Abstract
The microvasculature consists of endothelial cells and their surrounding pericytes. Few studies on the regulatory mechanisms of tumour angiogenesis have focused on pericytes. Here we report the identification of tumour-derived PDGFRβ+ (platelet-derived growth factor receptor β) progenitor perivascular cells (PPCs) that have the ability to differentiate into pericytes and regulate vessel stability and vascular survival in tumours. A subset of PDGFRβ+ PPCs is recruited from bone marrow to perivascular sites in tumours. Specific inhibition of PDGFRβ signalling eliminates PDGFRβ+ PPCs and mature pericytes around tumour vessels, leading to vascular hyperdilation and endothelial cell apoptosis in pancreatic islet tumours of transgenic Rip1Tag2 mice.
Blood vessels are composed of two interacting cell types: endothelial cells, which form the inner lining of the vessel wall, and perivascular cells (referred to as pericytes), vascular smooth muscle cells (vSMCs) or mural cells, which wrap around the vascular tube1–4. Owing to their contractile capabilities and their multiple cytoplasmic processes, pericytes have mainly been associated with stabilization and haemodynamic processes of blood vessels1,3. During blood vessel formation, pericytes first detach from the blood vessel wall that becomes degraded, thereby enabling endothelial cells to move into the surrounding matrix to form new blood vessels5,6. Immature blood vessels can form without or with pericyte contact7,8. Pericytes are recruited by PDGF-B-expressing endothelial cells to remodel, stabilize and mature the new vascular tube2,9,10. Indeed, mice that lack either PDGF-B or PDGFRβ show severe deficits in pericyte coverage of blood vessels11–13, leading to widespread microvascular leakage, haemorrhage and oedema formation10,14,15. It is believed that pericytes govern vessel stability and function through paracrine and cell–cell contact with endothelial cells by influencing each other’s mitotic rate, differentiation and growth arrest2,9,16,17.
During pathological angiogenesis in tumours, pericytes appear to have structural and morphological abnormalities. They are frequently more loosely associated with blood vessels, and reduced in number when compared with normal tissue18,19. For these reasons, pericytes are regarded as abnormal or dysfunctional in tumours but are rather neglected as important cell constituents in tumour angiogenesis.
Recent data from our group and others point in the opposite direction. During a study of the underlying mechanisms of stage-specific regulation of angiogenesis in the transgenic mouse model of pancreatic islet carcinogenesis (Rip1Tag2)20, we found that the broad spectrum receptor tyrosine kinase inhibitor SU6668, which preferably targets PDGFRβ, is able to regress blood vessels by detaching pericytes from tumour vessels in bulky tumours and thereby restricts tumour growth and stabilizes the cancer20. In addition, SU6668 diminished pericytes in xenograft tumours and restricted tumour growth21,22. Taken together, these data suggest that tumour pericytes are important vascular cell constituents that support the tumour vasculature. We report here the identification of PDGFRβ+ perivascular progenitor cells in tumours that are partly recruited from the bone marrow and differentiate into mature pericytes eliciting vascular stabilization, maturation and survival.
RESULTS
PDGFRβ+ perivascular cells are distinct from mature pericytes in tumours
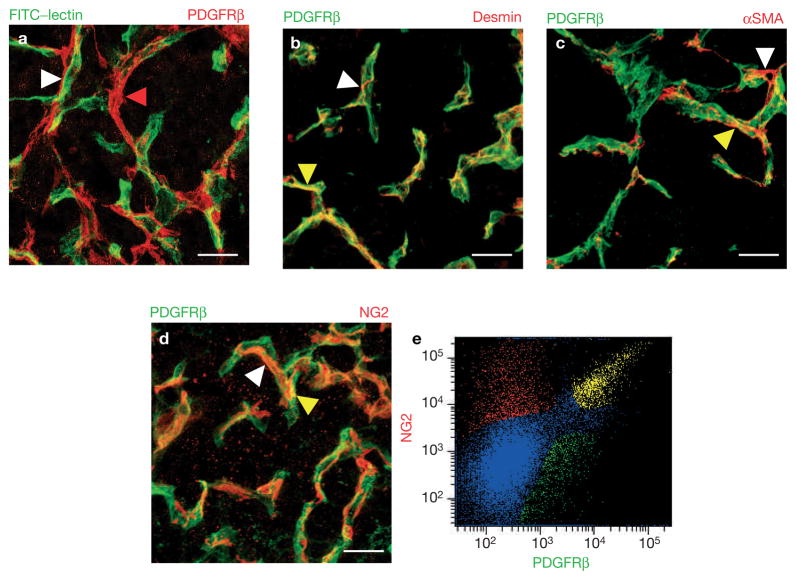
Previously, we reported that PDGFRβ+ cells in pancreatic islet tumours of Rip1Tag2 mice are closely associated with the tumour vasculature20 (Fig. 1a) and defined them as perivascular cells (PDGFRβ+ PVCs). We also demonstrated that the PDGF ligands B and D for PDGFRβ are expressed in tumour endothelial cells20, reflective of paracrine communication pathways similar to the vascular processes during development10,23,24. To elucidate the nature of PDGFRβ+ PVCs in islet tumours, we stained tumour tissue sections with an antibody for PDGFRβ and antibodies for mature pericyte markers (desmin, Fig. 1b; αSMA, Fig. 1c; or NG2, Fig. 1d; red) to visualize coexpression in vivo. Surprisingly, we observed only a partial overlap of PDGFRβ and the three mature pericyte markers, although all antibodies clearly stained cells that were in close contact with the vasculature (Fig. 1a–d). We quantified the percentage of PDGFRβ+ PVCs that expressed NG2 (Fig. 1e) by fluorescent activated cell sorting (FACS) in tumours. We obtained three distinct cell populations from pancreatic tumours: 46% were PDGFRβ-positive but NG2-negative; 26% were double-positive for NG2 and PDGFRβ; and 28% only expressed NG2 but not PDGFRβ (Fig. 1e). We then transferred FACS-isolated PDGFRβ+ PVCs onto tissue slides to determine the percentage of desmin+, NG2+ or αSMA+ cells (Fig. 2a, c, e) by immunocytochemistry. Congruently, only a subset of PDGFRβ PVCs expressed mature pericyte markers (Fig. 2a, c, e), as 14–15% were either positive for desmin, NG2, or αSMA by immunohistochemical staining (Fig. 2g; day 0). The slightly higher number of NG2+/PDGFRβ+ cells revealed by FACS is very probably attributable to the specific FACS gate setting.
Figure 1.
PDGFRβ+ cells are perivascular cells (PDGFRβ+ PVC) but are distinct from mature pericytes in tumours. (a) Blood vessels in pancreatic tumours were visualized with FITC-labelled tomato lectin (Lycopersicon esculentum) that was injected intravenously into 13-week-old Rip1Tag2 mice prior to killing them. Tumour sections were then stained with a red-labelled anti-PDGFRβ antibody. PDGFRβ+ cells are in close adjunction to blood vessels (white arrowhead) and can bridge between blood vessels (red arrowhead). (b–d) Immunohistochemical analysis of PDGFRβ+ cells24 and mature pericytes (red). Tumour sections were co-stained with anti-PDGFRβ24 and anti-desmin (b), anti-αSMA (c) or anti-NG2 (d) to reveal colocalization. Predominantly, PDGFRβ+ cells were distinct from mature pericytes (white arrowheads), but expression overlapped in a few areas (yellow arrowheads)20. (e) Quantification of NG2+/PDGFRβ+ cell populations in Rip1Tag2 pancreatic tumours. Tumours were dispersed into single cells, incubated with antibodies for PDGFRβ24 and NG2 (red) and then sorted by FAC. Three cell populations were revealed: 46% expressed PDGFRβ but not NG2, 26% were immunoreactive for both PDGFRβ and NG2, while 28% only expressed NG2. αSMA+ and desmin+ pericytes could not be isolated by FACS due to the nature of the commercially available antibodies. Scale bars, 8.7 mm.
