Abstract
Tissue engineering is expected to help us outlive the failure of our organs by enabling the creation of tissue substitutes capable of fully restoring the original tissue function. Degenerative joint disease, which affects one-fifth of the US population and is the country’s leading cause of disability, drives current research of actively growing, functional tissue grafts for joint repair. Toward this goal, living cells are used in conjunction with bio-material scaffolds (serving as instructive templates for tissue development) and bioreactors (providing environmental control and molecular and physical regulatory signals). In this review, we discuss the requirements for engineering customized, anatomically-shaped, stratified grafts for joint repair and the challenges of designing these grafts to provide immediate functionality (load bearing, structural support) and long-term regeneration (maturation, integration, remodeling).
Introduction
Cartilage provides load bearing and lubrication in our joints. Chondrocytes maintain the extracellular matrix (ECM) in healthy cartilage. However, when injured, cartilage has poor healing ability [1]. Joint disease and trauma often result in joint degeneration that requires surgical intervention, involving the use of artificial materials. Buckwalter and Lohmander stated that ‘no currently available synthetic material or combination of materials duplicates the ability of articular cartilage to provide a painless, low-friction gliding surface and to distribute loads across a synovial joint’ [2]. These limitations motivated the research of cell-based cartilage replacement. A major problem in implanting cartilage, either native or engineered, is its poor integration with the host. One approach to enhance graft–host integration is the implantation of osteochondral grafts –tissue composites containing cartilage that are layered over the underlying bone region (Box 1). We review here the engineering of osteochondral grafts with size, shape, structural and mechanical properties customized to the individual patient.
Box 1. Chondrogenic and osteogenic differentiation of mesenchymal stem cells
Formation of the skeleton requires the carefully coordinated interplay of many cell types, metabolic and regulatory factors. First, a loosely woven embryonic connective tissue, termed the mesenchyme, is formed with mesenchymal cells that can differentiate into many cell types, including chondrocytes and osteoblasts. In the areas of long bone formation, chondrocytes deposit a cartilaginous model of the skeletal elements. Chondrocytes within the centre of the shaft, the diaphysis, begin to increase in number and size. Hypertrophy of these chondrocytes is associated with apoptosis, matrix calcification and vascular invasion. Blood vessels bring stem cells that give rise to the bone-depositing osteoblasts and the bone-resorbing osteoclasts. The bone marrow cavity progressively expands toward the epiphyses – the ends of the bone – where the chondrocytes proliferate rapidly and form longitudinal columns of flattened cells. This is the primary growth plate with the characteristic zones of resting, proliferative, maturing and hypertrophic chondrocytes. Chondrocyte proliferation is balanced by chondrocyte apoptosis and the replacement of the calcified cartilage with bone. Later in development, secondary centres of ossification form within the cartilaginous epiphyses, and the longitudinal bone growth continues until puberty when chondrocyte proliferation ceases and the primary and secondary centres of ossification fuse. A thin layer of cartilage covering the joint surface remains, however, throughout adulthood, protecting the underlying bone and providing a smooth surface for articulation [68].
Tissue engineering is in essence an effort to recapitulate these developmental events. The actual ‘tissue engineers’ are living cells, and if placed into appropriate environments, the cells will proliferate and differentiate and assemble into appropriate tissue structures. For example, mesenchymal stem cells could be isolated for each patient and placed in a culture environment that would support cell differentiation and tissue development by providing a structural template, physiological conditions, local control of nutrients, growth factors and physical regulatory factors [12]. In general, there is a clear analogy between the conditions for normal tissue development and for the creation of functional tissue grafts (Figure I).
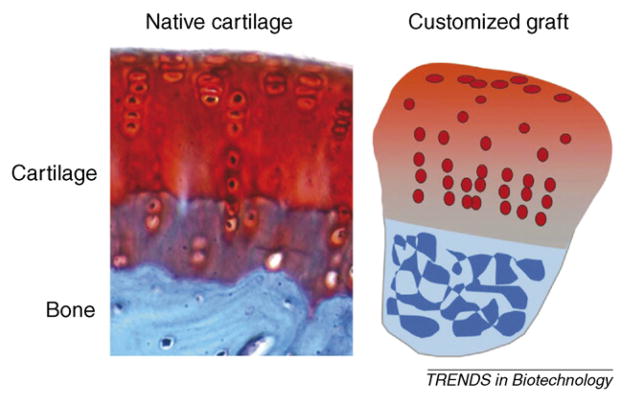
Figure I.
Native cartilage and a customized graft. Image of native tissues reproduced, with permission, from [69].
