassium <4 mEq/L was associated with increased mortality, with a trend toward increased hospitalization.
Keywords: Heart failure, potassium, mortality, hospitalization, propensity score
Potassium is an important determinant of myocardial function and low serum potassium may cause arrhythmias and sudden death.1–5 Diuretics are commonly used in HF and hypokalemia is an important complication of diuretic therapy.3, 6 Diuretic-associated increased mortality and morbidity may in part be attributed to low potassium.7 The effects of low potassium on cardiovascular morbidity and mortality are well known from studies in human hypertension and in animal models.1, 2, 8–11 However, the effects of low serum potassium on long-term outcomes in HF have not been well studied. The objective of this study was to determine the long-term effects of low serum potassium on mortality and hospitalization in a cohort of propensity score matched chronic systolic and diastolic HF patients.
Patients and methods
Study design
We conducted a non-randomized propensity-matched study of the DIG trial, which was a randomized clinical trial of digoxin in HF conducted in 302 centers (186 in the United States and 116 in Canada) over 32 months during 1991–1993.12, 13 Detailed descriptions of the rationale, design, implementation, and results of the DIG trial have been reported elsewhere.12, 13
Study patients
All of the 7788 DIG participants were ambulatory chronic systolic and diastolic HF patients in normal sinus rhythm. Of these, 6800 had left ventricular ejection fraction <=45%. Most DIG participants were receiving angiotensin-converting enzyme (ACE) inhibitors and diuretics. Beta-blockers were not approved for HF during the DIG trial and data on beta-blocker use were not collected. We restricted our analysis to a subset of 6857 patients with valid baseline data on serum potassium levels. Of these 6857 patients, 12 patients had serum potassium >5.5 mEq/L and were excluded. We restricted our main analysis to a subset of 1187 pairs of patients with normal and low serum potassium, who were matched by their propensities for low serum potassium.
Low serum potassium
Serum potassium values between 3.5 and 4 mEq/L have been used to define low serum potassium or hypokalemia.11, 14 However, values of serum potassium for hypokalemia in HF have not been clearly defined. Based primarily on effect of aldosterone antagonists in elevating serum potassium and in reducing mortality in HF,15, 16 Macdonald et al. suggested that a cutoff of serum potassium level 4 mEq/L should be used to define hypokalemia in HF and serum potassium should preferably be maintained at or above 4.5 mEq/L.1 Therefore, for the purpose of this analysis, we defined hypokalemia as serum potassium <4 mEq/L. Of the 6845 patients in our analysis, 1189 (17.4%) had serum potassium <4 mEq/L.
Study outcomes
The primary outcomes were all-cause mortality and all-cause hospitalization. We also studied mortality and hospitalizations due to cardiovascular causes and HF. All study outcomes were ascertained by blinded study investigators. DIG participants were followed for a median of 38 months and vital status data were complete for 99% of the patients.17
Propensity score methods
The propensity score is the conditional probability of receiving an exposure (e.g. having low potassium) given a set of measured covariates.18–21 Propensity score matching makes it possible to design observational studies like randomized clinical trials in several key ways.21 First, it allows investigators to assemble retrospectively a study cohort, in which patients are well balanced on all measured covariates. Second, it allows investigators to measure objectively the achieved balance (i.e. bias reduction) in the study cohort. Finally, and perhaps most importantly, it makes it possible do all these without knowledge of or access to outcomes data, as investigators of a randomized clinical trial would not know the outcomes of the trial during its design.21 Although, propensity score matching is often used to balance two treatment groups,7, 22–26 the method can also be used to balance patients across non-treatment exposures.27–30
Calculation of propensity scores
We estimated propensity scores for low serum potassium for each of the 6845 patients using a non-parsimonious multivariable logistic regression model. In the model, low serum potassium was used as the dependent variable, and all measured baseline patient characteristics shown in Table 1, except for glomerular filtration rate, chronic kidney disease, and ejection fraction >45% (which are derived values), were included as covariates. We also tested the following clinically plausible interaction terms: age and serum creatinine, age and potassium supplement use, serum creatinine and ACE inhibitor use, serum creatinine and diuretic (non-potassium-sparing) use, ACE inhibitors and potassium supplement use, and potassium-sparing diuretics and potassium supplement use. None of these interactions were significant and were dropped from the final model.31 The model was well-calibrated (Hosmer-Lemeshow test: p = 0.141) with reasonable discrimination (c statistic = 0.62).31
Table 1.