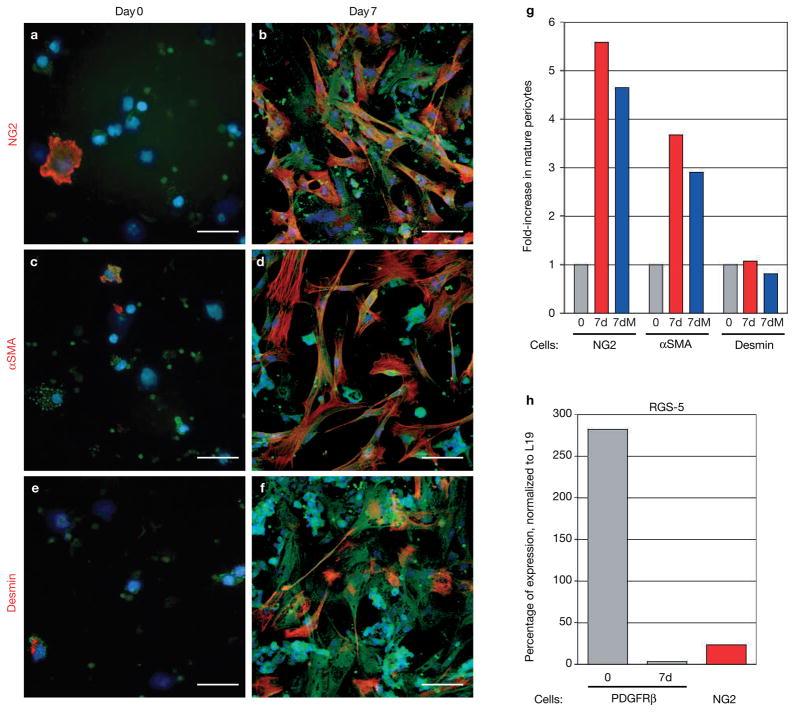
Figure 2.
PDGFRβ+ cells differentiate into mature pericytes in vitro. (a–f) PDGFRβ+ cells were isolated from tumours with a green fluorescent PDGFRβ antibody that was coated to magnetic beads and then cultured in vitro. Cells were immunolabelled with antibodies for the mature pericyte markers NG2 (a, b), αSMA (c, d) or desmin (e, f) immediately after the cells had settled onto the dish (day 0; a, c, e) or after 7 days in culture (day 7; b, d, f). (g) PDGFRβ+ cells that were positive for either NG2, αSMA or desmin, were counted at day 0 and day 7 to reveal the induction of mature pericytes. Whereas NG2+ and αSMA+ cells increased about fourfold after 7-day culture when compared with freshly isolated cells, desmin+ cells did not expand in number during the cell culture. Addition of the cell-cycle blocker mitomycin-C (5–10 μg ml−1; 7dM) during culture did not change the ratio of pericyte differentiation. (h) Quantitative Taqman RT–PCR analysis for RGS-5, a marker of developing and angiogenic pericytes, was performed on total RNA isolated from tumour-derived PDGFRβ+ (freshly isolated and cultured for 7 days) and NG2+ cells in Rip1Tag2 tumours. Expression levels were normalized to those of L19. RGS-5 levels were very high in isolated PDGFRβ+ cells (day 0) but markedly reduced in differentiated PDGFRβ+ cells in culture (day 7), and in mature tumour-derived NG2+ pericytes. Scale bars, 9 mm.
Thus, in Rip1Tag2 tumours, only a subset of PDGFRβ+ PVCs expressed mature pericyte markers and a subset of mature pericytes did not express detectable levels of PDGFRβ. How can we explain these three distinct populations? PDGFRβ is not exclusively expressed in pericytes, but also is present on stromal fibroblasts. Pancreatic Rip1Tag2 tumours are, however, stroma-poor and contain very few fibroblasts25,26. In addition, PDGFRβ+ cells are all closely associated with blood vessels in tumours. An alternative explanation is that the subset of cells positive for both PDGFRβ and mature pericyte markers could be an intermediate, more differentiated cell pool that is derived from a progenitor pool, that is, PDGFRβ+ PVCs, which then further mature into PDGFRβ-negative cells.
PDGFRβ+ PVCs express RGS-5, a marker for developing and angiogenic pericytes/vSMCs
If PDGFRβ+ PVCs in tumours are indeed activated progenitor cells, then they should reflect the expression profile of developing pericytes. We found mRNA of RGS-5 — a marker for developing activated pericytes/vSMCs in the embryo27,28 — to be highly expressed in PDGFRβ+ PVCs (Fig. 2h), but markedly downregulated in the more mature NG2-positive pericytes (Fig. 2h). Interestingly, RGS-5 is also expressed in activated pericytes in the adult at sites of physiological or pathological angiogenesis29.
Tumour-derived PDGFRβ+ PVCs differentiate into mature pericytes
To evaluate whether PDGFRβ+ PVCs could be pericyte progenitors, we induced differentiation of PDGFRβ+ PVCs in vitro. Tumour-derived isolated PDGFRβ+ cells were very small with endothelial cells or tumour cells (Fig. 2a, c, e) and grew very slowly, but after 2 days started to spread out and enlarge, resembling the in vivo morphology of a pericyte with extensive cellular protrusions (Fig. 2b, d, f). After 7 days of culture, we observed a five- to sixfold increase in the number of NG2+ and αSMA+ cells, but no increase in desmin+ cells, compared with the PDGFRβ+ cell population at day 0 (Fig. 2g). To rule out that the increase in NG2+ and αSMA+ cells is caused by selective growth of subpopulations, we cultured PDGFRβ+ PVCs in the presence of the cell-cycle blocker mitomycin-C (Fig. 2g; 7dM). We did not observe any changes in the number of differentiated cells when compared with cells that were grown without mitomycin-C. The fact that PDGFRβ+ PVCs are only able to differentiate into NG2+ or αSMA+ cells suggests that they are progenitors for these pericyte subpopulations, or that essential conditions are missing that allow the differentiation into desmin+ pericytes.