Clinical problem and current approaches
Degenerative joint diseases, mostly osteoarthritis, are the leading cause of chronic disability in the US, affecting ~20% of the adult population, with one-third of patients experiencing activity limitations and needing surgical intervention [3]. The total direct cost of osteoarthritis is estimated at US$28.6 billion dollars a year. Osteochondral defects resulting from traumatic injuries, osteochondritis dissecans and chondromalacia further contribute to the pool of patients that need surgical treatment. Standard procedures, such as total joint replacement using artificial materials, are generally successful in terms of pain relief and improved function but do not restore the articular cartilage and subchondral bone completely and degenerate over time [4]. Mosaicplasty involves taking osteochondral plugs from a non-load-bearing area of the patient’s own joint (autografts) and transplanting these plugs into the disease site. Both mosaicplasty and the transplantation of autologous chondrocytes (Carticel® procedure) treat focal defects and are limited by incomplete integration, conformity of the articular surface and donor-site morbidity [5,6].
Tissue engineering
Via tissue engineering, scientists aim to provide biological grafts of cartilage and osteochondral tissues for joint repair to help overcome the limitations of standard procedures. The promise of tissue engineering is rooted in the fact that engineered grafts can interact with and remodel their environment while providing structural and mechanical functionality and integration with the host. For cartilage, tissue engineering can involve cell seeding into a scaffold and cultivation of the resulting construct under conditions that promote cartilage formation (reviewed in Ref. [7]).
Young bovine chondrocytes have been used extensively to optimize the scaffold properties, culture conditions and duration of cultivation [8,9]. Lima and coworkers combined transient growth factor supplementation and mechanical stimulation to generate constructs that rapidly attained the mechanical properties of native articular cartilage (compressive moduli of up to 800 kPa within only five weeks of cultivation) [8]. At this time, we do not know ‘how much is enough’ and whether engineered cartilage needs to mimic the mechanical properties of native cartilage at the time of implantation. However, it is essential to have the ability to grow tissue constructs with mechanical functionality. In addition, engineered cartilage also needs to integrate with the host, and the intrinsic capacity of cartilage for integration is poor. One approach is to utilize osteochondral constructs and take advantage of the well-known healing ability of bone by using it as an anchor for graft fixation. Furthermore, for large anatomically correct grafts with shapes exactly matching those of the patient’s joint, the bone region is crucial for attachment and mechanical support. To engineer such constructs, the cartilage and bone components can either be generated independently in vitro and combined (e.g. sutured or glued) or fabricated as a single composite graft [10].
Custom design
Instead of repairing localized defects, custom-designed grafts could be engineered to replace whole regions of a joint and mimic the native tissues with respect to anatomical shape and structural and mechanical properties. In essence, this biomimetic approach aims at developing a biological alternative to artificial joint replacement therapy, with a focus on tissue functionality [11]. These grafts could be designed to provide immediate load bearing and structural support, as well as long-term regeneration (maturation, integration, remodeling; see Figure 1). Customization involves the determination of the cell source, scaffold material and culturing system.
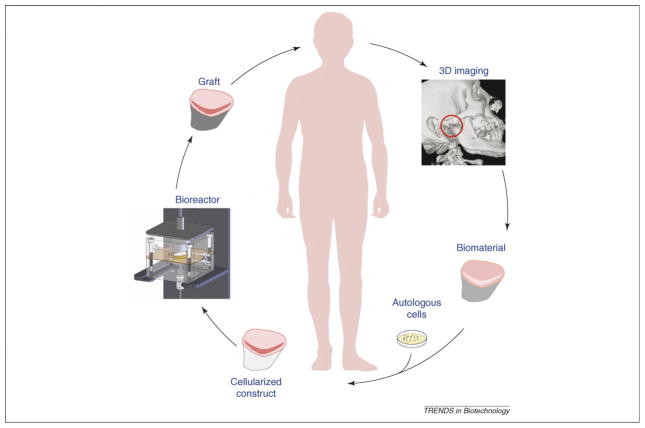
Figure 1.
Key steps in custom-designing osteochondral grafts. One approach to replace terminally damaged joint tissues is to use custom-designed grafts with high structural and functional fidelity for a large region or an entire joint surface. The example shown here is for engineering the temporomandibular joint (TMJ) condyle, the only articulating joint in the head, but the same approach could, in theory, be extended to other joints in the body. A clinical image is used first to obtain the exact graft geometry for the manufacture of an anatomically shaped biomaterial scaffold and culturing system. Cells are obtained from the patient and expanded in culture to form a cellularized construct. Scaffold seeded with cells is cultured in a bioreactor capable of providing the multi-parametric signals needed for graft development and maturation. Upon achieving targeted properties, the graft is implanted into the patient, where it will support the joint function and remodel, integrate and mature.
The cells need to be available in large numbers (or be readily expandable) and have a capacity for expressing cartilage and bone phenotype. To match the patient’s immunological makeup, an autologous cell source would be the best choice. Carticel® from Genzyme (Cambridge, MA; http://www.carticel.com/default.aspx) employs the patient’s own chondrocytes, expands them in culture and implants the cells into the joint defect under a sutured periosteal flap [5]. Mesenchymal stem cells (hMSCs) derived from an aspirate of the patient’s bone marrow or fat are additional examples of suitable cell sources [12,13].