Baseline patient characteristics, by serum potassium, before and after propensity score matching
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| N (%) or mean (±SD) | ≥4 mEq/L (N = 1189) | <4 mEq/L (N = 5656) | P | ≥4 mEq/L (N = 1187) | <4 mEq/L (N = 1187) | P |
| Age (years) | 63 (±11) | 64 (±11) | 0.035 | 63.5 (±11.1) | 63.4 (±11.3) | 0.859 |
| Age ≥65 years | 590 (50%) | 3001 (53%) | 0.041 | 604(51%) | 589 (50%) | 0.566 |
| Female | 363(31%) | 1318(23%) | <0.0001 | 367 (31%) | 362 (31%) | 0.859 |
| Non-white | 198 (17%) | 745 (13%) | 0.002 | 197 (17%) | 197 (17%) | 1.000 |
| Body mass index, kg/m2 | 27 (±6) | 27 (±5) | 0.645 | 27.3 (±5.5) | 27.3 (±5.6) | 0.822 |
| Duration of HF (months) | 30 (±37) | 29 (±36) | 0.333 | 28.8 (±36.0) | 29.8 (±36.3) | 0.477 |
| Primary cause of HF | ||||||
| Ischemic | 737 (62%) | 3981 (70%) | 750 (63%) | 737 (62%) | ||
| Hypertensive | 183 (15%) | 527 (9%) | <0.0001 | 153 (13%) | 183 (15%) | 0.067 |
| Idiopathic | 177 (15%) | 796 (14%) | 209 (18%) | 176 (15%) | ||
| Others | 92 (8%) | 352 (6%) | 75 (6%) | 91 (8%) | ||
| Prior myocardial infarction | 682 (57%) | 3620 (64%) | <0.0001 | 678 (57%) | 682 (58%) | 0.901 |
| Current angina pectoris | 316 (27%) | 1539 (27%) | 0.667 | 319 (30%) | 316 (27%) | 0.926 |
| Hypertension | 649 (55%) | 2604 (46%) | <0.0001 | 652 (55%) | 647 (55%) | 0.869 |
| Diabetes mellitus | 315 (27%) | 1639 (29%) | 0.090 | 302 (25%) | 315 (27%) | 0.574 |
| Chronic kidney disease | 527 (44%) | 2589 (46%) | 0.370 | 537 (45%) | 525 (44%) | 0.650 |
| Medications | ||||||
| Pre-trial digoxin use | 535 (45%) | 2346 (42%) | 0.026 | 533 (45%) | 533 (45%) | 1.000 |
| Trial use of digoxin | 601 (51%) | 2822 (50%) | 0.702 | 612 (52%) | 599 (51%) | 0.622 |
| ACE inhibitors | 1096 (92%) | 5283 (93%) | 0.129 | 1094 (92%) | 1095 (92%) | 1.000 |
| Hydralazine & nitrates | 26 (2%) | 80 (1%) | 0.053 | 26 (2%) | 25 (2%) | 1.000 |
| Diuretics | 987 (83%) | 4343 (77%) | <0.0001 | 986 (83%) | 985 (83%) | 1.000 |
| Potassium-sparing diuretics | 95 (8%) | 388 (7%) | 0.171 | 110 (9%) | 94 (8%) | 0.272 |
| Potassium supplement | 487 (41%) | 1638 (29%) | <0.0001 | 465 (39%) | 485 (41%) | 0.426 |
| Symptoms and signs of HF | ||||||
| Dyspnea at rest | 286 (24%) | 1223 (22%) | 0.070 | 292 (25%) | 286 (24%) | 0.811 |
| Dyspnea on exertion | 889 (75%) | 4264 (75%) | 0.657 | 894 (75%) | 887 (75%) | 0.776 |
| Limitation of activity | 892 (75%) | 4305 (76%) | 0.433 | 918 (77%) | 890 (75%) | 0.193 |
| Jugular venous distension | 172 (15%) | 690 (12%) | 0.034 | 183 (15%) | 171 (14%) | 0.526 |
| Third heart sound | 309 (26%) | 1312 (23%) | 0.043 | 328 (28%) | 308 (26%) | 0.379 |
| Pulmonary râles | 209 (18%) | 892 (16%) | 0.129 | 205 (17%) | 208 (18%) | 0.914 |
| Lower extremity edema | 292 (25%) | 1161 (21%) | 0.002 | 299 (25%) | 290 (24%) | 0.704 |
| NYHA functional class, % | ||||||
| Class I | 164 (14%) | 809 (14%) | 171 (14%) | 164 (14%) | ||
| Class II | 630 (53%) | 3076 (54%) | 0.125 | 624 (53%) | 629 (53%) | 0.899 |
| Class III | 362 (30%) | 1670 (30%) | 364 (31%) | 361 (30%) | ||
| Class IV | 33 (3%) | 101 (2%) | 28 (2%) | 33 (3%) | ||
| Heart rate (/minute), | 80 (±13) | 78 (±13) | <0.0001 | 80 (±13) | 80 (±13) | 0.783 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 129 (±22) | 127 (±20) | 0.038 | 128 (±21) | 129 (±21) | 0.620 |
| Diastolic | 76 (±11) | 75 (±11) | 0.001 | 76 (±12) | 76 (±12) | 0.363 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 182 (15%) | 753 (13%) | 0.070 | 194 (16%) | 182 (15%) | 0.536 |
| Cardiothoracic ratio >0.5 | 776 (65%) | 3366 (60%) | <0.0001 | 758 (64%) | 774 (65%) | 0.520 |
| Serum creatinine (mg/dL) | 1.27 (±0.4) | 1.3 (±0.4) | 0.047 | 1.3 (±0.4) | 1.3 (±0.4) | 0.568 |
| Estimated glomerular filtration rate, ml/min per 1.73 m2 | 64 (±21) | 63 (±21) | 0.177 | 63 (±20) | 64 (±21) | 0.357 |
| Ejection fraction (%) | 32 (±13) | 32 (±12) | 0.346 | 33 (±13) | 32 (±13) | 0.466 |
| Ejection fraction >45% | 162 (14%) | 704 (12%) | 0.270 | 154 (13%) | 161(51%) | 0.717 |
A random sample of 1187 patients were selected from 5656 patients with normal potassium and were paired with 1187 patients with low serum potassium in the matched cohort. This was done to assemble a pre-match cohort of the same size (n=2374) of that of the post-match cohort (n=2374) to avoid artificial inflation of the significance of intergroup differences in baseline covariates due to larger pre-match sample size (n=6845)
Assembly of study cohort: propensity score matching
We used an SPSS macro to match each low-potassium patient with a normal-potassium patient who had similar propensity scores to five, four, three, two and one decimal places in five repeated steps. In the first step, we multiplied the raw propensity scores (e.g. 0.57520576) by 100,000 (e.g. 57520.58), then rounded it to the nearest value divisible by 0.25 (e.g. 57520.50). We then matched low-potassium patients with normal-potassium patients who had similar propensity scores by this standard. The pairs of matched patients were removed from the file. In the second step, we multiplied the raw propensity scores by 10,000, rather than 100,000, and repeated the above process. This was repeated three more times, each time, multiplying by 1,000, 100 and finally 10. In all, we matched 1187 of the 1189 low-potassium patients with 1187 patients who had normal serum potassium, but had similar propensity for low potassium.32 This near 100% match is noteworthy given the modest discrimination of our propensity score model.31, 33
Assessment of bias reduction and balance
Balances in the distribution of baseline covariates between patients with normal and low potassium were assessed by estimating absolute standardized differences of covariates between the two groups, before and after matching.7, 26, 27, 29, 30 Standardized differences directly quantify biases in the means (or proportions) of covariates across the groups, and are expressed as percentages of the pooled standard deviations. Bias reduction was assessed by comparing the absolute standardized differences of covariates before and after matching. An absolute standardized difference of 0% on a covariate indicates no residual bias for that covariate, and an absolute standardized difference below 10% suggest inconsequential residual bias.26
Statistical analysis
We used Kaplan-Meier plots and matched Cox regression analysis to estimate associations of low potassium with various outcomes. We confirmed the assumption of proportional hazards by a visual examination of the log (minus log) curves. We then repeated our analyses using serum potassium as a continuous variable. To determine whether the loss of sample size in the matching process affected our results, we estimated the effect of low potassium on outcomes in the full pre-match cohort of 6845 patients using three different approaches: (1) unadjusted, (2) adjusted for raw propensity scores, and (3) adjusted for all covariates used in the propensity score model.