Tumour-derived PDGFRβ+ PVCs require endothelial cells to differentiate into desmin+ pericytes
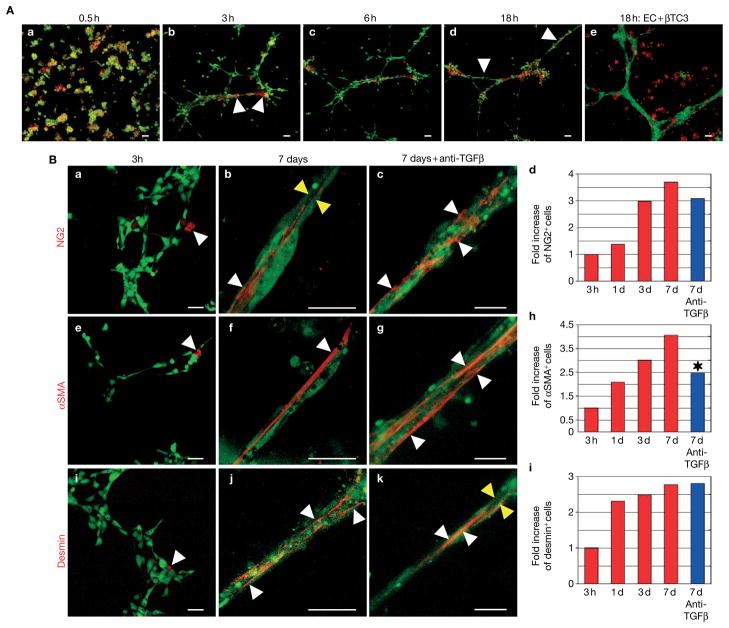
Pericytes are in close physical contact with endothelial cells in vivo but also communicate in a paracrine manner controlling each other’s growth and differentiation. We therefore determined whether PDGFRβ+ PVCs require endothelial cells for proper differentiation into pericytes. We pre-labelled primary human microvascular endothelial cells (HDMECs) with a green fluorescent vital dye and mixed them with PDGFRβ+ PVCs that were fluorescently labelled in red in a three-dimensional Matrigel assay in which endothelial cells form vascular tubes. We then observed vessel assembly with a confocal microscope over a period of 18 h using time-lapse imaging (Fig. 3A). Interestingly, endothelial cells and PDGFRβ+ PVCs were in close physical contact within 0.5–1 h. Vascular tube formation occurred rapidly after 5–6 h and complex aggregates were observed after 14–18 h. During the formation of the capillary network, PDGFRβ+ PVCs preferably clustered at branch points of endothelial tubes as seen in vivo in pancreatic islet tumours (Fig. 3A). Recruitment of PDGFRβ+ PVCs to the endothelial tubes was specific as βTC3 tumour cells, derived from Rip1Tag2 tumours, were unable to move towards blood vessels in vitro (Fig. 3A, e).
Figure 3.
PDGFRβ+ cells and endothelial cells in co-culture form pericyte-covered vascular tubes. (A) Time-lapse imagings of co-cultured endothelial cells24 and PDGFRβ+ cells (red) in 3D Matrigel at different time points. Human microvascular endothelial cells (HDMECs) were labelled with a green fluorescent vital dye and PDGFRβ+ cells, isolated from pancreatic tumours, with a red fluorescent vital dye and co-cultured in a 3D Matrigel. Vessel assembly was observed with a confocal microscope over a period of 18 h using time-lapse imaging. HDMECs were also cultured with the pancreatic islet tumour cells βTC3 over 18 h but these cells remained randomly distributed in the Matrigel (A, e). (B) Endothelial cell and PDGFRβ+ cell co-cultures form complex vascular tubes that are covered with mature periyctes leading to the induction of all three pericyte markers: NG2, desmin and αSMA. Endothelial cells24 and PDGFRβ+ cells (unlabelled) were co-cultured in Matrigel and the induction of NG2 (a–d), αSMA (e–h) and desmin (i–l) was visualized with red-labelled antibodies for the pericyte markers after 3 h (a, e, i) and 7 days in co-culture in the presence (b, f, j) or absence of TGFβ activity (c, g, k). Mature pericytes (red) elongate and wrap around endothelial tubes24 as indicated by white arrowheads. Endothelial tubes form a vessel lumen (yellow arrowheads). The increase in mature pericytes at 1-, 3- and 7-day cultures was quantified by comparing the ratio of pericytes at 1, 3 and 7 days to the numbers of cells at 3 h of incubation (d, h, l). In the presence of neutralizing TGFβ antibodies, αSMA+ cells, but not NG2+ or desmin+ cells, were reduced by 40% in a 7-day culture (*P = 0.0066). Scale bars, 2.3 mm (A), 5 mm (B, a, e, i), 14.9 mm (B, b, f, j), 9.9 mm (B, c, g, k).
We then monitored the vascular aggregates and quantified the number of mature pericytes at 1, 3 and 7 days of co-culture (Fig. 3B, d, h, l). After 1 day, we had already observed a twofold induction of NG2+, αSMA+ and desmin+ cells, which increased three- to fourfold after 7 days (Fig. 3B, d, h, l). These cells showed typical features of mature pericytes as they elongated along and wrapped around endothelial tubes (Fig. 3B). Notably, pericyte-covered vascular tubes with lumens formed when PDGFRβ+ PVCs were cultured with endothelial cells, closely resembling blood vessels in vivo (Fig. 3B, b, k; yellow arrowheads).
Which factors induce PDGFRβ+ PVC differentiation into pericytes? In vitro studies using mesenchymal and neural crest cell lines have implicated transforming growth factor-β (TGFβ) in smooth muscle cell and myofibroblast differentiation30–32. We found expression of TGFβ and its receptors TGFβRI and -RII on both tumour-derived endothelial cells and PDGFRβ+ PVCs as determined by polymerase chain reaction with reverse transcription (RT–PCR) analyses (data not shown). However, vessel assembly and pericyte coverage still occurred upon co-culture of endothelial cells and PDGFRβ+ PVCs in the presence of a neutralizing antibody for TGFβ (Fig. 3B, c, g, k), which successfully blocked differentiation of the mesenchymal 10T1/2 cell line into smooth-muscle-like cells31. Interestingly, whereas PDGFRβ+ PVCs matured into desmin+ and NG2+ pericytes in the absence of TGFβ activity (Fig. 3B, d, l), differentiation into αSMA+ cells was reduced by 40% (Fig. 3B, h). These data indicate that TGFβ affects PDGFRβ+ PVC differentiation into αSMA+ cells, but not differentiation into NG2+- and desmin+-positive pericytes. Independent of TGFβ activity, pericytes elongated and wrapped around endothelial tubes, forming stable aggregates (Fig. 3B, c, g, k). Taken together, our results demonstrate that PDGFRβ+ PVCs are a progenitor population of pericytes, and we therefore refer to them now as PDGFRβ+ PPCs (progenitor perivascular cells).