The scaffold needs to be biocompatible, have sufficient mechanical integrity to sustain habitual loading and have a controllable degradation rate. To induce the formation of a stratified tissue, it can be beneficial to design scaffolds with stratified molecular, structural and mechanical properties, for example by using composites. The scaffold material can also be functionalized with attached or encapsulated growth factors to enhance tissue development.
The size and shape of the scaffold should match the anatomy of the specific implantation site. To maintain and promote construct maturation, especially for anatomically shaped constructs of a large size, a bioreactor is necessary to provide efficient exchange of nutrients and metabolites. The bioreactor needs to be customized to support the cultivation of anatomically shaped grafts and provide gradients of physical and molecular cues controlling the spatial and temporal patterns of cell differentiation and assembly [14,15].
Cell sources
To avoid risks of immune rejection and infectious disease transmission, tissue grafts would ideally be prepared from the patient’s own (autologous) cells. To minimize the damage caused by biopsy, a small quantity of tissue from a single source would be used, and the cells would be expanded in vitro and induced towards appropriate phenotypes. One possibility includes the use of hMSCs, which can form several types of tissues, including bone and cartilage [13]. Thanks to their ability to adhere and grow on tissue culture plastic, hMSCs can be isolated from an aspirate of marrow and further purified by immunoselection [16]. The numbers of isolated stem cells vary between patients and aspirate preparations and tend to decline with the patient age. Investigations into culture conditions have indicated that the choice of the cell attachment substrate [17] and the supplementation of growth factors to the culture medium [18] can help maintain the cell differentiation potential during expansion in culture.
Studies of cell populations from different patients indicate common expression patterns of surface antigens, such as CD44, CD71, CD90 and CD105, with low expression levels of hematopoetic and endothelial lineage markers [19,20]. As there is no definitive set of markers that identify a stem cell, the differentiation potential is commonly determined using in vitro differentiation models. A frequently used model is the pellet culture, where aggregates of densely packed hMSCs are induced to differentiate by adding either osteogenic factors (dexamethasone, L-ascorbic acid, β-glycerophosphate) or chondrogenic factors (dexamethasone, transforming growth factor-β) to the culture medium [19]. To engineer tissue constructs, hMSCs were cultured on scaffolds and provided with enhanced mass transport, molecular and physical stimulating factors by bioreactors [21–23].
In recent years, adipose tissue has been extensively studied as an alternative, easily accessible and abundant source of multipotent human adult stem cells (hASCs) [24]. Surface antigen patterns expressed by hASCs resemble those of hMSCs, but the relative differentiation potential of these two cell types and the culture conditions supporting their differentiation are still under investigation [24]. Chondrogenic cells derived from hMSCs, as compared to adult chondrocytes, result in the less complete formation of cartilaginous tissue [25] and tend to undergo hypertrophy and calcification. Use of culture-expanded autologous chondrocytes for engineering cartilage regions of the graft [26] or co-cultures of primary chondrocytes and stem cells [27,28] could represent alternative strategies to improve chondrogenesis and graft properties.
The formation of osteochondral composites depends on our ability to induce and maintain osteogenesis and chondrogenesis in various regions of the scaffold (Figure 2). Quite a few approaches have been tested, including separate induction of cartilage and bone lineages in culture, apposition of scaffolds seeded with cartilage and bone forming cells and in vitro cultivation to promote integration and maturation [29,30]. The medium supplements, scaffold properties and bioreactor culture parameters varied with the cell source and differentiation stage [31].
Figure 2.
Potential of human mesenchymal stem cells to form osteochondral grafts. hMSCs can be isolated from the patient’s tissue, expanded in culture and used to form osteochondral composites. In one notable example, hMSCs were chondrogenically induced in pellets (medium supplemented with dexamethasone, L-ascorbic acid and transforming growth factor-β) and press-coated on porous polylactic blocks [30]. Confluent monolayers of the same cells were osteogenically induced (culture medium supplemented with dexamethasone, L-ascorbic acid and β-glycerophosphate) and seeded into the porous regions of the scaffolds from the opposite side. (a) Osteochondral composites with a 2 mm thick cartilaginous layer (CL) integrated with the underlying osteogenic layer (OL) were formed by cultivation in medium containing both chondrogenic and osteogenic factors. Scale bar represents 1.5 mm. (b) Deposition of cartilaginous matrix (blue glycosaminoglycans stain) was limited to the CL. Arrows indicate the boundary between CL and OL. (c) Mineralized matrix (red stain) was limited to the osteogenic layer. Images were reproduced with permission from [30].
Biomaterial scaffolds
For complex tissue grafts that require stratified structural and mechanical properties, a new generation of biomaterials is helping to reconstruct the anisotropy, nonlinearity and local mechanical properties of the native tissue. One emerging strategy for forming tailored composite scaffolds is to reinforce the hydrogels with encapsulated cells using textile-engineered fiber structures. Fibers can be spun, extruded or electrospun, offering a range of fiber diameters, mechanical features and fiber orientations to meet the tissue-specific needs. The fibers can be combined, wound, braided, woven or twisted to design structures with the desired transport properties, anisotropy and biomechanics. After this step, the fiber system can be embedded within a hydrogel or further modified by cross-linking to alter the mechanical properties, adjust degradation or enhance handling. These scaffolds were inspired by the architectures of native tissues. Examples include fiber composites for the reconstruction of ligaments, cartilage [32] and intervertebral discs [33] (Figure 3a–c).