Sensitivity analyses
Even though our matched cohort achieved excellent balance in all measured covariates between the two groups, we do not know if there was bias due to imbalances in unmeasured covariates. Therefore, we conducted a formal sensitivity analysis to quantify the degree of a hidden bias that would need to be present to invalidate our main conclusions.34
Subgroup analyses
We conducted subgroup analyses to determine the homogeneity of the associations of low potassium with all-cause mortality. We first calculated absolute risk differences, and then estimated the effect of low potassium on mortality in each subgroup using Cox regression model, in each case adjusting for propensity score for low potassium. Finally, we formally tested for first-order interactions using Cox proportional hazards models, entering interaction terms and adjusting for propensity scores, separately for each subgroup. All statistical tests were evaluated using two-tailed 95% confidence levels, and data analyses were performed using SPSS for Windows version 14.35
Results
Patient characteristics
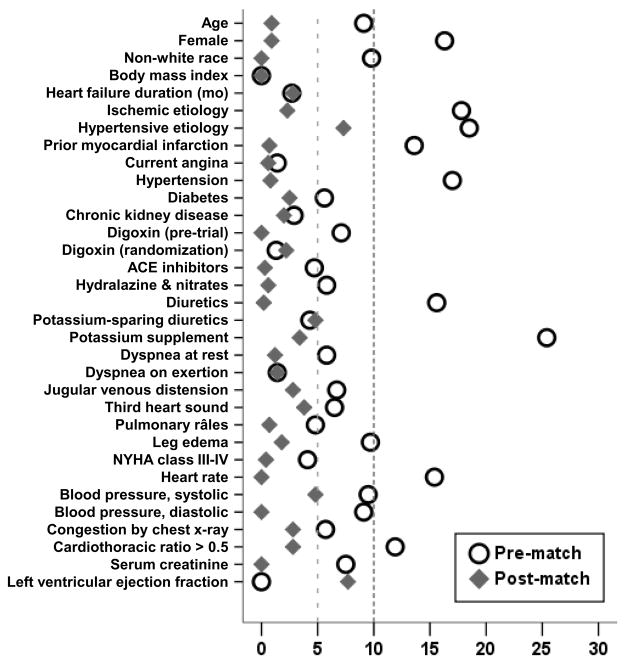
The mean (±SD) age of the 2374 matched patients was 63 (±11) years, (median 65; range 21–92), 729 (31%) were women and 394 (17%) were non-whites. Before matching, low-potassium patients were more likely to be women, non-whites, and have hypertension, elevated jugular venous pressure and leg edema, cardiomegaly, and be receiving diuretics and potassium supplements (Table 1). After matching, normal- and low-potassium patients were more similar in regards to all measured baseline covariates (Table 1 and Figure 1). Our propensity score matching reduced standardized differences for all observed covariates below 10% in absolute value, demonstrating substantial improvement in covariate balance across the treatment groups (Figure 1).
Figure 1.
Absolute standardized differences in covariates between patients with normal and low serum potassium, before and after propensity score matching
Association of low potassium with all-cause mortality
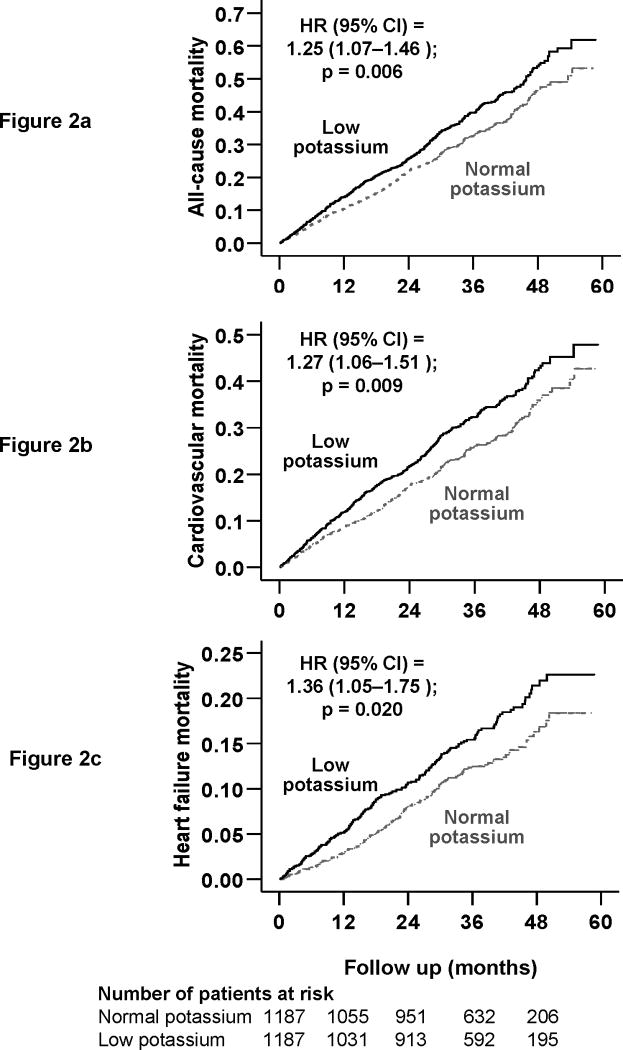
During a median follow up of 36.7 months, 820 (35%) patients in the matched cohort died from all causes, including 653 (28%) due to cardiovascular causes and 311 (13%) due to progressive HF. Kaplan-Meier survival curves for all-cause mortality are displayed in Figure 2a. All-cause mortality occurred in 379 (32%) of normal-potassium and 441 (37%) low-potassium patients respectively during 3437 and 3315 years of follow up. Mortality rates for normal- and low-potassium patients were respectively 1103 and 1330 per 10000 person-years of follow up [hazard ratio {HR} 1.25; 95% confidence interval {CI} 1.07–1.46; P=0.006; Table 2]. When we used serum potassium as a continuous variable, each unit increase in serum potassium was associated with 22% reduction in risk of total mortality (HR 0.78; 95% CI 0.66–0.92; p =0.003).