PDGFRβ+ PPCs originate from bone-marrow-derived haematopoietic Sca1+ stem cells
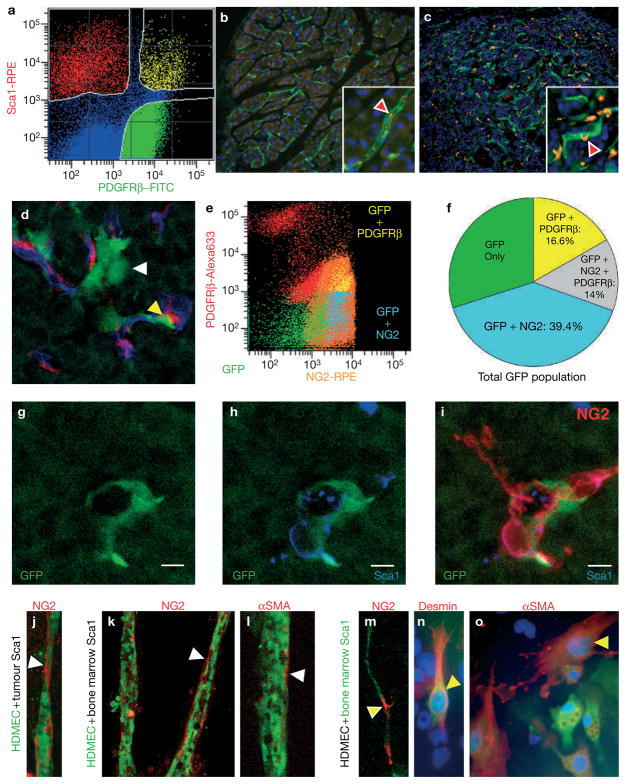
There are several mechanisms by which tumours could acquire PDGFRβ+ PPCs: tumours may activate quiescent PDGFRβ+ PPCs during angiogenesis, and/or may recruit them from other tissues. Recent findings point to recruitment from the bone marrow, since bone-marrow-derived haematopoietic cells expressing the pericyte marker NG2 are detected in close adjunction to blood vessels in a subcutaneous Bl6-F1 melanoma tumour model33. We tested PDGFRβ+ PPCs for the expression of several haematopoietic markers. By FACS analysis, we found that about 23% of PDGFRβ+ PPCs from pancreatic Rip1Tag2 tumours were immunoreactive for stem cell antigen-1 (Sca1), which is expressed on haematopoietic stem cells, and this accounted for about half of the Sca1+ cells in the tumours (Fig. 4a). Congruently, we found few small Sca1+ cells within blood vessels in the adjacent normal exocrine pancreas (Fig. 4b), but numerous Sca1+ cells in close association with tumour vessels in the Rip1Tag2 tumours (Fig. 4c). Furthermore, PDGFRβ+ PPCs expressed modest mRNA levels for c-Kit and VEGFR1 as determined by RT–PCR analysis, and 9.7% were immunoreactive for CD11b as quantified by FACS (see Supplementary Information, Fig. S1). To reveal whether pericyte progenitors are indeed recruited from a haematopoietic source such as the bone marrow, we transplanted GFP-expressing bone marrow cells from syngeneic actin–GFP mice into irradiated 10-week-old Rip1Tag2 mice and analysed the tumours 4 weeks after bone marrow transplantation (Fig. 4d–j). We identified about 1% of bone-marrow-derived GFP cells within the tumours of which many, but not all, were associated with the vasculature and expressed NG2 (Fig. 4d). FACS analysis further confirmed the identity of GFP cells that expressed PDGFRβ and/or NG2 (Fig. 4e): 16.6% were PDGFRβ-positive but NG2-negative, 14% were double-positive for NG2 and PDGFRβ, and about 40% expressed NG2 but not PDGFRβ (Fig. 4f). Interestingly, we found that GFP/NG2+ cells were a subset of Sca1+ cells (Fig. 4g–i), supporting our observation of perivascular Sca1+ cells covering blood vessels in the tumours (Fig. 4c). In contrast, we did not observe any CD45+ or CD11b+ cells that were positive for both NG2 and GFP, but identified a subset of CD45+ and CD11b+ GFP cells that are likely to be leukocytes and macrophages. If Sca1+ cells had been recruited specifically to the tumour at perivascular sites with the potential to differentiate into pericytes, then bone marrow and tumour-associated Sca1+ cells should have the propensity to differentiate into pericytes. We therefore isolated Sca1+ cells from the bone marrow or pancreatic tumours of Rip1Tag2 mice, and co-cultured them with HDMECs (Fig. 4). We then determined the incidence of NG2+, desmin+ or αSMA+ cells after 5-day co-culture with a red fluorescently labelled antibody. Regardless of whether Sca1+ cells were derived from the bone marrow (Fig. 4k–o) or from the tumour (Fig. 4j), associated with vascular tubes in a three-dimensional Matrigel culture (Fig. 4j–m) or were growing with endothelial cells as monolayers (Fig. 4n, o), we found that a subset of Sca1+ cells was induced to express NG2 (Fig. 4j, k, m) or αSMA (Fig. 4l, o), and to a much lesser extent desmin+ pericytes (Fig. 4n). Taken together, these data suggest that PDGFRβ+ PPCs are partly derived from bone-marrow-derived Sca1+ cells that are recruited to the angiogenic vasculature of pancreatic tumours.
Figure 4.
Tumour-associated PDGFRβ+ PPCs originate from bone-marrow-derived haematopoietic Sca1+ cells. (a) Quantification of Sca1+/PDGFRβ+ cell populations in Rip1Tag2 pancreatic tumours. Tumours were dispersed into single cells, incubated with antibodies for PDGFRβ and Sca1 (red) and then sorted by FACS. Three populations were revealed: 67% expressed PDGFRβ but not Sca1, 19% were immunoreactive for both Sca1 and NG2, while 17% expressed only Sca1. (b, c) Sca1+ cells (red) in normal exocrine pancreas (b) and in pancreatic islet tumours (c). The vasculature is visualized in green with FITC–tomato lectin. Higher magnifications in the boxes visualize Sca1+ cells within blood vessels in normal pancreas (b; red arrowhead), whereas numerous Sca1+ cells were observed in close association with tumour vessels in the perivascular space (c; red arrowhead). (d) Tumour sections of irradiated Rip1Tag2 mice that were reconstituted with bone marrow cells from syngeneic actin–GFP mice. Bone-marrow-derived cells are visualized in green, the vasculature was labelled with rhodamine–lectin and changed to false colour blue, and NG2+ cells were detected with an antibody for NG2 in red. Yellow arrowhead points to a GFP–NG2 double-positive cell. FACS analysis of GFP–bone-marrow-reconstituted tumours reveals GFP cells that are positive for PDGFRβ and/or NG2. (f) Quantitative analysis of GFP cells expressing PDGFRβ and/or NG2. (g–i) A subset of GFP cells in tumours (g) coexpress Sca1 (h) and NG2 (i). (j–o) Tumour- (j) and bone marrow (k–o)-isolated Sca1+ cells were co-cultured with HDMEC endothelial cells in a 3D Matrigel (j–m) or as monolayers on plastic dishes (n, o). The induction of NG2 (j, k, m), αSMA (l, o) and desmin (n) was visualized with red-labelled antibodies for the pericyte markers after 5 days in co-culture. Either isolated Sca1+ cells were co-cultured with pre-labelled HDMECs (green vital dye) (j–l), or Sca1+ cells were pre-labelled in green and cultured with unlabelled HDMEC (m–o). The latter allowed the identification of double-positive cells by their combined green and red colour when Sca1+ cells expressed a mature pericyte marker (m–o; yellow arrowheads). Sca1+ cells induced expression of NG2 (j, k, m), αSMA (l, n) and, to a lesser extent, desmin (o) after 5 days of culture. Scale bars, 7 mm (g–i).