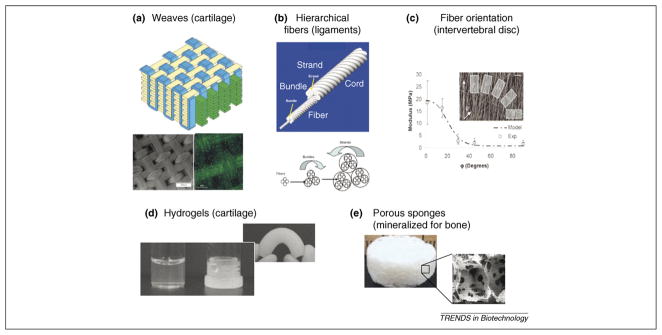
Figure 3.
Scaffold design: composite biomaterials for complex grafts. (a) Composite scaffold for cartilage tissue engineering made by microscale weaving of polycaprolactone fibers. Top: a schematic of the weave; bottom left: surface view of the weave by scanning electron microscopy (scale bar represents 500 μm); bottom right: fluorescence image of articular chondrocyte seeded weave within a 2% agarose gel, labeled with calcein-AM (scale bar represents 100 μm) (reproduced, with permission, from [32]). (b) Scaffolds for ligament and tendon engineering that were formed through the use of silk fibroin yarns in hierarchically organized forms of bundles, strands and cords. Top: a schematic of the yarn organization; bottom arrows show the twisting direction of the yarns at each level of organization (reproduced, with permission, from [43]). (c) Electrospun polycaprolactone fibres were used to generate scaffolds for engineering an intervertebral disc. Insert: this shows sections that were cut from mats of electrospun fibers to provide different fiber orientations within the sample for mechanical testing; graph: predictions of mechanical properties (modulus) versus experimental data from the samples of electropsun mats, where the modulus of the material was related to the fiber orientation. These relationships are important in terms of replicating the fiber orientations found in tissue structures, such as the intervertebral disc, to provide sufficient resistance to mechanical compression. (reproduced, with permission, from [33]). (d,e) Examples of key component parts for osteochondral systems. (d) Hydrogels for cartilage – the samples shown were generated from silk fibroin protein by controlling water content during self-assembly into gel states. (e) Porous sponges formed by porogen leaching from silk fibroin after solidification; these sponges are used for bone formation. This system can be pre-mineralized as needed to alter initial mechanical properties.
For bone, mechanically stiff biomaterials with options for medium perfusion and vascularization are required to support cell expansion, as well as the production of bone matrix rich in type I collagen and hydroxyapatite. The structural stability of bone scaffolds and scaffold mineralization are important to avoid premature collapse of the open porous structure and to maintain nutrient transport into the growing bone tissue [34]. Matrix-mediated or exogenous delivery of signaling molecules, such as bone morphogenetic protein-2 (BMP-2), are also important. Porous degradable polymers, including polylactic-co-glycolic acid (PLGA), collagen, polycaprolactone (PCL) and silk fibroin, are among several materials being studied that could meet these requirements.
By contrast, native cartilage matrix consists of a highly hydrated proteoglycan hydrogel embedded into a type II collagen network. It has been known for more than two decades that hydrogels support the spherical shape and normal phenotype of chondrocytes [35]. Based on these early findings, many studies have used hydrogels, such as agarose, alginate, chitosan and photopolymerizing systems, to engineer cartilage tissue [4,36,37]. In a study of human chondrocytes in six different scaffolds, the highest matrix production was recorded in collagen gel [38]. Notably, hydrogel systems yielded constructs with the highest compressive moduli among all scaffolds used: in fact, the moduli were similar to those for native articular cartilage [8].
Silk protein biomaterials are among those actively pursued for osteochondral tissue engineering [39]. Silk protein fibers are the strongest and toughest natural fibers known, providing an excellent starting point for use as scaffolds. Silk processing and self-assembly enable the formation of a range of silk-based scaffolds, including hydrogels, films, conformal coatings, porous matrices, nanoscale fibers and large fibers [40–42]. Importantly, the crystallinity (β sheet content) of silk can be controlled by the mode of processing, thereby providing an ability to modulate mechanical properties and degradation [42–44]. The presence of diverse amino acid side chains facilitates attachment of growth factors that are necessary for topological control of cell differentiation [45]. Controlled mineralization can also be achieved to generate highly porous and stiff matrices suitable for tissue engineering of bone [46]. Together, these features enable the design of silk-based scaffolds in the form of hydrogel for cartilage formation (Figure 3d), porous mineralized scaffold for bone formation (Figure 3e) and composite scaffolds for engineering stratified osteochondral tissues.