Figure 2.
Kaplan-Meier plots for mortality due to (a) all-causes, (b) cardiovascular causes, and (c) worsening heart failure
Table 2.
Cause-specific mortalities in heart failure patients for serum potassium ≤4 mEq/L
| Serum potassium ≥4 mEq/L (N = 1187) | Serum potassium ≤4 mEq/L (N = 1187) | Rate difference* (per 10,000 person-years) | Hazard ratio (95% confidence interval) | P value | ||
|---|---|---|---|---|---|---|
| Total follow up in years** | 3437 | 3315 | ||||
| All-cause | Events | 379 | 441 | + 228 | 1.25 (1.07–1.46) | 0.006 |
| Rate per 10,000 person-years | 1103 | 1330 | ||||
| Cardiovascular | Event | 297 | 356 | + 210 | 1.27 (1.06–1.51) | 0.009 |
| Rate per 10,000 person-years | 864 | 1074 | ||||
| Progressive heart failure‡ | Event | 137 | 174 | + 126 | 1.36 (1.05–1.75) | 0.020 |
| Rate per 10,000 person-years | 399 | 525 | ||||
| Other cardiac§ | Event | 137 | 160 | + 37 | 1.22 (0.94–1.58) | 0.131 |
| Rate per 10,000 person-years | 399 | 483 | ||||
| Other vascular¶ | Event | 23 | 22 | − 1 | 1.00 (0.50–2.00) | 1.000 |
| Rate per 10,000 person-years | 67 | 66 | ||||
| Non-cardiac-non--vascular | Event | 60 | 61 | + 9 | 1.30 (0.85–1.99) | 0.234 |
| Rate per 10,000 person-years | 175 | 184 | ||||
| Unknown | Event | 22 | 24 | + 8 | 0.94 (0.49–1.83) | 0.866 |
| Rate per 10,000 person-years | 64 | 72 | ||||
Absolute rate differences were calculated by subtracting the rates of death in the normal-potassium group from the rates of death in the low-potassium group
Hazard ratios and confidence intervals (CI) were estimated from matched Cox proportional-hazards models.
This category includes patients who died from progressive heart failure, even if the final event was an arrhythmia.
This category includes deaths presumed to result from arrhythmia without evidence of progressive heart failure and deaths due to atherosclerotic coronary disease, bradyarrhythmias, low-output states, and cardiac surgery.
This category includes deaths due to stroke, embolism, peripheral vascular disease, vascular surgery, and carotid endarterectomy.
Total follow up period is same for all cause-specific mortalities as for all-cause mortality
In the full pre-match cohort of 6845 patients, 2260 (33%) patients died from all causes. All-cause mortality occurred in 1818 (32%) of normal-potassium patients during 16391 years of follow up (rate, 1109/10000 person-years), and 442 (37%) of low-potassium patients during 442 years of follow up (rate, 1332/10000 person-years; unadjusted HR 1.20; 95% CI 1.08–1.33; p =0.001). When adjusted for all covariates (HR 1.21; 95% CI 1.09–1.35; p =0.001) or propensity scores (HR 1.19; 95% CI 1.07–1.32; p =0.001), the association remained essentially unchanged.
Results of sensitivity analyses
In the absence of hidden bias, a sign-score test for matched data with censoring provides modest evidence that low potassium was associated with increased mortality. Our sensitivity analysis suggests that an unmeasured binary covariate would need to increase the odds of low potassium by more than 6.7% to explain away this association (z-statistic=2.77; two-tailed p = 0.0056), suggesting that these results are sensitive to moderately strong hidden biases.
Low potassium and cause-specific mortalities
Kaplan-Meier survival curves for cardiovascular and HF mortalities are displayed in Figure 2b and 2c. Mortality due to cardiovascular causes occurred in 25% (rate, 858/10000 person-years) of normal-potassium patients and 30% (rate, 1070/10000 person-years) low-potassium patients (HR 1.19, 95% CI 1.00–1.41; P=0.056; Table 2). Mortality due to progressive HF occurred in 11% (rate, 365 per 10000 person-years) of normal-potassium patients and 15% (rate, 522 per 10000 person-years) low-potassium patients (HR 1.39, 95% CI 1.07–1.81; P=0.013; Table 2). Associations of low potassium and other cause-specific mortalities are displayed in Table 2.
Low potassium and hospitalizations
Hospitalizations due to all causes occurred in 1587 (67%) patients, including 1258 (53%) hospitalizations due to cardiovascular causes and 763 (32%) due to worsening HF. All-cause hospitalizations occurred in 67% each of normal- and low-potassium patients respectively during 1957 and 1879 years of follow up. Rates for all-cause hospitalization per 10000 person-years of follow up were 4047 and 4230 respectively for normal- and low-potassium patients (HR 1.11, 95% confidence interval {CI} 0.98–1.25; P=0.107).