Inhibition of PDGFRβ signalling depletes pericytes in tumours and increases endothelial cell apoptosis
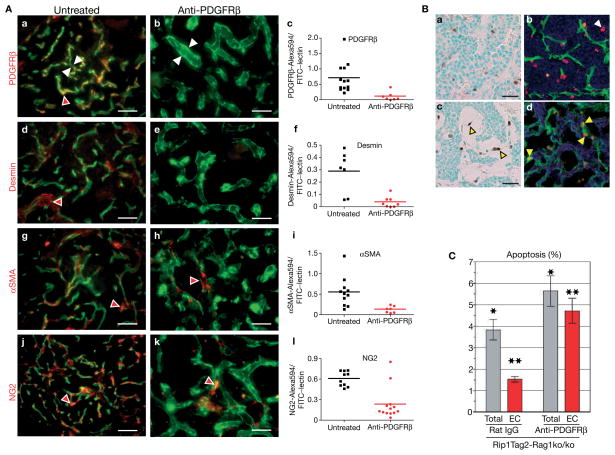
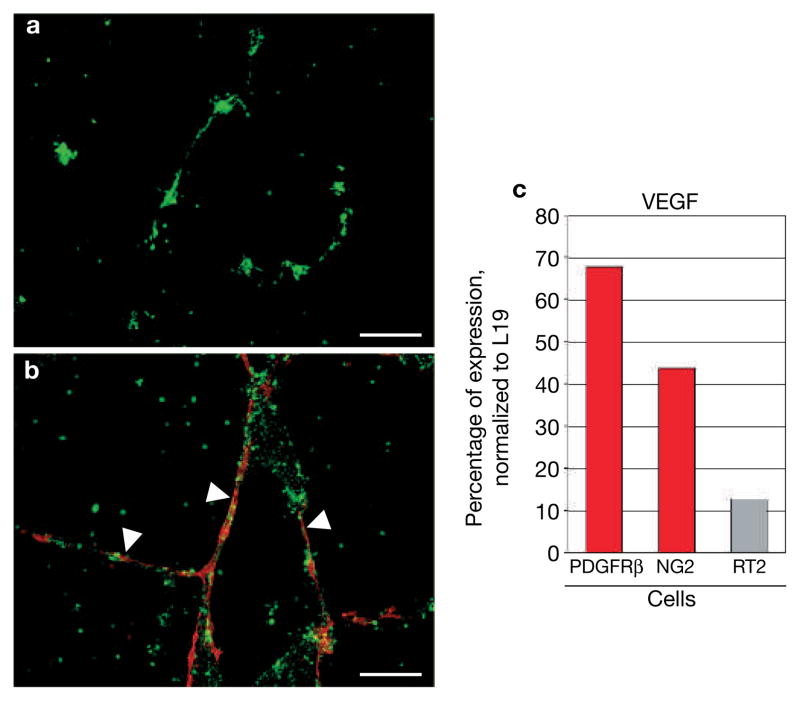
If PDGFRβ+ PPCs are able to mature into pericytes, then depletion of PDGFRβ+ PPCs should lead to an overall reduction of tumour pericytes. We treated 10-week-old tumour-bearing Rip1Tag2 mice, which were bred into an immunocompromised background (Rip1Tag2-Rag1ko/ko), daily with a neutralizing antibody for PDGFRβ or with control rat IgG for 3 weeks, then sacrificed the animals and analysed the morphology of the tumour vasculature and its pericyte coverage (Fig. 5A). The results were provocative because we not only depleted PDGFRβ+ PPCs from tumour vessels (Fig. 5A, a–c), but also markedly reduced the appearance of mature desmin+ (Fig. 5A, d–f), αSMA+ (Fig. 5A, g–i), and NG2+ pericytes (Fig. 5A, j–l). Quantitative evaluation of the number of pericytes by antibody staining (red) revealed an overall reduction of pericytes of 70–89% (Fig. 5A, c, f, i, l), compared with pericytes in untreated tumours. Tumours of transgenic mice treated with rat IgG, however, did not exhibit any pericyte detachment (see Supplementary Information, Fig. S2). In parallel with the absence of pericytes, tumour vessels were enlarged and hyperdilated, indicating that tumour pericytes have the capability to stabilize blood vessels (Fig. 5A, b, e, h, k). In addition, we found a 1.5-fold increase in apoptotic cells in tumours and a subsequent reduction in tumour growth (see Supplementary Information, Fig. S3) in anti-PDGFRβ-treated Rip1Tag2 mice when compared with rat IgG-treated mice (Fig. 5C). The apoptotic cells in treated tumours were predominantly located within the vascular lining (Fig. 5B, c), and co-staining of apoptotic cells with an antibody for CD31 identified them as endothelial cells (Fig. 5B, d), whereas apoptotic cells in control tumours were more randomly distributed (Fig. 5B, a, b). Quantitative analysis revealed that the majority of apoptotic cells were endothelial cells in tumours (85%) when mice were treated with anti-PDGFRβ-treated antibodies, whereas apoptotic cells in tumours of mice that had received rat IgG control consisted only of about 30% endothelial cells (Fig. 5C). It is important to note that neutralizing PDGFRβ antibodies did not affect pericyte numbers or attachment in normal organs such as the pancreas and liver (data not shown). The very high levels of apoptotic endothelial cells in the tumour vasculature devoid of pericytes further support the notion that tumour pericytes have protective functions for endothelial cells, probably by expressing important endothelial survival factors such as vascular endothelial growth factor (VEGF). Indeed, we found that VEGF expression levels were sevenfold and fourfold higher in isolated PDGFRβ+ and NG2+ pericytes, respectively, than in the whole tumour (Fig. 6c). In addition, vascular cord formation occurred quickly after 6–10 h in a Matrigel vascular tube assay in the absence (Fig. 6a) and presence of PDGFRβ+ cells (Fig. 6b), but the bare endothelial tubes started to break and clump after 48 h, whereas endothelial cords with surrounding PDGFRβ+ cells remained intact and stable until the experiment ended after 7 days of co-culture (Fig. 6a, b). Therefore, PDGFRβ+ PPCs and pericytes, which had differentiated from the PPCs during the 7-day incubation, stabilized and maintained the vascular tubes.
Figure 5.
Inhibition of PDGFRβ signalling depletes pericytes and increases endothelial cell apoptosis. (A) Anti-PDGFRβ treatment in immunocompromised Rip1Tag2-Rag1ko/ko mice depletes tumour pericytes. Ten-week-old Rip1Tag2-Rag1ko/ko mice (n = 8) were subjected to rat anti-mouse PDGFRβ or saline (control) for 3 weeks. Mice were injected intravenously with FITC-labelled tomato lectin prior to sacrifice to visualize the vasculature in green. Subsequently, tumour sections of control and treated mice were stained with a red-labelled antibody for PDGFRβ (a, b), desmin (d, e), αSMA (g, h) or NG2 (j, k). In contrast to control tumours, very few pericytes were observed in treated tumours (red arrowheads). Pericyte-depleted blood vessels were enlarged and hyperdilated with vessels in control tumours (white arrowheads). Quantitative evaluation of the number of PDGFRβ+ (c), desmin+ (f), αSMA+ (i) and NG2+ (l) cells was revealed on control and treated tumour sections by red antibody staining. The total area of red staining within the tumour boundaries within each image (7–13 images per set) was quantified using Improvision’s Volocity 2.6.1. Statistical analysis was performed with an unpaired t-test comparing the pericyte coverage of control-treated to anti-PDGFRβ tumours. P values were considered statistically strongly significant (P < 0.01).
(B) Anti-PDGFRβ treatment in Rip1Tag2-Rag1ko/ko mice increases endothelial cell apoptosis in tumours. Apoptotic cells in tumours of control (a, b) and anti-PDGFRβ-treated mice (c, d) were detected by TUNEL staining. Whereas apoptotic cells are more randomly distributed in control tumours (a), they are predominantly apparent in hyperdilated blood vessels of anti-PDGFRβ-treated tumours, reflecting endothelial cells undergoing apoptosis (c; yellow arrowheads). Co-staining of TUNEL-positive cells with an antibody against CD31 revealed the apoptotic index of endothelial cells in tumours of control rat IgG (b; white arrowhead) and anti-PDGFRβ-treated Rip1Tag2 mice (d; yellow arrowheads). (C) The apoptotic index of all cells (total) and endothelial cells (EC) in tumours of control rat IgG and anti-PDGFRβ-treated Rip1Tag2 mice. Six to seven tumour images containing a total of over 7,000 cells per group were used to determine the apoptotic index of the total cell population and the endothelial cell population within tumours. Statistical analysis comparing the rat IgG control group to the anti-PDGFRβ-treated group was performed with a two-tailed, unpaired t-test and P values were considered statistically significant (*P = 0.0038 for total apoptosis; **P = 0.0059 for endothelial cell apoptosis). Scale bars, 9.7 mm (A), 9.9 mm (B, a, c).
Figure 6.