For any designed scaffold system, integration between the cartilaginous and osseous parts and integration with native tissue are critical [39]. Integration can be approached via suturing, by cell-mediated ECM formation and by the use of fibrin and other glues. The biomaterial degradation also needs to be tailored to match the rate of tissue formation so that the development of new tissue is balanced with the degradation of the scaffold; this issue has not been explored in depth to date.
Anatomically shaped osteochondral grafts
For large osteochondral grafts replacing whole sections of the joint, it is important that the geometry of the joint is faithfully reconstructed to maintain the proper joint mechanics during articulation with adjacent surfaces. The temporomandibular joint (TMJ) has been a primary target for tissue-engineering applications because of the prevalence of TMJ disorders resulting from trauma, tumors, stiffening or fusion of the joint. Also, the small size of the TMJ minimizes difficulties of nutrient transport to cells, as compared to other, larger joints. One early study of a tissue-engineered TMJ utilized mature bovine chondrocytes and osteoblasts on a polymeric scaffold molded into the shape of a human mandibular condyle [10]. Scaffolds seeded with cells were placed subcutaneously (under the skin) in nude mice. This is not a load-bearing region and acts effectively as an in vivo bioreactor, providing nutrients and environmental control to the cells in the constructs. After 12 weeks, the explanted constructs contained discrete layers of cartilage and bone. This pioneering study highlighted the maintenance of specific osteogenic and chondrogenic zones in the resulting constructs and suggested the feasibility of a cell-based therapeutic approach.
This technique was further advanced by generating the osteogenic and chondrogenic regions from a single cell source [47–49]. Bone-marrow-derived rat mesenchymal stem cells (rMSCs) were isolated and induced into chondrogenic and osteogenic lineages during monolayer expansion. To obtain spatial resolution between osteo-induced and chondro-induced cells in the construct, the pre-differentiated rMSCs were then encapsulated into separate photo-polymerized hydrogels, and the composite constructs were again ‘cultivated’ in nude mice for 12 weeks. Upon removal from the mice, it was seen that, in addition to maintaining the overall anatomical morphology, the grafts demonstrated stratification and integration between the engineered bone and cartilage regions.
Because condyle geometry is patient specific, a therapeutic approach would necessarily utilize clinical imaging techniques to reconstruct its precise architecture. An image-based, micro-printing approach was used to design scaffolds with the required external and internal geometries of the TMJ [50]. Images obtained from computerized tomography (CT) scans of a pig mandible were digitized and used to design scaffolds with the overall morphology and specific internal architecture of the TMJ. This technique, known as solid free-form fabrication, lends itself to the formation of ‘designer scaffolds’ [51]. The method has also been extended to obtain biphasic constructs for osteochondral grafts [52]. A major advantage is that scaffolds could be generated that have desired mechanical properties, and ongoing research addresses the use of such scaffolds in a functional, load-bearing in vivo model of a pig, with promising initial results [53].
Bioreactors for anatomically shaped grafts
If large, anatomically shaped grafts are to be engineered to replace whole sections of the joint, a challenge is to develop appropriate in vitro culture systems for the maturation of the grafts before their in situ implantation. Bioreactor systems can be designed to control transport of nutrients and oxygen to cells in clinically sized constructs and provide lineage-specific biological stimuli in various regions of the graft [12]. Additionally, the development of functional, load-bearing characteristics of the graft would be enhanced by the application of biophysical stimulation to attain mechanical competence in both the cartilage and bone regions. For cartilage, dynamic compression can be applied to stimulate increased matrix organization by the cells [7]. For bone, medium perfusion can be applied to enable local control of mass transport and provide shear-stress-enhancing in vitro bone formation [54]. This is technically challenging for anatomically shaped constructs. Our laboratory has begun studies to generate mechanically competent, biological grafts derived from decellularized trabecular bone (Figure 4c) and porous mineralized scaffolds that faithfully recapitulate the anatomical shapes obtained from clinical images down to micrometer-scale resolution [12]. These scaffolds have been seeded with hMSCs and cultured to engineer bone via systems with medium perfusion through the constructs. Initial studies have shown that medium perfusion improved cell distribution and viability and promoted bone formation.
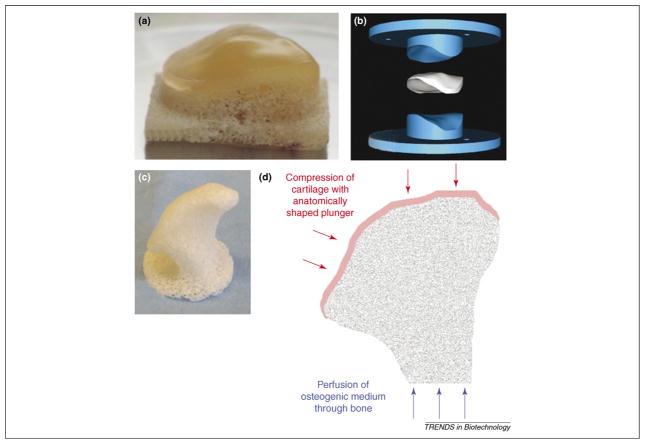
Figure 4.