Hospitalizations due to cardiovascular causes occurred in 52% (rate, 2680 per 10000 person-years) of normal-potassium patients and 54% (rate, 2848 per 10000 person-years) low-potassium patients (HR 1.05, 95% CI 0.91–1.20; P=0.516). Hospitalizations due to worsening HF occurred in 31% (rate, 365 per 10000 person-years) of normal-potassium patients and 33% (rate, 522 per 10000 person-years) low-potassium patients (HR 1.12, 95% CI 0.95–1.32; P=0.181).
Low potassium had no significant associations with hospitalizations due to supraventricular arrhythmias (HR 1.09, 95% CI 0.73–1.63; P=0.680) or ventricular arrhythmias (HR 0.94, 95% CI 0.58–1.53; P=0.806). It also had no significant association with myocardial infarction (HR 1.20, 95% CI 0.83–1.74; P=0.345), unstable angina pectoris (HR 1.11, 95% CI 0.79–1.57; P=0.541), or suspected digoxin toxicity (HR 0.58, 95% CI 0.23–1.48; P=0.257).
In the full pre-match cohort of 6482 patients, low potassium had no significant association with all-cause hospitalizations (unadjusted HR 1.08; 95% CI 1.01–1.18; p =0.031), which remained essentially unchanged after multivariable adjustment for all covariates (HR 1.07; 95% CI 0.99–1.16; p =0.087) or propensity scores (HR 1.06; 95%CI 0.98–1.14; p =0.155).
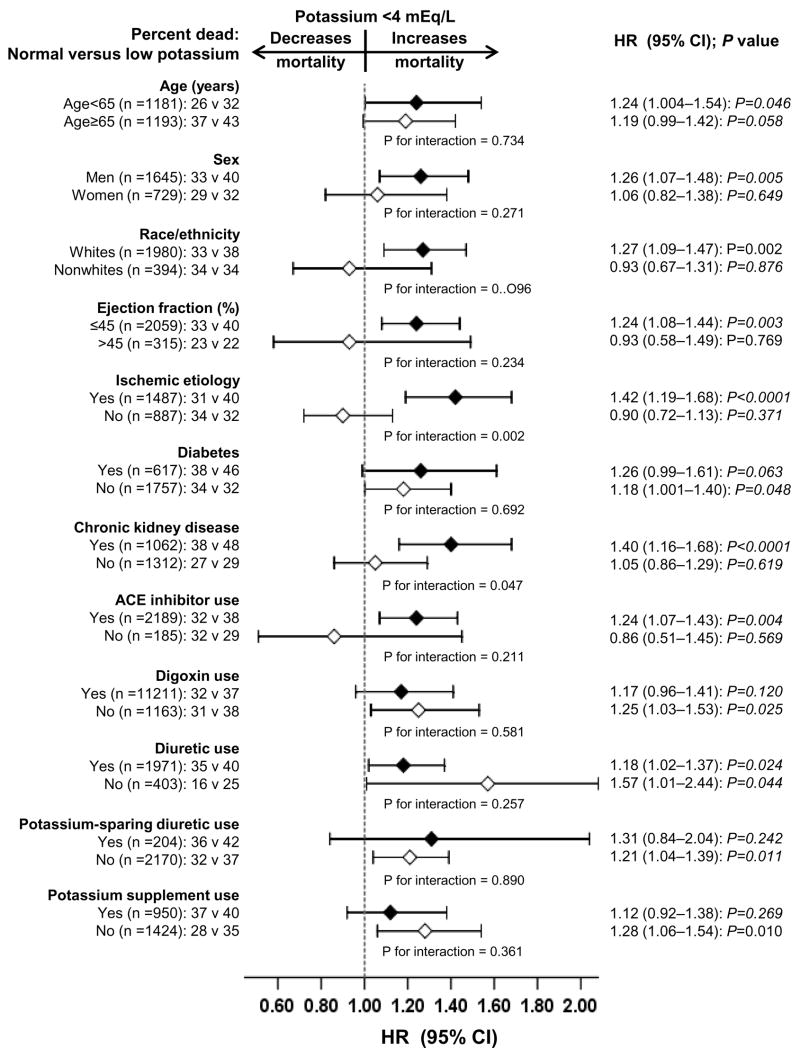
Subgroup analyses
The associations of low potassium and all-cause mortality were observed in a wide spectrum of HF patients (Figure 3). There were no significant interactions between low potassium and any of the subgroups, except for ischemic heart disease and chronic kidney disease (p for interactions respectively 0.002 and 0.047). Of interest, the association of low potassium and mortality was comparable, with 37% and 38% total mortality respectively among patients receiving and not receiving digoxin. The effects of low potassium on cardiovascular and HF mortality were also similar in patients receiving and not receiving digoxin.
Figure 3.
Hazard ratio and 95% confidence interval (CI) for all-cause mortality associated with low serum potassium in subgroups of patients with chronic heart failure
Discussion
The results of the current analysis demonstrate that in ambulatory chronic HF patients who were well-balanced in all measured baseline covariates, compared to serum potassium levels between 4 and 5.5, serum potassium levels, 4 mEq/L were associated with increased risk of mortality due to all causes, cardiovascular causes, and progressive HF, with trends toward increased hospital admissions due to all causes and cardiovascular causes. To the best of our knowledge, this is the first report of long-term effect of baseline serum potassium on outcomes in a propensity matched cohort of chronic HF. These findings are important as low serum potassium is relatively common in HF, often precipitated by diuretics, also commonly used in HF.
Possible mechanistic explanations
Low potassium may affect myocardial resting membrane potential, repolarization and relative refractory times, and conduction velocity.9 Data from animal models suggest that low potassium, even when within the conventional range of serum potassium, may cause lethal ventricular arrhythmias, impair myocardial responses to hypoxia, and may also impair myocardial contractile and relaxation responses to epinephrine.9, 10, 36
Low potassium may also be a marker of use of diuretics, and more symptomatic HF by indication. Use of non-potassium-sparing diuretics has been shown to increase mortality and hospitalization in HF.3, 7, 37 There were no imbalances in any of the measured covariates, including use of diuretics, between patients with normal and low potassium in the matched cohort. Diuretic-associated hypokalemia has been shown to more severe in patients receiving higher doses of diuretics.11 However, we had no data on diuretic dosage. We observed that low potassium was associated with increased cardiovascular and HF mortality, but not with hospitalization suggesting fatal arrhythmias as a possible mechanistic explanation of sudden cardiac deaths associated with low potassium. However, we had no data on sudden cardiac death.