PDGFRβ+ cells support vascular tube stability and survival. (a, b) Human microvascular endothelial cells (HDMECs) were labelled with a green fluorescent vital dye and cultured in a 3D Matrigel in the absence (a) or presence (b) of tumour-isolated PDGFRβ+ cells (pre-labelled in red). Vessel assembly occurred quickly in both situations, but bare endothelial tubes started to deteriorate after 2 days in culture resulting in endothelial cell clumps after 7 days (a). In contrast, PDGFRβ+ cell-covered tubes (white arrowheads) remained intact even after 7 days (b). (c) Quantitative RT–PCR analysis of VEGF from total RNA of tumour-isolated PDGFRβ+ cells, NG2+ cells and from total Rip1Tag2 tumours (RT2). VEGF transcription levels were normalized to levels of L19. VEGF transcription levels are highly enriched in PDGFRβ+ cells and NG2+ pericytes compared with VEGF levels in Rip1Tag2 tumours. Scale bars, 12.6 mm.
DISCUSSION
We have discovered a population of blood-vessel-associated PDGFRβ+ cells in pancreatic Rip1Tag2 tumours that elicit pericyte maturation and vascular stabilization and survival. Interestingly, only 15–20% express the mature pericyte markers desmin, NG2 or αSMA, whereas mature pericytes in these tumours are devoid of PDGFRβ. Our in vitro and in vivo evidence supports the conclusion that perivascular PDGFRβ+ cells are progenitors (PDGFRβ+ PPCs) that have the capacity to differentiate into desmin+, NG2+ and αSMA+ pericytes/vSMCs. Interestingly, we were able to dissect distinct differentiation pathways for pericytes. Whereas PDGFRβ+ PPCs induced NG2 and αSMA when cultured alone, co-culture of PDGFRβ+ PPCs with endothelial cells was crucial for the induction of desmin. We therefore propose that paracrine signalling circuits between endothelial cells and PDGFRβ+ PPCs drive induction to desmin+ pericytes in a cell-contact-dependent manner.
The proposition of distinct key factors triggering pathways that give rise to various pericyte subtypes raises the question of the identity of such factors. TGFβ seems to be required for differentiation into αSMA+ cells, because inhibiting TGFβ blocked differentiation to αSMA-positive pericytes, but not to desmin- and NG2-positive pericytes. Indeed, TGFβ appears to be involved in smooth muscle differentiation, since it induces αSMA in the immortalized neural crest stem cell line Monc-1 (ref. 32) and αSMA and SM22α, another contractile smooth muscle protein, in the mesenchymal cell line 10T1/2 (refs 31, 34). Another crucial factor is PDGF-B. When we abrogated the PDGFRβ-signalling pathway by treating tumour-bearing mice with a neutralizing PDGFRβ antibody, we depleted the tumours not only of PDGFRβ+ PPCs but also of mature pericytes.
Our data demonstrate that tumours share striking similarities with embryos. Similar to pancreatic islet tumours, PDGF-B is expressed by sprouting capillary endothelial cells during developmental angiogenesis, whereas its receptor PDGFRβ is localized on pericytes indicative of a paracrine signalling circuit. As with anti-PDGFRβ-treated tumours, PDGF-B- or PDGFRβ-deficient mice exhibit a marked reduction in the number of pericytes/vSMCs10,11,15,16, leading to hyperdilated blood vessels. In addition, fibrosarcomas transplanted into mice that cannot retain PDGF-B on the cell surface or in the extracellular matrix show fewer pericytes attached to the tumour vessels35, confirming the critical role of PDGFRβ signalling in pericyte activation and/or recruitment. In summary, these results support the hypothesis that pericytes in tumours, similar to those in the embryo, are formed de novo by maturation of undifferentiated perivascular cell progenitors recruited to the newly formed vasculature from bone marrow or from a pre-existing pool of pericytes. Abrogation of PDGFRβ signalling reduced the number of activated pericytes in tumours, whereas quiescent pericytes in normal tissues were not affected. A second implication from these data is that activated PDGFRβ+ PPCs are inhibited from multiplying and differentiating into mature pericytes. This hypothesis also reflects the situation in development where disruption of PDGF-B/PDGFRβ signalling hinders pericytes to expand and spread along the newly formed vessels due to their reduced proliferative and migratory capability2.
Where do PDGFRβ+ PPCs in the tumours come from? A small pool of these cells may exist in the tissue that then becomes activated when blood vessels need to be formed. Alternatively or additionally, a subset of PDGFRβ+ PPCs could be recruited from a different organ. Our novel finding that PDGFRβ+ PPCs are also bone-marrow-derived parallels the concept that tumours may recruit endothelial cell precursors from bone marrow36,37. PDGFRβ+ PPCs express haematopoietic stem cell markers including Sca1, CD11b and c-Kit. Bone marrow transplant experiments and cultures of bone-marrow- or tumour-derived Sca1+ cells with endothelial cells revealed that it is the Sca1+ cell population from the bone marrow that is recruited to angiogenic sites of the tumour and then matures into pericytes. Bone-marrow-derived cells that cover blood vessels and are either positive for NG2, CD11b or CD45 were detected in a subcutaneous B16-melanoma xenograft tumour model33 suggesting that the tumour type or location dictates which type of bone-marrow-derived cells are recruited to angiogenic sites. Taken together, these studies demonstrate that tumours are not only able to recruit endothelial precursor cells, but also pericyte precursor cells from the bone marrow during vascular remodelling.
Our results from anti-PDGFRβ-treated tumours also underline the functional importance of tumour pericytes. Pericyte-deprived tumour vessels were enlarged and hyperdilated, reminiscent of the phenotype in PDGF-B- or PDGFRβ-deficient embryos. These results imply that tumour pericytes, albeit less abundant or more loosely attached, still regulate vessel integrity, maintenance and function. The high increase in endothelial cell apoptosis, when pericytes are depleted from blood vessels, further strengthens the function of tumour pericytes in protecting endothelial cells, probably by expressing potent endothelial survival factors. One such factor, VEGF, which we found to be highly expressed in PDGFRβ+ PPCs, harks back to the observation that in vitro pericyte-associated endothelial tubes are more stable and viable than endothelial cords without them. In agreement with this finding, immature blood vessels without pericytes are more sensitive to anti-VEGF therapy38. Moreover, our group and others demonstrated that receptor tyrosine kinase inhibitors that target both pericytes and endothelial cells by combinatorial inhibition of PDGFR and VEGFR signalling, are very efficacious even in late-stage disease, disrupting the established tumour vasculature and effecting tumour regression20,22,39.
METHODS
Visualization of vasculature
To visualize blood vessels in tumours and normal tissue, mice were anaesthetized and injected intravenously with 0.05 mg FITC-labelled or rhodamine-labelled lectin (Lycopersicon esculentum; Vector Laboratories, Burlingame, CA) before the heart was perfused with 4% paraformaldehyde and the pancreas was frozen in OCT medium and sectioned at 25 and 50 μm.
Immunohistochemical analysis
Mice were anaesthetized, hearts perfused with 4% paraformaldehyde, pancreases collected, frozen in OCT medium, and 15 and 50 μm sections prepared. Pericytes were identified using the following antibodies: mouse anti-human desmin (1:100; DAKO Corp., Carpinteria, CA), mouse anti-human smooth muscle actin (1:500; DAKO Corp.), rabbit anti-mouse NG2 (1:500; Chemicon, Temecula, CA), mouse anti-human CD140b/PDGFRβ (1:200, BD Biosciences Pharmingen, San Diego, CA), rat anti-mouse PDGFRβ (1:100, eBiosciences, San Diego, CA), rabbit anti-mouse PDGFRβ 40 and R-Phycoerytherin (RPE)-conjugated rat anti-mouse Sca1 (1:200; BD Biosciences Pharmingen). Endothelial cells were visualized with a rat anti-mouse CD31 (1:100; BD Biosciences Pharmingen). For fluorescent visualization of the antibody reactions secondary AlexaFluor488- or AlexaFluor594-labelled secondary antibodies (1:200; Molecular Probes, Eugene, OR) were used. Photomicrographs were taken with a Zeiss LSM 510 confocal microscope, or a Zeiss Axiovert 2 microscope, using a Zeiss LSM 510 software (Zeiss, Thornwood, NY) or Openlab 3 software (Improvision, Lexington, MA) respectively. Levels in images were adjusted in Adobe Photoshop 7. The software program Volocity 2 (Improvision) was used to quantify pericyte coverage of blood vessels on tumour sections.