Engineering anatomically shaped tissue grafts. (a) Anatomically shaped osteochondral patella formed with a cartilage layer over unseeded trabecular bone substrate (reproduced, with permission, from [55]). (b) Digital image of a patella formed by using a two-part mold. The upper part has the ‘negative’ shape of the patella and the lower part has a ‘positive’ geometry. The spacing between these parts can be used to control the thickness of the cartilage layer (shown in white) (reproduced, with permission, from [55]). (c) Precisely shaped scaffold machined from decellularized trabecular bone using digital imaging data for a human temporomandibular joint (TMJ). (d) Schematic of the bioreactor with spatial control of lineage-specific stimuli to an anatomically shaped graft with cartilage and bone regions interpenetrating to form an ‘interface’. The system can provide dynamic loading of the cartilage layer (red arrows) and shear stress (via medium perfusion) to the bone region (blue arrows).
Bioreactor systems have also been developed to aid the cultivation of an anatomically shaped tissue-engineered patella [55]. Data for the patella geometry was obtained via stereophotogrammetry and used to drive a milling machine by means of computer-aided design (CAD) software. A positive and a negative mold of the patella were fabricated and positioned at a distance from each other to form a space for the formation of the cartilage layer of specific thickness in the osteochondral graft (Figure 4a,b). Mature chondrocytes were loaded into the cartilage layer but the bony substrate (also milled into the exact anatomical shape) remained acellular. Using this method, it was also possible to produce anatomically shaped loading platens for dynamic compression of the cartilage region during cultivation [7]. Dynamic compression has been shown to improve the mechanical properties of tissue-engineered cartilage grafts [56] and, for the anatomically-shaped patella, it also effectively increased nutrient transfer to regions within the constructs via compression-induced fluid flow.
Ideally, a bioreactor system should be capable of coordinating biological, physiological and mechanical stimuli and applying them in a spatially and temporally controlled manner to provide lineage-specific stimulation within the cartilage and bone regions. This includes the use of chondro-inductive growth factors combined with dynamic loading to the cartilage regions of the graft and osteo-inductive factors combined with medium perfusion (for nutrient transfer and shear stress) in the bone phase of the graft (Figure 4d). To stimulate osteochondral integration, the combined stimuli should be applied while the bone and cartilage phases are being grown in apposition. Clinically applicable custom-designed grafts will probably require the use of multi-parametric culture systems to ensure proper development of hierarchically organized and functional tissue grafts.
Summary and future directions
The clinical and scientific utility of tissue engineering depends on our ability to predictably direct cells to express the desired phenotype and thus generate functional tissues. Tissue engineering strives to recreate the native environment within the cell–scaffold–bioreactor system to promote the appropriate cell behavior. We discussed the engineering of osteochondral tissue grafts that are customized with respect to their component cells, shape and internal architecture, as well as the engineering of functional properties to resemble the hierarchy, anatomical shape and loading function of the native tissue. Our focus was on in vitro systems for engineering cell-based constructs that could provide a biological alternative to artificial joint replacement therapy and serve as a high fidelity model for controlled studies of development and disease. Toward this goal, living cells have been used in conjunction with biomaterial scaffolds (hydrogels for the cartilage region, porous mineralized matrix for the bone region) and bioreactors (environmental control, compressive loading for cartilage, perfusion for bone) to generate stratified grafts. Although studies conducted thus far have given promising results, much more needs to be done to better understand the regulation of graft development and maturation and to address important practical issues related to the engineering of clinically useful grafts.
Human cell source is one of the outstanding issues across all tissue engineering. hMSCs have advantages because of their availability and multipotency, but the effects of donor age and health are still not well understood [57], and these cells have less capacity to form cartilage than chondrocytes [58]. By contrast, harvesting of adult autologous articular chondrocytes involves joint biopsy, and the cells tend to dedifferentiate during expansion [35,59]. Approaches currently being studied include growth factor supplementation during cell expansion and culture [60,61], use of additional cell sources (such as nasal, ear and rib cartilage) [62], adipose-derived hMSCs [24] and cell co-culture [27,63]. Several products have utilized autologous cells in a synthetic matrix for focal cartilage repair, such as Bioseed®C (http://www.biotissue-tec.com/index.php?idcat=122) and Hyalograft® C (http://www.fidiapharma.com/files/index.cfm?id_rst=104&id_elm=31) [64]. Cell-free materials impregnated with growth factors are also under consideration for treating local defects [65,66]. The standardization of cell sourcing is also being addressed as one of the requirements for translation into clinical applications.