Aldosterone, a mineralocorticoid and a potent neurohormone that is activated in HF,38 stimulates exchange of sodium and potassium in distal renal tubules, resulting in increased excretion of potassium into the urine. Data from human hypertension suggest that non-potassium-sparing diuretics are more likely to cause hypokalemia when serum aldosterone is elevated.8, 39, 40 Thus, low potassium may be a marker of elevated aldosterone, which has bee shown to cause myocardial fibrosis and progression of HF.38, 41 Suppression of aldosterone, on the other hand, reduces mortality in HF.15, 16
Data from our subgroup analyses suggest that low serum potassium was significantly associated with increased all-cause mortality among patients with ischemic heart disease, but not among those without ischemic heart disease (Figure 3). Data from animal and human studies suggest that hypokalemia is associated with increased risk of ventricular arrhythmias in ischemic heart.42–44 We also observed low serum potassium associated increased all-cause mortality was only observed among patients with chronic kidney disease but not among those without chronic kidney disease (Figure 3). Diuretic use is a common cause of hypokalemia, and HF patients with chronic kidney disease are more likely to use diuretics.45 Yet, hyperkalemia, rather than hypokalemia, is more common in chronic kidney disease, and is a reason for concern for adverse outcomes in these patients.46 However, our data suggest that in the context of HF and chronic kidney disease, low serum potassium may be associated with increased adverse outcomes.
Clinical implications
Low potassium, well tolerated in healthy adults, is believed to increase risks of morbidity and mortality in patients with cardiovascular disease.44 However, the effect of low potassium on outcomes in HF is not well studied. Our findings suggest that maintaining serum potassium >4 mEq/L may improve survival in chronic HF. Serum potassium of <3.5 mEq/L is clinically considered low potassium. However, our findings suggest that serum potassium <4 mEq/L may be associated with increased mortality. Data from hypertension studies indicate that use of diuretics is an important cause of low potassium.8, 44 Diuretics are commonly used in HF and may be associated with increased mortality and morbidity in HF.7 Diuretics should be avoided in New York Heart Association (NYHA) class I and II patients who are euvolemic and are receiving appropriate neurohormonal blockade. For patients with NYHA class III–IV patients with fluid overload, who must be treated with diuretics and spironolactone (or eplerenone for post-myocardial infarction patients) may be used to antagonize the effects of aldosterone and prevent hypokalemia, carefully avoiding hyperkalemia.15, 16 Alternately, potassium supplement may also be used to avoid hypokalemia. Data from rat hypertension models suggest that a high-potassium diet may be protective in part via the inhibition of cardiac nicotinamide-adenine dinucleotide phosphate oxidase activities.47 However, no such data are available for human HF. Our findings also suggest that despite the known potential short-term adverse effects of digoxin in patients with low potassium, no such interaction of digoxin and low potassium on mortality were observed in our analysis.
Comparison with other published studies
The effects of low sodium on HF outcomes are well reported in the literature.48, 49 The effects of low potassium on outcomes in hypertension is also well reported.5, 8, 11 However, to the best of our knowledge, this is the first report of the long-term effect of low potassium on mortality and hospitalization in HF.
Strengths and limitations
Propensity score matching was an obvious strength of our study, which also provided the most conservative estimates. However, like any non-randomized design, propensity matching may not be able to balance unmeasured confounders. Even though a sensitivity analysis cannot confirm the presence of such an unmeasured confounder, the results of our sensitivity analysis suggests that our main conclusions may be sensitive to a hidden confounder.34 However, for any unmeasured or hidden covariate to become a confounder, it must be strongly associated with both low potassium and mortality, and not be strongly associated with any of the covariates used in the propensity score model.
We were able to match all but 2 patients with low potassium, in contrast to typical ~60% matching in other studies.26, 50 Our matching protocol also allowed near-exact matching by the propensity score. The results of our study are based on predominantly white, male, and relatively younger HF patients with normal sinus rhythm and not receiving beta-blockers, thus limiting their generalizability. Other limitations of our study include lack of data on dose of diuretics and sudden cardiac deaths. Finally, we had no data on serum magnesium, and thus do not know to what extent the observed effect may be caused by low magnesium.
Conclusions
We observed associations between low potassium and increased mortality in a wide spectrum of ambulatory patients with chronic mild to moderate systolic and diastolic HF. Low serum potassium in HF may be caused by diuretic therapy or may be marker of increased neurohormonal activity and disease progression. Diuretics should be avoided in HF patients who are asymptomatic or minimally symptomatic without fluid overload. In symptomatic HF patients with fluid overload and receiving diuretics, normal serum potassium should be maintained using potassium-sparing diuretics or potassium supplementation.
Table 3.