Apoptotic index
Apoptotic index was determined by TUNEL as previously described41. Reaction products were visualized with a red fluorescent antibody for Dixogenin (Roche, Alameda, CA). To reveal apoptotic endothelial cells, tumour tissues were labelled with FITC-tomato lectin. Eight fields of the respective sections were scored under immersion light microscopy (Zeiss Axiovert 2 microscope).
Isolation and preparation of pancreatic tumours
Mice were killed and tumours obtained from the pancreas. Tumours were minced with a razor blade and digested at 37 °C for 13 min with a 20 mL collagenase mix containing 0.2 g BSA (Sigma, St Louis, MO), 12,500 units Collagenase II, 12,500 units Collagenase IV, and 40 μl DNase I (Worthington Biochemical Corp., Lakewood, NJ). Cells from the digested tumours were passed through a 70 μm cell strainer and washed. Red blood cells were lysed with PharmLyse (BD Biosciences Pharmingen) and washed again. Cell pellets were resuspended in 1 × PBS plus 1% BSA. Cells were used for FACS analysis or for Dynal magnetic isolation.
FACS analysis
The cells were sorted on FACSVantage SE flow cytometer using the Cell Quest Pro software version 4 from Becton Dickinson Immunocytometry Systems (Franklin Lakes, New Jersey, MA) as described in ref. 20. Cells were pre-blocked with an Fc block (CD16/CD32; BD Biosciences Pharmingen) and then incubated with a primary antibody on ice. The following primary antibodies were used: CD31-FITC, 1:100; NG2, 1:200 (Chemicon); PDGFRβ, 1:100 (BD Biosciences Pharmingen); Sca1-RPE, 1:200 (BD Biosciences Pharmingen); and CD11b-AlexaFluor488, 1:100 (BD Biosciences Pharmingen). If the primary antibody was unlabelled, secondary fluorescently labelled antibodies with the fluorochromes R-Phycoerytherin, AlexaFluor488, AlexaFluor594, AlexaFluor633, or AlexaFluor647 (Molecular Probes) were added to the cell suspension.
RNA isolation and RT–PCR analysis
FACS-sorted cells were collected in a cell lysis solution containing β-mercaptoethanol (QIAGEN Inc., Valencia, CA). RNA was isolated following RNeasy Mini Kit protocols (QIAGEN Inc.) and transcribed into cDNA using iScript (Bio-Rad, Hercules, CA). cDNA was used for both quantitative PCR with iQ SYBR Green Supermix (Bio-Rad) and qualitative PCR (Eppendorf, Westbury, NY).
Dynal magnetic isolation, cell tracking and co-culture
Cells derived from tumours or bone marrow were incubated with primary PDGFRβ antibody (1:100; eBiosciences) or primary Sca1 antibody (1:100; BD Biosciences) respectively, washed, and then incubated with secondary sheep anti-rat M450 antibody labelled magnetic beads (Dynal, Lake Success, NY). Isolated cells were cultured in Nunc Lab-Tek Permanox culture slides (Nalge Nunc International, Rochester, NY) in stem cell or smooth-muscle cell medium with a variety of growth factors, including PDGF-B, bFGF and TGFβ, for observation. In some experiments, isolated cells were also labelled with CellTracker Red (Molecular Probes) and combined with CellTracker Green (Molecular Probes)-labelled cultured endothelial cells (primary HMDEC (Cambrex, Braintree, MA) or SV40Tag-immortalized HDMEC (CDC.EU/HMEC1))42 at a 1:3 ratio. We did not observe any differences in tube formation between primary and immortalized HDMEC. In addition, HDMEC were co-cultured with the pancreatic tumour cell line βTC3 that had been derived from RIP1TAG2 tumours43. Cells were placed onto Permanox culture slides (Nalge Nunc International) on a thin layer of growth-factor-reduced Matrigel (BD Discovery Labware, Bedford, MA). Co-cultures with normal HMDEC cells required EGM-MV2 (Cambrex), whereas immortalized CDC. EU/HMEC1 cells were grown in MCDB131 (Gibco/Invitrogen) supplemented with L-glutamine and 10% FBS. In addition, time points of 18 h, 3 days, and 7 days were taken as well. For co-cultures that were later stained with specific antibodies for mature pericyte markers, only one of either the magnetically isolated PDGFRβ+ cells, Sca1 cells or endothelial cells were stained with CellTracker Green (Invitrogen); primary and secondary antibodies mentioned above were used to identify the pericytes in the co-cultures. PDGFRβ+ PPC were also cultured in the presence of 5–10 μg ml−1 mitomycin-C (Sigma) without causing cell toxicity.
Time-lapse imaging
To determine the kinetics of pericyte-endothelial cell assembly and interaction, confocal time-lapse movies were acquired on the confocal microscope Zeiss LSM 510 using a ×10 Plan Neofluar lens. Co-cultures were placed in a 37 °C chamber while confocal movies were collected every 5 min for 18 h.
Quantification of pericyte maturation from in vitro cultures and tissue sections
For in vitro cultures, endothelial cells were labelled with a green tracking dye and co-cultured with unlabelled PDGFRβ+ perivascular cells. Mature pericytes were visualized with antibodies for desmin, NG2 or αSMA in red. Red and unlabelled cells were counted to reveal the percentage of red cells from the total cell population.
For tissue sections, fluorescent images of tumour sections were collected on a Zeiss Axioskop 2, with ×20 and ×40 Plan Neofluar lenses and a Zeiss Axiocam colour CCD. Red and green staining was quantitatively evaluated using Improvision’s Volocity 2.6.1. Classifiers were established for each image and were used to measure the total area of red and green staining within the tumour boundaries within each image. The tumour boundary was manually outlined using the Freehand Selection Tool and then Measure Objects was applied within that selection.
Treatment of transgenic mice
Ten-week-old male and female Rag1-deficient Rip1Tag2 transgenic mice (C57/Bl6J background) were injected with a neutralizing rat anti-mouse PDGFRβ antibody (eBiosciences; 0.5 mg per mouse per day, intraperitoneally (i.p.)) or with total rat IgG (Jackson ImmunoResearch, West Grove, PA; 0.5 mg per mouse per day, i.p.) for 3 weeks. Trials using the neutralizing PDGFRβ antibodies were repeated twice. Mice were maintained in accordance with the UCSF institutional guidelines governing the care of laboratory mice, killed after the treatment period, and tumours were analysed.
Bone marrow transplant
Ten-week old Rip1Tag2 transgenic mice (C57/Bl6J background) were irradiated with a lethal dose of 9 Gy and reconstituted with retro-orbital injection of 2 × 106 bone marrow cells isolated from the femur of syngeneic actin–GFP mice (C57/Bl6J6-TgN ACTbEGFP; Jackson Laboratory, Bar Harbor, Maine). Four weeks after transplantation, Rip1Tag2 transgenic mice were killed and pancreatic tumour tissues harvested. Tumour tissues from 70% of mice were used for immunohistochemical analyses and 30% of the tumours were taken for quantitative FACS analyses. The experiments were performed twice with a total of 16 mice.
Statistical analysis
All experiments were repeated two to four times. Statistical analyses were performed with a two-tailed unpaired Mann–Whitney test, or an unpaired t-test. P values < 0.01 were considered statistically significant. All calculations were performed using GraphPad Prism version 4.0a for Macintosh (GraphPad Software, San Diego, CA).