The design of tissue engineering systems for cartilage, bone and osteochondral grafts is largely determined by the need to restore ‘the ability of articular cartilage to provide a painless, low-friction gliding surface and to distribute loads across a synovial joint’ over a long term [2]. This translates into the requirement for a certain set of mechanical properties of cultured grafts at the time of implantation (to provide immediate load bearing at some level) and the capacity for integration and remodeling (to provide long-term function) [11,67]. It remains to be determined which exact criteria (molecular, structural and/or functional) predictably determine the clinical utility of engineered grafts, as well as ‘how much is enough’ – how closely the engineered graft needs to resemble the native tissue and which properties are more important than the others. To answer these questions, we are increasingly turning toward the use of engineered grafts themselves as tools for studies of development, disease and remodeling. Engineered osteochondral grafts (or cartilage and bone alone) can serve as high fidelity models for studying cartilage and bone development and repair. The control of environmental conditions provided through the design of scaffolds and bioreactors and the choice of cells can offer us more insight into the interplay of the molecular and physical factors that guide the development of bone, cartilage and their interface. Our understanding of the developmental process might then serve as a feedback for the optimization of engineering parameters toward better graft designs. These current developments might in fact help us reach the ultimate goal of tissue engineering – to overcome the failure of our tissues and organs.
Acknowledgments
Tissue engineering work in our laboratories has been supported by the National Institutes of Health (DE16525–01, HL076485 to G.V.N., EB002520 to G.V.N. and D.L.K.) and fellowships from the Arthritis Foundation (P.G.C.), Mandl Foundation (W.L.G.) and New York Stem Cell Foundation (D.M.).
References
- 1.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Lohmander S. Operative treatment of osteoarthrosis. Current practice and future development. J Bone Joint Surg Am. 1994;76:1405–1418. doi: 10.2106/00004623-199409000-00019. [DOI] [PubMed] [Google Scholar]
- 3.CDC (Center for Disease Control and Prevention) Targeting Arthritis: Reducing Disability for Nearly 19 Million Americans. US Department of Health and Human Services, CDC; 2007. ( http://www.cdc.gov/nccdphp/publications/aag/arthritis.htm) [Google Scholar]
- 4.Elisseeff J, et al. Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res. 2005;8:150–161. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 5.Brittberg M, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 6.Tuan RS. A second-generation autologous chondrocyte implantation approach to the treatment of focal articular cartilage defects. Arthritis Res Ther. 2007;9:109. doi: 10.1186/ar2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung CT, et al. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 8.Lima EG, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-[beta]3. Osteoarthritis Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freed LE, et al. Tissue engineering of cartilage in space. Proc Natl Acad Sci U S A. 1997;94:13885–13890. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng Y, et al. Tissue-engineered composites of bone and cartilage for mandible condylar reconstruction. J Oral Maxillofac Surg. 2001;59:185–190. doi: 10.1053/joms.2001.20491. [DOI] [PubMed] [Google Scholar]
- 11.Butler DL, et al. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 12.Chao P-HG, et al. Engineering cartilage and bone using human mesenchymal stem cells. J Orthop Sci. 2007;12:398–404. doi: 10.1007/s00776-007-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 14.Chang CH, et al. Cartilage tissue engineering on the surface of a novel gelatin-calcium-phosphate biphasic scaffold in a double-chamber bioreactor. J Biomed Mater Res B Appl Biomater. 2004;71:313–321. doi: 10.1002/jbm.b.30090. [DOI] [PubMed] [Google Scholar]
- 15.Lima EG, et al. Functional tissue engineering of free-swelling and dynamically loaded osteochondral constructs. Transactions of the Annual Meeting of the Orthopaedic Research Society; San Francisco. 2004. Paper number 0013. [Google Scholar]
- 16.Bianco P, et al. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 17.Mauney JR, et al. Matrix-mediated retention of osteogenic differentiation potential by human adult bone marrow stromal cells during ex vivo expansion. Biomaterials. 2004;25:3233–3243. doi: 10.1016/j.biomaterials.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Solchaga LA, et al. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 19.Meinel L, et al. Bone tissue engineering using human mesenchymal stem cells: Effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 20.Barrilleaux B, et al. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007–3019. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 21.Mauck RL, et al. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113–125. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 22.Marolt D, et al. Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials. 2006;27:6138–6149. doi: 10.1016/j.biomaterials.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Sikavitsas VI, et al. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A. 2003;100:14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gimble JM, et al. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauck RL, et al. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Redman SN, et al. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32. doi: 10.22203/ecm.v009a04. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks J, et al. A powerful tool in cartilage tissue engineering: coculturing primary chondrocytes with expanded chondrocytes enhances chondrogenesis. Transactions of the Annual Meeting of the Orthopaedic Research Society; Washington. 2005. Paper number 1792. [Google Scholar]