Cause-specific hospitalizations in heart failure patients for serum potassium ≤4 mEq/L
| Cause for hospitalization* | Rate/10,000 person-years follow up (number of events/follow-up in years) | Rate difference* (/10,000 person-years) | Matched hazard ratio (95% confidence interval)† | P value | |
|---|---|---|---|---|---|
| Serum potassium ≥4 mEq/L (N = 1187) | Serum potassium <4 mEq/L (N = 1187) | ||||
| All-cause | 3846 (768/1997) | 4243 (796/1876) | + 397 | 1.12 (0.99–1.27) | 0.073 |
| Cardiovascular | 3621 (865/2389) | 3980 (888/2231) | + 359 | 1.13 (0.99–1.29) | 0.082 |
| Worsening heart | 1766 (509/2883) | 2004 (546/2725) | + 238 | 1.14 (0.99–1.31) | 0.075 |
| Ventricular arrhythmia, | 172 (58/3369) | 169 (55/3261) | − 3 | 0.94 (0.59–1.52) | 0.808 |
| SV arrhythmias§ | 194 (65/3359) | 255 (82/3218) | + 61 | 1.20 (0.79–1.83) | 0.394 |
| AV block, bradyarrhythmia | 17 (6/3434) | 24 (8/3308) | +7 | 2.50 (0.49–12.89) | 0.273 |
| Suspected digoxin toxicity | 76 (26/3400) | 66 (22/3303) | −10 | 0.61 (0.29–1.29) | 0.198 |
| Myocardial infarction | 265 (89/3361) | 320 (104/3249) | + 55 | 1.40 (0.96–2.05) | 0.085 |
| Unstable angina | 683 (218/3193) | 644 (196/3042) | − 39 | 1.20 (0.92–1.57) | 0.176 |
| Stroke | 242 (81/3354) | 265 (86/3247) | + 23 | 0.85 (0.55–1.30) | 0.448 |
| Coronary revascularization¶ | 82 (28/3395) | 104 (34/3272) | +21 | 1.19 (0.61–2.31) | 0.613 |
| Cardiac transplantation | 53 (18/3421) | 21 (7/3300) | −32 | 0.86 (0.29–2.55) | 0.782 |
| Other cardiovascular** | 632 (203/3211) | 687 (209/3043) | + 55 | 1.11 (0.85–1.45) | 0.453 |
| Respiratory infection | 415 (138/3327) | 382 (122/3196) | −33 | 1.06 (0.76–1.50) | 0.728 |
| Other non-cardiovascular | 2054 (561/2731) | 2086 (554/2656) | + 32 | 1.03 (0.87–1.21) | 0.735 |
| Unspecified | 23 (8/3425) | 18 (6/3311) | −5 | 0.75 (0.17–3.35) | 0.706 |
| Number of hospitalizations | 14696 (3389/2306) | 13708 (3438/2508) | −988 | ||
Data shown include the first hospitalization of each patient due to each cause.
Absolute rate differences were calculated by subtracting the rates of hospitalization in the normal-potassium group from the rates of hospitalization in the low-potassium group
Hazard ratios and confidence intervals (CI) were estimated from a Cox proportional-hazards models that used the first hospitalization of each patient for each reason.
Supraventricular (SV) arrhythmias include Atrioventricular (AV) block and bradyarrhythmias
This category includes coronary-artery bypass grafting and percutaneous transluminal coronary angioplasty
This category includes embolism, venous thrombosis, peripheral vascular disease, hypertension, other vascular surgery, cardiac catheterization, other types of catheterization, pacemaker implantation, installation of automatic implantable cardiac defibrillator, electrophysiologic testing, transplant-related evaluation, nonspecific chest pain, atherosclerotic heart disease, hypotension, orthostatic hypotension, and valve operation
Acknowledgments
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Institute on Aging (5-K23-AG019211-04) and the National Heart, Lung, and Blood Institute (5-R01-HL085561-02 and P-50-HL077100).
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Footnotes
Conflict of Interest Disclosures: None
Author Contributions
A.A. conceived the study hypothesis and design, and wrote the first draft of the manuscript. A.A. conducted statistical analyses in consultation with T.E.L. All authors interpreted the data, participated in critical revision of the paper for important intellectual content, and approved the final version of the article. A.A. had full access to the data.
References
- 1.Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43:155–161. doi: 10.1016/j.jacc.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Young DB. Potassium depletion and diastolic dysfunction. Hypertension. 2006;48:201–202. doi: 10.1161/01.HYP.0000232615.73578.3d. [DOI] [PubMed] [Google Scholar]
- 3.Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation. 1999;100:1311–1315. doi: 10.1161/01.cir.100.12.1311. [DOI] [PubMed] [Google Scholar]
- 4.Multiple Risk Factor Intervention Trial Research Group. Multiple risk factor intervention trial, risk factor changes and mortality results. JAMA. 1982;248:1465–1477. [PubMed] [Google Scholar]
- 5.Siscovick DS, Raghunathan TE, Psaty BM, Koepsell TD, Wicklund KG, Lin X, Cobb L, Rautaharju PM, Copass MK, Wagner EH. Diuretic therapy for hypertension and the risk of primary cardiac arrest. N Engl J Med. 1994;330:1852–1857. doi: 10.1056/NEJM199406303302603. [DOI] [PubMed] [Google Scholar]
- 6.Cosin J, Diez J. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507–513. doi: 10.1016/s1388-9842(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laragh JH, Sealey JE. K(+) depletion and the progression of hypertensive disease or heart failure. The pathogenic role of diuretic-induced aldosterone secretion. Hypertension. 2001;37:806–810. doi: 10.1161/01.hyp.37.2.806. [DOI] [PubMed] [Google Scholar]
- 9.Fitzovich DE, Hamaguchi M, Tull WB, Young DB. Chronic hypokalemia and the left ventricular responses to epinephrine and preload. J Am Coll Cardiol. 1991;18:1105–1111. doi: 10.1016/0735-1097(91)90774-4. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro JI, Banerjee A, Reiss OK, Elkins N. Acute and chronic hypokalemia sensitize the isolated heart to hypoxic injury. Am J Physiol. 1998;274:H1598–1604. doi: 10.1152/ajpheart.1998.274.5.H1598. [DOI] [PubMed] [Google Scholar]
- 11.Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension. 2000;35:1025–1030. doi: 10.1161/01.hyp.35.5.1025. [DOI] [PubMed] [Google Scholar]
- 12.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 13.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 14.Walsh CR, Larson MG, Leip EP, Vasan RS, Levy D. Serum potassium and risk of cardiovascular disease: the Framingham heart study. Arch Intern Med. 2002;162:1007–1012. doi: 10.1001/archinte.162.9.1007. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 16.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 17.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 19.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Asso. 1984;79:516–524. [Google Scholar]