Supplementary Material
Figure S1 CD11b is expressed in a subset of PDGFRβ+ PPCs. Quantification of CD11b+/PDGFRβ+ cell populations in Rip1Tag2 pancreatic tumours. Tumours were dispersed into single cells, incubated with antibodies for PDGFRβ and CD11b and then sorted by FACS. Three populations were revealed: 18.1% expressed PDGFRβ but not CD11b, 9.7% were immunoreactive for both CD11b and PDGFRβ, while 72.2% expressed only CD11b.
Figure S2 Pericyte coverage of tumour vessels in Rip1Tag2-Ragko/ko mice treated with total rat IgG. Blood vessels in pancreatic tumours were visualised with FITC-labelled tomato lectin (Lycopersicon esculentum) that was injected intravenously into 13-week-old Rip1Tag2-Ragko/ko mice prior to sacrifice. Tumour sections were then stained with a red-labeled antibody for PDGFRβ (a), desmin (b), α-SMA (c), or NG2 (d). Pericytes remained attached to tumour vessels when Rip1Tag2 mice were treated with total rat IgG.
Figure S3 Anti-PDGFRb treatment in Rip1Tag2-Ragko/ko mice reduces tumour growth. 10-week-old Rip1Tag2-Ragko/ko mice bearing small tumours, were subjected to rat anti-mouse PDGFRβ (n=6), total rat IgG (n=4) or remained untreated (n=7) for 3 weeks. Mice were then euthanized and tumours microdissected from the excised pancreases. Tumour volume (cubic millimetres) was measured by using a ruler, applying the formula [volume=0.52 × (width)2 × (length)] for approximating the volume of a spheroid. Tumour burden per mouse was calculated by accumulating the tumour volume of every mouse.
Acknowledgments
We thank N. Boudreau for valuable discussions and advice, D. Hanahan for RipTag2-Rag1ko/ko mice, N. Korets for excellent technical assistance, A. McMillan for statistical analysis, and S. Reynolds for help with the manuscript preparation. This work was supported by grants from the National Institutes of Health (R01 CA109390, R01 CA99948, RO1 CA95287, P01 CA72006), by an NIH Institutional NRSA fellowship to A.J.E. (5T32HL007731), by a grant from the American Cancer Society, and by start-up funds to G.B. from the Department of Neurological Surgery at UCSF.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
References
- 1.Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- 2.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 3.Cleaver O, Melton DA. Endothelial signaling during development. Nature Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 4.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- 5.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 6.Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 7.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005;94:115–125. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 8.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-β, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 11.Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. Bioessays. 2001;23:494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- 12.Leveen P, et al. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 13.Lindahl P, Johansson B, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;126:3047–3055. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 14.Hellstrom M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enge M, et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- 17.Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and α3β1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin L, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa S, et al. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinmuth N, et al. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. Faseb J. 2001;15:1239–1241. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- 22.Shaheen RM, et al. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res. 2001;61:1464–1468. [PubMed] [Google Scholar]
- 23.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 24.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–218. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Parangi S, et al. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci USA. 1996;93:2002–2007. doi: 10.1073/pnas.93.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergers G, Hanahan D, Coussens LM. Angiogenesis and apoptosis are cellular parameters of neoplastic progression in transgenic mouse models of tumorigenesis. Int J Dev Biol. 1998;42:995–1002. [PubMed] [Google Scholar]
- 27.Bondjers C, et al. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162:721–729. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. Faseb J. 2003;17:440–442. doi: 10.1096/fj.02-0340fje. [DOI] [PubMed] [Google Scholar]
- 29.Kanamori M, Vanden Berg SR, Bergers G, Berger MS, Pieper RO. Integrin β3 overexpression suppresses tumor growth in a human model of gliomagenesis: implications for the role of β3 overexpression in glioblastoma multiforme. Cancer Res. 2004;64:2751–2758. doi: 10.1158/0008-5472.can-03-3354. [DOI] [PubMed] [Google Scholar]
- 30.Nishishita T, Lin PC. Angiopoietin 1, PDGF-B, and TGF-β gene regulation in endothelial cell and smooth muscle cell interaction. J Cell Biochem. 2004;91:584–593. doi: 10.1002/jcb.10718. [DOI] [PubMed] [Google Scholar]
- 31.Darland DC, D’Amore PA. Cell-cell interactions in vascular development. Curr Top Dev Biol. 2001;52:107–149. doi: 10.1016/s0070-2153(01)52010-4. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Lechleider RJ. Transforming growth factor-β-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res. 2004;94:1195–1202. doi: 10.1161/01.RES.0000126897.41658.81. [DOI] [PubMed] [Google Scholar]
- 33.Rajantie I, et al. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding R, Darland DC, Parmacek MS, D’Amore PA. Endothelial-mesenchymal interactions in vitro reveal molecular mechanisms of smooth muscle/pericyte differentiation. Stem Cells Dev. 2004;13:509–520. doi: 10.1089/scd.2004.13.509. [DOI] [PubMed] [Google Scholar]
- 35.Abramsson A, et al. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–117. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- 36.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 37.Rabbany SY, Heissig B, Hattori K, Rafii S. Molecular pathways regulating mobilization of marrow-derived stem cells for tissue revascularization. Trends Mol Med. 2003;9:109–117. doi: 10.1016/s1471-4914(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal [see comments] J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2004;23:939–952. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 40.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 41.Naik P, Karrim J, Hanahan D. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to progression from angiogenic progenitors. Genes Dev. 1996;10:2105–2116. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- 42.Ades EW, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 43.Radvanyi F, Christgau S, Baekkeskov S, Jolicoeur C, Hanahan D. Pancreatic β cells cultured from individual preneoplastic foci in a multistage tumorigenesis pathway: a potentially general technique for isolating physiologically representative cell lines. Mol Cell Biol. 1993;13:4223–4232. doi: 10.1128/mcb.13.7.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 CD11b is expressed in a subset of PDGFRβ+ PPCs. Quantification of CD11b+/PDGFRβ+ cell populations in Rip1Tag2 pancreatic tumours. Tumours were dispersed into single cells, incubated with antibodies for PDGFRβ and CD11b and then sorted by FACS. Three populations were revealed: 18.1% expressed PDGFRβ but not CD11b, 9.7% were immunoreactive for both CD11b and PDGFRβ, while 72.2% expressed only CD11b.
Figure S2 Pericyte coverage of tumour vessels in Rip1Tag2-Ragko/ko mice treated with total rat IgG. Blood vessels in pancreatic tumours were visualised with FITC-labelled tomato lectin (Lycopersicon esculentum) that was injected intravenously into 13-week-old Rip1Tag2-Ragko/ko mice prior to sacrifice. Tumour sections were then stained with a red-labeled antibody for PDGFRβ (a), desmin (b), α-SMA (c), or NG2 (d). Pericytes remained attached to tumour vessels when Rip1Tag2 mice were treated with total rat IgG.
Figure S3 Anti-PDGFRb treatment in Rip1Tag2-Ragko/ko mice reduces tumour growth. 10-week-old Rip1Tag2-Ragko/ko mice bearing small tumours, were subjected to rat anti-mouse PDGFRβ (n=6), total rat IgG (n=4) or remained untreated (n=7) for 3 weeks. Mice were then euthanized and tumours microdissected from the excised pancreases. Tumour volume (cubic millimetres) was measured by using a ruler, applying the formula [volume=0.52 × (width)2 × (length)] for approximating the volume of a spheroid. Tumour burden per mouse was calculated by accumulating the tumour volume of every mouse.