- 28.Ahmed N, et al. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem. 2007;20:665–678. doi: 10.1159/000107728. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer D, et al. In vitro generation of osteochondral composites. Biomaterials. 2000;21:2599–2606. doi: 10.1016/s0142-9612(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 30.Tuli R, et al. Human mesenchymal progenitor cell-based tissue engineering of a single-unit osteochondral construct. Tissue Eng. 2004;10:1169–1179. doi: 10.1089/ten.2004.10.1169. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoudifar N, Doran PM. Tissue engineering of human cartilage and osteochondral composites using recirculation bioreactors. Biomaterials. 2005;26:7012–7024. doi: 10.1016/j.biomaterials.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 32.Moutos FT, et al. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007;6:162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 33.Nerurkar NL, et al. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 34.Meinel L, et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A. 2004;71:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- 35.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 36.Awad HA, et al. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 37.Gleghorn J, et al. Adhesive properties of laminated alginate gels for tissue engineering of layered structures. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31565. ( http://www3.interscience.wiley.com/journal/30728/home) [DOI] [PubMed]
- 38.Gavenis K, et al. In vitro comparison of six different matrix systems for the cultivation of human chondrocytes. In Vitro Cell Dev Biol Anim. 2006;42:159–167. doi: 10.1290/0511079.1. [DOI] [PubMed] [Google Scholar]
- 39.Altman GH, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, et al. Biomaterial coatings by stepwise deposition of silk fibroin. Langmuir. 2005;21:11335–11341. doi: 10.1021/la051862m. [DOI] [PubMed] [Google Scholar]
- 41.Nazarov R, et al. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 42.Kim UJ, et al. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5:786–792. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 43.Horan RL, et al. Yarn design for functional tissue engineering. J Biomech. 2006;39:2232–2240. doi: 10.1016/j.jbiomech.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Jin HJ, et al. Water-stable silk films with reduced beta-sheet content. Adv Funct Mater. 2005;15:1241–1247. [Google Scholar]
- 45.Karageorgiou V, et al. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J Biomed Mater Res A. 2006;78:324–334. doi: 10.1002/jbm.a.30728. [DOI] [PubMed] [Google Scholar]
- 46.Li C, et al. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951–956. doi: 10.1177/154405910308201203. [DOI] [PubMed] [Google Scholar]
- 48.Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87:936–944. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 49.Alhadlaq A, et al. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–923. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 50.Feinberg SE, et al. Image-based biomimetic approach to reconstruction of the temporomandibular joint. Cells Tissues Organs. 2001;169:309–321. doi: 10.1159/000047896. [DOI] [PubMed] [Google Scholar]
- 51.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 52.Schek RM, et al. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Eng. 2004;10:1376–1385. doi: 10.1089/ten.2004.10.1376. [DOI] [PubMed] [Google Scholar]
- 53.Mao JJ, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holtorf HL, et al. Flow perfusion culture of marrow stromal cells seeded on porous biphasic calcium phosphate ceramics. Ann Biomed Eng. 2005;33:1238–1248. doi: 10.1007/s10439-005-5536-y. [DOI] [PubMed] [Google Scholar]
- 55.Hung CT, et al. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853–1864. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 56.Mauck RL, et al. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 57.Stenderup K, et al. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Mauck RL, et al. Cartilage tissue engineering with MSC-laden hydrogels: effect of seeding density, exposure to chondrogenic medium, and dynamic loading. Transactions of the Annual Meeting of the Orthopaedic Research Society; Chicago. 2006. Paper number 0336. [Google Scholar]
- 59.Binette F, et al. Expression of a stable articular cartilage phenotype without evidence of hypertrophy by adult human articular chondrocytes in vitro. J Orthop Res. 1998;16:207–216. doi: 10.1002/jor.1100160208. [DOI] [PubMed] [Google Scholar]
- 60.Jakob M, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 61.Martin I, et al. Osteochondral tissue engineering. J Biomech. 2007;40:750–765. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Indrawattana N, et al. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 63.Pound JC, et al. Strategies to promote chondrogenesis and osteogenesis from human bone marrow cells and articular chondrocytes encapsulated in polysaccharide templates. Tissue Eng. 2006;12:2789–2799. doi: 10.1089/ten.2006.12.2789. [DOI] [PubMed] [Google Scholar]
- 64.Tognana E, et al. Adjacent tissues (cartilage, bone) affect the functional integration of engineered calf cartilage in vitro. Osteoarthritis Cartilage. 2005;13:129–138. doi: 10.1016/j.joca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Huang X, et al. Osteochondral repair using the combination of fibroblast growth factor and amorphous calcium phosphate/poly(l-lactic acid) hybrid materials. Biomaterials. 2007;28:3091–3100. doi: 10.1016/j.biomaterials.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Fukuda A, et al. Enhanced repair of large osteochondral defects using a combination of artificial cartilage and basic fibroblast growth factor. Biomaterials. 2005;26:4301–4308. doi: 10.1016/j.biomaterials.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Vunjak-Novakovic G, Goldstein S. Biomechanical principles of cartilage and bone tissue engineering. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechanobiology. Lippincot-Williams and Wilkens; 2005. pp. 343–408. [Google Scholar]
- 68.White A, Wallis G. Endochondral ossification: a delicate balance between growth and mineralization. Curr Biol. 2001;11:R589–R591. doi: 10.1016/s0960-9822(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 69.Saadat E, et al. Long-term cyclical in vivo loading increases cartilage proteoglycan content in a spatially specific manner: an infrared microspectroscopic imaging and polarized light microscopy study. Arthritis Res Ther. 2006;8:R147. doi: 10.1186/ar2040. [DOI] [PMC free article] [PubMed] [Google Scholar]