- 20.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 22.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brener SJ, Lytle BW, Casserly IP, Ellis SG, Topol EJ, Lauer MS. Predictors of revascularization method and long-term outcome of percutaneous coronary intervention or repeat coronary bypass surgery in patients with multivessel coronary disease and previous coronary bypass surgery. Eur Heart J. 2006;27:413–418. doi: 10.1093/eurheartj/ehi646. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira-Gonzalez IJ, Ribera A, Cascant P, Permanyer-Miralda G. Outcomes in off-pump vs. on-pump coronary artery bypass grafting stratified by pre-operative risk profile: an assessment using propensity score. Eur Heart J. 2006;27:2473–2480. doi: 10.1093/eurheartj/ehl256. [DOI] [PubMed] [Google Scholar]
- 25.Lenderink T, Boersma E, Gitt AK, Zeymer U, Wallentin L, Van de Werf F, Hasdai D, Behar S, Simoons ML. Patients using statin treatment within 24 h after admission for ST-elevation acute coronary syndromes had lower mortality than non-users: a report from the first Euro Heart Survey on acute coronary syndromes. Eur Heart J. 2006;27:1799–1804. doi: 10.1093/eurheartj/ehl125. [DOI] [PubMed] [Google Scholar]
- 26.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed A. A Propensity Matched Study of New York Heart Association Class and Natural History End Points in Heart Failure. Am J Cardiol In Press. 2007 doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed A, Ali M, Lefante CM, Mullick MS, Kinney FC. Geriatric heart failure, depression, and nursing home admission: An observational study using propensity score analysis. Am J Geriatr Psychiatry. 2006;14:867–875. doi: 10.1097/01.JGP.0000209639.30899.72. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: A propensity score analysis. Am Heart J. 2006;152:956–966. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic Kidney Disease Associated Mortality in Diastolic Versus Systolic Heart Failure: A Propensity Matched Study. Am J Cardiol In Press. 2007 doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13:841–853. doi: 10.1002/pds.969. [DOI] [PubMed] [Google Scholar]
- 32.Levesque R. Macro. In: Levesque R, editor. SPSS® Programming and Data Management, 2nd Edition A Guide for SPSS® and SAS® Users. Chicago, IL: SPSS Inc; [Last access date: June 4, 2005]. Available online at: http://wwwspsscom/spss/data_management_bookhtm. [Google Scholar]
- 33.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–857. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum PR. Observational Studies. New York: Springer-Verlag; 2002. [Google Scholar]
- 35.SPSS. SPSS for Windows, Rel. 14. Chicago, IL: SPSS Inc., Chicago, IL; 2006. [Google Scholar]
- 36.Yano K, Hirata M, Matsumoto Y, Hano O, Mori M, Ahmed R, Mitsuoka T, Hashiba K. Effects of chronic hypokalemia on ventricular vulnerability during acute myocardial ischemia in the dog. Jpn Heart J. 1989;30:205–217. doi: 10.1536/ihj.30.205. [DOI] [PubMed] [Google Scholar]
- 37.Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 2003;42:705–708. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 38.Brilla CG, Rupp H, Funck R, Maisch B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodelling in congestive heart failure. Eur Heart J. 1995;16(Suppl O):107–109. doi: 10.1093/eurheartj/16.suppl_o.107. [DOI] [PubMed] [Google Scholar]
- 39.Sealey JE, Laragh JH. A proposed cybernetic system for sodium and potassium homeostasis: coordination of aldosterone and intrarenal physical factors. Kidney Int. 1974;6:281–290. doi: 10.1038/ki.1974.114. [DOI] [PubMed] [Google Scholar]
- 40.Weber MA, Drayer JI, Rev A, Laragh JH. Disparate patterns of aldosterone response during diuretic treatment of hypertension. Ann Intern Med. 1977;87:558–563. doi: 10.7326/0003-4819-87-5-558. [DOI] [PubMed] [Google Scholar]
- 41.Zannad F, Dousset B, Alla F. Treatment of congestive heart failure: interfering the aldosterone-cardiac extracellular matrix relationship. Hypertension. 2001;38:1227–1232. doi: 10.1161/hy1101.099484. [DOI] [PubMed] [Google Scholar]
- 42.Hohnloser SH, Verrier RL, Lown B, Raeder EA. Effect of hypokalemia on susceptibility to ventricular fibrillation in the normal and ischemic canine heart. Am Heart J. 1986;112:32–35. doi: 10.1016/0002-8703(86)90674-5. [DOI] [PubMed] [Google Scholar]
- 43.Nordrehaug JE, von der Lippe G. Serum potassium concentrations are inversely related to ventricular, but not to atrial, arrhythmias in acute myocardial infarction. Eur Heart J. 1986;7:204–209. doi: 10.1093/oxfordjournals.eurheartj.a062052. [DOI] [PubMed] [Google Scholar]
- 44.Gennari FJ. Hypokalemia. N Engl J Med. 1998;339:451–458. doi: 10.1056/NEJM199808133390707. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gennari FJ, Segal AS. Hyperkalemia: An adaptive response in chronic renal insufficiency. Kidney Int. 2002;62:1–9. doi: 10.1046/j.1523-1755.2002.00350.x. [DOI] [PubMed] [Google Scholar]
- 47.Matsui H, Shimosawa T, Uetake Y, Wang H, Ogura S, Kaneko T, Liu J, Ando K, Fujita T. Protective effect of potassium against the hypertensive cardiac dysfunction: association with reactive oxygen species reduction. Hypertension. 2006;48:225–231. doi: 10.1161/01.HYP.0000232617.48372.cb. [DOI] [PubMed] [Google Scholar]
- 48.Oren RM. Hyponatremia in congestive heart failure. Am J Cardiol. 2005;95:2B–7B. doi: 10.1016/j.amjcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Klein L, O’Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, Adams KF, Jr, Califf RM, Gheorghiade M. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation. 2005;111:2454–2460. doi: 10.1161/01.CIR.0000165065.82609.3D. [DOI] [PubMed] [Google Scholar]
- 50.Gum PA, Thamilarasan M, Watanabe J, Blackstone EH, Lauer MS. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: A propensity analysis. JAMA. 2001;286:1187–1194. doi: 10.1001/jama.286.10.1187. [DOI] [PubMed] [Google Scholar]